Introduction
Aging is a process of anatomical, functional and
metabolic changes that affects all systems, involves a process of
programmed cell death, and modulated by a subtle balance between
pro-and antiapoptotic molecules (1). Advancing age is a risk factor for a
variety of chronic health problems including cancer, diabetes,
cardiovascular, neurodegenerative and musculoskeletal diseases
(2). Physical activity and
high-Tryptophan diet may play important roles in aging-induced
diseases (3,4). Recent studies indicate that declined
autophagic capacity in aging cells impairs the process of cellular
housekeeping and increases the generation of reactive oxygen
species (ROS), which promotes oxidative stress. Oxidative stress
can induce the assembly of multiprotein inflammatory complexes
called the inflammasomes (5). A
growing body of evidence indicates that the aging process is
accompanied by a progressive chronic inflammatory response that are
mediated via chemokines, adhesion molecules, interleukin-l (IL-1)
and tumor necrosis factor-α (TNF-α). Indeed, the subclinical
chronic inflammatory process associated with the aging process has
been referred to as ‘inflamm-aging’ (6,7).
Studies have shown that these inflammatory factors are involved in
the initiation and regulation of most age-related diseases
(8).
Atherosclerosis (AS) is a manifestation of vascular
aging and is recognized as a chronic inflammatory disease of the
blood vessels. It is one of the underlying pathological changes
involved in age-related diseases (9). Inflammatory factors, such as TNF-α
and intercellular adhesion molecule-1 (ICAM-1) play important roles
in the pathogenesis and progression of AS (10,11).
Age-related oxidative stress mediates chronic
inflammation, which further accelerates AS (12). In particular, AS is the common
pathophysiological basis of cardiovascular and cerebrovascular
diseases. The arterial intima is the first site to be involved in
the early stages of AS (13).
Intima-media thickness (IMT) is widely used as a surrogate
indicator of asymptomatic AS. Increased IMT is an early sign of AS
(13). However, plaque formation
is the hallmark of AS.
Carotid artery has a greater predilection for
development of arteriosclerosis. Atherosclerotic changes in the
carotid artery tend to precede those in other parts of the systemic
vasculature. Studies have shown a close association of carotid AS
with coronary artery disease and ischemic cerebrovascular disease.
In addition, color Doppler ultrasound examination of carotid artery
is a convenient noninvasive and relatively straightforward
investigation. Therefore, assessment of carotid intima-media
thickness (CIMT) by ultrasonography can be used as a parameter to
assess carotid AS. Increased CIMT is an important risk factor for
cardiovascular and cerebrovascular diseases and has been used as a
noninvasive clinical indicator of early AS (13).
Transforming growth factor-β1 (TGF-β1) is implicated
in a wide array of cardiovascular pathological processes, including
hypertension, AS, myocardial hypertrophy and heart failure. It is a
pleiotropic cytokine which plays multiple roles in the regulation
of vascular function and hemostasis (14). In the context of AS, the role of
TGF-β1 is not well-characterized. On the one hand, TGF-β1 is
considered to be a protective cytokine (15), which has an antiatherogenic effect,
especially in the early stages of AS (16). On the other hand, proatherogenic
effects of TGF-β1 in the late stages of the disease has also been
reported; these include excessive formation of extracellular
matrix, promotion of in-stent restenosis and pathological vascular
remodeling (17,18).
Globule-epidermal growth factor-8 (MFG-E8) was
initially identified as a lactadherin with many discoidin domains
secreted in mouse mammary epithelial cells. Recently, studies have
shown that MFG-E8 is an element of the arterial inflammatory
signaling network which facilitates apoptosis in endothelial cells
(19). Studies have shown that
MFG-E8 is an inflammatory mediator that orchestrates diverse
cellular interactions involved in the pathogenesis of various
diseases, including hypertension and AS (19–21).
Recently, high-throughput proteomic screening has
indicated high expression levels of MFG-E8 and TGF-β1 in aging
arterial walls in both mice and humans. Both MFG-E8 and TGF-β1 are
essential elements of angiotensin II (Ang II) signaling.
Upregulation of MFG-E8 and TGF-β1 in arterial walls was shown to
accelerate age-associated arterial remodeling via activation of Ang
II signaling (19,22). In addition, high expression levels
of MFG-E8 and TGF-β1 have also been found in atherosclerotic
plaques (23). However, the
age-related differences in circulating MFG-E8, TGF-β1 have not been
investigated. Whether serum levels of MFG-E8, TGF-β1 affect the
process of AS is not clear. Further, the association of MFG-E8 and
TGF-β1 serum levels with severity of AS is not known. We
hypothesized that both MFG-E8 and TGF-β1 are age-related
inflammatory factors and that their expression levels are related
to the degree of AS.
In the present study, the primary objective was to
examine the association of serum levels of MFG-E8, TGF-β1, TNF-α
and ICAM-1 with age. The secondary objectives were to determine
serum levels of MFG-E8, TGF-β1 in patients with AS and to
investigate the correlation of serum levels of MFG-E8, TGF-β1 with
the severity of AS.
Materials and methods
Ethical approval
The aim of this study and all risks associated with
participation in the study were explained to all prospective
enrollees and written informed consent was obtained from all
subjects prior to their enrolment. The study protocol was approved
by the Medical Ethics Committee of the First Hospital of Jilin
University (ethical approval no. 2014-306; approval date, August 7,
2017) and was in compliance with the principles enshrined in the
Declaration of Helsinki.
Study population
A total of 157 study subjects (60 healthy
volunteers, 67 patients with carotid AS and 30 age-matched controls
without carotid AS) were recruited from the medical examination
center at The First Hospital of Jilin University between September
and December 2014. All study participants underwent following
investigations: Measurement of blood pressure, fasting blood
glucose (FBG), blood cell analysis, serum lipid profile [total
cholesterol (TC), high-density lipoprotein cholesterol (HDL-C),
low-density lipoprotein cholesterol (LDL-C), and triglycerides
(TG)], and serum alanine aminotransferase (ALT) levels.
Inclusion criteria
i) Sixty healthy volunteers (29 women and 31 men;
age range, 30–74 years) who showed no signs of carotid AS on
ultrasound were recruited from the medical examination center at
the First Hospital of Jilin University. None of the volunteers had
current or past history of hypertension, diabetes mellitus,
hyperlipidemia, smoking and other AS risk factors. ii) Sixty seven
patients (33 women and 34 men, age range, 40–86 years) with carotid
AS diagnosed by ultrasound, but who had normal serum lipid profile,
FBG and serum ALT levels.
Exclusion criteria
Patients with carotid AS who qualified any of the
following criteria were excluded: i) Use of hormones or other
immunosuppressive agents in the immediately preceding one month;
ii) abnormal thyroid function, connective tissue disease, Takayasu
arteritis or carotid aneurysm; iii) systemic immune disorders,
immunosuppressed patients; iv) history of infection of respiratory
tract, digestive tract or urinary tract within the last one month;
those having symptoms suggestive of infection such as fever, cough,
phlegm, nausea, vomiting, diarrhea, abdominal pain, frequent
urination, urgency or dysuria; patients with chronic infectious
diseases; v) severe anemia, primary hematological diseases; vi) any
tumor; vii) chronic hepatitis, liver cirrhosis, sever renal or
hepatic insufficiency, acute or chronic pancreatitis; viii) initial
stroke, pulmonary infarction or peripheral vascular disease; and
ix) organ or bone marrow transplant recipients.
Experimental groups
i) In order to investigate the age-related changes
in expressions of MFG-E8 and TGF-β1, 60 healthy volunteers without
carotid AS were divided into 3 groups according to age: Young group
(n=20, 10 men and 10 women; age range, 30–44 years); middle age
group (n=20, 10 men and 10 women; age range, 45–59 years) and old
group (n=20, 9 men and 11 women; age 60–74 years). ii) To examine a
potential association of MFG-E8 and TGF-β1 with the degree of AS,
97 patients with carotid AS were divided into 3 groups according to
the degree of carotid AS: IMT increased group (n=27, 10
hypertension, 5 diabetes, 14 without hypertension or diabetes, 2
concomitant hypertension and diabetes); plaque group (n=40, 15
hypertension, 12 diabetes, 21 without hypertension or diabetes, 8
concomitant hypertension and diabetes) and control group (n=30, 12
hypertension, 6 diabetes, 15 without hypertension or diabetes, 9
concomitant hypertension and diabetes).
Carotid ultrasound acquisition
protocol and diagnostic criteria for carotid AS
All enrolled participants underwent carotid
ultrasound examination with an ultrasonic probe frequency of 7.0
MHz by a trained senior technician from the color ultrasonic room,
Department of Neurology at the First Hospital of Jilin University
First Hospital. Carotid artery ultrasound examinations were
performed as previously described (24) with some modifications. In brief,
for each patient, ultrasonic images were obtained from 10 segments
of the bilateral carotid arteries: The common carotid artery, the
carotid bifurcation, the internal carotid artery, external carotid
artery and subclavian artery. CIMT of bilateral common carotid
arteries were measured. The maximal CIMT was measured on the near
and far wall at each segment. According to the diagnostic criteria
for carotid AS recommended by the Ultrasound Physicians Society of
China (2011), IMT <1.0 mm was defined as normal; 1.0 mm< IMT
<1.5 mm was defined as increased CIMT; local CIMT ≥1.5 mm or a
relative 50% increase of the local intima thickness was defined as
carotid atherosclerotic plaque. Therefore, patients with increased
CIMT or carotid atherosclerotic plaque or both were diagnosed as
CAS.
Evaluation of carotid plaque by Crouse
score
Leaving the length of carotid atherosclerotic
plaques out of account, Crouse scores for plaques were determined
as the sum of the maximum IMT of each plaque of both right and left
carotid arteries, as described elsewhere (25).
Enzyme-linked immunosorbent assays for
serum MFG-E8, TGF-β1, TNF-α and ICAM-1
Plasma concentrations of MFG-E8, TGF-β1, TNF-α and
ICAM-1 were determined by ELISA kits (cat. no. 2805-MF-050, MFG-E8,
Solid Phase Sandwich ELISA; R&D Systems, Minneapolis, MN, USA),
(cat. no. SEA010Hu, TGF-β1), (cat. no. SEA133Hu, TNF-α), (cat. no.
SEA548Hu, ICAM-1) (all from Uscn Life Science, Inc., Wuhan, China),
following the manufacturer's instructions. The minimal detectable
concentrations were: 0.5 pg/ml for MFG-E8; 1.0 pg/ml for TGF-β1;
<4.0 pg/ml for TNF-α; and 1.0 pg/ml for ICAM-1.
Laboratory measurements
TC, TGs, HDL-C, and FBG were quantified by routine
enzymatic laboratory methods using an automated biochemical
analyzer (Hitachi 7060; Hitachi, Tokyo, Japan).
Statistical analysis
A database was built using SPSS 20.0 for statistical
analysis. Normally distributed continuous variables are presented
as mean ± SD, while non-normally distributed variables are
presented as median and 25–75th interquartile range. Categorical
variables are presented as frequencies or percentages. For
quantitative continuous variables, the independent samples t-test
was performed. One-way ANOVA was used to assess the differences
between the three groups. Q test was used to assess between-group
differences. Chi-square test was used to compare categorical
variables. Pearson correlation test was used for normally
distributed continuous variables, while Spearman correlation test
was used for non-normally distributed continuous variables.
Logistic regression analysis was performed to describe the
relationship of MFG-E8 and TGF-β1 with the degree of AS. P<0.05
was considered to indicate a statistically significant
difference.
Results
Baseline characteristics of healthy
volunteers
There was a significant difference in ages of
healthy volunteers in the young, middle-age and old groups
(P<0.001 for all). Significant differences were found with
respect to TC and LDL-C levels between the young, middle-age and
old groups (P<0.05 for all). This suggested that TC, and LDL-C
levels increased with increase in age. There was no significant
difference with respect to other variables between the three groups
(P>0.05 for all; Table I).
 | Table I.Baseline characteristics of healthy
volunteers. |
Table I.
Baseline characteristics of healthy
volunteers.
|
| Groups |
|
|---|
|
|
|
|
|---|
| Indicators | Young group
(n=20) | Middle-age group
(n=20) | Old group (n=20) | P-value |
|---|
| Age (years) |
38.35±4.15 |
50.90±4.01b |
63.35±3.00b,c | <0.001 |
| Sex (male, %) | 10 (50.0%) | 10 (50.0%) | 9 (45.0%) | 0.935 |
| SBP (mmHg) |
121.9±9.0 |
129.4±11.7 |
128.7±12.2 | 0.067 |
| DBP (mmHg) |
78.7±5.9 |
78.1±8.1 |
80.2±8.0 | 0.665 |
| HR (beats/min) |
79.1±12.0 |
73.4±12.3 |
71.9±9.2 | 0.111 |
| WBC
(×109/l) |
6.48±1.10 |
6.39±1.19 |
6.84±1.37 | 0.463 |
| NE (%) |
0.58±0.07 |
0.57±0.06 |
0.59±0.08 | 0.712 |
| FPG (mmol/l) |
5.32±0.34 |
5.40±0.50 |
5.59±0.84 | 0.336 |
| TG (mmol/l) |
1.67±0.87 |
1.63±1.02 |
1.44±0.76 | 0.695 |
| TC (mmol/l) |
4.56±0.64 |
4.70±0.84 |
5.19±0.95a | 0.046 |
| HDL-C (mmol/l) |
1.29±0.35 |
1.35±0.34 |
1.46±0.35 | 0.313 |
| LDL-C (mmol/l) |
2.53±0.54 |
2.77±0.65 |
3.09±0.74a | 0.029 |
| Cr (µmol/l)
(µmol/l) |
64.52±13.69 |
70.27±15.92 |
63.46±18.19 | 0.359 |
| Uric acid
(µmol/l) |
327.10±83.86 |
357.45±98.97 |
315.05±95.00 | 0.337 |
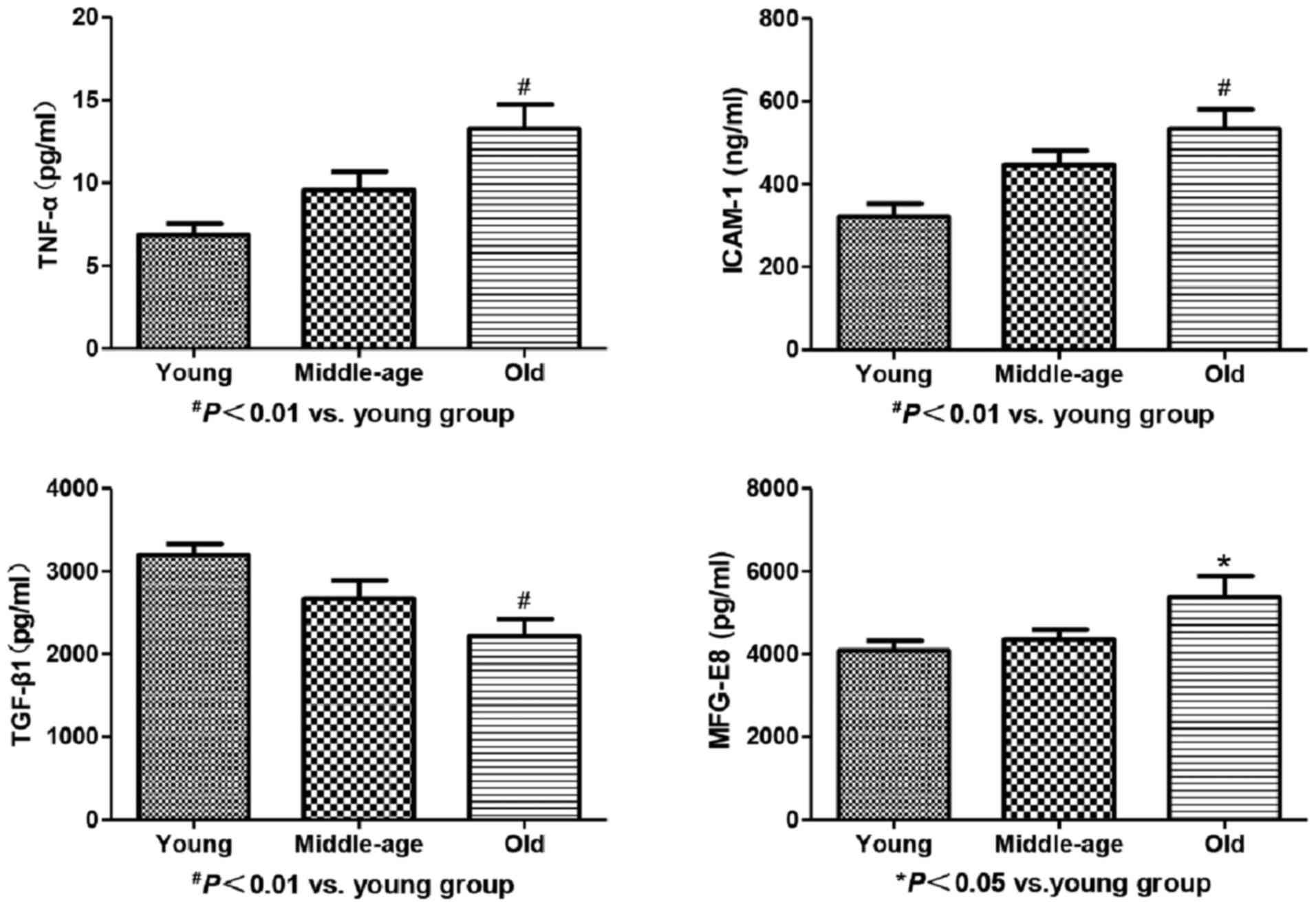
Serum levels of MFG-E8, TGF-β1, TNF-α
and ICAM-1 in healthy volunteers
Serum levels of MFG-E8, TNF-α and ICAM-1 increased
gradually with increase in age; however, statistically significant
differences in this respect were found only between the young and
old groups (P1<0.05, P2<0.01,
P3<0.01, respectively). In contrast, there was an
increasing trend in TGF-β1 level with increase in age; however, a
significant difference in this respect was observed only between
the young and old groups (P<0.01; Table II and Fig. 1).
 | Table II.Serum levels of MFG-E8, TGF-β1, TNF-α
and ICAM-1 in healthy volunteers disaggregated into three
age-groups. |
Table II.
Serum levels of MFG-E8, TGF-β1, TNF-α
and ICAM-1 in healthy volunteers disaggregated into three
age-groups.
|
| Groups |
|
|---|
|
|
|
|
|---|
| Indicators | Young group
(n=20) | Middle-age group
(n=20) | Old group
(n=20) | P-value |
|---|
| MFG-E8 (pg/ml) |
4080.5298±1090.88 |
4355.45±1037.28 |
5367.32±2297.79a | 0.032 |
| TGF-β1 (pg/ml) |
3201.33±576.05 |
2672.20±959.81 |
2219.81±918.19b | 0.002 |
| TNF-α (pg/ml) |
7.19±3.48 |
9.25±4.92 |
13.28±6.58b | 0.002 |
| ICAM-1 (ng/ml) |
321.95±138.90 |
428.38±155.63 |
534.18±210.89b | 0.001 |
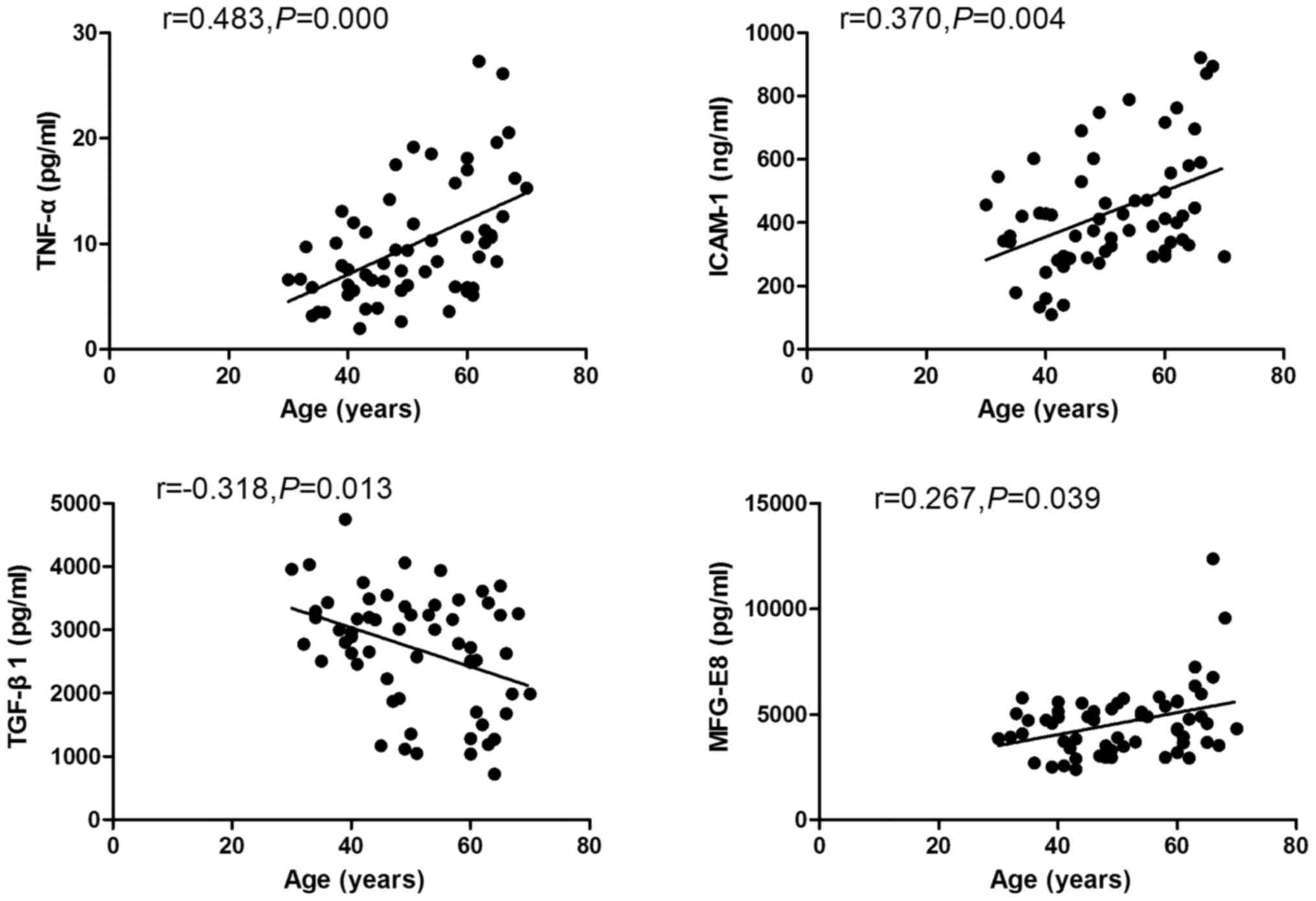
Correlation of peripheral MFG-E8,
TGF-β1, TNF-α and ICAM-1 with age
Spearman correlation analysis revealed a significant
positive correlation of serum levels of MFG-E8, TNF-α and ICAM-1
with age (r1=0.267, p1=0.039; r2=0.483, p2=0.000; r3=0.370,
p3=0.004, respectively). However, a negative correlation was
observed between serum levels of TGF-β1 and age (r=-0.318,
p=0.013). Besides, a negative relationship was found between serum
TNF-α and ICAM-1 (r=0.259, p=0.04; Fig. 2).
Baseline characteristics of patients
with carotid AS
Serum HDL-C level in plaque group of CAS patients
was significantly lower than that in the increased IMT group
(P<0.01). However, there were no significant differences between
the three groups with respect to age, sex, history of hypertension,
diabetes and smoking (P>0.05 for all; Table III).
 | Table III.Baseline characteristics of patients
with carotid atherosclerosis and controls. |
Table III.
Baseline characteristics of patients
with carotid atherosclerosis and controls.
|
| Groups |
|
|---|
|
|
|
|
|---|
| Indicators | Control (n=30) | Increased IMT
(n=27) | Plaques (n=40) | P-value |
|---|
| Age (years) | 61.36±9.01 | 61.44±8.14 | 64.78±7.78 | 0.148 |
| Sex (male, %) | 16 (53.3%) | 9 (33.3%) | 25 (62.5%) | 0.062 |
| Hypertension
(%) | 12 (40.0%) | 10 (37.0%) | 15 (37.5%) | 0.968 |
| Diabetes (%) | 6 (20.0%) | 5 (18.5%) | 12 (30%) | 0.471 |
| Smoking (%) | 6 (20.0%) | 4 (14.8%) | 13 (32.5%) | 0.210 |
| SBP (mmHg) | 133.1±14.3 | 133.8±18.4 | 137.7±20.5 | 0.512 |
| DBP (mmHg) | 80.0
(74.8,86.3) | 80.0
(72.0,90.0) | 80.0
(70.0,84.8) | 0.705 |
| HR (beat/min) | 70.0
(67.5,80.0) | 71.0
(66.0,77.0) | 70.0
(63.3,75.8) | 0.378 |
| WBC
(×109/l) | 6.35±1.45 | 6.63±1.40 | 6.65±1.61 | 0.689 |
| NE (%) | 0.58±0.06 | 0.60±0.07 | 0.60±0.08 | 0.312 |
| FPG (mmol/l) | 5.47
(5.01,6.05) | 5.26
(5.03,6.21) | 5.58
(5.19,6.86) | 0.326 |
| TG (mmol/l) | 1.40
(1.05,2.31) | 1.12
(0.85,1.86) | 1.28
(0.78,2.05) | 0.477 |
| TC (mmol/l) | 4.62±0.98 | 4.68±0.93 | 4.47±1.12 | 0.695 |
| HDL-C (mmol/l) | 1.27±0.32 | 1.40±0.42 |
1.15±0.33a | 0.021 |
| LDL-C (mmol/l) | 2.72±0.74 | 2.63±0.80 | 2.61±0.80 | 0.826 |
| Cr (µmol/l) | 63.63±20.17 | 61.76±12.38 | 68.20±12.44 | 0.208 |
| Uric acid
(µmol/l) | 373.29±121.15 | 325.35±97.30 | 326.34±89.49 | 0.205 |
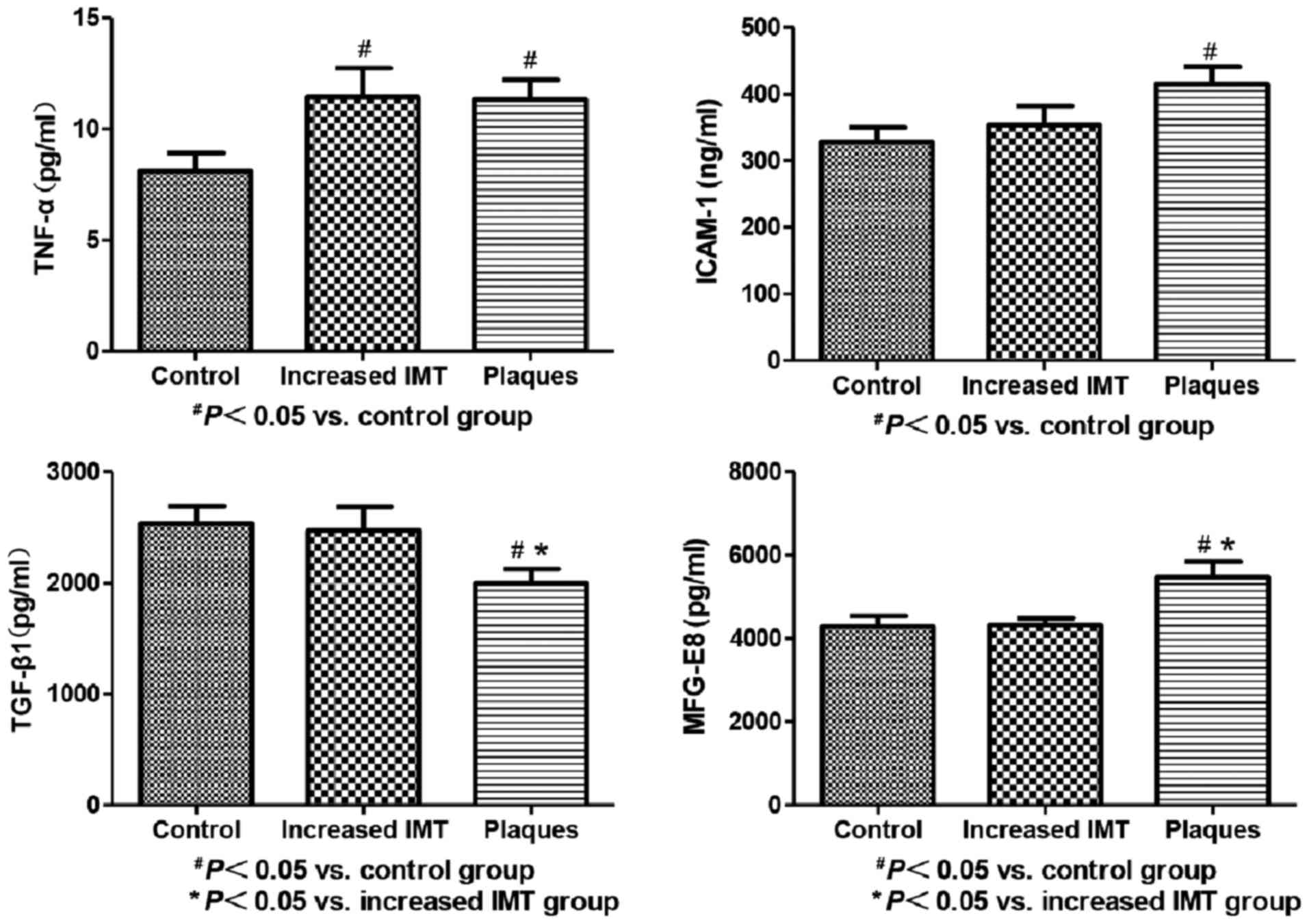
Serum levels of MFG-E8, TGF-β1, TNF-α
and ICAM-1 in patients with carotid AS
Serum levels of MFG-E8 in the plaques group were
significantly higher than those in the control and increased IMT
groups (P<0.05 for both). This finding suggested a significant
increase in MFG-E8 with increase in the degree of AS. On the
contrary, serum levels of TGF-β1 in the plaques group were
significantly lower than those in the control and increased IMT
groups (P<0.05 for both), which suggested that TGF-β1 level
decreased significantly with increase in the degree of AS. In
addition, serum levels of TNF-α in CAD patients, both in increased
IMT and plaques groups, were significantly higher than that in the
control group (P<0.05 for both); however, there was no
significant difference between the IMT and plaques groups in this
respect (P>0.05). Serum levels of ICAM-1 in the plaques group
were higher than those in the control group (P<0.05); however,
there was no significant difference in this respect between the IMT
and plaques groups (P>0.05; Fig.
3).
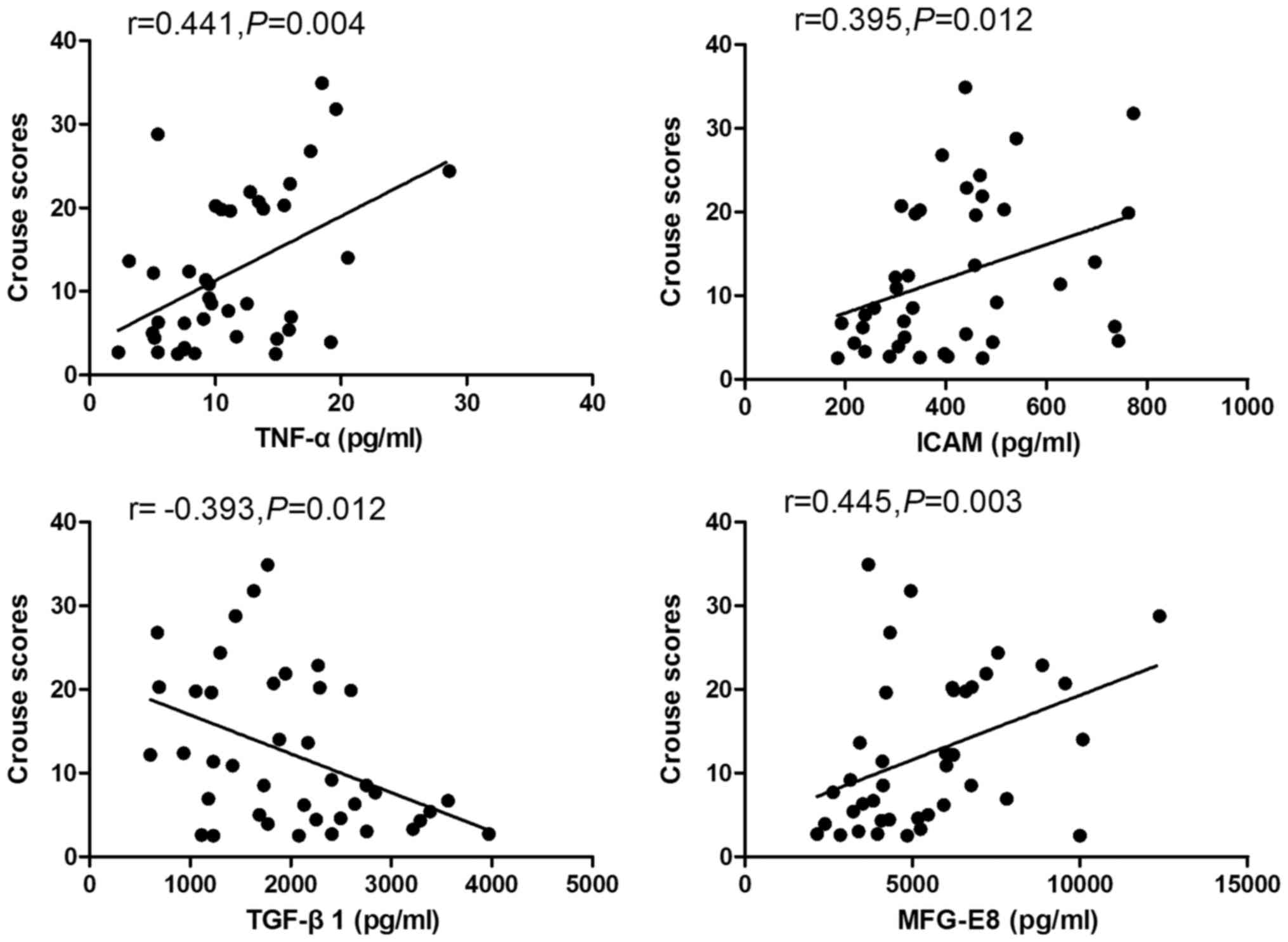
Correlation of Crouse scores with
serum levels of MFG-E8, TGF-β1, TNF-α and ICAM-1
Crouse scores for carotid artery intima-media
thickness were found to be associated with serum levels of MFG-E8,
TGF-β1 and other inflammatory factors on Spearman analysis. Serum
levels of MFG-E8, TNF-α and ICAM-1 showed a significant positive
correlation with Crouse scores for carotid artery intima-media
thicknesses (r1=0.455, r2=0.441 and r3=0.395, respectively;
P<0.05 for all). In contrast, a negative correlation was
observed between serum levels of TGF-β1 and Crouse scores
(r=-0.393, P<0.05; Fig. 4).
Discussion
AS is an age-related disease and several
inflammatory factors participate in the process of AS. Overall, our
findings indicate a direct association between serum levels of
MFG-E8, TNF-α and ICAM-1 with increase in age. Moreover, serum
levels of MFG-E8, TNF-α and ICAM-1 showed a positive correlation,
while those of TGF-β1 showed a negative correlation with age. In
addition, MFG-E8 serum levels in CAD patients were significantly
higher in the increased IMT and plaque groups. MFG-E8 levels in
plaque group were significantly higher than those in the increased
IMT group. On the contrary, serum levels of TGF-β1 in CAD patients
in the plaque group were lower than those in the control and
increased IMT groups. Serum TGF-β1 levels in the increased IMT
group were significantly lower than those in the control group. On
logistic regression analysis, serum levels of MFG-E8, TNF-α and
ICAM-1 showed a significant positive relation with Crouse score for
carotid artery intima-media thickness. In contrast, serum levels of
TGF-β1 showed a significant negative relation with Crouse
score.
Few studies have reported the correlation between
serum MFG-E8 and age. Cheng et al described a gradual
increase in MFG-E8 with age and a positive correlation between
serum MFG-E8 and pulse wave velocity in elderly patients with type
2 diabetes mellitus (26). The
findings are consistent with our results on the association between
MFG-E8 and increase in age. In this study, we observed a positive
correlation between serum levels of MFG-E8 and Crouse score for
carotid artery intima-media thickness in patients with carotid AS.
This suggested a positive correlation between serum levels of
MFG-E8 and the severity of carotid AS as assessed by ultrasound.
This finding provides a novel therapeutic target for AS. Based on
the previous studies, the likely mechanisms involved in the
age-related changes in serum MFG-E8 may be as follows:
age-associated activation of Ang II signaling. Upregulation of Ang
II signaling within the central arterial wall with increase in age
is well-documented (19). This may
contribute to upregulation of Ang II, angiotensin-converting enzyme
(ACE), Ang II receptor and AT1 receptor, which may induce vascular
smooth cells to secrete MFG-E8 (27,28).
A second mechanism may be the increased secretion of MFG-E8 by
activated macrophages. Studies have shown secretion of MFG-E8 by
activated macrophages in response to inflammatory reaction
(29,30). Subclinical chronic inflammation
with age would increase the pool of activated macrophages, which
may lead to increased production of MFG-E8.
In addition, increased MFG-E8 secretion could
stimulate MCP-1 expression, which accelerates the release of other
inflammatory factors such as TNF-α and ICAM-1 (19). All of these mechanisms may be
involved in the process of ‘inflammation-aging’.
TGF-β1 is secreted by various cells, and is
activated by plasmin cleavage. A previous study demonstrated that
expression levels of TGF-β1 in arterial wall were associated with
vascular AS and age. Redondo et al observed an
age-associated decrease in expression of TGF-β1 in serum and
vascular smooth muscle cells (VSMC) isolated from 169 patients who
underwent coronary artery bypass grafting (CABG) (31). The degree of cellular senescence
was closely related to lower TGF-β1 secretion and downregulation of
signaling pathway. Contrarily, Kanzaki et al reported
increase in TGF-β1 level with age. They reported over-expression of
TGF-β1 in atherosclerotic lesions, and exogenous TGF-β1 promoted
neointima formation in rabbit (32). Furthermore, TGF-β1 was shown to
enhance foam cell formation by inducing downregulation of CD36 and
scavenger receptor A in macrophages of atherosclerotic plaques via
a reparative response to vascular injury (33). The findings pertaining to TGF-β1 in
the present study are similar to those reported previously
(31). It may contribute to
vascular cells senescence. Age-induced senescence of vascular cells
resulted in decreased endogenous secretion of TGF-β1, although
compensatory effects also play a role in this process. Further
research is required to elucidate the underlying mechanisms. This
study is the first to demonstrate a negative correlation between
TGF-β1 and the degree of CAS.
Our study showed a gradual increase in TNF-α and
ICAM-1 levels with increase in age; further, their expression
levels in the CAS group were higher than those in healthy control,
while no significant difference was observed in this respect
between the IMT and plaques groups. The findings suggest that both
TNF-α and ICAM-1 are age-related inflammatory factors and are
associated with AS. Expression levels of both showed a relation
with the degree of AS. In a previous study, TNF-α was shown to
stimulate ICAM-1 gene transcription and protein expression via
activation of the nuclear factor-κB (NF-κB) (34). Our study revealed a positive
relation between serum levels of TNF-α and ICAM-1 in enrolled
healthy volunteers, which is consistent with previous studies.
Therefore, these results suggest that TNF-α and ICAM-1 are involved
in the age-induced chronic inflammation and interact with each
other in the aging process. Moreover, we also observed a positive
correlation between serum levels of TNF-α and ICAM-1 and Crouse
scores for carotid artery intima-media thickness in CAS group,
which is also consistent with the results of a previous study
(35). Serum levels of TNF-α and
ICAM-1 increased with the increase in the degree of AS, which is
consistent with previous researches (36). Based on these results, serum levels
of TNF-α and ICAM-1 may also be used to reflect the degree of
CAS.
In regards to the limitations of the present study,
our research verified the age-related changes in serum levels of
MFG-E8 and TGF-β1 and their correlation with the severity of CAS;
however, there were some study limitations. Firstly, the study
population comprised of residents of northeast China. Secondly, the
effect of blood lipid levels and drugs on the serum levels of
MFG-E8, TGF-β1 and other inflammatory factors were not considered.
Thirdly, the mechanisms by which MFG-E8 and TGF-β1 regulate AS were
not investigated. A further study will be needed to unravel their
mutual interactions and to explore the mechanisms by which these
contribute to the pathogenesis and development of AS.
In conclusion, the study demonstrated that both
MFG-E8 and TGF-β1 were age-related inflammatory factors through
examining the association of the serum levels of MFG-E8, TGF-β1,
TNF-α and ICAM-1 with age. Further, this is the first study to show
a positive correlation between serum MFG-E8 level and the degree of
AS as determined by ultrasound. In contrast, TGF-β1 level showed a
negative correlation with the degree of AS. Our findings are of
much clinical relevance and suggest that both MFG-E8 and TGF-β1 may
serve as quantitative indices of the severity of AS.
Acknowledgements
This study was supported in part by Natural
Foundation of China (project grant no. 51372096) and Natural
Foundation of Jilin China (project grant no. 201015143).
References
|
1
|
Green DR and Llambi F: Cell death
signaling. Cold Spring Harb Perspect Biol. 7:a0060802015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mobasheri A, Matta C, Zákány R and
Musumeci G: Chondrosenescence: Definition, hallmarks and potential
role in the pathogenesis of osteoarthritis. Maturitas. 80:237–244.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Musumeci G, Castrogiovanni P, Szychlinska
MA, Imbesi R, Loreto C, Castorina S and Giunta S: Protective
effects of high Tryptophan diet on aging-induced passive avoidance
impairment and hippocampal apoptosis. Brain Res Bull. 128:76–82.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Musumeci G, Castrogiovanni P, Trovato FM,
Imbesi R, Giunta S, Szychlinska MA, Loreto C, Castorina S and
Mobasheri A: Physical activity ameliorates cartilage degeneration
in a rat model of aging: A study on lubricin expression. Scand J
Med Sci Sports. 25:e222–e230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salminen A, Ojala J, Kaarniranta K and
Kauppinen A: Mitochondrial dysfunction and oxidative stress
activate inflammasomes: Impact on the aging process and age-related
diseases. Cell Mol Life Sci. 69:2999–3013. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franceschi C, Bonafè M, Valensin S,
Olivieri F, De Luca M, Ottaviani E and De Benedictis G:
Inflamm-aging. An evolutionary perspective on immunosenescence. Ann
NY Acad Sci. 908:244–254. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xia S, Zhang X, Zheng S, Khanabdali R,
Kalionis B, Wu J, Wan W and Tai X: An update on inflamm-aging:
Mechanisms, prevention, and treatment. J Immunol Res.
2016:84268742016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Martinis M, Franceschi C, Monti D and
Ginaldi L: Inflammation markers predicting frailty and mortality in
the elderly. Exp Mol Pathol. 80:219–227. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pelisek J, Wendorff H, Wendorff C, Kuehnl
A and Eckstein HH: Age-associated changes in human carotid
atherosclerotic plaques. Ann Med. 48:541–551. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Papa A, Danese S, Urgesi R, Grillo A,
Guglielmo S, Roberto I, Semeraro S, Scaldaferri F, Pola R, Flex A,
et al: Intercellular adhesion molecule 1 gene polymorphisms in
inflammatory bowel disease. Eur Rev Med Pharmacol Sci. 8:187–191.
2004.PubMed/NCBI
|
|
11
|
Mohammadpour AH, Falsoleiman H, Shamsara
J, Abadi G Allah, Rasooli R and Ramezani M: Pentoxifylline
decreases serum level of adhesion molecules in atherosclerosis
patients. Iran Biomed J. 18:23–27. 2014.PubMed/NCBI
|
|
12
|
Paneni F, Costantino S and Cosentino F:
Molecular pathways of arterial aging. Clin Sci (Lond). 128:69–79.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gujral DM, Shah BN, Chahal NS,
Bhattacharyya S, Hooper J, Senior R, Harrington KJ and Nutting CM:
Carotid intima-medial thickness as a marker of radiation-induced
carotid atherosclerosis. Radiother Oncol. 118:323–329. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schuliga M: The inflammatory actions of
coagulant and fibrinolytic proteases in disease. Mediators Inflamm.
2015:4376952015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pasterkamp G and Goumans MJ: The
microvasculature: The next battlefield where transforming growth
factor-β and endoglin draw their double-edged swords? Arterioscler
Thromb Vasc Biol. 37:10–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dhaouadi N, Li JY, Feugier P, Gustin MP,
Dab H, Kacem K, Bricca G and Cerutti C: Computational
identification of potential transcriptional regulators of TGF-β1 in
human atherosclerotic arteries. Genomics. 103:357–370. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Redondo S, Navarro-Dorado J, Ramajo M,
Medina Ú and Tejerina T: The complex regulation of TGF-β in
cardiovascular disease. Vasc Health Risk Manag. 8:533–539. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toma I and McCaffrey TA: Transforming
growth factor-β and atherosclerosis: Interwoven atherogenic and
atheroprotective aspects. Cell Tissue Res. 347:155–175. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang M, Wang HH and Lakatta EG: Milk fat
globule epidermal growth factor VIII signaling in arterial wall
remodeling. Curr Vasc Pharmacol. 11:768–776. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang M, Fu Z, Wu J, Zhang J, Jiang L,
Khazan B, Telljohann R, Zhao M, Krug AW, Pikilidou M, et al: MFG-E8
activates proliferation of vascular smooth muscle cells via
integrin signaling. Aging Cell. 11:500–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi YS: Functional role of milk fat
globule-epidermal growth factor viii in macrophage-mediated
inflammatory responses and inflammatory/autoimmune diseases.
Mediators Inflamm. 2016:56284862016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang M, Khazan B and Lakatta EG: Central
arterial aging and angiotensin II signaling. Curr Hypertens Rev.
6:266–281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bagnato C, Thumar J, Mayya V, Hwang SI,
Zebroski H, Claffey KP, Haudenschild C, Eng JK, Lundgren DH and Han
DK: Proteomics analysis of human coronary atherosclerotic plaque: A
feasibility study of direct tissue proteomics by liquid
chromatography and tandem mass spectrometry. Mol Cell Proteomics.
6:1088–1102. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Inci MF, Özkan F, Ark B, Vurdem ÜE, Ege
MR, Sincer I and Zorlu A: Sonographic evaluation for predicting the
presence and severity of coronary artery disease. Ultrasound Q.
29:125–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Crouse JR III, Grobbee DE, O'Leary DH,
Bots ML, Evans GW, Palmer MK, Riley WA and Raichlen JS; METEOR
Study Group, : Carotid intima-media thickness in low-risk
individuals with asymptomatic atherosclerosis: Baseline data from
the METEOR study. Curr Med Res Opin. 23:641–648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng M, Li BY, Li XL, Wang Q, Zhang JH,
Jing XJ and Gao HQ: Correlation between serum lactadherin and pulse
wave velocity and cardiovascular risk factors in elderly patients
with type 2 diabetes mellitus. Diabetes Res Clin Pract. 95:125–131.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu Z, Wang M, Gucek M, Zhang J, Wu J,
Jiang L, Monticone RE, Khazan B, Telljohann R, Mattison J, et al:
Milk fat globule protein epidermal growth factor-8: A pivotal relay
element within the angiotensin II and monocyte chemoattractant
protein-1 signaling cascade mediating vascular smooth muscle cells
invasion. Circ Res. 104:1337–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gao BB, Stuart L and Feener EP: Label-free
quantitative analysis of one-dimensional PAGE LC/MS/MS proteome:
Application on angiotensin II-stimulated smooth muscle cells
secretome. Mol Cell Proteomics. 7:2399–2409. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hanayama R, Tanaka M, Miwa K, Shinohara A,
Iwamatsu A and Nagata S: Identification of a factor that links
apoptotic cells to phagocytes. Nature. 417:182–187. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cunha C, Gomes C, Vaz A and Brites D:
Exploring new inflammatory biomarkers and pathways during
LPS-induced M1 polarization. Mediators Inflamm. 2016:69861752016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Redondo S, Navarro-Dorado J, Ramajo M,
Medina Ú, Molina-Sanchez P, Garces Z, García-Alonso M, Reguillo F,
Rodriguez E, Andres V and Tejerina T: Age-dependent defective
TGF-beta1 signaling in patients undergoing coronary artery bypass
grafting. J Cardiothorac Surg. 9:242014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanzaki T, Tamura K, Takahashi K, Saito Y,
Akikusa B, Oohashi H, Kasayuki N, Ueda M and Morisaki N: In vivo
effect of TGF-beta 1. Enhanced intimal thickening by administration
of TGF-beta 1 in rabbit arteries injured with a balloon catheter.
Arterioscler Thromb Vasc Biol. 15:1951–1957. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nabel EG, Shum L, Pompili VJ, Yang ZY, San
H, Shu HB, Liptay S, Gold L, Gordon D, Derynck R, et al: Direct
transfer of transforming growth factor beta 1 gene into arteries
stimulates fibrocellular hyperplasia. Proc Natl Acad Sci USA.
90:pp. 10759–10763. 1993; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen CC, Chow MP, Huang WC, Lin YC and
Chang YJ: Flavonoids inhibit tumor necrosis factor-alpha-induced
up-regulation of intercellular adhesion molecule-1 (ICAM-1) in
respiratory epithelial cells through activator protein-1 and
nuclear factor-kappaB: Structure-activity relationships. Mol
Pharmacol. 66:683–693. 2004.PubMed/NCBI
|
|
35
|
Ammirati E, Moroni F, Norata GD, Magnoni M
and Camici PG: Markers of inflammation associated with plaque
progression and instability in patients with carotid
atherosclerosis. Mediators Inflamm. 2015:7183292015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Steyers CM III and Miller FJ Jr:
Endothelial dysfunction in chronic inflammatory diseases. Int J Mol
Sci. 15:11324–11349. 2014. View Article : Google Scholar : PubMed/NCBI
|