Introduction
Colorectal cancer (CRC) is one of the most common
malignant cancers worldwide (1),
with considerable medical cost to society and suffering of patients
with CRC (2,3). As CRC is characterized by rapid
progression, majority of patients are diagnosed with advanced stage
at the first hospital visit and cannot undergo operation to remove
tumor (4). Patients that have had
the tumor removed surgically, still have high risk of recurrence
and metastasis postoperatively (5,6) due
to rapid growth (7), resistance to
apoptosis (8) and invasion of CRC
tumor cells (9). Thus, it is
important to identify the genes relevant to regulation of apoptosis
and invasion of CRC cells to improve the diagnosis and
comprehensive treatments for CRC.
Coiled-coil domain containing (CCDC) proteins, one
family of protein with coiled-coil structures, possess a wide range
of physiological functions (10),
and the potential associations between some members of CCDC family
and cancers have been previously reported. Previous studies have
confirmed that CCDC67, CCDC6 and CCDC134 were associated with
apoptosis and invasion of thyroid, lung and stomach cancer cells
(11–14). CCDC34 is also a member of CCDC
family, and has been reported to contribute to apoptosis and
invasion of bladder cancer cells (15); however, it is currently uncertain
whether CCDC34 can exacerbate this process in CRC cells. In the
current study, the expression of CCDC34 protein in
paraffin-embedded tissue samples was detected and clinical
pathological data of patients with CRC was also collected. The
association between CCDC34 expression and the biological
characteristics of patients with CRC was analyzed. Additionally,
endogenous CCDC34 expression in SW620 cells was suppressed with
small interfering RNA (siRNA) and the changes of cell metabolic
activity, apoptosis and invasion ability were detected following
the siRNA transfection. Subsequently, reverse-transcription
quantitative polymerase chain reaction (RT-qPCR) and western
blotting was used to detect the expression levels of apoptosis and
invasion-associated genes, including B cell leukemia/lymphoma 2
(Bcl-2), survivin, E-cadherin, N-cadherin and matrix
metallopeptidase-9 (MMP-9) after endogenous CCDC34 in CRC cells was
inhibited. The current findings suggested that the detection of
CCDC34 may be valuable for the evaluation of patients with CRC and
provided evidence for the investigation of its role in invasion and
metastasis of CRC.
Materials and methods
Patients and tissue specimens
In the current study, a total of 85 paraffin
specimens of tumor tissues were obtained from patients with CRC
diagnosed and received surgical treatment at Hebei General Hospital
(Shijiazhuang, China) between January 2015 and June 2016. In
addition, 60 paraffin specimens of paracancerous tissues were
selected as controls, which were >3 cm from edge of cancer and
no cancer cells were observed with microscopic examination. In
total, 59 male patients and 26 female patients were recruited, and
the patients aged from 41–76 (mean age, 57.29±7.49). All enrolled
patients did not suffer from other cancers and were pathologically
confirmed as adenocarcinoma with no preoperative treatments such as
radiotherapy, chemotherapy or targeted therapy. The current study
has been approved by the Medical Ethics Committee of Hebei General
Hospital and informed consent was obtained from all
participants.
Cell lines and reagents
HCT8, HCT116, SW620, SW480, LS-174T, HT29 human
colon cancer cell lines were purchased from the Cell Resource
Centre of Shanghai Institutes for Biological Sciences, Chinese
Academy of Sciences (Shanghai, China), and passaged and preserved
in Hebei General Hospital. Cells after 5–8 passages were used for
the study. Rabbit anti-human polyclonal antibodies against CCDC34
(SAB1303750) were from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany), and antibodies against Bcl-2 (sc-783), survivin
(sc-10811), E-cadherin (sc-7870), N-cadherin (sc-7939), MMP-9
(sc-10737) and β-actin (sc-8432) were all from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The immunohistochemistry
(IHC) kit was purchased from Fuzhou Maixin Biotechnology Co., Ltd.
(Fuzhou, China). Culture medium Dulbecco's modified Eagle's medium
(DMEM), fetal bovine serum (FBS) and trypsin were purchased from
Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Primers
for qPCR and siRNA for CCDC34 were synthesized by Shanghai Sangon
Biotech, Co., Ltd. (Shanghai, China). Lipofectamine 2000 reagent
was obtained from Invitrogen; Thermo Fisher Scientific, Inc.
Apoptosis detection kits containing Annexin V-FITC and PI were
purchased from Wuhan Boster Biological Technology, Ltd., (Wuhan,
China). Caspase-3 and −8 Activity Assay kits were obtained from EMD
Millipore (Billerica, MA, USA).
IHC analysis
Sections were fixed with 4% neutral buffered
formalin for 24 h, and cut from each paraffin tissue block of CRC
with 4 µm thickness, and antigen retrieval was performed according
to following steps: Sections were immersed in citrate buffer
(pH=6.0), and the pressure cooker antigen retrieval method was
utilized for 4 min. Sections were removed, the buffer was allowed
to come to room temperature and the sections were rinsed 3 times (5
min each time). They were subjected to the IHC procedure following
the manufacturer's protocol and the results were examined by a
professional pathologist. The number and the percentage of positive
cells were calculated in 100 cells from randomly chosen 5 fields in
each section using a TE2000-U microscope (×400; Nikon Corporation,
Tokyo, Japan). The dilutions of antibodies were as following:
CCDC34 (1:200), Bcl-2 (1:400), survivin (1:400), E-cadherin
(1:400), N-cadherin (1:250), MMP-9 (1:400). The duration of each
incubation step was performed at 37°C for 3 h according to the
relevant manufacturer's instructions. The cells with yellow or
brown particles in the cytoplasm were considered to be positive for
CCDC34, Bcl-2, survivin, E-cadherin, N-cadherin and MMP-9. The
scoring was performed with the percentage of positive cells (0,
0–25%; 1, 25–50%; 2, 51–75%; 3, 76–100%) and intensity of staining
(0, no staining; 1, pale yellow; 2, yellow; 3, brown). The
percentage of positive cells and intensity of staining were added
up to produce the result for each case: 0, Negative staining (−),
1–2 as mild staining (+), 3–4, moderate staining (++), 5–6, intense
staining (+++). A result of (−) was recorded as negative and
results of (+), (++), and (+++) were recorded as positive.
Cell culture
HCT8, HCT116, SW620, SW480, LS-174T, HT29 human
colon cancer cell lines which were cultured in DMEM containing 10 %
FBS and incubated at 37°C supplemented with 5% CO2 was
passaged every 2–3 days. The cells at logarithmic growth phase were
used for the subsequent experiments. This procedure was repeated
three times.
CCDC34-siRNA transfection and
groups
The synthesized CCDC34-siRNA sequence was
5′-GCCUGAGAGGAAUGGAGUUTT-3′ and negative control (NS) siRNA
sequence 5′-UUCUCCGAACGUGUCACGUTT-3′. SW620 cells were transfected
with various concentrations of CCDC34-siRNA or NS-siRNA using
Lipofectamine 2000 and used for subsequent experiments after 48 h.
The cells were assigned into CCDC34-siRNA group, NS-siRNA group and
blank control group (treated only with Lipofectamine 2000
reagent).
MTT assay
Tumor single-cell suspensions were prepared and
seeded in 96-well plate (105 cells/well). MTT (20 µl)
solution (5 mg/ml) was added to each well 4 h prior the end of the
experiment and then following a 4 h incubation at room temperature,
the culture medium was removed and 150 µl DMSO was added to each
well. The absorbance values were determined following shaking at
room temperature for 15 min at 490 nm using a microplate
reader.
Cell apoptosis assay
The cells of all groups transfected for 48 h were
collected by trypsinization after rinsed with PBS and the cell
density was adjusted to 5×105 cells/ml. Then, 500 µl
binding buffer was added to suspend cells and 10 µl Annexin V-FITC
or PI was added and mixed respectively. The cells were counted by
FACS flow cytometry after incubation in dark for 15 min and
subjected to calculate percentage of apoptotic cells.
Cell invasion ability assay
SW620 cells were seeded in 24-well plates with
105 cells/well and treated as the different groups,
which were transfected with CCDC34-siRNA or NS-siRNA. Transwell
chambers were coated with 100 µl Matrigel was treated with
ultraviolet radiation. SW620 cells from each group were seeded in
200 µl in the upper chamber with serum free DMEM, and DMEM medium
containing 20% FBS was added to the lower chamber. After 24 h,
Matrigel glue and extra SW620 cells in the upper chamber were wiped
with cotton swabs. Methanol was utilized to fix the membranes for
10 min. The cells penetrating to the lower membrane were counted
following crystal violet staining. Each experiment was repeated 3
times.
RT-qPCR
The cells were collected 48 h after transfection and
total RNA was isolated using the TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
RNA (2 µg) was reverse transcribed to synthesize first-strand cDNA
according to the instruction of Reverse Transcription System kit
(Promega Corporation. Madison, WI, USA) according to following
steps: 25°C for 5 min, 42°C for 60 min, 70°C for 15 min. cDNA
templates (2 µg) were amplified by qPCR using SYBR Green PCR kit
(Promega Corporation) to establish a 25 µl reaction system on an
RT-qPCR machine. PCR reaction started with 1 cycle of 95°C for 5
min, followed by 45 cycles of 2 steps as 95°C for 30 sec, 72°C for
30 sec and 72°C for 5 min. The quantification cycle (Cq) (16) was obtained from the reaction curve
and the expression of targeted genes was normalized to the
housekeeping gene GAPDH. The primers used are listed in Table I.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| CCDC34 |
ACAGAAACAGGTGCGCTTACC |
CAGCCGGTCACGTTCTTCTTT |
| Bcl-2 |
TGTGTGGAGAGCGTCAACC |
TGGATCCAGGTGTGCAGGT |
| Survivin |
GCCAGATTTGAATCGCGGGA |
GCAGTGGATGAAGCCAGCCT |
| E-cadherin |
CACTCGTCGCTGGATCTGTCA |
CACAGCCTACTGCATGGCTCA |
| N-cadherin |
GAGATCCTACTGGACGGTTCG |
TCTTGGCGAATGATCTTAGGA |
| MMP-9 |
AGAACCAATCTCACCGACAGG |
CGACTCTCCACGCATCTCT |
| GAPDH |
GACCCCTTCATTGACCTCAAC |
CGCTCCTGGAAGATGGTGAT |
Western blotting
Cell protein was extracted with cell lysis buffer
(Sigma-Aldrich; Merck KGaA) 48 h after transfection and protein
concentrations were determined by Bradford assay. Protein (40 µg)
was loaded and separated on a 12% SDS-PAGE gel and transferred to a
polyvinylidene fluoride membrane. Membranes were blocked with 5%
fetal bovine serum (Thermo Fisher Scientific, Inc.) in
Tris-buffered saline with 0.05% Tween-20 (TBST) for 1 h at room
temperature and incubated with primary antibodies of interest
overnight at 4°C. The dilutions of antibodies were as following:
CCDC34 (1:800), Bcl-2 (1:1,000), survivin (1:400), E-cadherin
(1:800), N-cadherin (1:1,000), MMP-9 (1:400). After being washed
with TBST 3 times, membranes were incubated with IgG secondary
antibody (1:1,000, sc-2007; Santa Cruz Biotechnology, Inc.) for 1 h
at room temperature and subjected to color development. The
absorbance of target bands was detected with an Odyssey Infrared
Imaging System (9120; Li-COR Biosciences, Lincoln, NE, USA) to
determine the relative expression intensity of the proteins.
Caspase-3 and −8 activity assays
The activity of caspase-3 and −8 was detected by
spectrophotometry. Cells were collected, lysed for 20 min using the
cold lysis buffer, and centrifuged for 10 min at 12,500 × g. The
supernatant was transferred to test the protein concentration. The
analysis was performed according to the manufacturer's protocol of
the activity detection kits. The absorbance values at the
wavelength of 405 nm were obtained from a microplate reader. The
caspase activity was presented as caspase enzyme units in per unit
cell protein.
Statistical analysis
Statistical analyses were performed with SPSS
software version 19.0 (IBM Corp., Armonk, NY, USA). Chi-squared
test, Mann-Whitney rank sum test, Spearman correlation, one-way
analysis of variance and Dunnett's test were used for data
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
CCDC34 protein expression in CRC
tissues and adjacent normal mucosa
The IHC results illustrated the CCDC34-positive rate
in the 85 CRC tissues was 74.12% (63/85) and 28.33% (17/60) in
paracancerous tissues. As presented in Fig. 1, the expression of CCDC34 was
increased in CRC tissues compared with adjacent tissues
(χ2=29.810, P<0.001).
Association between CCDC34 protein
expression and CRC clinical pathology parameters
Findings are presented in Table II and revealed that the protein
expression of CCDC34 was related to invasion depth of the tumor,
differentiation and metastasis in the lymph node. CCDC34-positive
CRC tissues were characterized with deeper infiltrating of the
tumor and higher positive rate of lymphatic metastasis (P<0.05).
No significant association was identified between CCDC34 protein
expression and the remaining biological characteristics
(P>0.05).
 | Table II.Relationship between coiled-coil
domain containing 34 protein expression and clinicopathological
characteristics of patients with colorectal cancer. |
Table II.
Relationship between coiled-coil
domain containing 34 protein expression and clinicopathological
characteristics of patients with colorectal cancer.
| Clinicopathological
parameters | Positive (63) | Negative (22) | Total | χ2 | P |
|---|
| Sex |
|
Male | 45 | 14 | 59 | 0.466 | 0.495 |
|
Female | 18 | 8 | 26 |
|
|
| Age (years) |
|
≥60 | 18 | 9 | 27 | 1.145 | 0.285 |
|
<60 | 45 | 13 | 58 |
|
|
| Tumor
differentiation |
|
Well-differentiated | 45 | 17 | 62 | 0.282 | 0.595 |
| Poorly
differentiated | 18 | 5 | 23 |
|
|
| Depth of
invasion |
| Serosal
infiltration | 49 | 12 | 61 | 4.343 | 0.037 |
| No
serosal infiltration | 14 | 10 | 24 |
|
|
| Lymphatic
metastasis |
|
Positive | 51 | 11 | 62 | 7.915 | 0.005 |
|
Negative | 12 | 11 | 23 |
|
|
| TNM stages |
| I | 5 | 4 | 9 | 2.012 | 0.570 |
| II | 10 | 4 | 14 |
|
|
|
III | 43 | 12 | 55 |
|
|
| IV | 5 | 2 | 7 |
|
|
| Distant
metastasis |
|
Positive | 5 | 2 | 7 | 0.029 | 0.865 |
|
Negative | 58 | 20 | 78 |
|
|
Alteration of CCDC34 protein
expression in SW620 cells post CCDC34-siRNA treatment
The protein expression levels of CCDC34 in SW620
cells transfected with CCDC34-siRNA for 48 h was detected with
western blotting. As demonstrated in Fig. 2, CCDC34 protein expression in the
CCDC34-siRNA transfected group was significantly lower than the
negative control and blank control groups (P<0.05), whereas
there was no obvious difference between the NS-siRNA group and the
blank control groups (P>0.05).
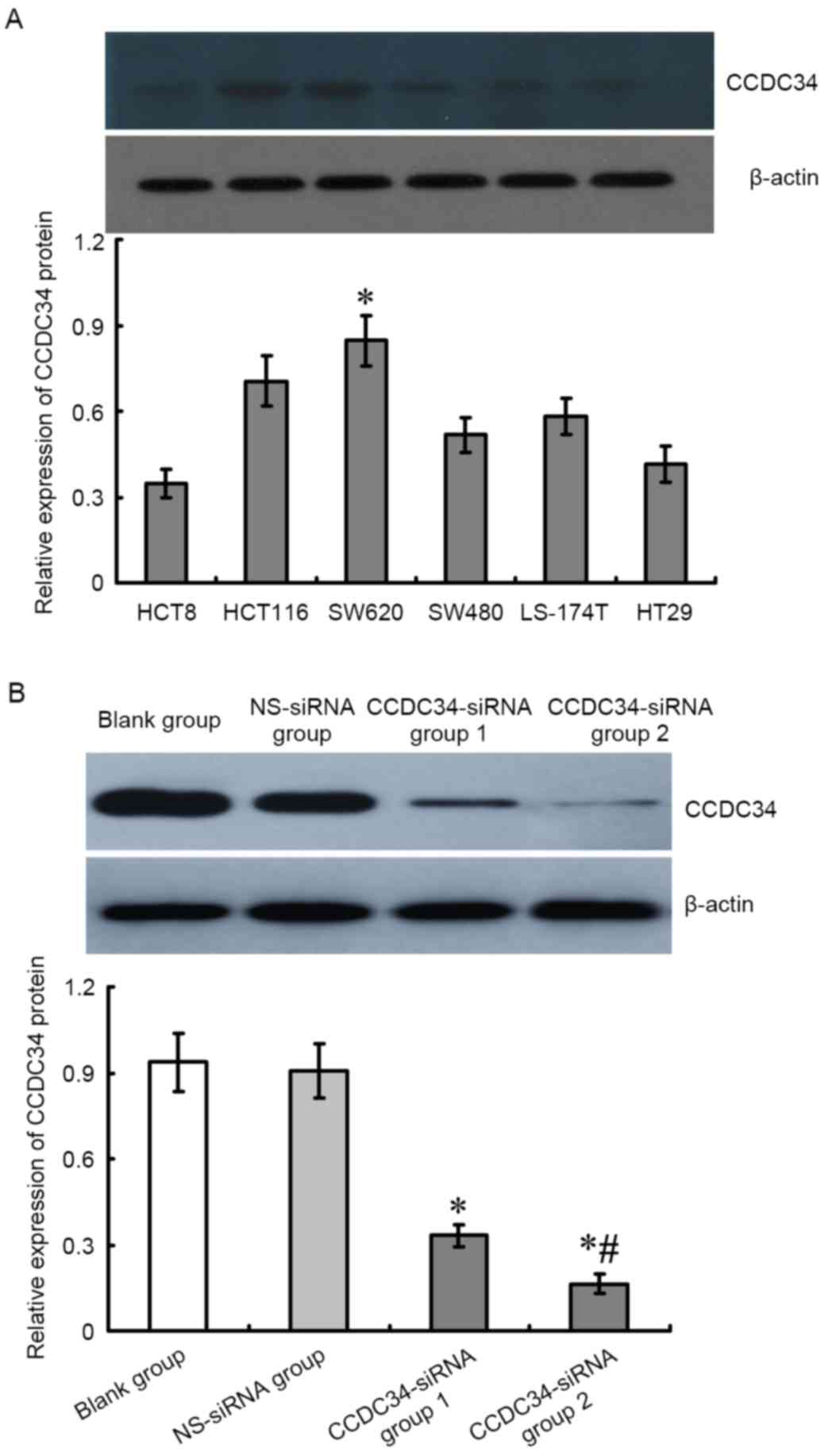 | Figure 2.Different expression levels of CCDC34
protein in CRC cell lines and inhibitory effect of CCDC34-siRNA on
CCDC34 expression in SW620 cells. (A) From the 6 CRC cell lines,
the highest CCDC34 protein expression was detected in the SW620
cells; therefore, the SW620 cells were used for subsequent
experiments. *P<0.05 vs. HCT8, HCT116, SW480, LS-174T, HT29
cells. (B) CCDC34-siRNA was transfected into SW620 cells, CCDC34
expression level was significantly downregulated following
CCDC34-siRNA transfection. Group 1, 20 µmol/l; Group 2, 80 µmol/l;
*P<0.05 vs. blank or NS-siRNA groups, #P<0.05 vs.
CCDC34-siRNA group 1. CCDC34, coiled-coil domain containing 34; NS,
negative control; siRNA, small interfering RNA; CRC, colorectal
cancer. |
Metabolic activity of SW620 cells
following CCDC34-siRNA treatment
In Fig. 3,
metabolic activity of SW620 cells transfected with CCDC34-siRNA for
48 h was 0.232±0.031, and was significantly lower compared with the
NS-siRNA group (0.283±0.029) and the blank group (0.306±0.041;
P<0.05), whereas there was no difference observed between
con-siRNA group and the blank control group (P>0.05).
Apoptosis rate of SW620 cells post
CCDC34-siRNA treatment
In Fig. 4, the
apoptotic rate of SW620 cells transfected with CCDC34-siRNA was
28.62±4.57% compared with 12.65±3.05% in the NS-siRNA group and
11.26±2.26% in the blank group. This indicated that the apoptotic
rate of the CCDC34-siRNA group was evidently elevated when compared
with the two other groups (P<0.01).
Cell invasion ability of SW620 cells
following CCDC34-siRNA treatment
As presented in Fig.
5, Transwell assay results highlighted the lower number of
SW620 cells crossing the Transwell chamber membrane in the
CCDC34-siRNA group (50.17±6.15) compared with the NS-siRNA group
(83.50±6.47) and the blank group (85.67±5.05; P<0.05), whereas
there was no obvious difference between the NS-siRNA and blank
groups (P>0.05).
Alteration of the expression of
apoptosis-associated BCl-2 and caspase-3, −8 activity in SW620
cells following CCDC34-siRNA treatment
In Fig. 6A-C, mRNA
and protein expression levels of Bcl-2 and survivin were
significantly decreased in cells of CCDC34-siRNA group when
compared with the NS-siRNA and blank groups (P<0.05). No obvious
difference was identified between the NS-siRNA group and the blank
group (P>0.05). Additionally, the activity of caspase-3 and −8
in cells of CCDC34-siRNA group was higher compared with the control
groups (P<0.05), whereas there was no obvious difference between
the NS-siRNA and blank groups (P>0.05; Fig. 6D).
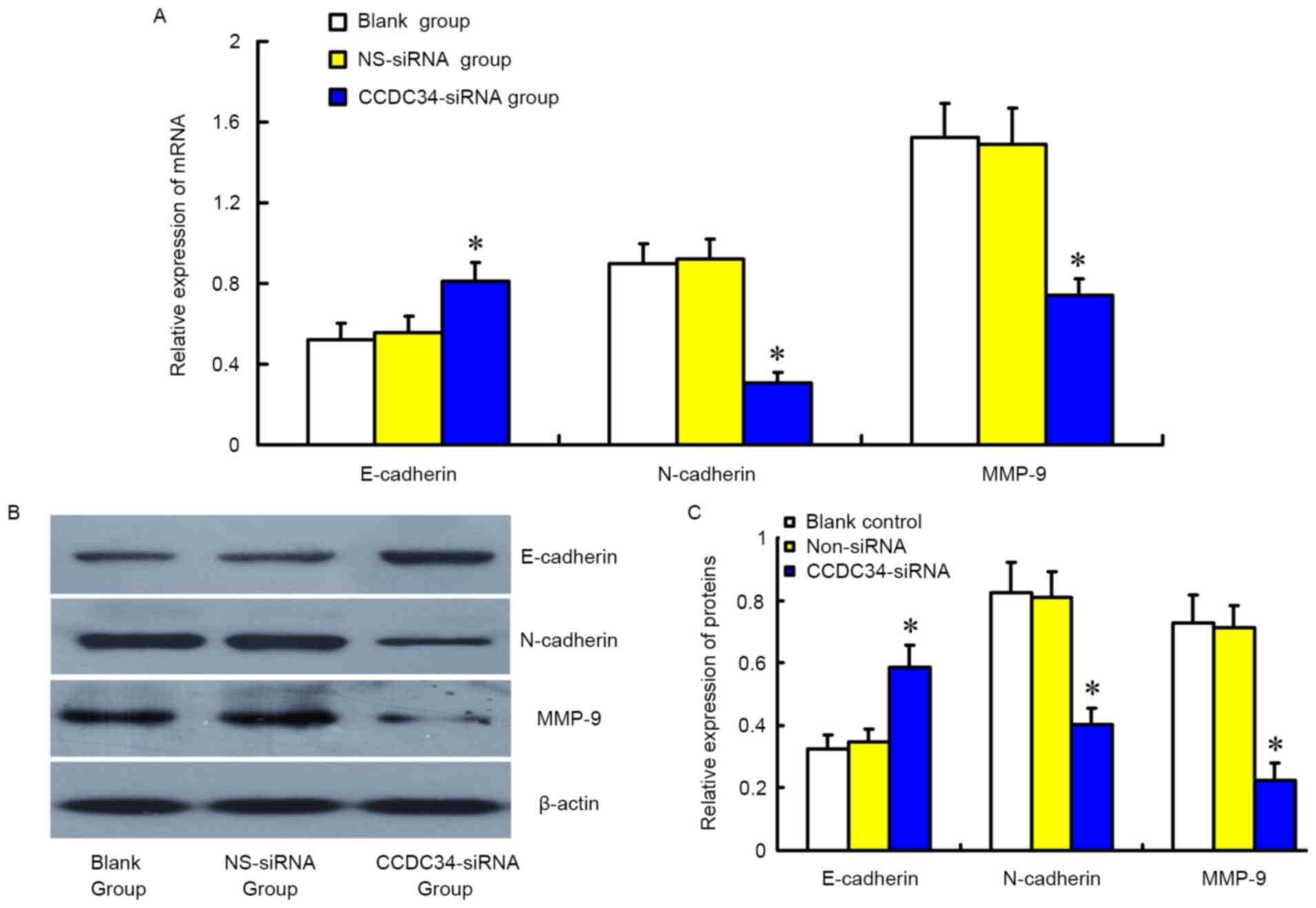
Alteration of the expression of
invasion-associated genes in SW620 cells following CCDC34-siRNA
treatment
As presented in Fig.
7, western blotting and RT-qPCR revealed mRNA and protein
expression of E-cadherin was significantly increased in cells of
the CCDC34-siRNA group compared with the control groups
(P<0.05), whereas that of N-cadherin and MMP-9 were
significantly reduced (P<0.05). No obvious difference between
the NS-siRNA group and the blank group was identified
(P>0.05).
Discussion
CRC is one of the most common cancers globally
(17). Although the treatments
have improved in terms of surgical technique (18), radiotherapy (19), chemotherapeutics and targeted drugs
for patients CRC (20,21), the overall therapeutic efficacy is
unsatisfactory with a high mortality rate, which leads to suffering
patients and also a considerable burden to society (22). Therefore, investigating the
pathogenesis and developing novel therapies have been hotspots in
CRC research. Recurrence and metastasis are primary causes of death
for CRC patients; therefore, methods blocking this process may
potentially improve CRC treatment. Previous studies have reported
that CRC cells which have high anti-apoptotic and invasion
abilities are prone to rapid progression and metastasis (23,24).
The anti-apoptosis and invasion of CRC cells may be due to the
combined effects of multiple genes, and considering that, it is
important to identify the key genes relevant to this process. Some
genes have been previously identified associated with
anti-apoptosis and invasion of CRC cells; however, specific
mechanisms remain to be elucidated. CCDC, a protein with a
coiled-coil structure, has the functions of metabolism regulation,
cell membrane channel and molecular chaperone (25,26).
The association between some members of the CCDC family and cancers
has been investigates previously. Gong et al (15) revealed that abnormal high
expression of CCDC34, a member of CCDC family, was detected in
bladder cancer cells, and suppression of CCDC34 contributed to the
reduced proliferation and invasion and increased apoptosis of
cancer cells. The present study observed high expression of CCDC34
in CRC tissues, particularly in tissues with deep tumor invasion
and lymphatic metastasis. This infers that CCDC34 contributed to
CRC progression and metastasis, and detection of CCDC34 in CRC
tissues may provide information to evaluate patients' condition.
Anti-apoptosis, invasion and metastasis of cancer cells have
important roles in progression of CRC. As CRC cells have
anti-apoptotic ability, previous studies regarding CRC treatment
are focused on how to suppress anti-apoptotic ability of CRC cells
(27,28). In addition, considering CRC cells
have strong invasive and metastatic ability, suppression of these
abilities may control tumor development (29,30).
In the in vitro experiments in the present study, reduced
cell metabolic activity, increased apoptotic rate and decreased
invasion were observed following the suppression of the expression
of CCDC34 in the SW620 cell line, which may indicate that CCDC34
may have the ability to regulate cell apoptosis and invasion. In
order to determine the role of CCDC34 in apoptosis and invasion of
CRC cells, the expression levels of apoptosis and
invasion-associated genes in CRC cells were detected following the
suppression of CCDC34 expression and the role of CCDC34 in CRC was
investigated.
Bcl-2 is an important gene that regulates apoptosis
through the mitochondrial pathway and it is able to suppress cell
apoptosis in various ways (31,32).
Survivin, a member of inhibitor of apoptosis proteins family, is
able to suppress apoptosis by suppressing caspase-3 and −8 activity
which are apoptosis promoting molecules (33,34).
In the current study, reduced expression of Bcl-2 and survivin was
detected in the SW620 cell line following CCDC34 inhibition,
whereas the activity of caspase-3 and −8 was increased. This
suggested that CCDC34 increased apoptosis resistance by activating
Bcl-2 and survivin and suppressing caspase-3 and caspase-8.
Epithelial-mesenchymal transition (EMT) has been
identified to participate in cancer invasion and metastasis
(35). In the current study, the
changes of EMT-associated genes in SW620 cell line following
suppression of CCDC34 was also detected. E-cadherin, a
transmembrane glycoprotein in epithelial cells, is essential for
cell junction and integrity of structure (36,37).
Previous studies have revealed that the downregulation of
E-cadherin expression may trigger the invasion and expansion of
basement membrane, which may lead to tumor invasion and metastasis
(38,39). N-cadherin is one of the important
mesenchymal markers and its upregulated expression is the hallmark
of EMT, as well as an indicator of tumor invasion and metastasis
(40,41). MMP-9, one of the important members
of the MMPs family is involved in the degradation of extracellular
matrix and contribution to metastasis in tumors (7,8,42,43)
and regulated by E-cadherin (44).
The present study determined that E-cadherin expression was
significantly increased following the inhibition of the endogenous
CCDC34 expression by RNA interference, whereas expression of
N-cadherin and MMP-9 was decreased. This indicates that CCDC34 is
involved in CRC EMT, which may lead to cancer invasion and
metastasis by suppressing E-cadherin and promoting N-cadherin and
MMP-9. However, the corresponding molecular mechanisms should be
further clarified by future studies.
In conclusion, the present study demonstrated
increased expression of CCDC34 protein in CRC tissues was
associated with reduced apoptosis and increased metastasis in CRC
cell line. CCDC34 may promote anti-apoptosis and invasion by
regulating Bcl-2, survivin, E-cadherin, N-cadherin and MMP-9.
However, the sample size in the present study was limited and the
in vitro experiments are insufficient. Despite the
limitations, it may be concluded that CCDC34 had an important role
in CRC invasion and metastasis. Further investigation of the
functions of CCDC34 may be beneficial to CRC evaluation and CCDC34
may also be regarded as the target gene for controlling CRC
progression and metastasis.
References
|
1
|
Hashim D, Boffetta P, La Vecchia C, Rota
M, Bertuccio P, Malvezzi M and Negri E: The global decrease in
cancer mortality: Trends and disparities. Ann Oncol. 27:926–933.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Favoriti P, Carbone G, Greco M, Pirozzi F,
Pirozzi RE and Corcione F: Worldwide burden of colorectal cancer: A
review. Updates Surg. 68:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schreuders EH, Ruco A, Rabeneck L, Schoen
RE, Sung JJ, Young GP and Kuipers EJ: Colorectal cancer screening:
A global overview of existing programmes. Gut. 64:1637–1649. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simpkins SJ, Pinto-Sanchez MI, Moayyedi P,
Bercik P, Morgan DG, Bolino C and Ford AC: Poor predictive value of
lower gastrointestinal alarm features in the diagnosis of
colorectal cancer in 1981 patients in secondary care. Aliment
Pharmacol Ther. 45:91–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tokodai K, Narimatsu H, Nishida A, Takaya
K, Hara Y, Kawagishi N, Hashizume E and Ohuchi N: Risk factors for
recurrence in stage II/III colorectal cancer patients treated with
curative surgery: The impact of postoperative tumor markers and an
infiltrative growth pattern. J Surg Oncol. 114:368–374. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vatandoust S, Price TJ and Karapetis CS:
Colorectal cancer: Metastases to a single organ. World J
Gastroenterol. 21:11767–11776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen GQ, Tang CF, Shi XK, Lin CY, Fatima
S, Pan XH, Yang DJ, Zhang G, Lu AP, Lin SH and Bian ZX:
Halofuginone inhibits colorectal cancer growth through suppression
of Akt/mTORC1 signaling and glucose metabolism. Oncotarget.
6:24148–24162. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
He G, Feng C, Vinothkumar R, Chen W, Dai
X, Chen X, Ye Q, Qiu C, Zhou H, Wang Y, et al: Curcumin analog EF24
induces apoptosis via ROS-dependent mitochondrial dysfunction in
human colorectal cancer cells. Cancer Chemother Pharmacol.
78:1151–1161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sathyanarayanan A, Chandrasekaran KS and
Karunagaran D: microRNA-146a inhibits proliferation, migration and
invasion of human cervical and colorectal cancer cells. Biochem
Biophys Res Commun. 480:528–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Modjtahedi N, Tokatlidis K, Dessen P and
Kroemer G: Mitochondrial proteins containing
Coiled-Coil-Helix-Coiled-Coil-Helix (CHCH) domains in health and
disease. Trends Biochem Sci. 41:245–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yin DT, Xu J, Lei M, Li H, Wang Y, Liu Z,
Zhou Y and Xing M: Characterization of the novel tumor-suppressor
gene CCDC67 in papillary thyroid carcinoma. Oncotarget.
7:5830–5841. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morra F, Luise C, Visconti R, Staibano S,
Merolla F, Ilardi G, Guggino G, Paladino S, Sarnataro D, Franco R,
et al: New therapeutic perspectives in CCDC6 deficient lung cancer
cells. Int J Cancer. 136:2146–2157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong J, Zhao M, Luo Q, Ma Y, Liu J, Wang
J, Yang M, Yuan X, Sang J and Huang C: CCDC134 is down-regulated in
gastric cancer and its silencing promotes cell migration and
invasion of GES-1 and AGS cells via the MAPK pathway. Mol Cell
Biochem. 372:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park SJ, Jang HR, Kim M, Kim JH, Kwon OH,
Park JL, Noh SM, Song KS, Kim SY, Kim YH and Kim YS: Epigenetic
alteration of CCDC67 and its tumor suppressor function in gastric
cancer. Carcinogenesis. 33:1494–1501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong Y, Qiu W, Ning X, Yang X, Liu L, Wang
Z, Lin J, Li X and Guo Y: CCDC34 is up-regulated in bladder cancer
and regulates bladder cancer cell proliferation, apoptosis and
migration. Oncotarget. 6:25856–25867. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bode AM, Dong Z and Wang H: Cancer
prevention and control: Alarming challenges in China. Natl Sci Rev.
3:117–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ratti F, Catena M, Di Palo S, Staudacher C
and Aldrighetti L: Impact of totally laparoscopic combined
management of colorectal cancer with synchronous hepatic metastases
on severity of complications: A propensity-score-based analysis.
Surg Endosc. 30:4934–4945. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ahmed I, Howard M, Rehman Z, Ofar F,
Marley P, O'Doherty E and Martin MJ: A comparison of overall and
disease-specific survivals following adjuvant radiotherapy with
neo-adjuvant radiotherapy for rectal cancer. J Clin Oncol. 27 15
suppl:e150082009.
|
|
20
|
Bartoş A, Bartoş D, Szabo B, Breazu C,
Opincariu I, Mironiuc A and Iancu C: Recent achievements in
colorectal cancer diagnostic and therapy by the use of
nanoparticles. Drug Metab Rev. 48:27–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Verdaguer H, Tabernero J and Macarulla T:
Ramucirumab in metastatic colorectal cancer: Evidence to date and
place in therapy. Ther Adv Med Oncol. 8:230–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen W, Zheng R, Zeng H and Zhang S: The
updated incidences and mortalities of major cancers in China, 2011.
Chin J Cancer. 34:53–507. 2015. View Article : Google Scholar :
|
|
23
|
Kawakami H, Huang S, Pal K, Dutta SK,
Mukhopadhyay D and Sinicrope FA: Mutant BRAF upregulates MCL-1 to
confer apoptosis resistance that is reversed by MCL-1 antagonism
and cobimetinib in colorectal cancer. Mol Cancer Ther.
15:3015–3027. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Feng Y, Feng L, Yu D, Zou J and Huang Z:
srGAP1 mediates the migration inhibition effect of Slit2-Robo1 in
colorectal cancer. J Exp Clin Cancer Res. 35:1912016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Truebestein L and Leonard TA:
Coiled-coils: The long and short of it. Bioessays. 38:903–916.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peralta S, Clemente P, Sánchez-Martínez A,
Calleja M, Hernández-Sierra R, Matsushima Y, Adán C, Ugalde C,
Fernández-Moreno MÁ, Kaguni LS and Garesse R: Coiled coil
domain-containing protein 56 (CCDC56) is a novel mitochondrial
protein essential for cytochrome c oxidase function. J Biol Chem.
287:24174–24185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan XJ, Wang Y, Wang L and Zhu M:
Salidroside induces apoptosis and autophagy in human colorectal
cancer cells through inhibition of PI3K/Akt/mTOR pathway. Oncol
Rep. 36:3559–3567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jayathilake AG, Senior PV and Su XQ: Krill
oil extract suppresses cell growth and induces apoptosis of human
colorectal cancer cells. BMC Complement Altern Med. 16:3282016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Z, Han S, Huang W, Wu J, Liu Y, Cai
S, He Y, Wu S and Song W: MicroRNA-215 suppresses cell
proliferation, migration and invasion of colon cancer by repressing
Yin-Yang 1. Biochem Biophys Res Commun. 479:482–488. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jing X, Wu H, Ji X, Wu H, Shi M and Zhao
R: Cortactin promotes cell migration and invasion through
upregulation of the dedicator of cytokinesis 1 expression in human
colorectal cancer. Oncol Rep. 36:1946–1952. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Merino D, Lok SW, Visvader JE and Lindeman
GJ: Targeting BCL-2 to enhance vulnerability to therapy in estrogen
receptor-positive breast cancer. Oncogene. 35:1877–1887. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kvansakul M and Hinds MG: The Bcl-2
family: Structures, interactions and targets for drug discovery.
Apoptosis. 20:136–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feng W, Yoshida A and Ueda T: YM155
induces caspase-8 dependent apoptosis through downregulation of
survivin and Mcl-1 in human leukemia cells. Biochem Biophys Res
Commun. 435:52–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sam MR, Ahangar P, Nejati V and Habibian
R: Treatment of LS174T colorectal cancer stem-like cells with n-3
PUFAs induces growth suppression through inhibition of survivin
expression and induction of caspase-3 activation. Cell Oncol
(Dordr). 39:69–77. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen X, Bode AM, Dong Z and Cao Y: The
epithelial-mesenchymal transition (EMT) is regulated by oncoviruses
in cancer. FASEB J. 30:3001–3010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Petrova YI, Schecterson L and Gumbiner BM:
Roles for E-cadherin cell surface regulation in cancer. Mol Biol
Cell. 27:3233–3244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Chen XY, Huang KJ, Wu WD, Jiang
T, Cao J, Zhou LS, Qiu ZJ and Huang C: Expression of FoxM1 and the
EMT-associated protein E-cadherin in gastric cancer and its
clinical significance. Oncol Lett. 12:2445–2450. 2016.PubMed/NCBI
|
|
38
|
Iseki Y, Shibutani M, Maeda K, Nagahara H,
Ikeya T and Hirakawa K: Significance of E-cadherin and CD44
expression in patients with unresectable metastatic colorectal
cancer. Oncol Lett. 14:1025–1034. 2017.PubMed/NCBI
|
|
39
|
Sheng L, Zhang S and Xu H: Effect of
slug-mediated down-regulation of E-cadherin on invasiveness and
metastasis of anaplastic thyroid cancer cells. Med Sci Monit.
23:138–143. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang H, Svoboda RA, Lazenby AJ, Saowapa
J, Chaika N, Ding K, Wheelock MJ and Johnson KR: Up-regulation of
N-cadherin by collagen I-activated discoidin domain receptor 1 in
pancreatic cancer requires the adaptor molecule Shc1. J Biol Chem.
291:23208–23223. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fernández NB, Lorenzo D, Picco ME, Barbero
G, Dergan-Dylon LS, Marks MP, García-Rivello H, Gimenez L, Labovsky
V, Grumolato L and Lopez-Bergami P: ROR1 contributes to melanoma
cell growth and migration by regulating N-cadherin expression via
the PI3K/Akt pathway. Mol Carcinog. 55:1772–1785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rabkin SW: Differential expression of
MMP-2, MMP-9 and TIMP proteins in thoracic aortic
aneurysm-comparison with and without bicuspid aortic valve: A
meta-analysis. Vasa. 43:433–442. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Araújo RF Jr, Lira GA, Vilaça JA, Guedes
HG, Leitão MC, Lucena HF and Ramos CC: Prognostic and diagnostic
implications of MMP-2, MMP-9, and VEGF-α expressions in colorectal
cancer. Pathol Res Pract. 211:71–77. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Guo JQ, Zheng QH, Chen H, Chen L, Xu JB,
Chen MY, Lu D, Wang ZH, Tong HF and Lin S: Ginsenoside Rg3
inhibition of vasculogenic mimicry in pancreatic cancer through
downregulation of VE-cadherin/EphA2/MMP9/MMP2 expression. Int J
Oncol. 45:1065–1072. 2014. View Article : Google Scholar : PubMed/NCBI
|