Introduction
Parkinson's disease (PD) is a neurodegenerative
disorder with high incidence among the elderly (1,2).
Symptoms of PD include motor defects and other non-motor
disabilities. The main motor impairments are resting tremor,
postural instability and bradykinesia. Non-motor disabilities
include depression, anxiety, cognitive impairment and sleep
disorders (3). One of the symptoms
of PD is pathological selective degeneration of dopaminergic
neurons (4).
Epidemiological studies demonstrated that exposure
to pesticides increases the risk of PD (5). Paraquat (PQ) is an organic herbicide
that can affect energy metabolism by inhibiting mitochondrial
respiratory chain reactions (6).
PQ can control certain intracellular signaling cascades (7). Maneb (MB) is a fungicide widely used
in crop production that can influence dopamine (DA) homeostasis and
mitochondrial function. It has been proposed that PQ, in the
presence of MB, alters the DA metabolism by enhancing the exposure
of neurons to reactive oxygen species and reactive nitrogen
clusters. A previous report, using rats as model organisms,
demonstrated that combined exposure to PQ and MB is more toxic
compared with exposure to PQ alone (8). MB can increase the content of PQ in
the brain by changing the metabolic dynamics of PQ and therefore
converting non-toxic PQ into a toxic form (9).
PQ and MB are lethal pesticides frequently used in
combination for crop production in China, including potatoes,
tomatoes and other vegetables. Residual PQ and MB are frequently
detected in China (10,11).
The wingless (Wnt) protein family primarily includes
secreted factors (12). Wnt1 and
Wnt5a serve a role in the regulation of proliferation and
differentiation of midbrain dopaminergic neurons (13). It has been demonstrated that Wnt1
and Wnt5a can interact to regulate the homeostasis of the nuclear
receptor related factor 1 (Nurr1), and that Nurr1+ precursors
promote formation of dopaminergic neurons in the midbrain (14,15).
Previous research has indicated that exposure to
environmental toxins can cause permanent damages to biochemical
homeostasis and behavior of adult rats (16). The present study investigated the
hypothesis that combined exposure to PQ and MB during the gestation
and lactation period of rats can lead to alterations in Wnt1 and
Wnt5a.
Materials and methods
Chemicals and reagents
PQ was purchased from J&K Chemical Technology,
Inc. (Beijing, China) and MB was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany; both 98% pure). Rabbit anti-Wnt1
(cat. no. ab85060; 1:1,000), anti-Nurr1 (cat. no. ab9332; 1:1,000),
anti-Tyrosine hydroxylase (TH; cat. no. ab6221; 1:1,000) and
anti-actin (cat. no. ab179467; 1:1,000) antibodies were purchased
from Abcam (Cambridge, UK). An anti-Wnt5a antibody (cat. no.
55184-1-AP; 1:1,000) was purchased from Wuhan Sanying Biotechnology
(Wuhan, China), and the goat anti-rabbit IgG antibody (cat. no.
ZB-2305; 1:5,000) was purchased from Beijing Zhongshan Golden
Bridge Biotechnology Co., Ltd. (Beijing, China).
Animals and treatments
The present study was approved by the Ethical
Committee of the Harbin Medical University (Harbin, China). A total
of 40 virgin female and 20 male Sprague-Dawley rats were purchased
from Vital River Laboratories (Beijing, China). The animals were
reared for1 week in barrier facilities and then female and male
rats were mated at 2:1 proportion. Vaginal smear was examined the
next day and when sperm was identified, rats were categorized as
day 0 subjects of the study. Pregnant rats were randomly divided
into three groups: i) The saline treatment group, 1 mg/kg body
weight saline, (n=10); ii) the high dose treatment group, 15 mg/kg
body weight PQ + 45 mg/kg body weight MB, (n=15); and iii) the low
dose treatment group, 10 mg/kg body weight PQ + 30 mg/kg body
weight MB, (n=15). Administration of saline, or PQ and MB was
performed by gavaging twice a week from gestation to weaning. Rats
were sacrificed following weaning.
Body weight and food consumption
Virgin female (230±20 g) and male Sprague-Dawley
rats (310±20 g) used in the present study were 8 weeks old. The
animals were provided water and food ad libitum. The animal
cages were maintained at a constant 12 h light/dark cycle,
temperature 22±2°C and relative humidity at 50±15%. There was no
significant difference in body weight and food consumption among
females and offspring once a week during the experiment (data not
shown).
High performance liquid chromatography
with a fluorescence detector (HPLC-FL) determination of DA
The DA content was detected by HPLC-FL. The corpus
striatum of the rats in each group were homogenized in 0.1 M
perchloric acid and centrifuged at 2,000 × g for 20 min at 4°C. The
supernatant was then filtered through a 0.2 µm cellulose membrane.
The following chromatography conditions were used: 45×150 mm
VERIANODSCI8 column (5 µm; Nacalai Tesque, Inc., Kyoto, Japan); The
mobile phase was composed of 20 mM trisodium citrate and 50 mM
sodium hydrogen phosphate. The following chromatography conditions
were used: Temperature, 35°C; flow rate, 1.0 ml/min. A fluorescence
detector (Agilent Technologies, Inc., Santa Clara, CA, USA) was set
at an excitation wavelength of 285 nm and an emission wavelength of
333 nm. The data were quantified using the area under the peaks and
external standards. The quantification was verified using
calibration curves obtained from individual monoamine
standards.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
The midbrain tissue was dissolved in TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
reagent. Total RNA was extracted from the treated midbrain tissue
according to the manufacturer's protocol. Primers for rat Wnt1,
Wnt5a, Nurr1 and TH were designed using the Primer Premier software
(version, 5.0; Premier Biosoft Internetional, Palo Alto, CA, USA;
Table I), based on Gene Bank
sequences of these genes. Synthesis of cDNA was performed using
PrimeScript RT reagent kit and gDNA Eraser (both from Takara
Biotechnology Co., Ltd., Dalian, China), according to the
manufacturer's protocol. qPCR reactions were performed using
SYBR® Premix Ex Taq™ II kit (Takara
Biotechnology Co., Ltd.) in 20 µl reactions containing 2 µg cDNA
template and 10 µM forward and reverse primers in an ABI 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The following thermocycling conditions were used for the
qPCR: Initial denaturation at 95°C for 30 sec; 35 cycles of 94°C
for 5 sec; 57.4°C for 20 sec and 72°C for 20 sec; and final
extension at 72°C for 1 min. The threshold cycle was determined and
the results are expressed as relative expression ratio. The
relative expression ratio of a target gene was calculated as
previously described (17).
 | Table I.Primer sequences for the reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for the reverse
transcription-quantitative polymerase chain reaction.
| Target | Forward 5′→3′ | Reverse 5′→3′ | Length (bp) | Annealing
temperature (°C) |
|---|
| Wnt1 |
TTTTCTCTCCGTGTCCCT |
GCTCCCCAACCTTATTTC | 227 | 60 |
| Wnt5a |
GACTTACCTCGGGACTGG |
GACCTGCTTCATTGTTGTG | 166 | 58 |
| Nurr1 |
CCAATCCGGCAATGACCAG |
TGATGATCTCCATAGAGCCAGTCAG | 129 | 60 |
| TH |
AGCTGTGCAGCCCTACCAAGA |
GTGTGTACGGGTCAAACTTCACAGA | 140 | 62 |
| β-actin |
GGAAATCGTGCGTGACATTAAAG |
CGGCAGTGGCCATCTCTT | 74 | 60 |
Western blotting
Western blot analysis was used to quantify the
protein amount of Wnt1, Wnt5a, Nurr1 and TH. Midbrain tissue was
lysed in Radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology, Shanghai, China) for 2 h on ice. The
protein concentrations in the supernatants were determined with a
Bicinchoninic Acid protein assay kit (Beyotime Institute of
Biotechnology). Protein samples, 4 µl each protein per lane, were
separated on 10% SDS-PAGE gels (80 V, 20 min). Proteins were
electrophoretically transferred to polyvinylidene difluoride (PVDF)
membranes. PVDF membranes were blocked in 5% skimmed dry milk in
Tris-buffered saline (TBS) at room temperature for 30 min.
Membranes were then incubated with primary antibodies at 4°C
overnight. The following day, PVDF membranes were washed with TBS
containing Tween-20 four times for 5, 10, 10 and 15 min. PVDF
membranes were then incubated with a goat anti-rat horseradish
peroxidase secondary antibody at room temperature for 60 min. The
PVDF membranes were then washed with TBS containing Tween-20 four
times as previously. Antibody binding was detected using an
enhanced chemiluminescence system (Tanon Science and Technology
Co., Ltd., Shanghai, China). Density of the specific protein bands
was standardized to β-actin with the Image J software (version 1.5;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation.
Data were analyzed using the SPSS software (version 17.0; SPSS,
Inc., Chicago, IL, USA). The difference between groups was analyzed
using analysis of one way analysis of variance and Bonferroni's
multiple comparison was used as a post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of combined exposure to PQ and
MB during development on DA levels
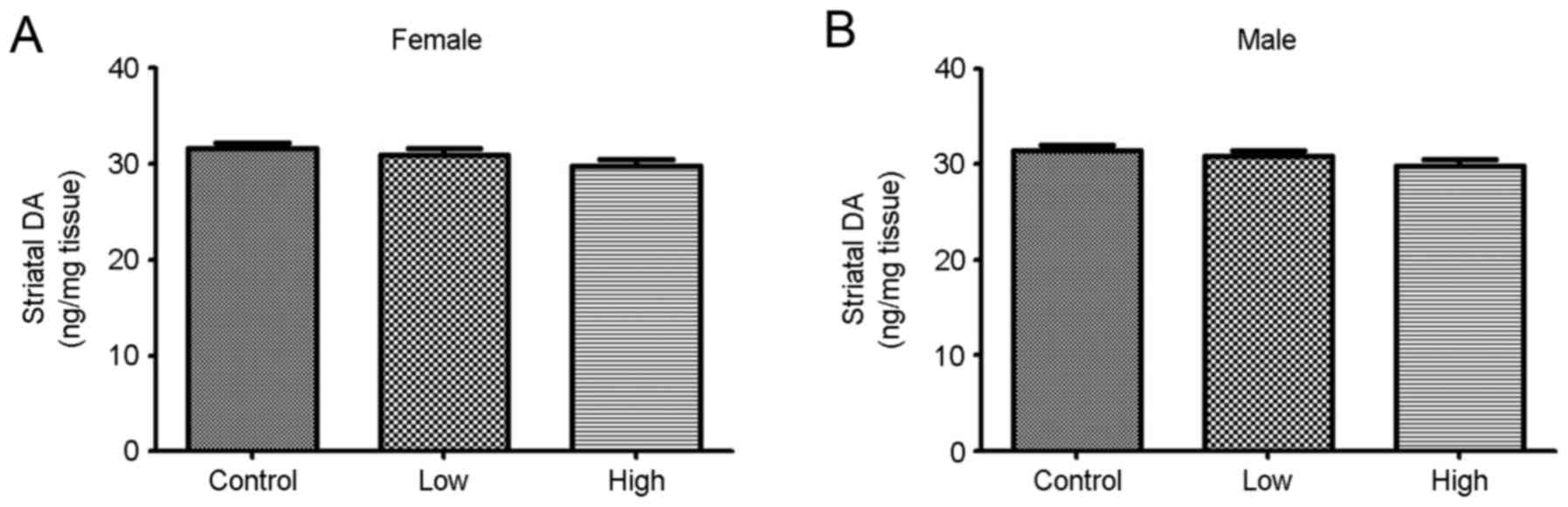
DA levels were measured following combined exposure
to PQ and MB during gestation and lactation periods. The DA levels
decreased upon exposure to PQ and MB, but the differences were not
statistically significant (Fig.
1).
Effects of combined exposure to PQ and
MB during development on Wnt1 mRNA and protein expression
levels
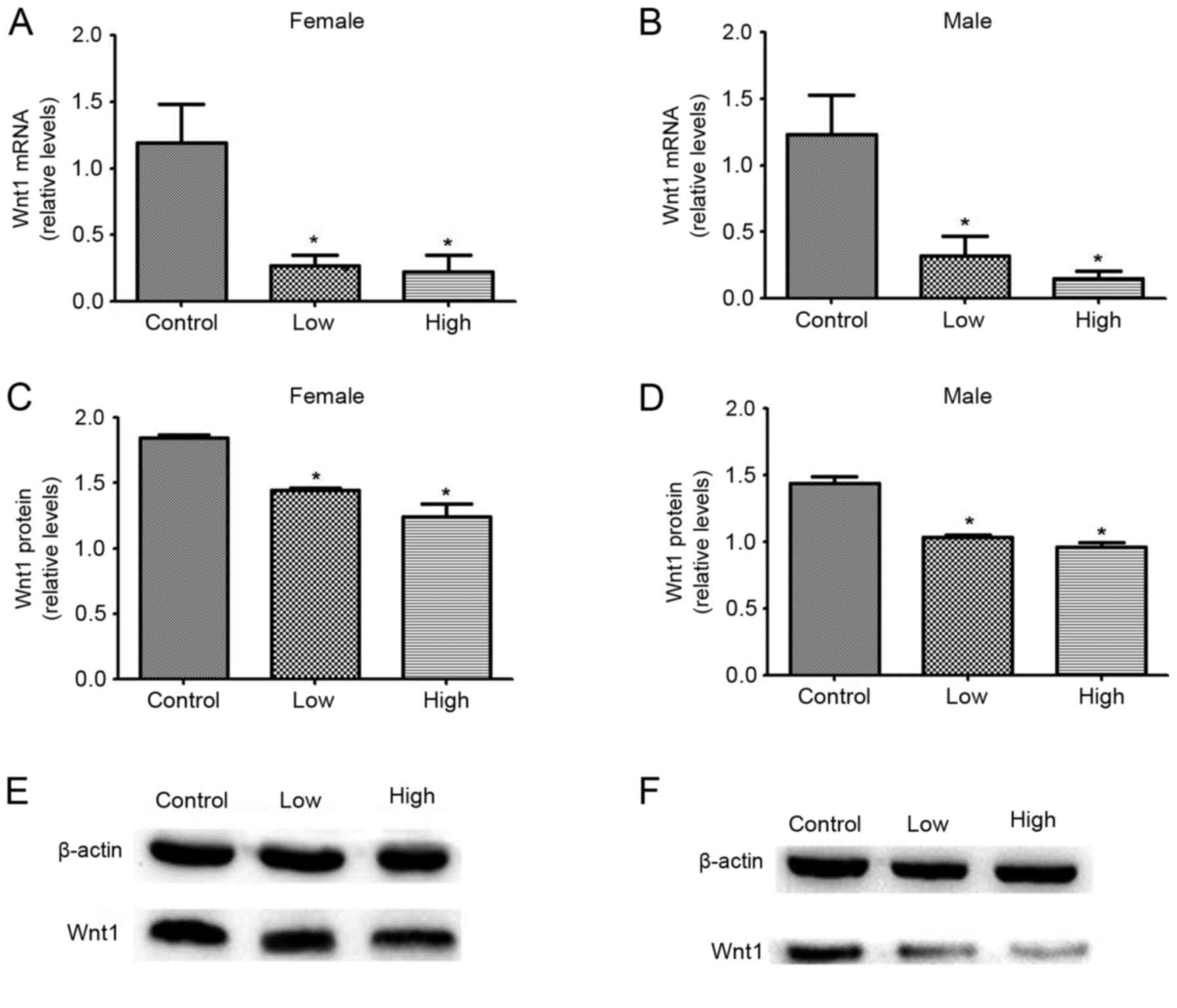
The effects of combined exposure to PQ and MB during
development on midbrain Wnt1 protein and mRNA expression levels
were studied in female and male offspring. mRNA and protein
expression levels of Wnt1 decreased significantly upon combined
exposure to PQ and MB at both high and low doses, compared with the
unexposed control group. Among female offspring, mRNA expression
levels decreased by 77.40 and 81.02%, and protein expression levels
decreased by 21.69 and 32.75% in the low and high dose groups,
respectively. Among male offspring, mRNA expression levels
decreased by 74.00 and 87.62%, and protein expression levels
decreased by 28.23 and 33.00% in the low and high dose groups,
respectively (Fig. 2).
Effects of developmental exposure to
PQ and MB on Wnt5a mRNA and protein expression levels
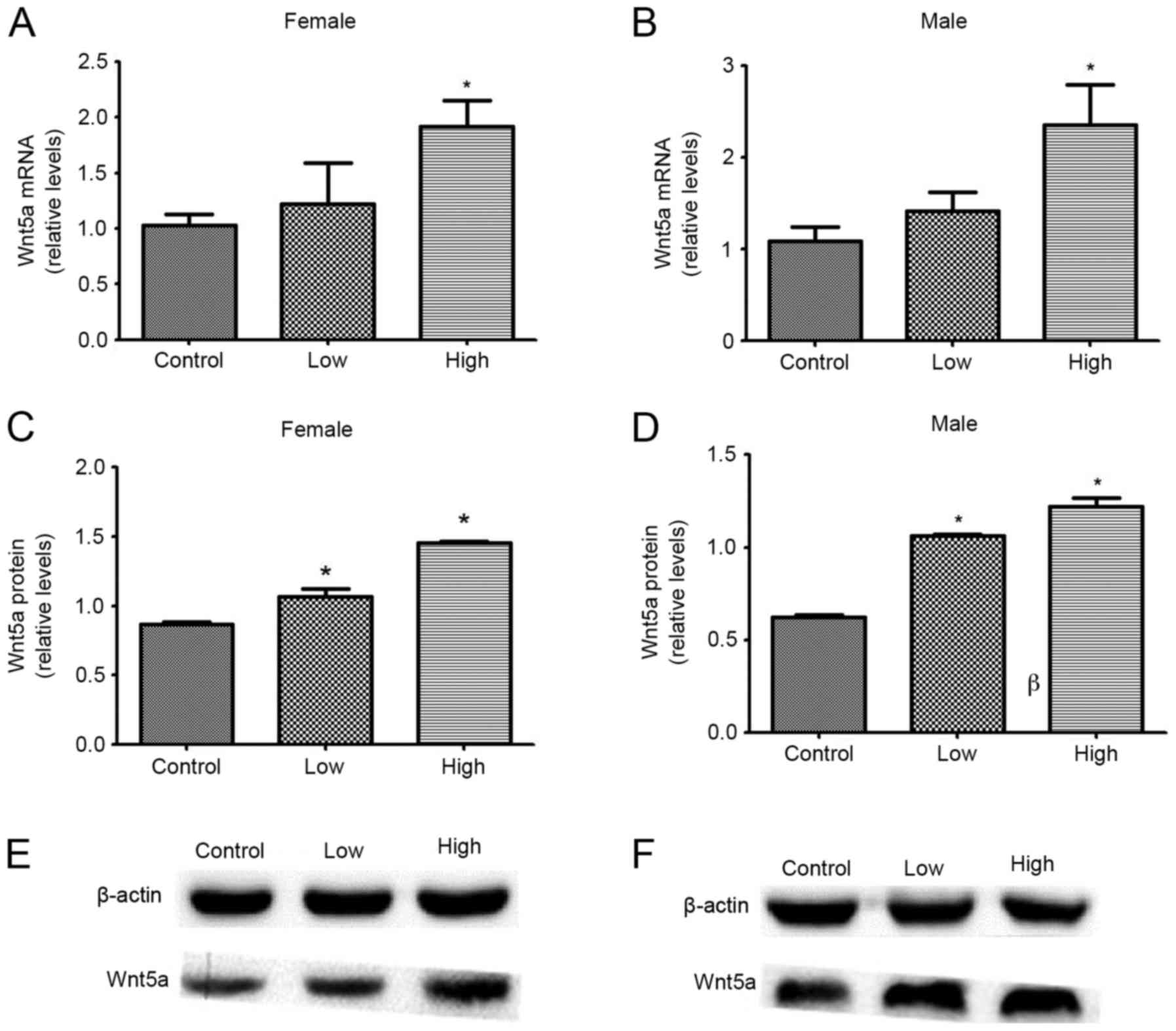
The effect of combined exposure to PQ and MB during
development on Wnt5a protein and mRNA expression levels in the
midbrain, was investigated. mRNA and protein levels of Wnt5a were
significantly increased following exposure to a high dose of PQ and
MB, compared with the control group (all, P<0.05). Only protein
expression of Wnt5a in both males and females was significantly
increased upon exposure to low doses of PQ and MB, compared with
the control group (P<0.05). Among female offspring, mRNA
expression levels increased by 18.94 and 86.68% and protein
expression levels increased by 22.67 and 66.94%, in the low and
high dose groups, respectively. Among male offspring, mRNA
expression levels increased by 30.61 and 117.03% and protein
expression levels increased by 70.70 and 95.87%, in the low and
high dose groups, respectively (Fig.
3).
Effects of developmental exposure to
PQ and MB on Nurr1 mRNA and protein expression levels
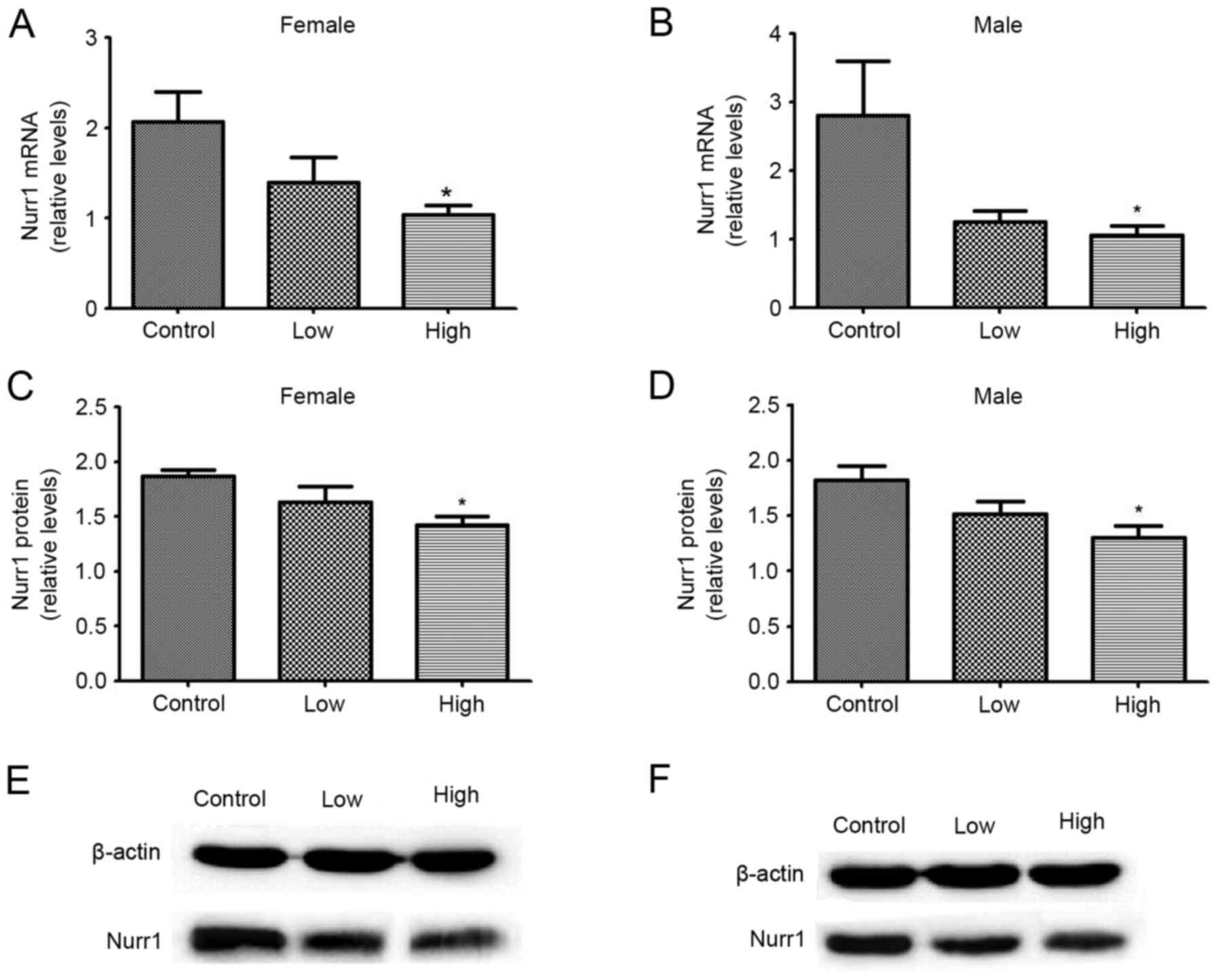
As presented in Fig.
4, the effect of combined exposure to PQ and MB during
development on midbrain Nurr1 protein and mRNA expression levels
was studied in female and male offspring. mRNA and protein
expression levels of Nurr1 were significantly decreased following
exposure to high doses of PQ and MB, compared with the control
group (P<0.05). Among female offspring, mRNA expression levels
decreased by 32.29 and 49.84%, and protein expression levels
decreased by 12.93 and 23.90%, in the low and high dose groups,
respectively. Among male offspring, mRNA expression levels
decreased by 55.57 and 62.50%, and protein expression levels
decreased by 16.55 and 28.47%, in the low and high dose groups,
respectively.
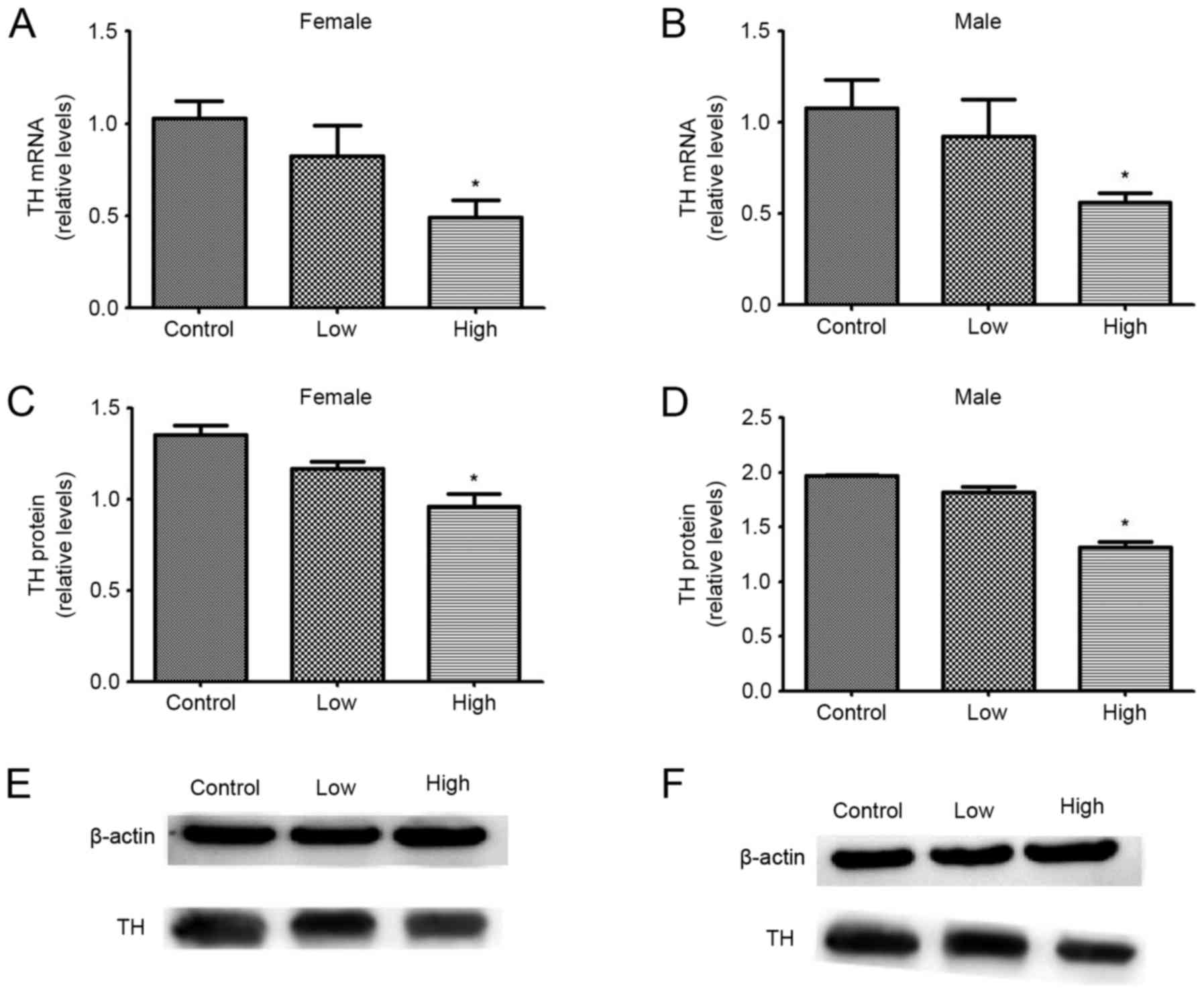
Effects of exposure to PQ and MB
during development on TH mRNA and protein expression levels
The effects of combined exposure to PQ and MB during
development on midbrain TH protein and mRNA expression levels in
female and male offspring are summarized in Fig. 5. mRNA and protein expression levels
of TH were significantly decreased following exposure to PQ and MB
compared with the control group, but only when administrered at a
high dose (P<0.05). Among female offspring, mRNA levels
decreased by 19.90 and 52.43% and protein levels decreased by 13.68
and 28.79%, in the low and high dose groups, respectively. Among
male offspring, mRNA expression levels decreased by 14.21 and
48.14% and protein expression levels decreased by 7.86 and 33.35%,
in the low and high dose groups, respectively.
Discussion
PQ can cross the brain blood barrier directly as it
has a similar structure to the toxic 1-methyl-4-phenylpyridinium
(MPP+), but the process is less efficient.
MPP+ can be bound by a transport protein of the
dopaminergic neurons and transported to mitochondria. This process
promotes excessive free oxygen radical secretion and induction of
oxidative stress responses, which inhibit the activity of
mitochondrial respiratory chain complex I and the synthesis of ATP,
leading to denaturation and death of dopaminergic neurons (18). A number of studies investigated the
effects of PQ on the Wnt signaling pathway. L'Episcopo et al
(19) demonstrated that exposure
to MPP+ decreased protein expression of β-catenin and
led to overexpression of phosphorylated glycogen synthase kinase 3β
in mice, affecting the Wnt signaling pathway. According to the
results obtained by Gollamudi et al (20), combined treatment with PQ and MB
affects Wnt pathways more significantly compared with the effect of
PQ alone. MB selectively inhibits mitochondrial complex III which
may lead to a reduction in DA release, and therefore an increase in
DA concentration in synaptic vesicles (21). Therefore, combined treatment with
MB and PQ can induce oxidative stress, and dopaminergic neurons can
be more susceptible to injury caused by the oxidative stress
(22). PQ can induce the injury of
PC-12 cells in dopaminergic neurons through Wnt signaling pathways
(23). Investigation of the
combined effect of PQ and MB can have toxicological
implications.
Previous studies have demonstrated that PQ and MB
can permeate into embryos through the placental barrier during the
early stage of brain development, affecting the development of
dopaminergic neurons (24). Wnt1
and Wnt5a are secreted glycoproteins that serve a role on the
formation and differentiation of midbrain dopaminergic neurons
(25). Wnt1 activates the Wnt
signaling pathway to promote the formation of midbrain dopaminergic
neurons (26). Knockdown of Wnt1
impairs proliferation of dopaminergic precursors and leads to the
death of dopaminergic neurons in the midbrain (27). Wnt5a promotes differentiation of
neural cells into dopaminergic precursors (28). The knockdown of Wnt5a leads to
enhanced proliferation of progenitor cells (29).
In the present study, pregnant rats were exposed to
PQ and MB from the 5th day of gestation to weaning, a period during
which Wnt proteins begin to be expressed (30). In the present study, it has been
observed that exposure to PQ and MB during gestation and lactation
leads to reduced and increased expression of Wnt1 and Wnt5a mRNA
and protein expression, respectively, in both female and male
offspring. Wnt1 and Wnt5a coordinate to promote the formation and
proliferation of midbrain dopaminergic precursors (31).
Nurr1 is expressed in the central nervous system and
promotes the differentiation of neural precursors into dopaminergic
neurons (32–34). Nurr1 expression begins at day 10 of
embryo development, reaches peak 1–2 days following birth, and then
gradually decreases, but its expression remains high in the
midbrain (35). Knock out of Nurr1
during embryonic development can result in incomplete development
of dopaminergic neurons (36). In
the present study, exposure to PQ and MB during gestation and
lactation led to reduced expression of Nurr1 mRNA and protein
expression, in both female and male offspring.
Previous studies have demonstrated that Nurr1
directly regulates the promoter of TH gene (37). Upon activation, Nurr1 translocates
to the nucleus, where it binds to the promoter region and activates
the transcription of TH (38). TH
is a marker for dopaminergic neurons and a rate limiting enzyme in
the synthesis of dopamine (39–41).
TH is only expressed in mature dopaminergic neurons, when enzymes
and transporters specific for dopaminergic neurons are fully
expressed and functional. Combined exposure to PQ and MB during
gestation and lactation reduced TH mRNA and protein expression in
both female and male offspring.
The effects of combined exposure to PQ and MB on DA
levels in the striatum of offspring were investigated in the
present study. DA levels in the striatum of offspring were not
significantly decreased, even though TH expression was
downregulated. It has been previously hypothesized that in order to
maintain homeostasis, the synthesis, release, degradation and
re-uptake of DA are regulated by multiple mechanisms (42). A plausible explanation of the
results of the present study is that the transportation and
re-uptake of DA were altered in order to compensate for the lack of
DA synthesis and to maintain the function of the DA system. In
theory, it is possible that the DA content in the striatum
decreases with age and this decrease can cause symptoms of PD.
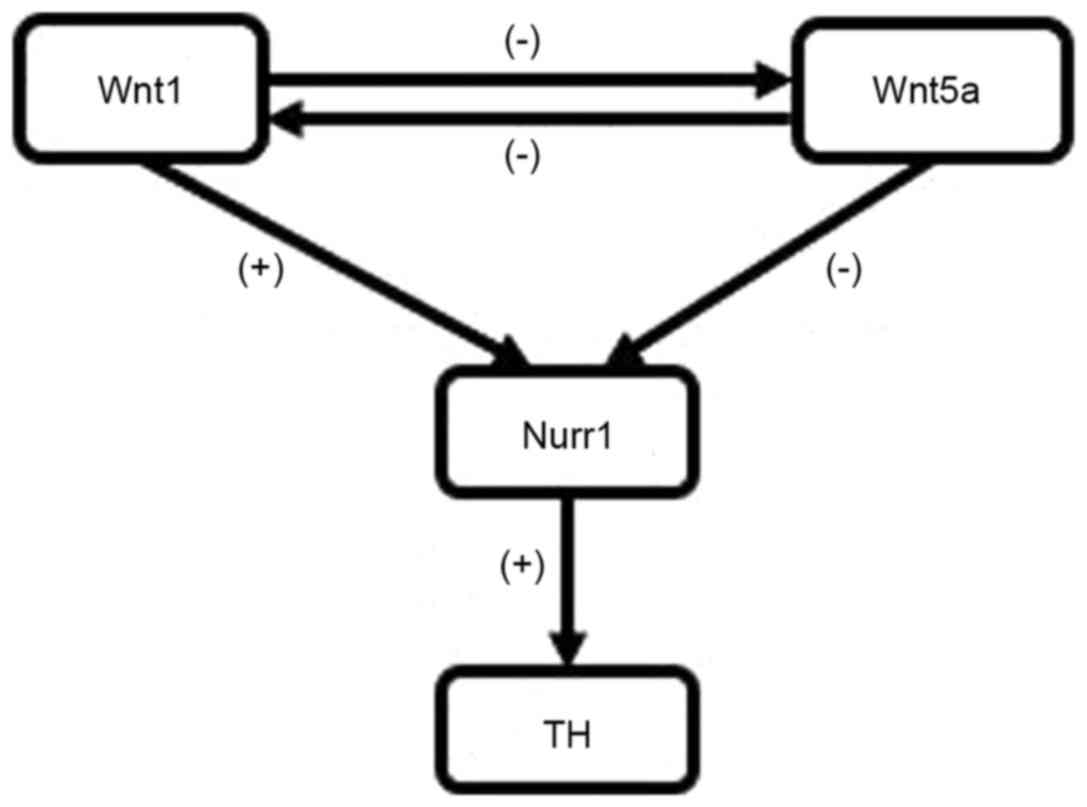
In conclusion, the present study demonstrated that
combined exposure to PQ and MB during gestation and lactation
alters the expression of proteins associated with the formation and
development of dopaminergic neurons in offspring. It has been
demonstrated that exposure to PQ and MB during gestation and
lactation leads to upregulation of Wnt5a and down-regulation of
Wnt1, Nurr1 and TH (Fig. 6).
Future studies should investigate whether exposure to PQ and MB
during gestation and lactation followed by a subsequent re-exposure
during adulthood would enhance susceptibility of dopaminergic
neurons to environmental risk factors.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402711).
Glossary
Abbreviations
Abbreviations:
|
PQ
|
paraquat
|
|
MB
|
maneb
|
|
TH
|
tyrosine hydroxylase
|
|
Wnt1
|
wingless 1
|
|
Wnt5a
|
wingless 5a
|
|
PD
|
Parkinson's disease
|
|
HPLC-FL
|
high performance liquid chromatography
with a fluorescence detector
|
|
DA
|
dopamine
|
|
Nurr1
|
nuclear receptor related factor 1
|
|
MPP+
|
1-methyl-4-phenylpyridinium
|
References
|
1
|
Hauser RA, Heritier S, Rowse GJ, Hewitt LA
and Isaacson SH: Droxidopa and reduced falls in a trial of
parkinson disease patients with neurogenic orthostatic hypotension.
Clin Neuropharmacol. 39:220–226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldman JG: Neuropsychiatric issues in
Parkinson disease. Continuum. 22:1086–1103. 2016.PubMed/NCBI
|
|
3
|
Savica R, Grossardt BR, Bower JH, Ahlskog
JE and Rocca WA: Time trends in the incidence of parkinson disease.
JAMA Neurol. 73:981–989. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pathak K and Akhtar N: Nose to brain
delivery of nanoformulations for neurotherapeutics in parkinson's
disease: Defining the preclinical, clinical and toxicity issues.
Curr Drug Deliv. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ritz B and Yu F: Parkinson's disease
mortality and pesticide exposure in California 1984–1994. Int J
Epidemiol. 29:323–329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shimizu K, Matsubara K, Ohtaki K and
Shiono H: Paraquat leads to dopaminergic neural vulnerability in
organotypic midbrain culture. Neurosci Res. 46:523–532. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Finkbeiner S, Tavazoie SF, Maloratsky A,
Jacobs KM, Harris KM and Greenberg ME: CREB: A major mediator of
neuronal neurotrophin responses. Neuron. 19:1031–1047. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thiruchelvam M, Prokopenko O, Cory-Slechta
DA, Buckley B and Mirochnitchenko O: Overexpression of superoxide
dismutase or glutathione peroxidase protects against the paraquat +
maneb-induced Parkinson disease phenotype. J Biol Chem.
280:22530–22539. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Prakash J, Yadav SK, Chouhan S and Singh
SP: Neuroprotective role of Withania somnifera root extract in
maneb-paraquat induced mouse model of parkinsonism. Neurochem Res.
38:972–980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumari R, Jha RR, Singh MP and Patel DK:
Whirling agitated single drop microextraction technique for the
simultaneous analysis of paraquat and maneb in tissue samples of
treated mice. J Sep Sci. 39:1725–1733. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caputi FF, Carretta D, Lattanzio F,
Palmisano M, Candeletti S and Romualdi P: Proteasome subunit and
opioid receptor gene expression down-regulation induced by paraquat
and maneb in human neuroblastoma SH-SY5Y cells. Environ Toxicol
Pharmacol. 40:895–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni W, Zeng S, Li W, Chen Y, Zhang S, Tang
M, Sun S, Chai R and Li H: Wnt activation followed by Notch
inhibition promotes mitotic hair cell regeneration in the postnatal
mouse cochlea. Oncotarget. 7:66754–66768. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kriks S, Shim JW, Piao J, Ganat YM,
Wakeman DR, Xie Z, Carrillo-Reid L, Auyeung G, Antonacci C, Buch A,
et al: Dopamine neurons derived from human ES cells efficiently
engraft in animal models of Parkinson's disease. Nature.
480:547–551. 2011.PubMed/NCBI
|
|
14
|
Alavian KN, Jeddi S, Naghipour SI, Nabili
P, Licznerski P and Tierney TS: The lifelong maintenance of
mesencephalic dopaminergic neurons by Nurr1 and engrailed. J Biomed
Sci. 21:272014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kitagawa H, Ray WJ, Glantschnig H,
Nantermet PV, Yu Y, Leu CT, Harada S, Kato S and Freedman LP: A
regulatory circuit mediating convergence between Nurr1
transcriptional regulation and Wnt signaling. Mol Cell Biol.
27:7486–7496. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee PC, Bordelon Y, Bronstein J and Ritz
B: Traumatic brain injury, paraquat exposure and their relationship
to Parkinson disease. Neurology. 79:2061–2066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yuan JS, Reed A, Chen F and Stewart CN Jr:
Statistical analysis of real-time PCR data. BMC Bioinformatics.
7:852006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schober A: Classic toxin-induced animal
models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res.
318:215–224. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
L'Episcopo F, Tirolo C, Testa N, Caniglia
S, Morale MC, Deleidi M, Serapide MF, Pluchino S and Marchetti B:
Plasticity of subventricular zone neuroprogenitors in MPTP
(1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) mouse model of
Parkinson's disease involves cross talk between inflammatory and
Wnt/β-catenin signaling pathways: Functional consequences for
neuroprotection and repair. J Neurosci. 32:2062–2085. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gollamudi S, Johri A, Calingasan NY, Yang
L, Elemento O and Beal MF: Concordant signaling pathways produced
by pesticide exposure in mice correspond to pathways identified in
human Parkinson's disease. PloS One. 7:e361912012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Desplats P, Patel P, Kosberg K, Mante M,
Patrick C, Rockenstein E, Fujita M, Hashimoto M and Masliah E:
Combined exposure to Maneb and Paraquat alters transcriptional
regulation of neurogenesis-related genes in mice models of
Parkinson's disease. Mol Neurodegener. 7:492012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Barlow BK, Thiruchelvam MJ, Bennice L,
Cory-Slechta DA, Ballatori N and Richfield EK: Increased
synaptosomal dopamine content and brain concentration of paraquat
produced by selective dithiocarbamates. J Neurochem. 85:1075–1086.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan M, Wang X, Zhao L, Chang X and Zhou Z:
The effect of Wnt signaling pathway on paraquat induced PC12 cells
damage. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 33:806–811.
2015.(In Chinese). PubMed/NCBI
|
|
24
|
Kumar A, Leinisch F, Kadiiska MB, Corbett
J and Mason RP: Formation and implications of alpha-synuclein
radical in maneb- and paraquat-induced models of Parkinson's
disease. Mol Neurobiol. 53:2983–2994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sousa KM, Villaescusa JC, Cajanek L, Ondr
JK, Castelo-Branco G, Hofstra W, Bryja V, Palmberg C, Bergman T,
Wainwright B, et al: Wnt2 regulates progenitor proliferation in the
developing ventral midbrain. J Biol Chem. 285:7246–7253. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Götz S, Weisenhorn DM Vogt,
Simeone A, Wurst W and Prakash N: A WNT1-regulated developmental
gene cascade prevents dopaminergic neurodegeneration in adult
En1(+/-) mice. Neurobiol Dis. 82:32–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Barlow BK, Lee DW, Cory-Slechta DA and
Opanashuk LA: Modulation of antioxidant defense systems by the
environmental pesticide maneb in dopaminergic cells.
Neurotoxicology. 26:63–75. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andersson ER, Prakash N, Cajanek L, Minina
E, Bryja V, Bryjova L, Yamaguchi TP, Hall AC, Wurst W and Arenas E:
Wnt5a regulates ventral midbrain morphogenesis and the development
of A9-A10 dopaminergic cells in vivo. PloS One. 3:e35172008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Andersson ER, Saltó C, Villaescusa JC,
Cajanek L, Yang S, Bryjova L, Nagy II, Vainio SJ, Ramirez C, Bryja
V and Arenas E: Wnt5a cooperates with canonical Wnts to generate
midbrain dopaminergic neurons in vivo and in stem cells. Proc Natl
Acad Sci U S A. 110:pp. E602–E610. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fischer T, Guimera J, Wurst W and Prakash
N: Distinct but redundant expression of the Frizzled Wnt receptor
genes at signaling centers of the developing mouse brain.
Neuroscience. 147:693–711. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gao H, Sun B, Fu H, Chi X, Wang F, Qi X,
Hu J and Shao S: PDIA6 promotes the proliferation of HeLa cells
through activating the Wnt/β-catenin signaling pathway. Oncotarget.
7:53289–53298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zetterstrom RH, Solomin L, Jansson L,
Hoffer BJ, Olson L and Perlmann T: Dopamine neuron agenesis in
Nurr1-deficient mice. Science. 276:248–250. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Saucedo-Cardenas O, Quintana-Hau JD, Le
WD, Smidt MP, Cox JJ, De Mayo F, Burbach JP and Conneely OM: Nurr1
is essential for the induction of the dopaminergic phenotype and
the survival of ventral mesencephalic late dopaminergic precursor
neurons. Proc Natl Acad Sci USA. 95:pp. 4013–4018. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Castillo SO, Baffi JS, Palkovits M,
Goldstein DS, Kopin IJ, Witta J, Magnuson MA and Nikodem VM:
Dopamine biosynthesis is selectively abolished in substantia
nigra/ventral tegmental area but not in hypothalamic neurons in
mice with targeted disruption of the Nurr1 gene. Mol Cell Neurosci.
11:36–46. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao Q, Castillo SO and Nikodem VM:
Distribution of messenger RNAs for the orphan nuclear receptors
Nurr1 and Nur77 (NGFI-B) in adult rat brain using in situ
hybridization. Neuroscience. 75:221–230. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hermanson E, Joseph B, Castro D, Lindqvist
E, Aarnisalo P, Wallén A, Benoit G, Hengerer B, Olson L and
Perlmann T: Nurr1 regulates dopamine synthesis and storage in MN9D
dopamine cells. Exp Cell Res. 288:324–34. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ding Y, Zhang Z, Ma J, Xia H, Wang Y, Liu
Y, Ma Q, Sun T and Liu J: Directed differentiation of postnatal
hippocampal neural stem cells generates nuclear receptor related1
protein and tyrosine hydroxylaseexpressing cells. Mol Med Rep.
14:1993–1999. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim KS, Kim CH, Hwang DY, Seo H, Chung S,
Hong SJ, Lim JK, Anderson T and Isacson O: Orphan nuclear receptor
Nurr1 directly transactivates the promoter activity of the tyrosine
hydroxylase gene in a cell-specific manner. J Neurochem.
85:622–634. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Huang L, Deng M, He Y, Lu S, Liu S and
Fang Y: β-asarone increases MEF2D and TH levels and reduces
α-synuclein level in 6-OHDA-induced rats via regulating the
HSP70/MAPK/MEF2D/Beclin-1 pathway: Chaperone-mediated autophagy
activation, macroautophagy inhibition and HSP70 up-expression.
Behav Brain Res. 313:370–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Medeiros HH, Santana MA, Leite MD, Aquino
LA, de Barros MA, Galvão NT, Ladd FV, Cavalcante JC, Costa MS,
Cavalcante JS and Nascimento ES Jr: The cytoarchitectonic and
TH-immunohistochemical characterization of the dopamine cell groups
in the substantia nigra, ventral tegmental area and retrorubral
field in a bat (Artibeus planirostris). Neurosci Res. 112:37–46.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nagatsu T and Nagatsu I: Tyrosine
hydroxylase (TH), its cofactor tetrahydrobiopterin (BH4), other
catecholamine-related enzymes and their human genes in relation to
the drug and gene therapies of Parkinson's disease (PD): Historical
overview and future prospects. J Neural Transm (Vienna).
123:1255–1278. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yu J, Li X, Yang J, Wu Y and Li B: Effects
of simazine exposure on neuronal development-related factors in
mn9d cells. Med Sci Monit. 22:2831–2838. 2016. View Article : Google Scholar : PubMed/NCBI
|