Introduction
Gastric carcinoma is one of the most common
gastrointestinal malignancies worldwide. Gastric cancer has been
reported as the fourth leading cause of cancer-associated morbidity
and the third most common cause of cancer-associated mortality
worldwide in 2012, as it accounts for 8.8% of all cancer death
cases (1). Eastern Asia, including
Japan, Korea and China, is a geographical region with one of the
highest incidence rates of gastric cancer, accounting for ~2/3
gastric cancer cases worldwide (2).
Integrins are a group of transmembrane receptors
that mediate adhesion between cells and components of the
extracellular matrix. Integrins have been reported to interact with
focal adhesion kinase (FAK), leading to the activation of
p130Crk-associated substrate and paxillin-mediated signaling
pathways to modulate the expression of the extracellular
signal-regulated kinase (ERK) gene. This leads to modulation of
tumor angiogenesis, invasion, metastasis and apoptosis (3–5). In
addition, integrins have been demonstrated to be highly expressed
in several types of malignant tumors, including gastric and
squamous cell carcinoma, and melanoma (6,7). The
ERK intracellular signal transduction pathway has been implicated
in numerous cellular processes. The ERK signaling cascade
communicates extracellular signals from surface receptors to the
cell nucleus; a process which has been reported to regulate various
cellular functions, including cell growth, development,
differentiation, division and death (8,9).
Phosphorylated (p)-ERK is the active form of ERK (10). ERK has been demonstrated to inhibit
apoptosis through activation of p90 ribosomal s6 kinase (11,12).
In addition, p-ERK promotes cellular proliferation by activating
various transcription factors, including CCAAT-enhancer-binding
protein, Elk-1, c-Jun, c-Myc and c-Fos, which promote cell cycle
progression from the G1 to the S phase, thereby
facilitating malignant tumor growth (13). Furthermore, the activation of
ERK1/2 has been reported to enhance the protein expression of
matrix metalloproteinase (MMP)-2 and MMP-9, which degrade the
basement membrane barrier (14),
and thus enhance epithelial-to-mesenchymal transition to promote
tumor cell invasion and metastasis (15).
Positron emission tomography (PET) is one of the
most common non-invasive molecular imaging methods that is used to
observe tumor development in vivo, through the observation
of metabolic activity. Small animal PET scans (MicroPET) have been
designed to study human diseases using in vivo experimental
animal models (16). MicroPET
allows continuous longitudinal monitoring of the same animal, and
the collection of data in real-time. In addition, it may be used
for the non-invasive, dynamic and quantitative observation of
physiological and pathological alterations in vivo, thus
facilitating the study of disease pathogenesis and the evaluation
of drug efficacy (17,18). The 18F-labeled
Arg-Gly-Asp (RGD) peptide has been demonstrated to specifically
target the vitronectin receptor αvβ3, a
member of the integrin superfamily (19). Targeting the vitronectin
αvβ3 integrin receptor could be used as a
tool to visualize and quantify integrin αvβ3
expression levels, thus facilitating observation of the
distribution of integrin αvβ3 in the body
using MicroPET.
WD-3 also known as Weitiao No. 3 is a formula
developed by Professor Jingfang Zhao in 1997 (20). Previous studies have suggested that
treatment with WD-3 may improve the quality of life in patients
with advanced colon cancer and gastric carcinoma. Among patients
with advanced gastric cancer receiving treatment with WD-3, the
disease control rate (88.16%) and the 3-year overall survival rate
(61.18%) were significantly improved when compared with untreated
patients (20,21). However, the molecular mechanisms
underlying the inhibitory effects of WD-3 on tumor cells have yet
to be elucidated. The present study aimed to investigate the
putative effects of WD-3 on vitronectin receptor
αvβ3 in a nude mouse-human gastric cancer
xenograft model using 18F-RGD PET/computerized
tomography (CT). Immunohistochemistry was performed to examine the
effects of WD-3 administration on p-ERK1/2 protein expression, and
thus the implication of the integrin
αvβ3/FAK/mitogen-activated protein kinase/ERK
signaling pathway in the mechanism of action of WD-3.
Materials and methods
Tumor cell line and animals
A total of 24 male BALB/c nude mice (age, 6 weeks;
weight, 18–24 g) were purchased from Shanghai SLAC Laboratory
Animal Co., Ltd. [Shanghai, China; certificate no. SCXK (Shanghai)
2012-0002] and maintained in a specific pathogen-free animal
facility. They were housed with free access to water and food in a
specific pathogen-free facility under a 12-h light/dark cycle at
50±10% humidity and 21±2°C. The SGC-7901 human gastric cancer cell
line was purchased from the Chinese Academy of Sciences Cell
Bank.
The experimental protocols used in the present study
were approved by the Ethics Committee of the Jiangsu Institute of
Nuclear Medicine (Wuxi, China). Ether was used for mouse anesthesia
and euthanasia.
Experimental drugs and reagents
WD-3 was composed of 10 g Condonopsis
pilosula root, 10 g Atractylodes macrocephalae, 10 g
Wolfiporia extensa, 10 g Polyporus umbrellatus, 10 g
oryzae germinatus, fructus, 10 g wheat germ, 6 g Pinellia
ternata, 6 g Citrus tangerina-Peel, 30 g Semen
coicis, 10 g Dioscorea polystachya, 10 g poria with
hostwood, 10 g Eriobotrya japonica leaf and 3 g radix
liquiritiae. Raw herbs were provided by the Traditional
Medicine Pharmacy of the Wuxi Traditional Chinese Medicine Hospital
(Wuxi, China). Herbs were placed into a decocting pot with a 5-fold
volume of water and soaked for 30 min. The mixture was boiled over
a strong flame before herbs were decocted over a reduced flame for
30 min and filtered. Any residue was decocted continuously for 30
min with a 5-fold volume of water and filtered. Filtrates were
combined and concentrated into a crude drug solution with a
concentration of 2.85 g/ml and refrigerated at 2–8°C. Albumin-bound
paclitaxel (100 mg aliquots for injection) was purchased from
Abraxis BioScience, Inc. (Los Angeles, CA, USA).
Herbs were placed into a decocting pot with a 5-fold
volume of water and soaked for 30 min. The mixture was boiled over
a strong flame before herbs were decocted over a reduced flame for
30 min and filtered. Any residue was decocted continuously for 30
min with a 5-fold volume of water and filtered. Filtrates were
combined and concentrated into a crude drug solution with a
concentration of 2.85 g/ml and refrigerated at 2–8°C.
Tumor xenografts in nude mice
SGC-7901 human gastric cancer cells were
conventionally cultured (RPMl-1640 nutrient solution containing 100
IU/ml penicillin and 100 µg/ml streptomycin (HyClone Laboratories,
Inc., Logan, UT, USA), cultivated with 5% CO2 at 37°C),
and the cell concentration was adjusted to 1×107
cells/ml and cells were resuspended in phosphate-buffered saline.
Under aseptic conditions, SGC-7901 cells (~2×106
cells/mouse) were implanted subcutaneously into the right forelimb
of nude BALB/c mice. After 7 days, tumors that grew to a measurable
size with a tumor diameter of ~0.5 cm were selected to establish
the transplanted tumor model, and were randomly assigned into the
following 4 groups (n=6 mice/group): Control group (CG), Chinese
medicine group (CMG), Western medicine group (WMG) and Chinese and
Western medicine combination group (WMG + CMG). Mice in the CG
received daily intragastric injections of 0.5 ml saline; mice in
the CMG received daily intragastric injections of 0.5 ml WD-3
(containing 2.85 g/ml crude drug); mice in the WMG + CMG received
daily intragastric injections of 0.5 ml WD-3, and intravenously
administered with albumin-bound paclitaxel (25 mg/kg) via the tail
vein on days 0, 2 and 4; mice in the WMG received intravenous
injections of albumin-bound paclitaxel (25 mg/kg) on days 0, 2 and
4. The duration of treatment was 30 days.
Tumor assessment
Tumor growth was assessed using standard caliper
measurement twice a week for 4 weeks. The following formula was
used to calculate tumor volume: Tumor volume
(mm3)=(tumor length × width × height)/2, with all
measurements in mm. Following 30 days of treatment, mice were
sacrificed and tumor xenografts were harvested. The rate of tumor
growth inhibition was calculated following the evaluation of tumor
weight, according to the following formula: Tumor inhibition rate
(%)=[(tumor weight (g) in the control group-tumor weight (g) in
treatment group)/tumor weight (g) in the control group] ×100.
MicroPET was used to assess tumor
growth
18F-RGD PET scans were performed prior to
the initiation of drug treatment (day 0) and at 3, 7, 18 and 24
days post-drug administration. The MicroPET protocol was as
follows: 1 h prior to scanning, a single tracer 18F-RGD
injection of 100±20 µCi (100–200 µl; Jiangsu Institute of Nuclear
Medicine, Jiangsu, China) was intravenously administered via the
lateral tail vein. Mice were not required to fast and were
administered with drugs while conscious. Normal eating continued
following drug administration. A total of 1 h following
administration, 10-min static MicroPET was performed. PET scans and
image analysis were performed using an Inveonmicro PET (Siemens
Healthineers, Erlangen, Germany).
MicroPET data processing
Scans were reconstructed using Inveon Acquisition
Workplace software (version 1.4; Siemens Healthineers), using a
three-dimensional ordered-subset expectation maximization/maximum a
posteriori algorithm with the following parameters: matrix,
128×128×159; pixel size, 0.86×0.86×0.8 mm, and β-value, 1.5, with
uniform resolution. Acquisition time, 10 min; acquisition energy
window, 350–650 keV. The Micro PET Analysis software (version ASI
Pro 6.7.1.1; Siemens AG, Munich, Germany) was used to outline the
brain, heart, liver, kidney and tumor tissue as the regions of
interest (ROIS). The mean uptake value of radioactive material (PET
units/g) of the region of interest was obtained. The radioactivity
concentration (accumulation) within a tumor or an organ was
obtained from mean pixel values within the multiple ROI volume,
which had been converted to MBq/ml/min using a conversion factor.
The conversion to MBq/g/min assumed a tissue density of 1 g/ml.
Imaging ROI-derived %ID/g was calculated by dividing the ROIs by
the administered activity injected dose per gram.
p-ERK1/2 protein expression
Following 30 days of treatment, mice were
sacrificed, and tumor tissue samples were harvested and fixed in 4%
paraformaldehyde for 24 h at 25°C. Immunohistochemistry was used to
detect the expression of p-ERK1/2 protein. Tissue sections (4-µm)
were prepared from 10% formalin-fixed (for 24 h at 25°C) and
paraffin-embedded tissues. Following deparaffinization and
rehydration with ethanol (70–100%), the slides were heated to 100°C
in 10 mmol/l sodium citrate buffer (pH, 6) for 15 min to for
antigen retrieval. Endogenous peroxidase activity was blocked by
incubating at 25°C with 0.6% H2O2 in methanol
for 20 min. Sections were subsequently blocked with 10% normal
horse serum (Wuhan Boster Biological Technology, Ltd., Wuhan,
China) for 5 min at 25°C. Following blocking, sections were
incubated with the Rabbit monoclonal anti-p-ERK1 and p-ERK2
(1:1,000; cat. no. sc-20147; Santa Cruz Biotechnology, Inc.),
Sections were incubated with primary antibodies at room temperature
for 2 h. The slides were incubated with streptavidin-horseradish
peroxidase conjugated biotinylated secondary antibodies Biotin Goat
Anti-Rabbit immunoglobulin G (IgG; 1:2,000; cat. no. K4009; Dako;
Agilent Technologies, Inc.) for 30 min at room temperature.
Following incubation, an avidin/strepavidin complex (Dako; Agilent
Technologies, Inc.) was added. A non-specific staining blocker
(GeneTex Biotechnology Co., Ltd., Shanghai, China) and
enzyme-labeled sheep anti-rabbit IgG polymer reagent (GeneTex
Biotechnology Co., Ltd.,) were added according to the
manufacturer's protocol. The antigen detection was conducted via a
color reaction with 3,3′-diaminobenzidine (Dako; Agilent
Technologies, Inc.). Sections were counterstained using hematoxylin
(AppliChem Inc., St Louis, MO, USA) and mounted with Aquatex (Merck
KGaA, Darmstadt, Germany). Stained samples were observed under a
light microscope. p-ERK1/2 staining was yellow, brown or tan in
color, primarily localized to the nucleus and partly in the
cytoplasm. For each tissue section, a total of 4 fields of view
were analyzed under an inverted microscope at ×400 magnification,
and 1,500 cells were randomly chosen to counted by 2 independent
blinded investigators (authors 1 and 2) to calculate the percentage
of p-ERK1/2-positive cells by IPP software (Image-Pro Plus version
6.0, Media Cybernetics, Inc., Rockville, MD, USA) and the results
were consistent between the two readings. As previously described
by Watanabe et al (22),
the immunohistochemical semi-quantitative scoring criteria that
were used were as follows: <5% positive cells, negative (0
points); 5–20% positive cells, weakly positive (1 point); 20–50%
positive cells, positive (2 points); 50–75% positive cells, strong
positive (3 points); >75% positive cells, very strong positive
(4 points). Color intensity scoring criteria were as follows: No
color, 0 points; light color, 1 point; medium color, 2 points;
darker color, 3 points; deep color, 4 points. The overall rating
was calculated according to the following formula: Overall
rating=(positive cell percentage score × color intensity score)/4.
A high overall rating suggested that p-ERK1/2 protein expression
was high, whereas a low overall rating suggested low p-ERK1/2
protein expression.
Statistical analysis
Statistical analysis of differences among groups was
assessed by one-way analysis of variance followed by a post hoc
least significant difference (equal variances) or Tamhane's T2 test
(unequal variances) for multiple comparisons. Statistical analysis
was performed using SPSS software (version 15.0; SPSS, Inc.,
Chicago, IL, USA). The measurement data were presented as the mean
± standard deviation of three independent experiments (n=6 per
group). P<0.05 was considered to indicate a statistically
significant difference.
Results
Effects of WD-3 on gastric tumor
volume in vivo
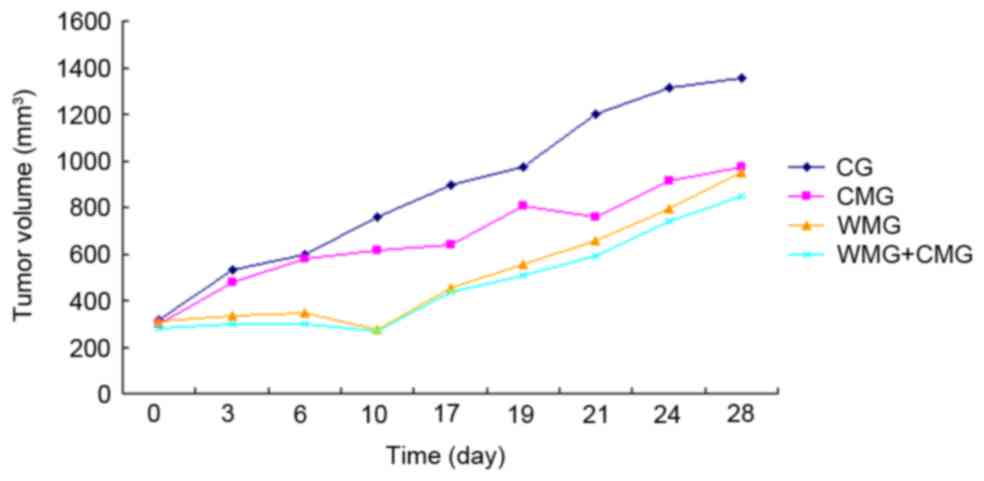
During the initial 10 days following the initiation
of drug treatment, mice in the WMG and CMG + WMG exhibited a
decrease in tumor volume; however, from day 10 onwards, tumor
volumes followed an increasing trend (Fig. 1). Notably, mice in the CMG
exhibited tumor growth rates similar to mice in the CG for the
initial 10 days of treatment; however, from day 15 onwards, tumor
growth in the CMG appeared to slow when compared with the CG
(Fig. 1).
Effects of WD-3 on gastric tumor
weight in vivo
Following 30 days of treatment, tumors were
collected and weighed. The results demonstrated that tumor weight
in the CMG, WMG and CMG + WMG was significantly reduced (P<0.05)
when compared with in the CG (Table
I). In addition, no statistically significant difference in
tumor weight was detected among mice in the CMG, WMG and CMG + WMG
(Table I).
 | Table I.Tumor weight and rate of growth
inhibition. |
Table I.
Tumor weight and rate of growth
inhibition.
| Group | Tumor weight
(g) | Tumor inhibition
rate (%) |
|---|
| CG | 0.83±0.20 | – |
| CMG |
0.72±0.26a | 12.97±1.21 |
| WMG |
0.58±0.41a | 30.61±2.52 |
| WMG + CMG |
0.56±0.23a | 32.71±1.43 |
18F-RGD PET/CT results
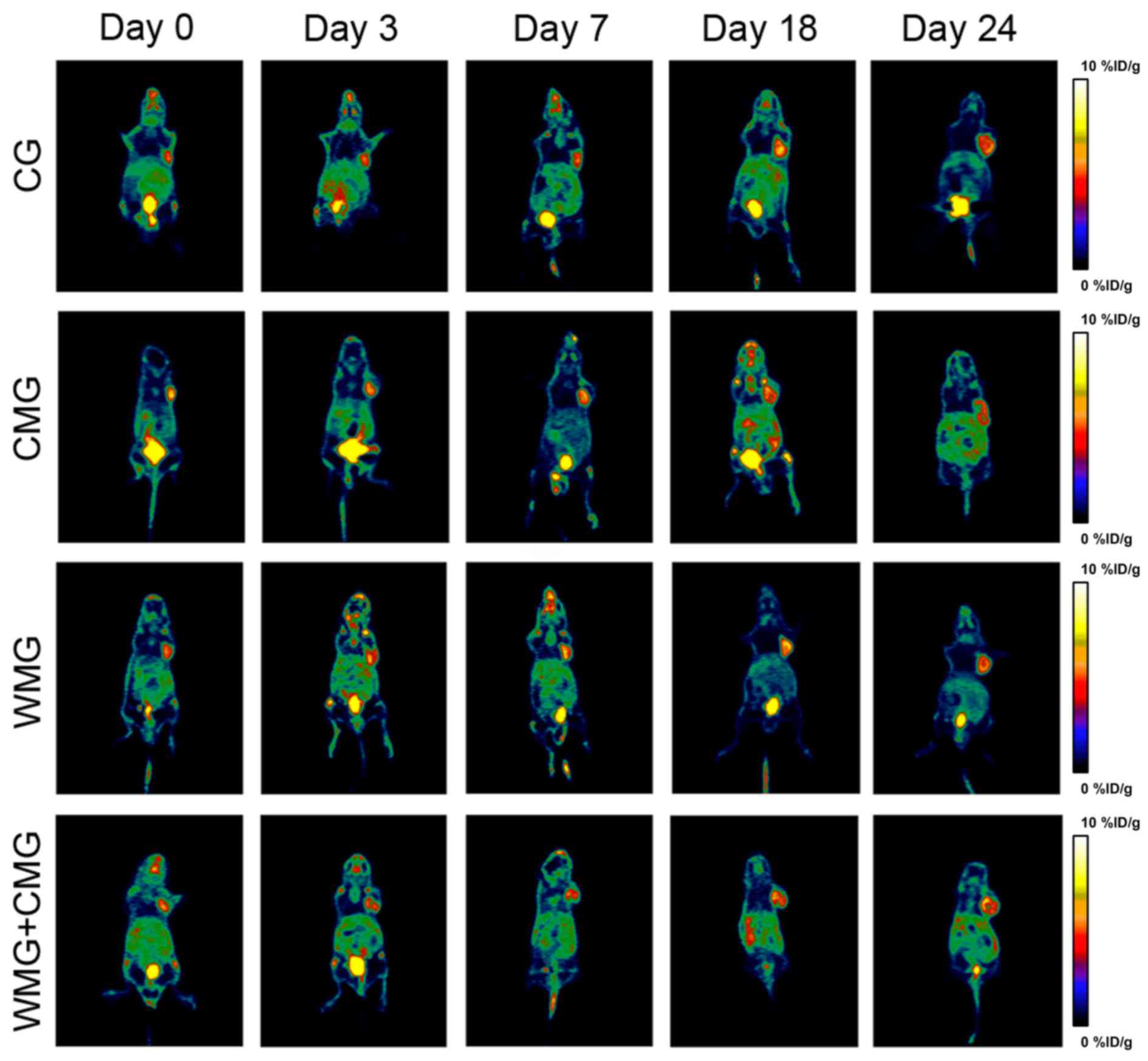
The results of the PET/CT scans indicated increased
uptake of radioactive material was observed at days 3, 7, 18 and 24
in the CG when compared with day 0 (Figs. 2 and 3). In the WMG, %ID/g values decreased on
days 3 and 7 of treatment, and on day 3 the %ID/g value was
significantly lower when compared with the CG (P<0.05; Fig. 2). However, on days 18 and 24 of
treatment radioactivity uptake was increased in mice in the WMG
when compared with 3 and 7 days. In addition, mice in the CMG + WMG
demonstrated significantly decreased radioactivity uptake on
treatment days 3, 18 and 24 compared with mice in the CG (Fig. 2). As treatment progressed,
radioactivity uptake in CMG mice gradually decreased; notably mice
in the CMG exhibited significantly decreased %ID/g values on days
18 and 24 when compared with mice in the CG (Figs. 2 and 3).
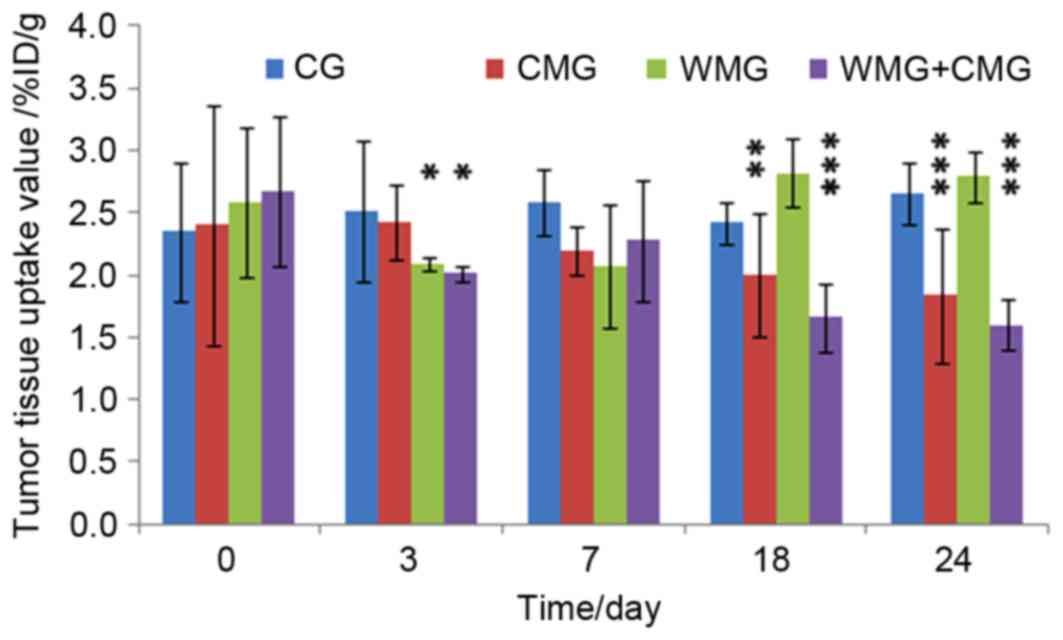 | Figure 2.Radioactivity uptake was measured on
days 0, 3, 7, 18 and 24 of treatment using
18F-Arg-Gly-Asp positron emission
tomography/computerized tomography, and expressed as the %ID/g.
Data are expressed as the mean ± standard deviation of three
independent experiments (n=6 per group). *P<0.05, **P<0.01,
***P<0.001 vs. the CG. CG, control group mice treated with
saline; WMG, Western medicine group mice treated with albumin-bound
paclitaxel; CMG, Chinese medicine group mice treated with WD-3; CMG
+ WMG, mice were treated with a combination of albumin-bound
paclitaxel and WD-3; %ID/g, percentage of injected dose per tissue
weight. |
Effects of WD-3 on p-ERK1/2 protein
expression levels in tumor tissue samples
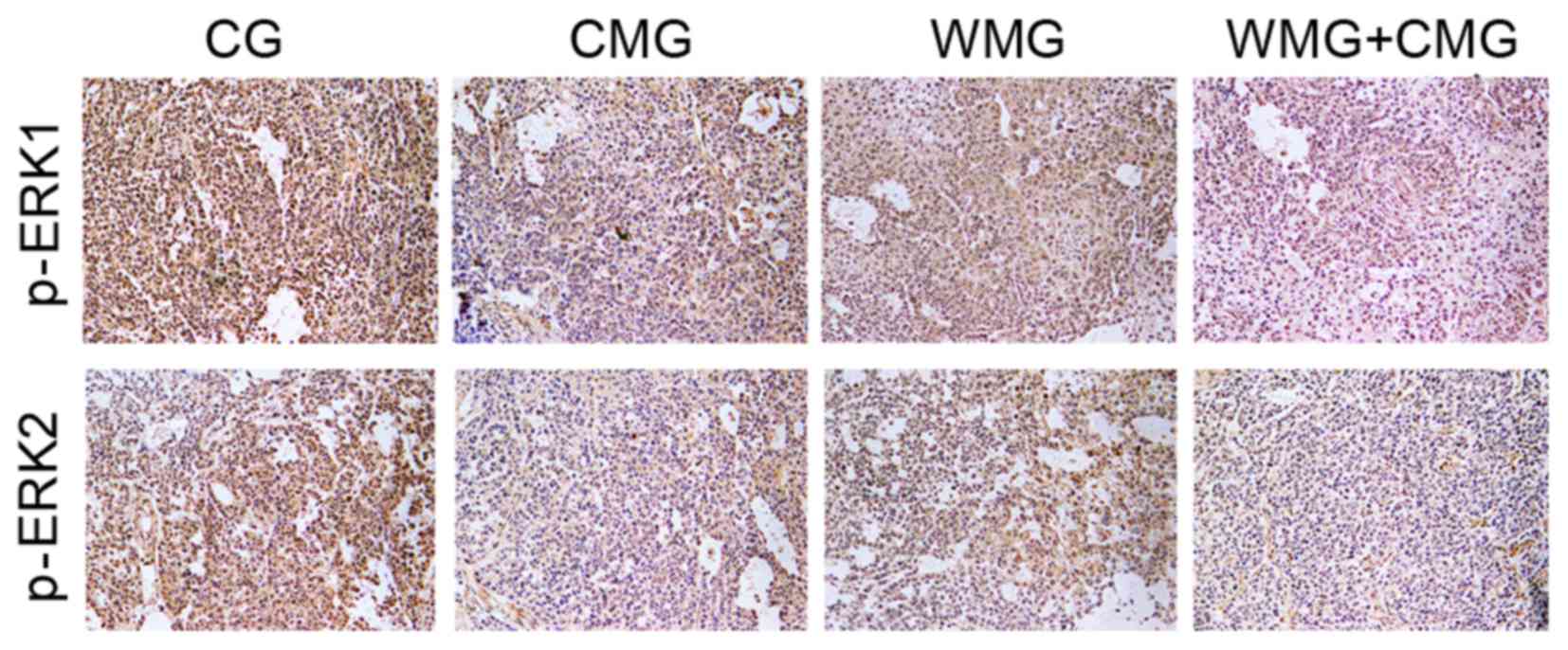
p-ERK1/2 proteins are primarily expressed in the
nucleus (23). In the present
study, immunohistochemical examination and semi-quantification
suggested that mice in the CMG, WMG and CMG + WMG exhibited
significantly reduced p-ERK1/2 protein expression, indicated by
weak p-ERK staining and significantly lower comprehensive scores
when compared with mice in the CG (Fig. 4; Table II).
 | Table II.Comprehensive scores of
immunohistochemical analysis of p-ERK1/2 protein expression. |
Table II.
Comprehensive scores of
immunohistochemical analysis of p-ERK1/2 protein expression.
|
| Score |
|---|
|
|
|
|---|
| Group (n=6
mice/group) | p-ERK1 | p-ERK2 |
|---|
| CG |
3.33±0.52 |
3.50±0.56 |
| CMG |
2.55±0.84a |
2.67±0.82a |
| WMG |
2.33±0.52a |
2.50±0.55a |
| WMG + CMG |
2.00±0.26a |
2.17±0.41a |
Discussion
Clinical studies have reported that albumin-bound
paclitaxel inhibit gastric cancer development in humans (24). The present study demonstrated that
nude mice bearing human gastric tumor xenografts treated with
albumin-bound paclitaxel (WMG), as well as with a combination of
albumin-bound paclitaxel and WD-3 (CMG + WMG) exhibited
significantly reduced gastric tumor mass when compared with control
mice, thus confirming the antitumor efficacy of paclitaxel. A
previous study demonstrated that gastric cancer cells could be
characterized by high integrin αvβ3
expression, which suggests that this factor may be a potential
biomarker for the evaluation of tumor prognosis in patients with
gastric cancer (25). The
18F-RGD PET results revealed that radioactivity SUVs
were decreased during the early stages of treatment in xenografted
mice in the WMG and CMG + WMG groups. In addition, the results of
the present study suggest that paclitaxel may inhibit tumor
angiogenesis via downregulation of integrin
αvβ3 expression, thereby inhibiting tumor
growth. Nevertheless, in the groups that weren't treated with
albumin-bound paclitaxel the inhibition of integrin
αvβ3 decreased and SUV values increased. WD-3
administration exerted no significant effects on tumor volume when
compared with the CG at the beginning of treatment, conversely,
tumor weight was significantly reduced in mice in the CMG when
compared with those of the CG at last. In addition,
18F-RGD PET revealed that on day 18 and 24 of treatment,
SUVs in CMG mice were significantly decreased when compared with CG
mice. These results suggested that WD-3 may inhibit tumor
angiogenesis and consequently tumor growth; however, its inhibition
of tumor volume's effects were less pronounced when compared with
conventional chemotherapy in the early stages of treatment (days 3
and 7). However, taking into consideration the reduced toxicity
associated with traditional Chinese medicine compared with
antineoplastic drugs, WD-3 may have potential as an alternative
therapeutic strategy for the long-term treatment of patients with
cancer (21). The present study
demonstrated that mice in the CMG + WMG displayed increased energy
and average body weights when compared with mice in the WMG (data
not shown). These results suggested that WD-3 may be associated
with fewer toxic adverse events compared with chemotherapeutic
agents.
The present study demonstrated that paclitaxel
administered in combination with WD-3 effectively inhibited
integrin αvβ3 expression and tumor growth,
which suggests that the traditional Chinese formula WD-3 may
potentially enhance the efficacy of chemotherapeutic agents when
used as adjuvant treatment. Immunohistochemistry demonstrated that
mice in the CMG and the WMG + CMG were characterized by reduced
p-ERK1/2 expression when compared with the CG. These results
suggested that WD-3 may interfere with the FAK/MAPK/ERK signaling
pathway through the downregulation of integrin
αvβ3 expression, ultimately inhibiting ERK
phosphorylation and subsequent tumor growth. However, the exact
molecular mechanisms underlying the effects of WD-3 observed in the
present study remain unclear. Integrin receptors have been reported
to induce kinase activation and initiate downstream signal
transduction cascades following mechanical stimulation (26). Further studies are required to
investigate whether WD-3 may be able to interfere with FAK
phosphorylation through mechanotransduction pathways, and thus
inhibit tumor growth.
In conclusion, the results of the present study
suggest that the traditional Chinese medicine agent, WD-3, may
inhibit tumor angiogenesis by decreasing the expression of receptor
αvβ3 in vivo, and may have potential
as an adjuvant agent to be used in combination with chemotherapy
for the treatment of patients with gastric cancer. In addition,
WD-3 was revealed to inhibit the gastric cancer cell growth
potentially via inhibition of ERK1/2 phosphorylation. Furthermore,
PET/CT scan results suggested that WD-3 may inhibit tumor
angiogenesis in mice bearing human gastric cancer xenografts in
vivo.
Acknowledgements
The present study was supported by the Science and
Technology Development Foundation of Wuxi City (grant no.
0302-B010507-130006-PB).
Glossary
Abbreviations
Abbreviations:
|
ERK
|
extracellular signal-regulated
kinase
|
|
RGD
|
Arg-Gly-Asp
|
|
PET
|
positron emission tomography
|
|
CT
|
computerized tomography
|
|
SUV
|
standardized uptake value
|
|
FAK
|
focal adhesion kinase
|
|
MMP
|
matrix metalloproteinase
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan JL: Integrin signaling through FAK in
the regulation of mammary stem cells and breast cancer. IUBMB Life.
62:268–276. 2010.PubMed/NCBI
|
|
4
|
Yun SP, Ryu JM and Han HJ: Involvement of
β1-integrin via PIP complex and FAK/paxillin in
dexamethasone-induced human mesenchymal stem cells migration. J
Cell Physiol. 226:683–692. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li D, Ding J, Wang X, Wang C and Wu T:
Fibronectin promotes tyrosine phosphorylation of paxillin and cell
invasiveness in the gastric cancer cell line AGS. Tumori.
95:769–779. 2009.PubMed/NCBI
|
|
6
|
Missan DS, Mitchell K, Subbaram S and
DiPersio CM: Integrin α3β1 signaling through MEK/ERK determines
alternative polyadenylation of the MMP-9 mRNA transcript in
immortalized mouse keratinocytes. PLoS One. 10:e01195392015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pickarski M, Gleason A, Bednar B and Duong
LT: Orally active αvβ3 integrin inhibitor MK-0429 reduces melanoma
metastasis. Oncol Rep. 33:2737–2745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Osório-Costa F, Rocha GZ, Dias MM and
Carvalheira JB: Epidemiological and molecular mechanisms aspects
linking obesity and cancer. Arq Bras Endocrinol Metabol.
53:213–226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Neil E and Kolch W: Conferring
apccificity on the ubiquitous Ras/MEK signaling pathway. Br J
Cancer. 90:283–288. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steelman LS, Abrams SL, Whelan J, Bertrand
FE, Ludwig DE, Bäsecke J, Libra M, Stivala F, Milella M, Tafuri A,
et al: Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and
Jak/STAT pathways to leukemia. Leukemia. 22:686–707. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheung KL, Lee JH, Shu L, Kim JH, Sacks DB
and Kong AN: The Ras GTPase-activating-like protein IQGAPI mediates
Nrf2 protein activation via the mitogen-activated protein
kinase/extracellular signal regulated kinase (ERK) kinase (MEK)-ERK
pathway. J Biol Chem. 288:22378–22386. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bai Y, Cui W, Xin Y, Miao X, Barati MT,
Zhang C, Chen Q, Tan Y, Cui T, Zheng Y and Cai L: Prevention by
sulforaphane of diabetic cardiomyopathy is associated with
up-regulation of Nrf2 expression transcription activation. J Mol
Cell Cardiol. 57:82–95. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Muslin AJ: MAPK signalling in
cardiovascular health and disease: Molecular mechanisms and
therapeutic targets. Clin Sci (Lond). 115:203–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guruvayoorappan C and Kuttan G:
Amentoflavone inhibits experimental tumor metastasis through a
regulatory mechanism involving MMP-2, MMP-9, prolyl hydroxylase,
lysyl oxidase, VEGF, ERK-1, ERK-2, STAT-1, nm23 and cytokines in
lung tissues of C57BL/6 mice. Immunopharmacol Immunotoxicol.
30:711–727. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Kuiatse I, Lee AV, Pan J, Giuliano
A and Cui X: Sustained c-Jun-NH2-kinase activity promotes
epithelial mesenchymal transition, invasion, and survival of breast
cancer cells by regulating extracellular signal-regulated kinase
activation. Mol Cancer Res. 8:266–277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chatziioannou AF: PET scanners dedicated
to molecular imaging of small animal models. Mol Imaging Biol.
4:47–63. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kosugi C, Saito N, Murakami K, Ochiai A,
Koda K, Ono M, Sugito M, Ito M, Oda K, Seike K and Miyazaki M:
Positron emission tomography for preoperative staging in patients
with locally advanced or metastatic colorectal adenocarcinoma in
lymphnode metastasis. Hepatogastroentero logy. 55:398–402.
2008.
|
|
18
|
Nahas CS, Akhurst T, Yeung H, Leibold T,
Riedel E, Markowitz AJ, Minsky BD, Paty PB, Weiser MR, Temple LK,
et al: Positron emission tomography detection of distant metastatic
or synchronous disease in patients with locally advanced rectal
cancer receiving preoperative chemoradiation. Ann Surg Oncol.
15:704–711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Meerovitch K, Bergeron F, Leblond L,
Grouix B, Poirier C, Bubenik M, Chan L, Gourdeau H, Bowlin T and
Attardo G: A novel RGD antagonist that targets both alphavbeta3 and
alpha5beta1 induces apoptosis of angiogenic endothelial cells on
type I collagen. Vascul Pharmacol. 40:77–89. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jianliang You, Liuyong Zhou and Ming Xu:
Clinical research of the treatment of advanced gastric cancer using
Chinese herbal medicine WD-3. Hubei J Traditional Chinese Med.
26:8–9. 2004.
|
|
21
|
Zhou LY, Shan ZZ and You JL: Clinical
observation on treatment of colonic cancer with combined treatment
of chemotherapy and chinese herbal medicine. Chin J Integr Med.
15:107–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe H, Kanzaki H, Narukawa S, Inoue
T, Katsuragawa H, Kaneko Y and Mori T: Bcl 2 and Fas expression in
eutopic and ectopic human endometrium during the menstrual cycle in
relation to endometrial cell apoptosis. Am J Obstet Gynecol.
176:360–368. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zehorai E, Yao Z, Plotnikov A and Seger R:
The subcellular localization of MEK and ERK-a novel nuclear
translocation signal (NTS) paves a way to the nucleus. Mol Cell
Endocrinol. 314:213–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sasaki Y, Nishina T, Yasui H, Goto M, Muro
K, Tsuji A, Koizumi W, Toh Y, Hara T and Miyata Y: Phase II trial
of nanoparticle albumin-bound paclitaxel as second-line
chemotherapy for unresectable or recurrent gastric cancer. Cancer
Sci. 105:812–817. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Böger C, Warneke VS, Behrens HM, Kalthoff
H, Goodman SL, Becker T and Röcken C: Integrins αvβ3 and αvβ5 as
prognostic, diagnostic, and therapeutic targets in gastric cancer.
Gastric Cancer. 18:784–795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Friedland JC, Lee MH and Boettiger D:
Mechanically activated integrin switch controls alpha5beta1
function. Science. 323:642–644. 2009. View Article : Google Scholar : PubMed/NCBI
|