Introduction
Inflammation-related damage can occur in any tissue
following infection, exposure to toxins, after ischemia, and in
allergic and auto-immune reactions. Inflammatory processes are
systematized by inflammatory cells, such as mast cells (1). Mast cells are widely present in the
connective tissues of mammals and other vertebrates and are
physically in close proximity to blood vessels (2). The cells are significant effector
cells in inflammation as well as allergic reactions, because they
secrete various cytokines (3).
Regulation of cytokine secretion from mast cells could be a useful
therapeutic strategy for allergic inflammatory diseases. The
signaling pathway inducing mast cell degranulation has been
characterized (4). Mast cell
activation induces phosphorylation of tyrosine kinase and migration
of calcium ion (Ca2+) in the body (5). Tyrosine phosphorylation is an
important event in intracellular signal transduction induced
activation of protein kinase C and secretion through
Ca2+ influx (6).
Calcium acts as a second messenger in mast cell activation, with
activation in response to increased intracellular Ca2+
(7). The release of intracellular
Ca2+ is essential for the activation of
mitogen-activated protein kinase (MAPK) (8,9).
MAPK participates in the mast cell regulation of cytokine
production in response to particular extracellular stimuli, which
subsequently begins the biological responses that drive cell
differentiation, proliferation, and apoptosis. These include
extracellular signal-regulated kinase (ERK), c-Jun N-terminal
kinase (JNK), and p38 mitogen-activated protein kinase (p38)
(10). Phsophorylation-mediated
ERK activation regulates cytoplasmic and nuclear targets, and
various cell responses including proliferation, migration,
differentiation and death (11).
JNK is involved in a signaling pathway concerned with the
inflammatory response. Activated JNKs affect the formation of the
activator protein 1 (AP-1) transcription factor complex that
participates in the expression of many inflammatory factors and
controls the synthesis of many inflammatory cytokines (12). P38 is involved in the production of
pro-inflammatory cytokines by regulating the expression of nuclear
factor-κ-light-chain-enhancer of activated B cells (NF-κB)
(13). In addition, ERKs, JNKs,
and p38 are involved in control of interleukin (IL)-6 mRNA or
protein production in response to FcεRI aggregation (14). Activated NF-κB regulates the
expression of cytokines, chemokines, and cell adhesion molecules
(CAM). The activity of NF-κB occurs when IκB is phosphorylated and
disassociated by IκB kinase (IKK) catalysis (15).
Curcuma longa L. is a perennial herb in
family Zingiberaceae. It is a traditional medicine used to treat
disorders, anorexia, diabetic wounds, hepatic disorders,
rheumatism, and sinusitis in China and India (16). The three main ingredients of
Curcuma longa L. are curcumin (diferuloylmethane),
demethoxycurcumin (DMC), and bisdemethoxycurcumin (BDMC), with
curcumin being most abundant (17). Curcumin has physiological
activities including antioxidant, anti-inflammation, and anticancer
activities (18–20). However, curcumin is unstable and
easily degrades in vivo, so more stable curcuminoids are
needed to replace it (21). BDCM
is more comparatively stable in vivo, is more readily taken
up into the cell nucleus (22,23),
and possesses anticancer, antioxidant and antibacterial activities
(24–26). BDCM has anti-inflammatory activity
in lipopolysaccharide-induced RAW 264.7 macrophages, with the
inhibition of inducible nitric oxide synthase (iNOS),
cyclooxygenase-2 (COX-2) and NF-κB. BDCM also suppresses
carrageenan-induced paw edema in mice (27,28).
The anti-inflammatory effect of BDCM in human mast
cells is unknown. The present study investigated the influence of
BDCM on cytokine, MAPK, and NF-κB activity in HMC-1 induced with
phorbol-12-myristate-13-acetate (PMA) and A23187.
Materials and methods
Reagents and antibodies
Bisdemethoxycurcumin (Fig. 1) was purchased from Tokyo Chemical
Industry Co., Ltd. (Tokyo, Japan). PMA and A23187 (calcymycin;
C29H37N3O6) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Iscove's modified Dulbecco's medium (IMDM) was
obtained from Welgene (Daegu, Korea). Anti-human tumor necrosis
factor-α (TNF-α; 555212), anti-IL-6 (555220), anti-IL-8 (555244),
biotinylated anti-human TNF-α (51-26372E), anti-IL-6 (51-26452E),
IL-8 (51-26542E), and anti-recombinant human TNF-α (51-26376E),
anti-IL-6 (51-26456E), and anti-IL-8 (51-26546E) antibodies were
obtained from BD Pharmingen (San Diego, CA, USA). The reverse
transcription kit was purchased from Qiagen (Valencia, CA, USA).
Nuclear and cytoplasmic extraction reagents were purchased from
Thermo Fisher Scientific, Inc., (Waltham, MA, USA).
Anti-phosphorylated (p-)ERK1/2 (sc-7383), anti-p-JNK1/2 (sc-6254),
anti-p-p38 (sc-7973), anti-ERK1/2 (sc-93), anti-JNK1/2 (sc-571)
anti-p38 (sc-535), anti-β-actin (sc-47,778), anti-NF-κB (sc-8008),
anti-mouse, and anti-rabbit antibodies were obtained from Santa
Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture and cell viability
The human mast cell line 1 (HMC-1) was cultured in
IMDM supplemented with 10% fetal bovine serum (FBS), 100 IU/ml
penicillin, 50 µg/ml streptomycin, and 1.2 mM α-thioglycerol at
37°C in an incubator with an atmosphere of 5% CO2. Cell
viability was evaluated by the MTS
[3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]
(Promega, Madison, WI, USA) assay following incubation in the
presence of 25 or 50 µM BDCM for 24 h at the aforementioned
temperature and CO2 conditions using an Epoch microplate
spectrophotometer (BioTek, Winooski, VT, USA) at an optical density
of 490 nm.
Analysis of cytokine production
The OptEIA™ human enzyme-linked
immunosorbent assay (ELISA; BD Bioscience, San Jose, CA, USA) was
used to assay culture supernatants to measure TNF-α, IL-6, and IL-8
secretion. HMC-1 cells were seeded at 5×105 cells per
well in 24-well plates and treated with 25 or 50 µM BDCM for 30
min. The cells were then stimulated for 8 h with 50 nM PMA and 1 µM
A23187 (Sigma-Aldrich). Cytokines in the supernatant were measured
using ELISA. Each well of the 96-well microplate was coated with
capture antibody diluted in coating buffer (0.1 M carbonate, pH
9.5). Each plate was sealed and incubated overnight at 4°C. After
washing three times with phosphate-buffered saline (PBS) containing
0.05% Tween-20, non-specific binding sites were blocked with PBS
containing 10% FBS (pH 7.0) for 1 h. A total of 100 µl of each
sample, or TNF-α, IL-6, and IL-8 standards were added to wells and
incubated for 2 h at room temperature. A total of 100 µl of
detection antibody conjugated with and avidin-horseradish
peroxidase (HRP) diluted in assay buffer was applied for 1 h. A
total of 100 µl of substrate solution (tetramethylbenzidine, TMB)
was added to each wells and incubated for 30 min at room
temperature in the dark. A total of 50 µl of stop solution (2 M
H2SO4) was added and the absorbance was
determined at 450 nm.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from HMC-1 cells using easy
BLUE™ Total RNA Extraction kit (iNtRon, Seongnam, Korea). The total
RNA was dissolved in DEPC-treated distilled water. A
spectrophotometer (Biotek) was used to evaluate RNA purity by
measuring the ratio of the absorbance at 260 and 280 nm.
Complementary deoxyribonucleic acid (cDNA) was synthesized using
the QuantiTect Reverse Transcription kit (Qiagen, Seoul, Korea)
according to the manufacturer's instructions. RT-qPCR was performed
in triplicate using power SYBR-Green PCR master mix (Applied
Biosystems, Foster City, CA, USA) in a StepOne PLus Real-Time-PCR
system (Applied Biosystems). The amplification was 1 cycle of 95°C
for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1
min, followed by 1 cycle 95°C for 15 sec and 60°C for 1 min. The
expression levels of the target genes relative to the endogenous
reference gene, β-actin, were calculated using the ΔΔCq method
using StepOne software v2.3 (Applied Biosystems). The primer
sequences are listed in Table
I.
 | Table I.Sequences of oligonucleotide primers
designed for reverse transcription-quantitative polymerase chain
reaction. |
Table I.
Sequences of oligonucleotide primers
designed for reverse transcription-quantitative polymerase chain
reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| hTNF-α |
|
|
Forward |
GACAAGCCTGTAGCCCATGTTGTA |
|
Reverse |
CAGCCTTGGCCCTTGAAGA |
| hIL-6 |
|
|
Forward |
AAGCCAGACTGTGCAGATGACTA |
|
Reverse |
TGTCCTGCAGCCACTGGTTC |
| hIL-8 |
|
|
Forward |
ACACTGCGCCAACACAGAAATTA |
|
Reverse |
TTTGCTTGAAGTTTCACTGGCATC |
| β-actin |
|
|
Forward |
ATTGCCGACAGGATGCAGAAC |
|
Reverse |
ATGGAGCCACCGATCCACA |
Western blot analysis
BDCM-pretreated and 1-h PMA and A23187 stimulated
cells were collected and lysed with ice-cold lysis buffer (iNtRon).
Following centrifugation at 13,000 rpm for 20 min, the supernatant
was transferred to a 1.5 ml microtube. A total of 20–30 µl of
denatured protein lysate was separated by 12% sodium dodecyl
sulfate-polyacrylamide electrophoresis and the resolved proteins
were transferred to polyvinylidene fluoride membranes. The
membranes were incubated with anti-human-phsopho-ERK antibody,
anti-human-phsopho-JNK antibody, or anti-human-phsopho-p38 antibody
(Santa Cruz Biotechnology, Inc.) overnight at 4°C. HRP-conjugated
antibody against mouse IgG (Santa Cruz Biotechnology, Inc.) diluted
1:2,500 in 3% BSA was used as the secondary antibody. The proteins
were detected using EzWestLumi plus luminal substrate (ATTO Co.,
Tokyo, Japan). After stripping, the membranes were reprobed with
anti-human ERK antibody, anti-human JNK antibody, or anti-human p38
antibody (Santa Cruz Biotechnology, Inc.) as respective loading
controls.
Cytoplasmic and nuclear protein
extraction
Cytoplasmic and nuclear proteins were extracted from
BDCM-pretreated and 2-h PMA and A23187 stimulated HMC-1 cells.
Nuclear extraction was performed according to the manufacturer's
instructions using NE-PER® Nuclear and Cytoplasmic
Extraction Reagents (Thermo Fisher Scientific, Inc.). Cell volume
of 20 µl corresponded to a volume ratio of CER I:CER II:NER
(200:11:100 µl). A tube containing CER I was first vortexed
vigorously at the highest speed setting for 1 sec to fully suspend
the cell pellet. The tube was then incubated on ice for 10 min.
Ice-cold CER II was then added to the tube followed by vortexing
for 5 sec at the highest speed setting. The tube was then incubated
on ice for 1 min before being vortexed for 5 sec at the highest
speed setting. The tube was then centrifuged at 13,000 rpm for 5
min in a microcentrifuge. The supernatant (cytoplasmic extract) was
then immediately transferred to a clean and pre-chilled tube and
stored until use. Ice-cold NER was added to the pellet and the tube
was vortexed for 15 sec at the highest speed setting. The sample
was placed on ice and vortexed for 15 sec every 10 min for a total
of 40 min. The tube was then centrifuged at 13,000 rpm for 5 min in
a microcentrifuge. The supernatant (nuclear extract) was then
immediately transferred to a clean and pre-chilled tube. The
extracted cytoplasmic and nuclear protein was detected by western
blot analysis.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Student's t-test for multiple comparisons was performed
using SPSS Ver. 23 software (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
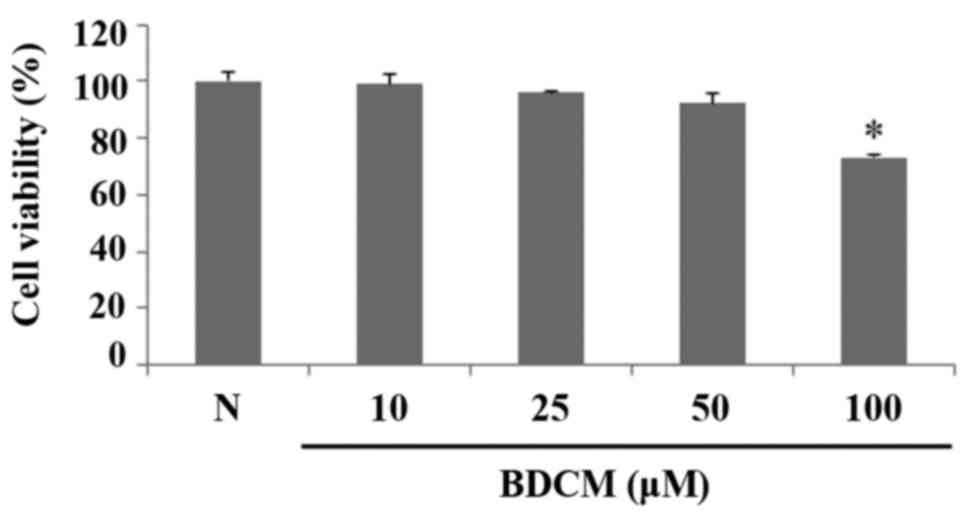
Cell viability measurement
MTS assay was performed to measure cell viability of
HMC-1 treated with BDCM. BDCM did not affect the viability of HMC-1
cells at concentrations of 10, 25, and 50 µM. The cytotoxicity of
BDCM was observed at concentrations of 100 µM (Fig. 2).
Effect of BDCM on production of
pro-inflammatory cytokines
ELISA was used to evaluate the effect of BDCM on the
production of IL-6, IL-8, and TNF-α. The production of all three
cytokines in HMC-1 cells considerably increased after stimulation
with PMA and A23187 (Fig. 1).
However, the productions of these cytokines were decreased by
pretreatment with either concentration of BDCM (Fig. 3).
Effect of BDCM on pro-inflammatory
cytokine gene expression
RT-qPCR was used to determine the expression levels
of the genes encoding IL-6, IL-8, and TNF-α. Expression of the
three genes in HMC-1 cells were significantly increased after
stimulation with PMA and A23187, but were suppressed by
pretreatment with either concentration of BDCM (Fig. 4).
Effect of BDCM on activation of
MAPKs
Western blots were used to investigate the effect of
BDMC on activation of MAPKs (ERK, JNK, and p38). Phosphorylation of
the three MAPKs was increased by stimulation of PMA and A23187 in
HMC-1 cells. Both concentrations of BDCM reduced JNK
phosphorylation induced by PMA and A23187. Phosphorylation of ERK
and p38 was reduced by BDCM at 50 µM (Fig. 5).
Effects of BDCM on NF-κB activation,
IκBα phosphorylation, and degradation
The expression of NF-κB signaling molecules and
NF-κB transcriptional activity was investigated at the protein
level. NF-κB and p-IκBα expressions in HMC-1 cells were increased
by stimulation with PMA and A23187. Expressions of NF-κB and p-IκBα
were decreased in BDCM-pretreated cells and IκBα degradation was
inhibited (Fig. 6).
Discussion
Mast cells are influential effector cells in the
immune system. The importance of mast cells in allergic diseases,
anaphylaxis, and autoimmunity is established. Mast cell-related
diseases result from increased mast cell number and/or
activity.
Activity of genes encoding several cytokines and
their protein production were increased in HMC-1 cells stimulated
with by PMA and A23187, as was phosphorylation of MAPKs and
activity NF-κB. These observations were consistent with the
degranulation of HMC-1 cells in response to increased intracellular
Ca2+. The inflammatory reaction caused by the mast cell
degranulation was alleviated if HMC-1 cells were first pretreated
with BDCM. Phosphorylation of JNK, ERK, and p38 was also inhibited
by BDCM as was NF-κB and p-IκBα production.
p38 regulates cell proliferation, apoptosis,
environmental stress, and neuropathic pain. It is activated by
various stress conditions and inflammatory cytokines, such as
ultraviolet irradiation, osmotic and oxidative stress, heat shock,
IL-1β, TNF-α, and transforming growth factor-β. The activated p38
transfers from the cytosol into the nucleus or to the other regions
of the cell, activates downstream kinases, and regulates
inflammatory processes, such as those involving iNOS, TNF-α, IL-1β,
and COX-2 (29).
Many recent studies have described the interaction
of Curcuma longa L. and NF-κB. Because this transcription
factor is closely related to inflammatory and immune responses,
Curcuma longa L. mediates its effects, at least in part,
through the inhibition of NF-κB activation. NF-κB activation is
regulated by MAPK via several mechanisms, but accumulated evidence
shows that inhibitory proteins called I-κB regulate NF-κB
activation by MAPKs that induce specific phosphorylation. In
addition, previous studies have demonstrated a role for NF-κB
activation and inflammatory cytokine production regulation in
inflammatory responses (30). The
expression of TNF-α, IL-6, and IL-8 genes is dependent on the
activation of transcription factor NF-κB in mast cells.
This means that BDCM, one of the major components of
Curcuma longa L., can inhibit the expression of inflammatory
mediators by inhibiting NF-κB activation and I-κB depression in
HMC-1. Therefore, BDCM suppressed the nuclear translocation of
NF-κB, phosphorylation of I-κB, and degradation of I-κB. These
results indicate that the molecular mechanism by which BDCM
suppresses the expression of pro-inflammatory cytokines and
inflammatory mediators is through NF-κB inactivation. Specifically,
I-κB degradation and translocation are blocked. Inhibition of NF-κB
and MAPK affects the expression of cytokines and
cytokine-associated genes.
In summary, BDCM regulates the expression of IL-6,
IL-8, and TNF-α in PMA and A23187-induced HMC-1 cells, and inhibits
the ERK, JNK, p38 MAPK, and NF-κB pathways. These results implicate
BDCM as a valuable compound to suppress the NF-κB signaling pathway
in mast cell-mediated inflammatory diseases.
Acknowledgements
This study was supported by Wonkwang University
2017.
References
|
1
|
Jeong HJ, Na HJ, Kim SJ, Rim HK, Myung NY,
Moon PD, Han NR, Seo JU, Kang TH, Kim JJ, et al: Anti-inflammatory
effect of Columbianetin on activated human mast cells. Biol Pharm
Bull. 32:1027–1031. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galli SJ, Zsebo KM and Geissler EN: The
kit ligand, stem cell factor. Adv Immunol. 55:1–96. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Metcalfe DD, Baram D and Mekori YA: Mast
cells. Physiol Rev. 77:1033–1079. 1997.PubMed/NCBI
|
|
4
|
Yoo JM, Yang JH, Kim YS, Yang HJ, Cho WK
and Ma JY: Inhibitory effects of viscum coloratum extract on
IgE/antigen-activated mast cells and mast cell-derived inflammatory
mediator-activated chondrocytes. Molecules. 22:pii: E37. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Spalinger MR, McCole DF, Rogler G and
Scharl M: Protein tyrosine phosphatase non-receptor type 2 and
inflammatory bowel disease. World J Gastroenterol. 22:1034–1044.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hamawy MM, Mergenhagen SE and Siraganian
RP: Protein tyrosine phosphorylation as a mechanism of signalling
in mast cells and basophils. Cell Signal. 7:535–544. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Petrou T, Olsen HL, Thrasivoulou C,
Masters JR, Ashmore JF and Ahmed A: Intracellular calcium
mobilization in response to ion channel regulators via a calcium
induced calcium release mechanism. J Pharmacol Exp Ther.
360:378–387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crossthwaite AJ, Hasan S and Williams RJ:
Hydrogen peroxide-mediated phosphorylation of ERK1/2, Akt/PKB and
JNK in cortical neurones: Dependence on Ca (2+) and PI3-kinase. J
Neurochem. 80:24–35. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wong CK, Tsang CM, Ip WK and Lam CW:
Molecular mechanisms for the release of chemokines from human
leukemic mast cell line (HMC)-1 cells activated by SCF and
TNF-alpha: Roles of ERK, p38 MAPK, and NF-kappaB. Allergy.
61:289–297. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Zhang XH, Liu GR, Liu C and Dong YM:
Isoquercitrin suppresses the expression of histamine and
pro-inflammatory cytokines by inhibiting the activation of MAP
Kinases and NF-κB in human KU812 cells. Chin J Nat Med. 14:407–412.
2016.PubMed/NCBI
|
|
11
|
Cagnol S and Chambard JC: ERK and cell
death: Mechanisms of ERK-induced cell death-apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Wang L, Dong X, Hu X, Zhou L, Liu
Q, Song B, Wu Q and Li L: The c-Jun N-terminal kinase (JNK) pathway
is activated in human interstitial cystitis (IC) and rat protamine
sulfate induced cystitis. Sci Rep. 6:196702016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Y, Yang Q, Xu H, Zhang J, Deng H, Gao
H, Yang J, Zhao D and Liu F: miRNA-221-3p Enhances the
Secretion of Interleukin-4 in Mast Cells through the Phosphatase
and Tensin Homolog/p38/Nuclear Factor-kappaB Pathway. PLoS One.
11:e01488212016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kandere-Grzybowska K, Kempuraj D, Cao J,
Cetrulo CL and Theoharides TC: Regulation of IL-1-induced selective
IL-6 release from human mast cells and inhibition by quercetin. Br
J Pharmacol. 148:208–215. 2016. View Article : Google Scholar
|
|
15
|
Schuliga M: NF-kappaB signaling in chronic
inflammatory airway disease. Biomolecules. 5:1266–1283. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ooko E, Kadioglu O, Greten HJ and Efferth
T: Pharmacogenomic characterization and isobologram analysis of the
combination of ascorbic acid and curcumin-two main metabolites of
Curcuma longa-in cancer cells. Front Pharmacol. 8:382017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chearwae W, Anuchapreeda S, Nandigama K,
Ambudkar SV and Limtrakul P: Biochemical mechanism of modulation of
human P-glycoprotein (ABCB1) by curcumin I, II and III purified
from Turmeric powder. Biochem Pharmacol. 68:2043–2052. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jagetia GC and Rajanikant GK: Curcumin
stimulates the antioxidant mechanisms in mouse skin exposed to
fractionated γ-irradiation. Antioxidants (Basel). 4:25–41. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chin KY: The spice for joint inflammation:
Anti-inflammatory role of curcumin in treating osteoarthritis. Drug
Des Devel Ther. 10:3029–3042. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deka SJ, Mamdi N, Manna D and Trivedi V:
Alkyl cinnamates induce protein kinase C translocation and
anticancer activity against breast cancer cells through induction
of the mitochondrial pathway of apoptosis. J Breast Cancer.
19:358–371. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J, Yang H, Zhou X, Wang H, Gong L and
Tang C: Bisdemethoxycurcumin suppresses migration and invasion of
highly metastatic 95D lung cancer cells by regulating E-cadherin
and vimentin expression, and inducing autophagy. Mol Med Rep.
12:7603–7608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gordon ON, Luis PB, Ashley RE, Osheroff N
and Schneider C: Oxidative transformation of demethoxy- and
bisdemethoxycurcumin: Products, mechanism of formation, and
poisoning of human topoisomerase IIα. Chem Res Toxicol. 28:989–996.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ramezani M, Hatamipour M and Sahebkar A:
Promising Anti-tumor properties of bisdemethoxycurcumin: A
naturally occurring curcumin analogue. J Cell Physiol. Jan
11–2017.(Epub ahead of print). PubMed/NCBI
|
|
24
|
Xu JH, Yang HP, Zhou XD, Wang HJ, Gong L
and Tang CL: Role of wnt inhibitory factor-1 in inhibition of
bisdemethoxycurcumin mediated epithelial-to-mesenchymal transition
in highly metastatic lung cancer 95D cells. Chin Med J (Engl).
128:1376–1383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li YB, Gao JL, Zhong ZF, Hoi PM, Lee SM
and Wang YT: Bisdemethoxycurcumin suppresses MCF-7 cells
proliferation by inducing ROS accumulation and modulating
senescence-related pathways. Pharmacol Rep. 65:700–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haukvik T, Bruzell E, Kristensen S and
Tønnesen HH: A screening of curcumin derivatives for antibacterial
phototoxic effects studies on curcumin and curcuminoids. XLIII.
Pharmazie. 66:69–74. 2011.PubMed/NCBI
|
|
27
|
Guo LY, Cai XF, Lee JJ, Kang SS, Shin EM,
Zhou HY, Jung JW and Kim YS: Comparison of suppressive effects of
demethoxycurcumin and bisdemethoxycurcumin on expressions of
inflammatory mediators in vitro and in vivo. Arch Pharm Res.
31:490–496. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim AN, Jeon WK, Lee JJ and Kim BC:
Up-regulation of heme oxygenase-1 expression through
CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect
of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic
Biol Med. 49:323–331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun J and Nan G: The mitogen-activated
protein kinase (MAPK) signaling pathway as a discovery target in
stroke. J Mol Neurosci. 59:90–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blackwell TS, Blackwell TR and Christman
JW: Impaired activation of nuclear factor-kappaB in
endotoxin-tolerant rats is associated with down-regulation of
chemokine gene expression and inhibition of neutrophilic lung
inflammation. J Immunol. 158:5934–5940. 1997.PubMed/NCBI
|