Introduction
Patients with type 2 diabetes mellitus (T2DM) have
an increased risk of cardiovascular disease (CVD), which remains
the leading cause of morbidity and mortality worldwide (1). A major risk factor of CVD in T2DM is
dyslipidaemia, which is characterised by reduced high-density
lipoprotein (HDL), and increased triglyceride (TG) and small
density low-density lipoprotein (sd-LDL; Fig. 1) (2). Apolipoproteins are the protein
portion of lipoproteins, and mainly comprise six species called
apolipoprotein (apo)A, apoB, apoC, apoD, apoE and apoM, some of
which have several subtypes. Apolipoproteins are important
components of plasma lipoproteins and are expressed mainly in the
liver and partly in the intestine and other tissues (3). Their basic function is to carry
lipids and to stabilise the structure of lipoproteins; some
apolipoproteins also activate enzymes that participate in the
metabolism of lipoproteins and recognise endothelial receptors
associated with inflammatory signaling pathways (3). Previously reported epidemiological
data suggested that the metabolic disorders of apolipoproteins are
associated with the pathophysiological processes of T2DM (Fig. 1) (3,4). The
present review described the metabolic disorders of apolipoproteins
in T2DM and the relationship between each major apolipoprotein and
T2DM, associated complications and the effects of apolipoprotein
polymorphisms on diabetic susceptibility.
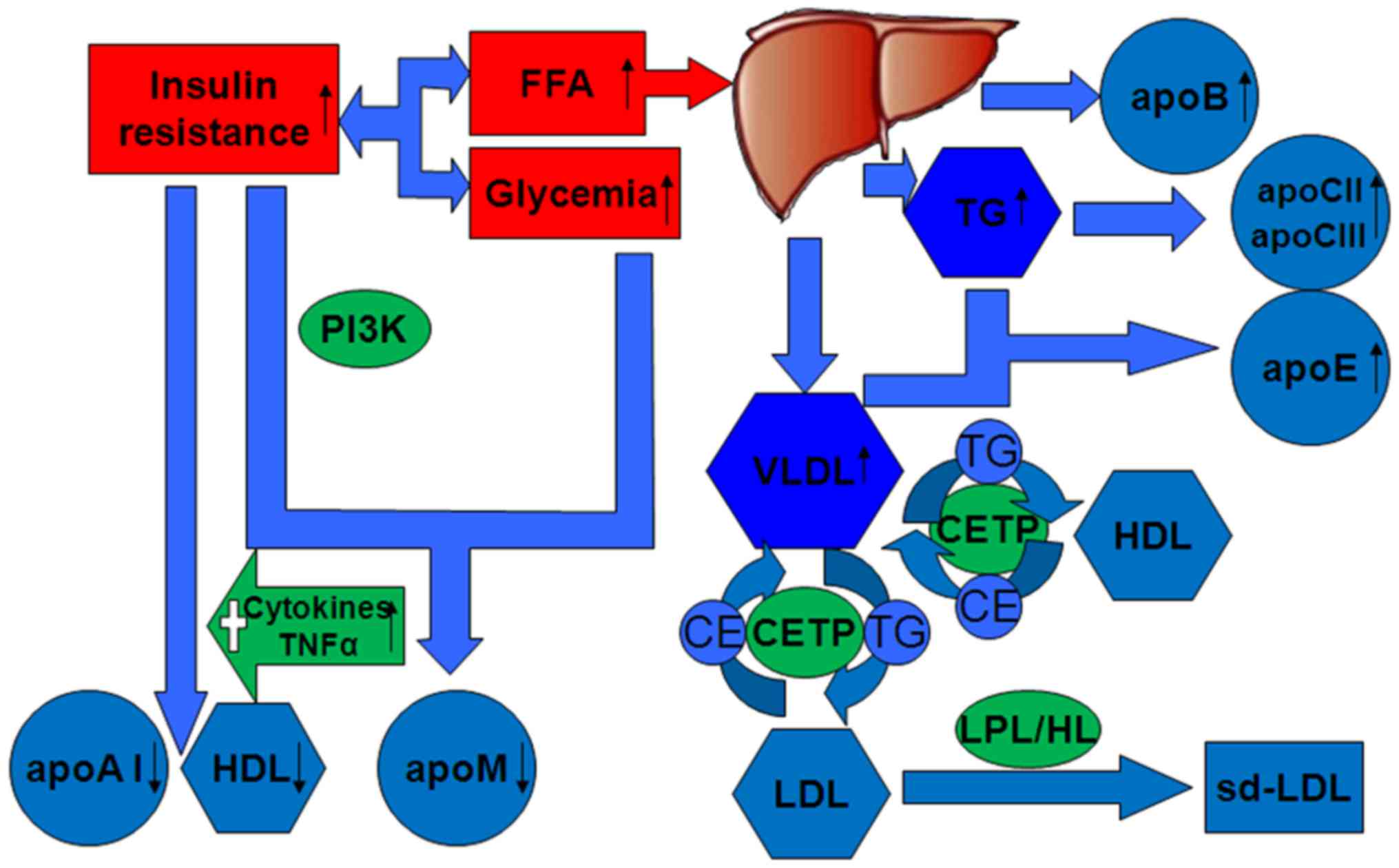 | Figure 1.Altered expression of apolipoproteins
and lipoproteins in type 2 diabetes mellitus. Insulin resistance
initiates the characteristic of high TG level, low HDL cholesterol
level and high sd-LDL level, as well as high levels of apoB,
apoC-II, apoC-III and apoE, and low levels of apoA-1 and apoM. When
the concentrations of VLDL-transported TG are high, CETP promotes
the transfer of LDL CE in exchange for TG. TG-rich LDL can undergo
hydrolysis by HL or LPL. The rate of HDL degradation is increased
and its production is not altered, resulting in decreased plasma
HDL. apo, apolipoprotein; CE, colesteryl ester; CETP, cholesteryl
ester transfer protein; FFA, free fatty acid; HDL, high-density
lipoprotein; HL, hepatic lipase; LDL, low-density lipoprotein; LPL,
lipoprotein lipase; PI3K, phosphatidylinositol 3-kinase; sd-LDL,
high small low-density lipoprotein; TG, triglyceride; VLDL, very
low-density lipoprotein. |
Relationship between apoA and T2DM
Introduction to apoA
The human apoA family includes apoA-I, apoA-II,
apoA-IV and apoA-V. ApoA-I is mainly distributed in the plasma
chylomicrons (CMs), HDL2 and HDL3, and is the major component of
HDL, comprising ~70% total protein content of HDL (5). Plasma HDL concentration is determined
by the fractional catabolic rate of apoA-I and apoA-II, as well as
HDL reduction with apoA-I deficiency. Therefore, apoA serves an
important role in the metabolism of HDL (6). ApoA may enhance the hydrolysis of TGs
with endothelial lipoprotein lipase (LPL) by stabilising the
structure of LPL dimers or TG-rich lipoproteins; as such, apoA
deficiency has been associated with atherosclerosis (6). ApoA-I serves an important role in
glucose stabilisation and the function of mitochondria in muscle
(7). Lipid-free and
lipid-associated apoA-I and apoA-II concentrations increase β-cell
insulin secretion and reduce the plasma level of glucose (8); thus, they may serve a protective role
in T2DM. However, the fractional catabolic rate of apoA-I is
significantly higher, and the absolute production rate of apoA-I is
inhibited in patients with T2DM compared with healthy individuals;
this inhibition may lead to a decrease in plasma apoA-I levels,
which may contribute to low HDL (Fig.
1; Table I) (9). In addition, increased production of
inflammatory cytokines, such as tumour necrosis factor α, may
increase insulin resistance and directly downregulate apoA-I
expression in T2DM (Fig. 1)
(10).
 | Table I.Altered plasma concentrations of
apolipoproteins in T2DM and its complications. |
Table I.
Altered plasma concentrations of
apolipoproteins in T2DM and its complications.
| Apo | T2DM vs.
healthy | T2DM with CVD vs.
T2DM | T2DM with other
diseases |
|---|
| apoA | apoA-I ↓
(3,50) | apoA-I ↓ (3) | 1. T2DM with MetS
vs. only MetS:apoA-I ↓; apoA-I:apoB ↓ (17) |
|
| Glycated apoA-I ↑
(3) | Glycated apoA-I ↑
(3) | 2. T2DM on
haemodialysis vs. healthy people:apoA-IV ↓ (13) |
|
|
|
| 3. PDR vs.
NPDR:apoA-I ↓; apoA-I:B ↓ (14) |
|
|
|
| 4. T2DM with MOP
vs. MOP:apoA-IV ↑ in jejunum (15) |
| apoB | apoB ↑ (3,50) | apoB ↑ (3) | 1. T2DM with MOP
vs. only MOP:apoB48 ↑ in jejunum (15) |
|
| Glycated apoB ↑
(15) | apoB:A-I ↑
(30) | 2. Albuminuric T2DM
vs. normoalbuminuric T2DM:apoB ↑ (28) |
|
| apoB:apoA-I ↑
(15) |
|
|
| apoC | apoC-II ↑; apoC-III
↑ | LDL-apoC-III ↑
(38) | 1. T2DM with MetS
vs. only MetS:apoC-III unchanged (17) |
|
| apoC-III:C-II ↓
(34) | apoC-II ↑ (39) |
|
| apoD | no research | no research | No research |
| apoE | apoE ↑ (50) |
|
|
| apoM | apoM ↓ (63) |
| 1. T2DM with
hyperlipidaemia vs. only T2DM:apoM unchanged (59) |
Metabolism of apoA in T2DM and its
complications
T2DM leads to arteriosclerosis and reduces the
antioxidant properties of human apoA-I, which inhibits
hyperglycaemia-induced oxidative stress and the production of
NADPH-mediated reactive oxygen species (ROS) in human macrophages
(11). Nobécourt et al
(3) concluded that the
non-enzymatically glycated apoA-I was attributed to a reduced
ability to inhibit nuclear factor-κB activation and ROS formation.
However, glycated apoA-I plasma concentration is usually increased
in patients with T2DM, leading to a reduction in anti-inflammatory
effects, which may enhance the inflammatory response (3). apoA-I levels are low when apoA-I
glycation is significantly elevated in patients with T2DM and
significant coronary artery disease (CAD). Therefore, the baseline
relative intensity of apoA-I glycation is an independent
determinant of CAD and plaque progression in T2DM (12). The plasma apoA-IV concentration was
reported to be significantly lower in patients with T2DM, and low
apoA-IV concentration was strongly associated with the risk of
all-cause mortality and cardiac disease-related mortality,
particularly sudden cardiac death (13). Compared with patients with mild
non-proliferative diabetic retinopathy, those with proliferative
diabetic retinopathy are characterised by decreased serum apoA-I
levels and decreased apoA-I/apo-B ratio (14). Furthermore, the apoA-IV content may
be significantly higher in morbidly obese persons (MOPs) with T2DM
compared with MOPs without T2DM (15). Women with polycystic ovary syndrome
were indicated to have an increased risk of developing T2DM;
therefore, apoA-I may be used to identify high-risk subgroups
(16). ApoA-I expression is lower
in persons with combined metabolic syndrome (MetS) and T2DM
compared with those with MetS alone, which suggested that diabetes
may adversely influence plasma apoA-I levels (Table I) (17).
apoA gene mutations and polymorphisms
in T2DM
Gene mutations and polymorphisms may affect plasma
lipid levels and may lead to CVD and atherosclerosis (18). The apoA-V 1131T>C
single-nucleotide polymorphism (SNP) has been associated with
increased plasma TG in both healthy people and those with T2DM
(19). The hypertriglyceridaemic
effect of high retinol-binding protein (RBP)4 expression levels was
demonstrated to be enhanced by the presence of the apoA-V
1131T>C genetic variant, indicating that this variant increases
plasma TG by regulating RBP4 (20). However, previous studies that did
not observe the aforementioned association concluded that a higher
TG level in T2DM may be the result of insulin resistance and
reduced lipolysis rather than genetic polymorphisms in apoA-V
1131T>C (21). Genotyping of
apoA-V 1131C was linked to lower LDL levels, and SW19 polymorphisms
were linked to higher TG levels in patients with T2DM (Table II) (22). However, another study reported that
the apoA-V 1131T>C SNP does not affect LDL levels in healthy
people (23).
 | Table II.Polymorphisms of apolipoproteins with
the susceptibility of T2DM. |
Table II.
Polymorphisms of apolipoproteins with
the susceptibility of T2DM.
| apo | Apolipoprotein
polymorphism |
|---|
| apoA | apoA-V 1131T>C
polymorphisms influence plasma TG levels and LDL levels in T2DM
(19); other studies do not find the association (21,23) |
| apoB | No research |
| apoC | 1. apoC-III 482T
allele is associated with increased risk of T2DM (41) |
|
| 2. apoC-III
1100C>T is associated with increased risk of T2DM in Caucasian
Europeans (42) |
|
| 3. apoC-III
P1-S2-X1 is a susceptibility haplotype that increases the risk of
coronary heart disease in T2DM (39) |
| apoD | apoD TaqI
polymorphism is associated with susceptibility of T2DM in South
Indians, Nauruans and British Caucasians (47,48) |
| apoE | 1. apoE ε4 allele
is associated with increased risk of T2DM (57) and severe
neuropathy, ischemic heart disease (55), coronary artery disease
(56) and Alzheimer disease in T2DM (59) |
|
| 2. apoE ε4 allele
is associated with increased risk of cardiovascular events in
patients with T2DM and end-stage renal disease (58) |
|
| 3. apoE ε2 allele
is associated with increased risk of T2DM (51) |
|
| 4. apoE ε2/3
genotype increase the risk for diabetic nephropathy (53) |
| apoM | 1. apoM T-778C is
associated with increased risk of T2DM (69) |
|
| 2. apoM C-1065
allele is associated with T2DM duration of >10 years (71) |
|
| 3. apoM C-724del is
associated with T2DM (67) |
Relationship between apoB and T2DM
Introduction to apoB
ApoB is a component of CMs, very low-density
lipoprotein (VLDL), intermediate-density lipoprotein and LDL
(24). ApoB48 is 48% of the
full-length protein; however, VLDL contains only full-length apoB
in humans. In T2DM, the increased flux of free fatty acids promotes
hepatic TG production, which subsequently induces apoB and VLDL
secretion (2). TGs transported by
VLDL are exchanged for HDL-transported cholesteryl esters through
the action of cholesteryl ester transfer protein (CETP) (2). As a result of this exchange, the
concentration of both atherogenic cholesterol-rich VLDL remnant
particles and TG-rich, cholesterol-depleted HDL particles are
increased. In addition, TG transfer from VLDL to LDL may be through
CETP, in exchange for LDL-transported cholesteryl ester (2). TG-rich LDL may be hydrolysed by
hepatic lipases or LPLs, which may result in lipid-depleted sd-LDL
(Fig. 1) (2). Then, Forkhead box (Fox) O1 becomes
inhibited, leading to increased expression of microsomal
triglyceride transfer protein and apoC-III. Meanwhile, the
multicomponent mechanistic target of rapamycin complex 1 remains
activated, suppressing sortilin, which can decrease apoB and
triglyceride secretion; subsequently, the ability of insulin to
suppress apoB secretion is diminished and, thus, apoB secretion is
increased (24).
Metabolism of apoB in T2DM and its
complications
ApoB clearance is decreased and the levels of plasma
apoB are increased in patients with T2DM (Fig. 1; Table
I) (12). CETP protein
expression level and activity, and HDL levels are significantly
increased, whereas apoB levels are significantly decreased
following insulin treatment (25).
These results suggested that insulin may reduce apoB concentrations
and serve an antiatherosclerotic role. Dyslipidaemia (higher TG and
VLDL), abnormal gene expression (glucose transporters 1 and 2, and
glycogen synthase kinase 3), abnormal protein expression (tumor
necrosis factor-a, interleukin-6, retinol binding protein 4 and
soluble cluster of differentiation 36), and phosphorylation of
multiple pathways components (insulin receptor substrate 2/Akt
protein/protein tyrosine phosphatase-1B) related to inflammatory
insulin signalling may be associated with VLDL-apoB100 particle
overproduction in T2DM (26).
Increased levels of circulating glycated apoB in T2DM are probably
linked with greater susceptibility of sd-LDL to glycation (4). The apoB/apoA-I ratio was revealed to
be independently associated with T2DM (27). Patients with T2DM and albuminuria
exhibit greater levels of circulating apoB (28), which indicated that apoB may also
be associated with diabetic nephropathy. A strong independent
negative correlation between the total fractional catabolic rate of
VLDL-apoB100 and plasma RBP4 concentration in T2DM has been
reported previously (29), which
suggested that RBP4 may reduce VLDL-apoB100 catabolism.
apoB/apoA-I ratio and serum apoB concentration were
revealed to be higher in patients with T2DM and CVD compared with
those with T2DM without CVD (3,30),
which suggested that the apoB/apoA-I ratio may be an indicator of
CVD risk in patients with T2DM (Table
I). Significant correlations have also been made between apoB48
and carotid intima-media thickness (31), which indicated that fasting apoB48
levels may aid in predicting arterial stiffness in middle-aged
patients with T2DM. Plasma apoB48 concentration may also be an
independent predictor of vasodilator function in the brachial
artery (32), and fasting apoB48
may be an independent marker of peripheral arterial disease in
patients with T2DM (33). Data
from these previous studies indicated that plasma apoB
concentrations may be associated with cardiovascular events in T2DM
patients.
Relationship between apoC and T2DM
Introduction to apoC
ApoC comprises apoC-I, apoC-II and apoC-III, which
are mainly components of CMs, VLDL and HDL, and participates in the
metabolism of these lipoprotein particles (34). ApoC-I is a potent activator of
lecithin-cholesterol acyltransferase (LCAT), and excess LCAT
results in increased total cholesterol (TC) and TG levels (34). At intermediate concentrations and
in normolipidaemic individuals, apoC-II activates LPL. However,
both very high and very low concentrations of apoC-II have been
associated with decreased LPL activity and hypertriglyceridaemia
(34). Overproduction of apoC-II
was associated with increased TG-rich particles and alterations in
the distribution of HDL particle, both of which are factors that
may increase the risk of CVD (34).
Metabolism of apoC in T2DM and its
complications
Plasma concentration levels of both apoC-II and
apoC-III, as well as the apoC-II/apoC-III ratio, were reported to
be markedly higher in patients with T2DM, and this increase was
associated with elevated TG (Fig.
1; Table I) (34). Glucose induces apoC-III
transcription, which may represent a mechanism that links
hyperglycaemia, hypertriglyceridaemia and CVD in patients with
T2DM; this process is inhibited by treatment with agonists of
farnesoid X receptor and peroxisome proliferator-activated
receptor-α (35). The highest
tertile of HDL apoC-III was reported to be a major independent
predictor of new-onset T2DM in the Turkish population, particularly
in women in which the middle tertile was also indicated to be
highly predictive of T2DM; however, non-HDL apoC-III does not
independently predict T2DM (36).
Plasma apoC-III levels may be altered in individuals with a family
history of diabetes (34),
indicating that the latter is an important factor for apoC-III.
ApoC-III delays TG-rich lipoprotein lipolysis by
inhibiting the expression of LPL and the hepatic uptake of TG-rich
lipoproteins by remnant receptors, and is strongly associated with
hypertriglyceridaemia and CVD progression (37). apoC-III plasma concentration may
strongly and independently predict coronary events in T2DM
(37). In patients with T2DM, high
levels of apoC-III increase the susceptibility of LDL to hydrolysis
and aggregation by sphingomyelinases (38). The sialylation of apoC-III, which
increases with increased apoC-III concentration, are essential for
its pro-inflammatory properties, could increase CVD risk (38). Chronic underexpression of apoC-III
may also affect heart functions (39). apoC-II levels are higher in
patients with T2DM and CVD compared with patients without CVD
(39). These results indicated
that apoC-II and apoC-III serve important roles in cardiac
events.
Gene mutations and polymorphisms of
apoC in T2DM
Circulating apoC-III is an independent determinant
of both incident T2DM and CVD. T2DM patients with apoC-III 482TT
homozygotes have higher apoC-III levels compared with C-allele
carriers (40). In lean patients,
the apoC-III 482TT allele was associated with an increased risk of
T2DM, but no association was made in overweight patients. Lean
patient carriers of the 482C>T allele may require more frequent
insulin therapy, which may be an effect of apoC-III variants on
β-cell function. This genetic overlap also occurs in T1DM (41). ApoC-III 1,100C>T was reported to
be associated with an increased risk of T2DM and this effect was
independent of the effects on TG levels (42). A previous study investigated
genetic variations in the 3′flanking region of apoA-I
(PstI), the 3′untranslated region of apoC-III (SstI)
and intron 2 of apoA-IV (XbaI) in 435 patients with T2DM and
revealed that the P1-S2-X1 haplotype increased the risk of CVD in
patients with T2DM (Table II)
(39). Patients with T2DM and the
apoC-III m482 AA polymorphism exhibited lower cognitive functions
and significantly higher glucose and TC levels compared with
patients with the AG or GG genotypes. Patients with T2DM and the
apoC-III 3u386 GC or GG polymorphism exhibited significantly higher
TG, TC and glucose levels compared with Caribbean Hispanic patients
with the CC genotype (43).
Relationship between apoD and T2DM
ApoD is expressed in numerous tissues, including
brain, intestine, liver, cardiac and skeletal muscle, adipose
tissue and pancreas. In plasma, apoD is mainly bound to HDL, with a
low level of apoD bound to VLDL and LDL, which suggested that apoD
may serve an important role in the metabolism of both TG and TC
(44). ApoD is a potent activator
of LCAT. apoA-I and apoC-I can also modulate LCAT activity by
stabilizing the enzyme on HDL (44), indicating that apoD may have some
interaction with apoA-I and apoC-I in HDL metabolization, although
further studies are required. ApoD also serves a role in HDL
remodelling through covalent crosslinking with apoA-II, a
structural component of HDL (44).
Previous studies investigating the association between apoD and
T2DM are limited; however, it has been reported that apoD serves an
important role in oxidative stress, which is closely associated
with insulin resistance and diabetes (45). Another study demonstrated that apoD
functions to protect against lipid peroxidation and oxidative
stress (46). ApoD expression was
also revealed to be upregulated in cultured myotubes from patients
with T2DM (46). A linkage has
been observed between the TaqI polymorphism of apoD and T2DM
in South Indians, Nauruans and British Caucasian populations
(Table II) (47,48).
T2DM may influence the expression levels of apoD mRNA in the
hypothalamus of obese db/db mice (46); however, the underlying
pathophysiological role of apoD in T2DM is not clear. Therefore,
further research is required.
Relationship between apoE and T2DM
Introduction to apoE
ApoE possesses three major alleles (ε2, ε3 and ε4)
and encodes a 299 amino-acids-long protein that has three isoforms
(E2, E3 and E4). ε3 is the most common isoform and has a frequency
of 70–80%; whereas the frequency of ε2 and ε3 are 5–10 and 10–15%,
respectively (49). ApoE is a
component of VLDL, CMs and HDL; increased production and secretion
of apoE by the liver, or accumulation of apoE in the plasma has
been associated with increased VLDL synthesis and secretion
(50). ApoE serves an important
role in regulating plasma and cellular lipid concentrations
(50).
Metabolism of apoE in T2DM
As insulin resistance is associated with metabolic
dyslipidaemia, the function of apoE isoforms in lipid metabolism
may serve an important role in T2DM pathogenesis (51). Serum apoE concentrations were
previously demonstrated to be elevated in patients with T2DM
(Fig. 1; Table I) (52). The serum levels of apoE are
independently associated with urinary albumin excretion in patients
with T2DM, and levels are significantly higher in patients with
albuminuria and T2DM compared with T2DM alone (51), which indicated that apoE may be
related to T2DM and its complications.
Association between apoE genotype and
T2DM
A meta-regression analysis suggested that carriers
of the apoE ε2 allele have an increased risk for T2DM, and the apoE
ε2/3 genotype may increase the risk of diabetic nephropathy
(53). The apoE ε4 allele is has
been associated with the development of T2DM and severe peripheral
neuropathy in patients with T2DM (54). In addition, the ε4 allele may be
linked with the development of ischemic heart disease in patients
with T2DM (55). ApoE ε4 has also
been associated with the development of T2DM with CAD (Table II) (56,57),
as well as increased risk of cardiovascular events and related
mortalities in patients with T2DM and end-stage renal disease
(Table I) (58). A number of previous human and
animal studies have reported a causal link between aberrant insulin
metabolism, both hypoinsulinaemia and insulin resistance, and the
pathogenesis of Alzheimer's disease (AD) (49). T2DM and prediabetic states, such as
abnormal glucose tolerance and insulin resistance, have been
implicated as risk factors of AD. Compared with those who have
neither T2DM nor the apoE ε4 allele, patients with both factors
have a higher risk of developing AD, mixed AD (59) and cognitive dysfunction (60), which suggested that apoE may be a
risk factor of AD. A strong association has been made between T2DM
and the development of AD in patients with T2DM and the apoE ε4
allele that also have numerous neuritic plaques and neurofibrillary
tangles in the cortex and hippocampus, and with a high burden of
cerebral amyloid angiopathy (61).
In addition, protein and mRNA expressions of insulin-degrading
enzyme are significantly reduced in the hippocampus in patients
with AD that carry the apoE ε4 genotype (49). These data suggested that carrying
the apoE ε4 allele may increase the risk of developing AD in
patients with T2DM (Table I).
Relationship between apoM and T2DM
Introduction to apoM
ApoM expression is highly tissue specific; it is
mainly expressed in liver and kidney, and weakly expressed in
embryonic liver and kidney, stomach, muscle cells, heart,
intestine, brain, spleen and testes; however, expression has not
been detected in muscle tissue, duodenum and ovaries (62). ApoM is present in HDL and, to a
lower degree, in TG-rich lipoproteins and LDL in plasma. ApoM is
crucial for pre-b-HDL formation and cholesterol efflux to HDL; it
protects against atherosclerosis (63). Previous studies have indicated that
apoM may be associated with both apoA-I and apoE (64,65).
For example, ApoM may be an independent predictor of apoA-I and
apoA-II catabolism in overweight/obese, insulin-resistant men
(64). ApoM expression is reduced
in diabetic mice and its synthesis may be regulated by insulin
(62). Furthermore,
phosphatidylinositol 3-kinase may also control the expression of
apoM in HepG2 human liver carcinoma cells. apoM may serve a role in
the metabolism of glucose and lipids by regulating peroxisome
proliferator-activated receptor γ (66).
Metabolism of apoM in T2DM
Plasma concentrations of apoM are ~9% lower in
patients with T2DM compared with healthy control individuals in
Caucasians (Fig. 1; Table I) (67). Our recent research also
demonstrated that Chinese T2DM patients also had lower apoM levels
than healthy controls (68). Of
note, no differences of plasma apoM concentrations were observed
between T2DM patients and healthy controls, indicating that low
plasma apoM in T2DM may be the accompanying effect of HDL (68).
Gene mutations and polymorphisms of
apoM in T2DM
The apoM T-778C SNP was notably associated with T2DM
in the Han Chinese population (69). Plasma TC levels were demonstrated
to be significantly higher in individuals with apoM T-778C CC or CT
genotype compared with TT genotype in healthy people (70). However, the apoM T-778C TT genotype
was significantly associated with elevated plasma TC and LDL levels
in patients with T2DM (67). In
vitro experiment demonstrated that apoM T-778C T allele could
led to ectopic expression of apoM transcript, which can modify
hepatic cell cholesterol content (67). In addition, the allele C of the
apoM C-1065A SNP was significantly increased in patients with T2DM
>10 years compared with those in T2DM duration <10 years
(Table II) (71). A recent study reported that the
apoM C-724del SNP was associated with CVD and myocardial infarction
(72), and another study
demonstrated that this polymorphism was also related to T2DM
(67).
Conclusions and prospects
Dyslipidaemia in patients with T2DM is characterised
by high plasma TG, reduced HDL and increased sd-LDL. Dyslipidaemia
may lead to atherosclerosis and other complications, and is closely
associated with insulin resistance (Fig. 1) (2). Complex metabolic disorders of
apolipoproteins in T2DM occur, such as high plasma concentrations
of apoB, apoC-II, apoC-III and apoE, and low plasma concentrations
of apoA-I and apoM, which are associated with dyslipidaemia and the
pathophysiology of complications (Fig.
1; Table I) (3,34,50,63).
T2DM combined with CVD and other complications also affect plasma
apolipoprotein concentrations (Table
I) (3,38,39).
Certain apolipoprotein polymorphisms are related to the
susceptibility of T2DM, as well as complications and lipid
metabolism (19,21,23,39,41,42,47,48,51,53,55–59,66,68,70);
however, the complex mechanisms have not been fully elucidated
(Table II). In addition, few
studies have been conducted on the interactions between each
apolipoprotein. Apolipoproteins are closely related to diabetes and
other metabolic diseases; however, the complex mechanisms of
insulin and glucose regulation of apolipoproteins are not well
understood. Thus, the association between apolipoproteins and the
pathogenesis of diabetes requires further research.
Acknowledgements
The authors would like to acknowledge the support of
The Anhui Province Key Laboratory of Biological Macro-molecules
Research (Wannan Medical College). This study was supported by
grants from The Science and technology project in Wuhu (grant no.
2013cxy04), The Anhui Provincial Natural Science Foundation (grant
no. 1508085MH149), The Natural Science Research Project of Anhui
Colleges and Universities (grant no. KJ2016A737) and The National
Natural Science Foundation of China (grant no. 81200632).
References
|
1
|
Ingelfinger JR and Rosen CJ: Cardiac and
renovascular complications in type 2 diabetes-is there hope? N Engl
J Med. 375:380–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shulman GI: Ectopic fat in insulin
resistance, dyslipidemia, and cardiometabolic disease. N Engl J
Med. 371:1131–1141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nobécourt E, Tabet F, Lambert G, Puranik
R, Bao S, Yan L, Davies MJ, Brown BE, Jenkins AJ, Dusting GJ, et
al: Nonenzymatic glycation impairs the anti-inflammatory properties
of apolipoprotein A-I. Arterioscler Thromb Vasc Biol. 30:766–772.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Younis NN, Soran H, Pemberton P,
Charlton-Menys V, Elseweidy MM and Durrington PN: Small dense LDL
is more susceptible to glycation than more buoyant LDL in Type 2
diabetes. Clin Sci (Lond). 124:343–349. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Viney NJ, van Capelleveen JC, Geary RS,
Xia S, Tami JA, Yu RZ, Marcovina SM, Hughes SG, Graham MJ, Crooke
RM, et al: Antisense oligonucleotides targeting apolipoprotein(a)
in people with raised lipoprotein(a): Two randomised, double-blind,
placebo-controlled, dose-ranging trials. Lancet. 388:2239–2253.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rye KA, Barter PJ and Cochran BJ:
Apolipoprotein A-I interactions with insulin secretion and
production. Curr Opin Lipidol. 27:8–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lehti M, Donelan E, Abplanalp W,
Al-Massadi O, Habegger KM, Weber J, Ress C, Mansfeld J, Somvanshi
S, Trivedi C, et al: High-density lipoprotein maintains skeletal
muscle function by modulating cellular respiration in mice.
Circulation. 128:2364–2371. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fryirs MA, Barter PJ, Appavoo M, Tuch BE,
Tabet F, Heather AK and Rye KA: Effects of high-density
lipoproteins on pancreatic beta-cell insulin secretion.
Arterioscler Thromb Vasc Biol. 30:1642–1648. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duvillard L, Dautin G, Florentin E,
Jeannin A, de Barros JP Pais, Lagrost L, Petit JM, Gambert P and
Vergès B: Increased apolipoprotein AI production rate and
redistribution of high-density lipoprotein size induced by estrogen
plus progestin as oral contraceptive. J Clin Endocrinol Metab.
94:4891–4897. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bisoendial R, Tabet F, Tak PP, Petrides F,
Torres LF Cuesta, Hou L, Cook A, Barter PJ, Weninger W and Rye KA:
Apolipoprotein A-I limits the negative effect of tumor necrosis
factor on lymphangiogenesis. Arterioscler Thromb Vasc Biol.
35:2443–2450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tabet F, Lambert G, Torres LF Cuesta, Hou
L, Sotirchos I, Touyz RM, Jenkins AJ, Barter PJ and Rye KA:
Lipid-free apolipoprotein A-I and discoidal reconstituted
high-density lipoproteins differentially inhibit glucose-induced
oxidative stress in human macrophages. Arterioscler Thromb Vasc
Biol. 31:1192–1200. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pu LJ, Lu L, Zhang RY, Du R, Shen Y, Zhang
Q, Yang ZK, Chen QJ and Shen WF: Glycation of apoprotein A-I is
associated with coronary artery plaque progression in type 2
diabetic patients. Diabetes Care. 36:1312–1320. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kollerits B, Krane V, Drechsler C, Lamina
C, März W, Ritz E, Wanner C and Kronenberg F: German Diabetes and
Dialysis Study Investigators: Apolipoprotein A-IV concentrations
and clinical outcomes in haemodialysis patients with type 2
diabetes mellitus-a post hoc analysis of the 4D Study. J Intern
Med. 272:592–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hu A, Luo Y, Li T, Guo X, Ding X, Zhu X,
Wang X and Tang S: Low serum apolipoprotein A1/B ratio is
associated with proliferative diabetic retinopathy in type 2
diabetes. Graefes Arch Clin Exp Ophthalmol. 250:957–962. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soriguer F, Garcia-Serrano S,
Garrido-Sánchez L, Gutierrez-Repiso C, Rojo-Martínez G,
Garcia-Escobar E, García-Arnés J, Gallego-Perales JL, Delgado V and
García-Fuentes E: Jejunal wall triglyceride concentration of
morbidly obese persons is lower in those with type 2 diabetes
mellitus. J Lipid Res. 51:3516–3523. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galazis N, Afxentiou T, Xenophontos M,
Diamanti-Kandarakis E and Atiomo W: Proteomic biomarkers of type 2
diabetes mellitus risk in women with polycystic ovary syndrome. Eur
J Endocrinol. 168:R33–R43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang W, Khan S, Blackett P, Alaupovic P
and Lee E: Apolipoproteins A-I, B, and C-III in young adult
Cherokee with metabolic syndrome with or without type 2 diabetes. J
Clin Lipidol. 7:38–42. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mendes-Lana A, Pena GG, Freitas SN, Lima
AA, Nicolato RL, Nascimento-Neto RM, Machado-Coelho GL and Freitas
RN: Apolipoprotein E polymorphism in Brazilian dyslipidemic
individuals: Ouro Preto study. Braz J Med Biol Res. 40:49–56. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Charriere S, Bernard S, Aqallal M, Merlin
M, Billon S, Perrot L, Le Coquil E, Sassolas A, Moulin P and
Marcais C: Association of APOA5-1131T>C and S19W gene
polymorphisms with both mild hypertriglyceridemia and
hyperchylomicronemia in type 2 diabetic patients. Clin Chim Acta.
394:99–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cabré A, Lázaro I, Girona J, Manzanares
JM, Marimón F, Plana N, Guardiola M, Heras M and Masana L: The
APOA5-1131 T>C variant enhances the association between RBP4 and
hypertriglyceridemia in diabetes. Nutr Metab Cardiovasc Dis.
20:243–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Celap I, Simundic AM, Nikolac N, Kackov S
and Katalinic D: Association of APOA5-1131T>C polymorphism and
serum lipid levels in patients with type 2 diabetes. DNA Cell Biol.
32:589–593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sóter MO, Gomes KB, Fernandes AP, Carvalho
Md, Pinheiro PS, Bosco AA, Silva DD and Sousa MO: -1131T>C and
SW19 polymorphisms in APOA5 gene and lipid levels in type 2
diabetic patients. Mol Biol Rep. 39:7541–7548. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brito DD, Fernandes AP, Gomes KB, Coelho
FF, Cruz NG, Sabino AP, Cardoso JE, Figueiredo-Filho PP, Diamante
R, Norton CR and Sousa MO: Apolipoprotein A5-1131T>C
polymorphism, but not APOE genotypes, increases susceptibility for
dyslipidemia in children and adolescents. Mol Biol Rep.
38:4381–4388. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sørensen LP, Andersen IR, Søndergaard E,
Gormsen LC, Schmitz O, Christiansen JS and Nielsen S: Basal and
insulin mediated VLDL-triglyceride kinetics in type 2 diabetic men.
Diabetes. 60:88–96. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aslan I, Kucuksayan E and Aslan M: Effect
of insulin analog initiation therapy on LDL/HDL subfraction profile
and HDL associated enzymes in type 2 diabetic patients. Lipids
Health Dis. 12:542013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qin B, Anderson RA, Kuzuya T, Kitaura Y
and Shimomura Y: Multiple factors and pathways involved in hepatic
very low density lipoprotein-apoB100 overproduction in Otsuka
Long-Evans Tokushima Fatty rats. Atherosclerosis. 222:409–416.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hwang YC, Ahn HY, Kim WJ, Park CY and Park
SW: Increased apoB/A-I ratio independently associated with Type 2
diabetes mellitus: Cross-sectional study in a Korean population.
Diabet Med. 29:1165–1170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan J, Gao F, Bao Y, Zhang L, Tu Y and Jia
W: Non-high-density lipoprotein cholesterol is associated more
closely with albuminuria in Chinese type 2 diabetic patients with
normal renal function, compared with traditional lipid parameters.
J Clin Lipidol. 6:382–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vergès B, Guiu B, Cercueil JP, Duvillard
L, Robin I, Buffier P, Bouillet B, Aho S, Brindisi MC and Petit JM:
Retinol-binding protein 4 is an independent factor associated with
triglycerides and a determinant of very low-density
lipoprotein-apolipoprotein B100 catabolism in type 2 diabetes
mellitus. Arterioscler Thromb Vasc Biol. 32:3050–3057. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taskinen MR, Barter PJ, Ehnholm C,
Sullivan DR, Mann K, Simes J, Best JD, Hamwood S and Keech AC:
FIELD study investigators: Ability of traditional lipid ratios and
apolipoprotein ratios to predict cardiovascular risk in people with
type 2 diabetes. Diabetologia. 53:1846–1855. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dahlén EM, Bjarnegard N, Länne T, Nystrom
FH and Ostgren CJ: Sagittal abdominal diameter is a more
independent measure compared with waist circumference to predict
arterial stiffness in subjects with type 2 diabetes-a prospective
observational cohort study. Cardiovasc Diabetol. 12:552013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chan DC, Wong AT, Yamashita S and Watts
GF: Apolipoprotein B-48 as a determinant of endothelial function in
obese subjects with type 2 diabetes mellitus: Effect of fenofibrate
treatment. Atherosclerosis. 221:484–489. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mancera-Romero J, Sánchez-Chaparro MA,
Rioja J, Ariza MJ, Olivecrona G, González-Santos P and Valdivielso
P: Fasting apolipoprotein B48 is a marker for peripheral arterial
disease in type 2 diabetes. Acta Diabetol. 50:383–389. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Béliard S, Nogueira JP, Maraninchi M,
Lairon D, Nicolay A, Giral P, Portugal H, Vialettes B and Valéro R:
Parallel increase of plasma apoproteins C-II and C-III in Type 2
diabetic patients. Diabet Med. 26:736–739. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Caron S, Verrijken A, Mertens I, Samanez
CH, Mautino G, Haas JT, Duran-Sandoval D, Prawitt J, Francque S,
Vallez E, et al: Transcriptional activation of apolipoprotein CIII
expression by glucose may contribute to diabetic dyslipidemia.
Arterioscler Thromb Vasc Biol. 31:513–519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Onat A, Hergenc G, Ayhan E, Uğur M, Kaya
H, Tuncer M and Can G: Serum apolipoprotein C-III in high-density
lipoprotein: A key diabetogenic risk factor in Turks. Diabet Med.
26:981–988. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee SJ, Campos H, Moye LA and Sacks FM:
LDL containing apolipoprotein CIII is an independent risk factor
for coronary events in diabetic patients. Arterioscler Thromb Vasc
Biol. 23:853–858. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hiukka A, Stahlman M, Pettersson C, Levin
M, Adiels M, Teneberg S, Leinonen ES, Hultén LM, Wiklund O, Oresic
M, et al: ApoCIII-enriched LDL in type 2 diabetes displays altered
lipid composition, increased susceptibility for sphingomyelinase,
and increased binding to biglycan. Diabetes. 58:2018–2026. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Singh P, Singh M, Gaur S and Kaur T: The
ApoAI-CIII-AIV gene cluster and its relation to lipid levels in
type 2 diabetes mellitus and coronary heart disease: Determination
of a novel susceptible haplotype. Diab Vasc Dis Res. 4:124–129.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Onat A, Erginel-Unaltuna N, Coban N, Cicek
G and Yüksel H: APOC3-482C>T polymorphism, circulating
apolipoprotein C-III and smoking: Interrelation and roles in
predicting type-2 diabetes and coronary disease. Clin Biochem.
44:391–396. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
van Hoek M, van Herpt TW, Dehghan A,
Hofman A, Lieverse AG, van Duijn CM, Witteman JC and Sijbrands EJ:
Association of an APOC3 promoter variant with type 2 diabetes risk
and need for insulin treatment in lean persons. Diabetologia.
54:1360–1367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dorfmeister B, Cooper JA, Stephens JW,
Ireland H, Hurel SJ, Humphries SE and Talmud PJ: The effect of
APOA5 and APOC3 variants on lipid parameters in European Whites,
Indian Asians and Afro-Caribbeans with type 2 diabetes. Biochim
Biophys Acta. 1772:355–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Smith CE, Tucker KL, Scott TM, Van Rompay
M, Mattei J, Lai CQ, Parnell LD, Junyent M, Lee YC, Garcia-Bailo B
and Ordovás JM: Apolipoprotein C3 polymorphisms, cognitive function
and diabetes in Caribbean origin Hispanics. PLoS One. 4:e54652009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lim W, Bae H and Song G: Differential
expression of apolipoprotein D in male reproductive system of rats
by high-fat diet. Andrology. 4:1115–1122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Dassati S, Waldner A and Schweigreiter R:
Apolipoprotein D takes center stage in the stress response of the
aging and degenerative brain. Neurobiol Aging. 35:1632–1642. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hansen L, Gaster M, Oakeley EJ, Brusgaard
K, Nielsen EM Damsgaard, Beck-Nielsen H, Pedersen O and Hemmings
BA: Expression profiling of insulin action in human myotubes:
Induction of inflammatory and pro-angiogenic pathways in
relationship with glycogen synthesis and type 2 diabetes. Biochem
Biophys Res Commun. 323:685–695. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Baker WA, Hitman GA, Hawrami K, McCarthy
MI, Riikonen A, Tuomilehto-Wolf E, Nissinen A, Tuomilehto J, Mohan
V, Viswanathan M, et al: Apolipoprotein D gene polymorphism: A new
genetic marker for type 2 diabetic subjects in Nauru and south
India. Diabet Med. 11:947–952. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vijayaraghavan S, Hitman GA and Kopelman
PG: Apolipoprotein-D polymorphism: A genetic marker for obesity and
hyperinsulinemia. J Clin Endocrinol Metab. 79:568–570. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miranda LF, Gomes KB, Tito PA, Silveira
JN, Pianetti GA, Byrro RM, Peles PR, Pereira FH, Santos TR, Assini
AG, et al: Clinical response to donepezil in mild and moderate
dementia: Relationship to drug plasma concentration and CYP2D6 and
APOE genetic polymorphisms. J Alzheimers Dis. 55:539–549. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Okamoto N, Morikawa M, Amano N, Yanagi M,
Takasawa S and Kurumatani N: Effects of tooth loss and the
apolipoprotein E ε4 Allele on mild memory impairment in the
Fujiwara-kyo study of Japan: A Nested Case-Control Study. J
Alzheimers Dis. 55:575–583. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Anthopoulos PG, Hamodrakas SJ and Bagos
PG: Apolipoprotein E polymorphisms and type 2 diabetes: A
meta-analysis of 30 studies including 5423 cases and 8197 controls.
Mol Genet Metab. 100:283–291. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tan KC, Shiu SW, Wong Y, Wong WK and Tam
S: Plasma apolipoprotein E concentration is an important
determinant of phospholipid transfer protein activity in type 2
diabetes mellitus. Diabetes Metab Res Rev. 22:307–312. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Reis KA, Ebinc FA, Koc E, Demirci H, Erten
Y, Güz G, Derici UB, Bali M, Söylemezoğlu O, Arınsoy T and Sindel
S: Association of the angiotensinogen M235T and APO E gene
polymorphisms in Turkish type 2 diabetic patients with and without
nephropathy. Ren Fail. 33:469–474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Monastiriotis C, Papanas N, Trypsianis G,
Karanikola K, Veletza S and Maltezos E: The ε4 allele of the APOE
gene is associated with more severe peripheral neuropathy in type 2
diabetic patients. Angiology. 64:451–455. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Al-Majed HT, Qasem JA, Al-Sherifi AK,
Al-Attar AA, Qasem AA and Abdullah SA: Association between
apolipoprotein E-polymorphism and Ischemic heart disease patients
with or without type 2 diabetes mellitus: A preliminary study in
Kuwait. Arch Iran Med. 14:385–388. 2011.PubMed/NCBI
|
|
56
|
Vaisi-Raygani A, Rahimi Z, Tavilani H and
Pourmotabbed T: Butyrylcholinesterase K variant and the APOE-ε4
allele work in synergy to increase the risk of coronary artery
disease especially in diabetic patients. Mol Biol Rep.
37:2083–2091. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chaudhary R, Likidlilid A, Peerapatdit T,
Tresukosol D, Srisuma S, Ratanamaneechat S and Sriratanasathavorn
C: Apolipoprotein E gene polymorphism: Effects on plasma lipids and
risk of type 2 diabetes and coronary artery disease. Cardiovasc
Diabetol. 11:362012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Winkler K, Hoffmann MM, Krane V, März W,
Drechsler C and Wanner C: Apolipoprotein E genotype predicts
cardiovascular endpoints in dialysis patients with type 2 diabetes
mellitus. Atherosclerosis. 208:197–202. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Irie F, Fitzpatrick AL, Lopez OL, Kuller
LH, Peila R, Newman AB and Launer LJ: Enhanced risk for Alzheimer
disease in persons with type 2 diabetes and APOE epsilon4: The
Cardiovascular Health Study Cognition Study. Arch Neurol. 65:89–93.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Dore GA, Elias MF, Robbins MA, Elias PK
and Nagy Z: Presence of the APOE epsilon4 allele modifies the
relationship between type 2 diabetes and cognitive performance: The
Maine-Syracuse Study. Diabetologia. 52:2551–2560. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kempf SJ, Janik D, Barjaktarovic Z,
Braga-Tanaka I III, Tanaka S, Neff F, Saran A, Larsen MR and Tapio
S: Chronic low-dose-rate ionising radiation affects the hippocampal
phosphoproteome in the ApoE-/-Alzheimer's mouse model. Oncotarget.
7:71817–71832. 2016.PubMed/NCBI
|
|
62
|
Zhang P, Gao J, Pu C, Feng G, Wang L,
Huang L, Tao Q and Zhang Y: Effects of hyperlipidaemia on plasma
apolipoprotein M levels in patients with type 2 diabetes mellitus:
An independent case-control study. Lipids Health Dis. 15:1582016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang H, Pluhackova K, Jiang Z and
Böckmann RA: Binding Characteristics of Sphingosine-1-Phosphate to
ApoM hints to assisted release mechanism via the ApoM
Calyx-opening. Sci Rep. 6:306552016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ooi EM, Watts GF, Chan DC, Nielsen LB,
Plomgaard P, Dahlbäck B and Barrett PH: Association of
apolipoprotein M with high-density lipoprotein kinetics in
overweight-obese men. Atherosclerosis. 210:326–330. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kurano M, Tsukamoto K, Hara M, Ohkawa R,
Ikeda H and Yatomi Y: LDL receptor and ApoE are involved in the
clearance of ApoM-associated sphingosine 1-phosphate. J Biol Chem.
290:2477–2488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Qu X, Zhao S, Gao J, Hu M, Dong L and
Zhang X: Reduced expression and secretion of apolipoprotein M in
fat-fed, streptozotocin-diabetic rats is partially reversed by an
artificial ligand of PPARγ. Zhong Nan Da Xue Xue Bao Yi Xue Ban.
37:796–801. 2012.(In Chinese). PubMed/NCBI
|
|
67
|
Zhang PH, Gao JL, Pu C, Feng G, Wang LZ,
Huang LZ and Zhang Y: A single-nucleotide polymorphism C-724/del in
the proter region of the apolipoprotein M gene is associated with
type 2 diabetes mellitus. Lipids Health Dis. 15:1422016. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang PH, Gao JL, Pu C, Feng G, Wang L,
Huang L and Zhang Y: ApoM/HDL-C and apoM/apoA-I ratios are
indicators of diabetic nephropathy in healthy controls and type 2
diabetes mellitus. Clin Chim Acta. 466:31–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Niu N, Zhu X and Liu Y, Du T, Wang X, Chen
D, Sun B, Gu HF and Liu Y: Single nucleotide polymorphisms in the
proximal promoter region of apolipoprotein M gene (apoM) confer the
susceptibility to development of type 2 diabetes in Han Chinese.
Diabetes Metab Res Rev. 23:21–25. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang Z, Chu G and Yin RX: Apolipoprotein
M T-778C polymorphism is associated with serum lipid levels and the
risk of coronary artery disease in the Chinese population: A
meta-analysis. Lipids Health Dis. 12:1352013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhou JW, Tsui SK, Ng MC, Geng H, Li SK, So
WY, Ma RC, Wang Y, Tao Q, Chen ZY, et al: Apolipoprotein M gene
(APOM) polymorphism modifies metabolic and disease traits in type 2
diabetes. PLoS One. 6:e173242011. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Guo H, Zhao XX, Zhang XJ, Chen W and Zhang
J: Functional study of −724I/D polymorphism in apolipoprotein M
(apoM) gene promoter region and its association with myocardial
infarction. Med Sci Monit. 21:371–375. 2015. View Article : Google Scholar : PubMed/NCBI
|















