Introduction
Osteosarcoma is a relatively common primary bone
malignancy and tends to occur in young people (1). The incidences of osteosarcoma in
children and adolescents are high and accounts for approximately
20% of all primary bone cancers; the incidence of osteosarcoma is
around 0.2–3/100,000 per year, which is even higher in the age
group of 15–19 years (0.8–11/100,000 per year) (2). Despite the fact that several
improvements have been made in the curative protocols, osteosarcoma
remains a devastating disease with poor early diagnosis as well as
a low long-term survival rate (3),
and the 5-year survival rate obtained by traditional chemotherapy
in combination with surgical resection is still below 70% (4), mainly due to the resistance to
chemotherapy and resulting failure of osteosarcoma treatment
(5). Therefore, a deeper
understanding of osteosarcoma development on the molecular level
may pave the way for future therapy development. In addition, the
process of tumorigenesis, i.e., a cumulative occurrence of gene
mutations that influence the expression of both oncogenes and tumor
suppressor genes, may also affect osteosarcoma development, where
the osteoprogenitor cells acquire the ability of uncontrolled
proliferation and bone formation (6). Therefore, using genome-wide
sequencing, many studies have been carried out to identify genes
responsible for the development of osteosarcoma (7–9).
Dermatopontin (DPT), initially found
by purification of dercoin in calf skin, is an extracellular matrix
(ECM) protein with a tyrosine residue and a molecular weight of 22
kDa, and can promote cell adhesion and ECM assembly by closely
interacting with other ECM proteins (10). Since ECM degradation is an
important prerequisite for tumor metastasis (11,12),
DPT can affect tumor prognosis by regulating tumor invasion
and metastasis (13,14). The expression of DPT is
decreased in systemic sclerosis and hypertrophic scaring (15) as well as uterine leiomyomas and
keloids (16) and may play an
important role in wound healing (17). Recently, it has been shown that
DPT can mediate in vivo prostate cell growth
(18) and inhibit the metastasis
of human oral cancer (13).
Unfortunately, the DPT expression in osteosarcoma and its
functional characteristics have not yet been thoroughly studied.
Therefore, in this paper, we will analyze the effect of DPT
during apoptosis and proliferation of osteosarcoma MG-63 cells,
thus providing a potential therapeutic target and new ideas for
chemotherapy and gene therapy of osteosarcoma.
Materials and methods
Cell culture and treatment
The osteosarcoma MG-63 cells were purchased from
Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai,
China). The cells were cultured in 1640 media supplemented with 10%
inactivated fetal calf serum plus 100 units/ml penicillin and 100
mg/ml streptomycin (all from Gibco, Thermo Fisher, Waltham, MA,
USA) at 37°C and 5% CO2. When the cells grew to 80%
confluency, they were digested using 0.25% trypsin and
passaged.
Establishment of DPT gene silencing
model
According to the design principle of shRNA, the
sequence of the DPT gene was obtained from GenBank
(NM_001937.4), and RNA interference sites provided by Invitrogen
on-line (Thermo Fisher) were used to design 3 interference
sequences, i.e., shRNA-a, shRNA-b and shRNA-c. Blast software was
used for comparison on the human genome, and the sequence of
DPT gene was identified as unique. In the same way, a
non-specific shRNA-control was designed as a negative sequence
without any matching sequences in the human genome. Four shRNA
sequences were synthesized by Sangon (Shanghai, China):
ShRNA-control: 5′-ACTACCGTTGTTATAGGTG-3′; DPT-shRNA-a:
5′-TGGCGGGAAGTGTGAAGCG-3′; DPT-shRNA-b: 5′-CATCAACGAGTGCTCCAGC-3′;
DPT-shRNA-c: 5′-GGTGTCTTCCAGATCCTGA-3′. BamHI and HindIII
restriction enzyme cutting sites (TaKaRa Bio, Tokyo, Japan) were
added to both ends of the sequences, and each designed
oligonucleotide sequence was inserted into a pSIREN-RetroQ-TetH
vector (TaKaRa Bio). The recombinant plasmids were amplified by
Escherichia coli (E. coli) DH5α (Tiangen, Beijing, China)
and cultured overnight in LB medium containing Ampicilin. The
plasmids were extracted using a plasmid miniprep kit (Beyotime,
Shanghai, China) and were sequenced by Invitrogen (Invitrogen,
Thermo Fisher, Waltham, MA, USA) to check whether the base
sequences of the shRNA fragments were correct.
Cell grouping and transfection
In the experiment, the cells were assigned to blank
group, shRNA-control group, DPT-shRNA-a group, DPT-shRNA-b group
and DPT-shRNA-c group. The blank group received no treatment. The
shRNA-control group was transfected with shRNA-control plasmids.
DPT-shRNA-a, DPT-shRNA-b and DPT-shRNA-c groups were transfected
with DPT-shRNA-a, DPT-shRNA-b and DPT-shRNA-c plasmids,
respectively. According to the instructions of Fugene transfection
reagent (Invitrogen, Thermo Fisher), the MG-63 cells were seeded on
6-well plates the day prior to cell transfection and routinely
incubated. The culture media were changed at 2 h before the
transfection, followed by adding 2 ml of 1640 culture media into
each well. For each well, 8 µl Fugene reagent and 3.2 µg of
plasmids were mixed and incubated for 20 min at room temperature.
The 1640 culture media in each well were aspirated and 200 µl of
Opti-MEN media were added into each well, followed by 800 µl of
Fugene/plasmid mixture. The plates were incubated at 37°C for 5 h.
After the transfection, 2 ml of 1640 media were added into each
well and the plates were incubated at 37°C. After 24, 36, 48 and 72
h, the transfection efficiency was observed by fluorescence
microscopy.
Quantitative real time polymerase
chain reaction (qRT-PCR)
After 36 h of transient shRNA transfection, the
cells were collected in a 15 ml centrifuge tube and the total RNA
of osteosarcoma MG-63 cells was extracted according to the
instruction of a RNA extraction kit (Promega Corp., Madison, WI,
USA). The optic density (OD) 260/280 ratio of the extracted RNA
samples was analyzed by an ultraviolet spectrophotometer, and the
RNA concentration was calculated. The RNA samples were then stored
at −80°C for future use. PCR primers were designed using the
Primer6.0 primer design software, according to the gene sequences
in the Genebank database: β-actin: Forward (5′-3′) TGG CAT TGC CGA
CAG GAT GCA GCAA; reverse (5′-3′) CTC CTC ATA CTC CTG CTT GCT GAT;
DPT: Forward (5′-3′) GGT GGC TAC GGG TAC CCA TA; reverse (5′-3′)
GTC AGA GCC TTC CTT CTT GC. The primers were synthesized by Sangon
(Shanghai, China). Reverse transcription (RT) was performed
according to the protocol provided with the RNA Reverse
Transcription Kit (Promega Corp.). PCR was carried out in a
two-step reaction: Pre-denaturation at 95°Cfor 15 min, followed by
40 cycles of denaturation at 95°Cfor 10 sec and annealing/extension
at 60°Cfor 30 sec. The qRT-PCR reaction system consisted of 12.5 µl
of SYBR Green Mix, 1 µl of forward primer, 1 µl of reverse primer,
2 µl of DNA template, and 8.5 µl of ddH2O (Promega
Corp.). β-actin was used as an internal reference. The reliability
of the PCR was evaluated by the dissolution curve and the cycle
quantification (Cq) value was obtained. The relative expression of
the target gene was calculated according to 2−ΔΔCq, and
each measurement was repeated three times.
Western blot analysis
The protein in each cell group was extracted at 36 h
after transient shRNA transfection. The protein concentration was
determined by according to the manual of a BCA kit (Boster, Wuhan,
Hubei, China). The extracted protein was added to the loading
buffer and then boiled at 95°C for 5 min. A total of 30 µg samples
were added into each well of a 10% polyacrylamide gel (Boster,
Wuhan, Hubei, China), and the protein was then separated by
electrophoresis. The electrophoresis voltage was 80 V changed to
120 V with wet transfer. The membrane transfer voltage was 100 mV,
and the duration was 45–70 min. After transfer using a
polyvinylidene fluoride (PVDF) membrane, the membrane was mounted
in 5% bovine serum albumin (BSA) for 1 h at room temperature,
followed by addition of primary anti-DPT (1:1,000, Abcam,
Cambridge, UK) antibody and incubation at 4°C overnight. TBST
solution was used to rinse the membrane (3 times of 5 min each),
and the membrane was incubated with the corresponding secondary
antibodies at room temperature for 1 h. After additional membrane
washing (3 times of 5 min each), chemiluminescence reagent was
added and the membrane was developed. β-actin was used as the
internal reference (1:5,000, Kangchen, Shanghai, China). Each
measurement was repeated 3 times. A Bio-rad Gel DOC EZ imager
(Bio-Rad, Hercules, CA, USA) was used to image the membrane. The
gray value analysis of the target band was conducted using the
Image J software.
Cell Counting Kit-8 (CCK-8) assay
After the normal MG-63 cells and the cells
transfected with DPT-shRNA-a and shRNA-control reached the phase of
logarithmic growth, they were collected and seeded into 96-well
plates at a concentration of 103 cells/well. In each
well, 200 µl of cell culture media were added. Three duplicated
wells were set up for each cell group, and the experiment was
repeated 3 times. After culturing for 24, 48, 72, and 96 h, 20 µl
of CCK-8 reagent were added into each well of the 96-well plates,
which were returned to an incubator for cell culture. Subsequently,
the cells were removed from the incubator 4 h later and the
absorbance (A) value at 490 nm was measured with a microplate
reader. The cell growth curve was plotted using time as the X-axis
and the value of A490 nm as the Y-axis.
Flow cytometry
Cells were harvested after 36 h of transfection and
washed once with 1× PBS, and then resuspended in PBS containing 75%
ethanol and 0.5 mmol/l LEDTA. After fixing for 1 h at 4°C, the
cells were centrifuged at 2000 rpm for 5 min, and the supernatant
was discarded. The cells were washed once with 1× PBS and
resuspended in 500 µl of PBS containing 0.1% Triton X-100 and 50
µg/ml RNase, and 90 µl of 0.5 mg/ml propidium iodide (PI) were
added into the suspension rapidly. The suspension was then mixed
well using a pipette, and placed in the dark for 30 min of reaction
at room temperature. The solution was then filtered with a nylon
membrane and analyzed by an EPICS XL-4 flow cytometer (Beckman
Coulter, Brea, CA, USA). Each measurement was repeated 3 times.
Annexin V-FITC was used to detect
apoptosis
After 36 h of transfection, the cells were taken out
from the incubator, washed twice with PBS, digested with 0.25%
trypsin and collected by centrifugation at 1,000 rpm for 10 min.
The cells were then washed 3 times with PBS and resuspended. The
number of cells was counted and the cell concentration was adjusted
to 5×105 cells/ml. A volume of 5 µl Annexin V-FITC was
added into 100 µl cells, gently mixed, and incubated in the dark at
room temperature for 10 min. The suspension was then centrifuged at
1000 rpm for 5 min and the supernatant was discarded. A volume of
10 µl PI staining solution was added and gently mixed before
analyzing the cells by flow cytometry. The percentage of Annexin
V+/PI- cells in the total cell number was used as the apoptosis
rate, and each measurement was repeated 3 times.
Statistical analysis
The data were analyzed by SPSS20.0 statistical
software. The measurement data were expressed as mean ± standard
deviation (SD). The differences among multiple groups were analyzed
by analysis of variance (ANOVA). The difference between two groups
was verified using t test. A P-value of <0.05 was
considered statistically significant.
Results
DPT gene silencing in osteosarcoma
MG-63 cells
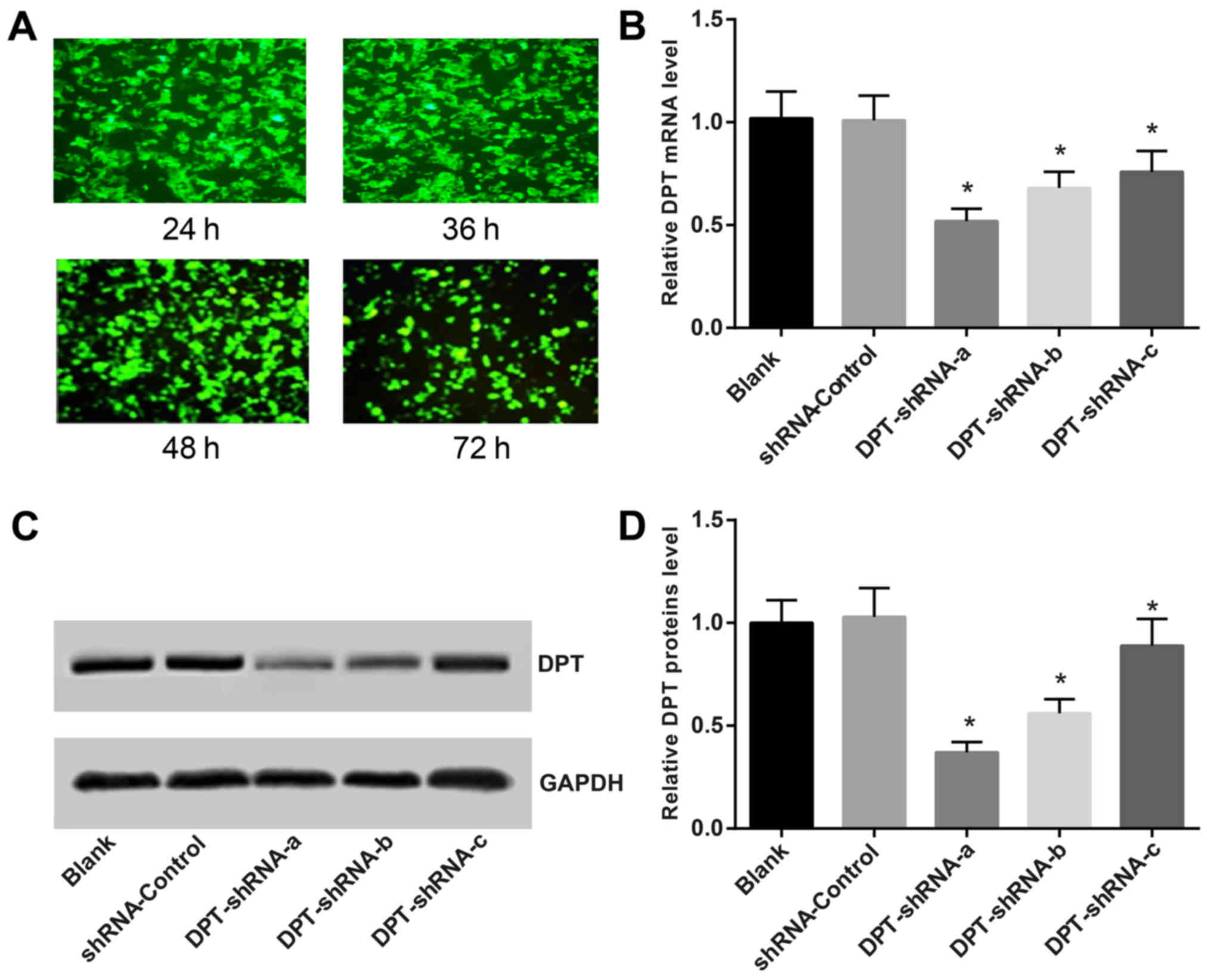
After transfection with DPT-shRNA, the percentage of
MG-63 cells with green fluorescence was 91, 97, 92 and 87%,
respectively, at 24, 36, 48 and 72 h post transfection. At 36 h
after transfection, the proportion of green fluorescent cells
reached the highest level (Fig.
1A). The expression of DPT was detected by qRT-PCR. The
results showed that the expression of DPT in
DPT-shRNA-transfected cells was significantly lower than that in
the shRNA-control group (P<0.05), while the expression of
DPT in the shRNA-control group was similar to that in the
blank group (P>0.05). This indicates that the DPT-shRNA
recombinant plasmids can significantly inhibit the expression of
DPT gene, whose expression was the lowest in the DPT-shRNA-a
group (Fig. 1B). Western blotting
showed that the expression of DPT protein in cells
transfected with DPT-shRNA-a, b and c was significantly attenuated
compared to that in the shRNA-control group (all P<0.05),
especially for the DPT-shRNA-a group. There was no significant
difference between the DPT-shRNA-c group and the blank group
(Figs. 1C and 1D). Therefore, DPT-shRNA-a recombinant
plasmid was selected for subsequent experiments.
Effects of DPT gene silencing on the
proliferation of MG-63 cells
MG-63 cells were transfected with DPT-shRNA-a
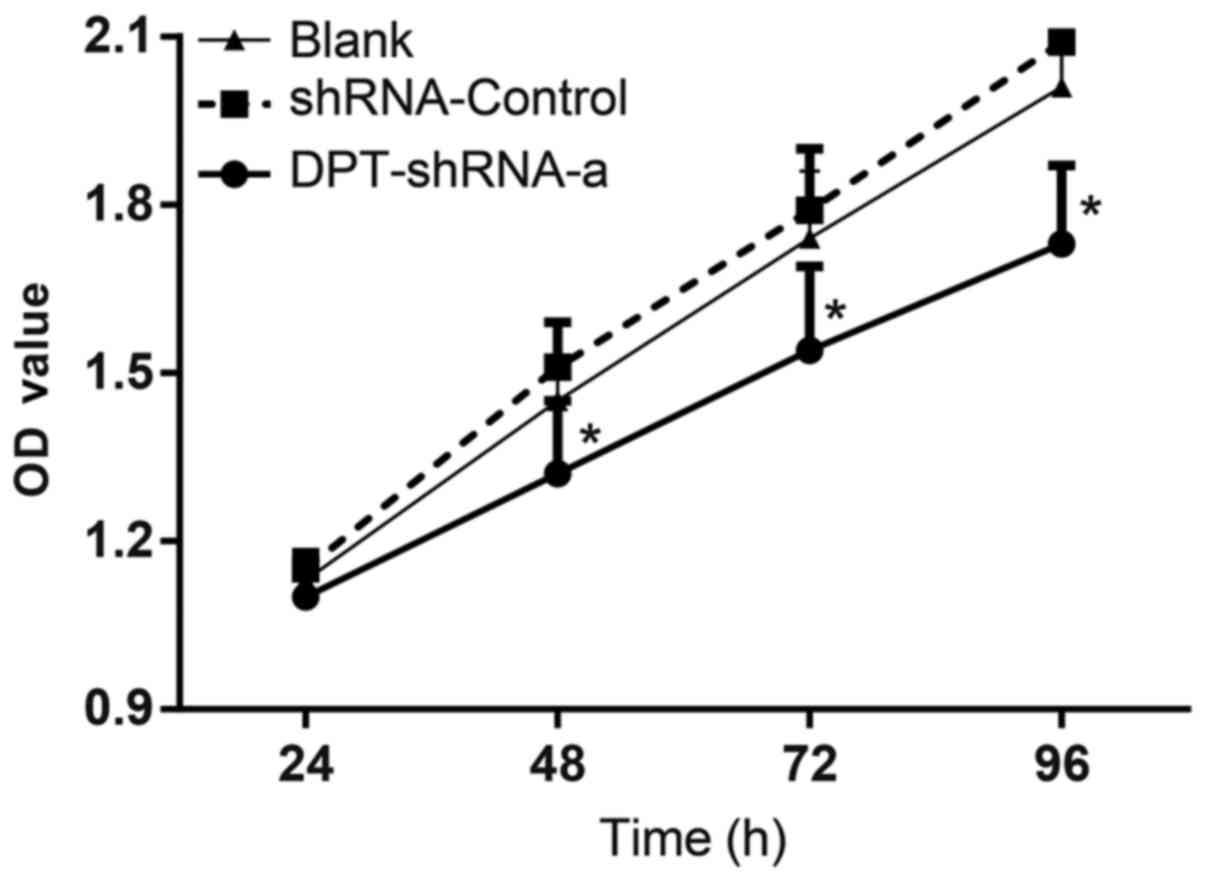
plasmid and the cell proliferation was detected by the CCK-8 assay.
The OD values of cells in the blank group were 1.13±0.04,
1.45±0.07, 1.74±0.12 and 2.01±0.18, respectively, at 24, 48, 72 and
96 h post transfection. The OD values of cells in the shRNA-control
group were 1.15±0.03, 1.51±0.08, 1.79±0.11 and 2.09±0.17,
respectively, at 24, 48, 72 and 96 h post transfection. The OD
values of cells in the DPT-shRNA-a group were 1.10±0.05, 1.32±0.13,
1.54±0.15 and 1.73±0.14, respectively, at 24, 48, 72 and 96 h post
transfection. There were significant differences between cells in
the DPT-shRNA-a group and cells in the shRNA-control, the blank
groups at 48, 72 and 96 h (all P<0.05), while the OD values in
the shRNA-control and blank groups were not significantly different
(P>0.05) (Fig. 2). These
results showed that DPT silencing could significantly
inhibit the proliferation of MG-63 cells.
Effects of DPT gene silencing on cell
cycle of MG-63 cells
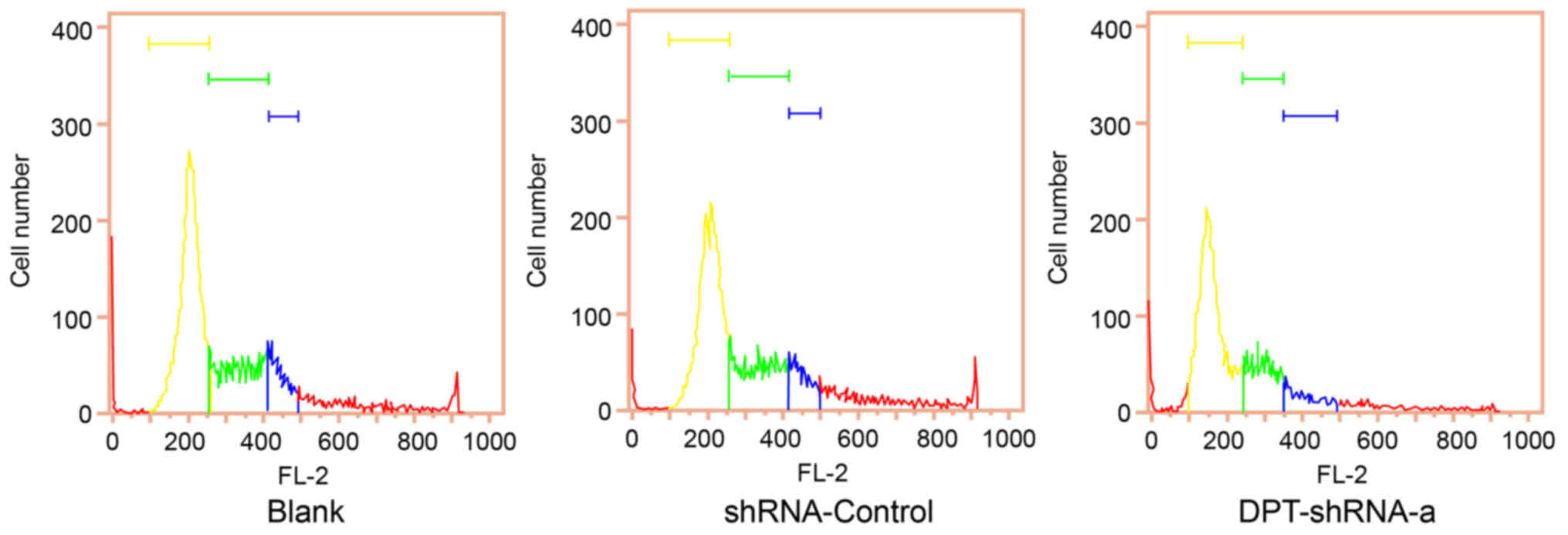
Flow cytometry analysis showed that the proportion
of cells at G0/G1 and G2/M phases in the DPT-shRNA-a group was
significantly higher than that in the blank group (both P<0.05),
while the proportion of cells at S phase in the DPT-shRNA-a group
was significantly lower than that in the blank group (P<0.05),
indicating that DNA replication in DPT-shRNA-a cells decreased and
their cell cycle progression was slowed down. In addition, there
was no significant difference between the shRNA-control group and
the blank group (P>0.05) (Fig.
3, Table I).
 | Table I.Effects of DPT gene silencing
on cell cycle of osteosarcoma MG-63 cells, as determined by flow
cytometry. |
Table I.
Effects of DPT gene silencing
on cell cycle of osteosarcoma MG-63 cells, as determined by flow
cytometry.
| Group | G0/G1 | S | G2/M |
|---|
| Blank |
61.8±3.1 |
26.3±0.7 |
11.9±3.6 |
| shRNA-Control |
62.3±
1.0 |
26.8±0.5 |
10.9±1.5 |
| DPT-shRNA-a |
68.7±3.8a |
14.2±0.2a |
17.1±3.9a |
Effects of DPT gene silencing on
apoptosis of MG-63 cells
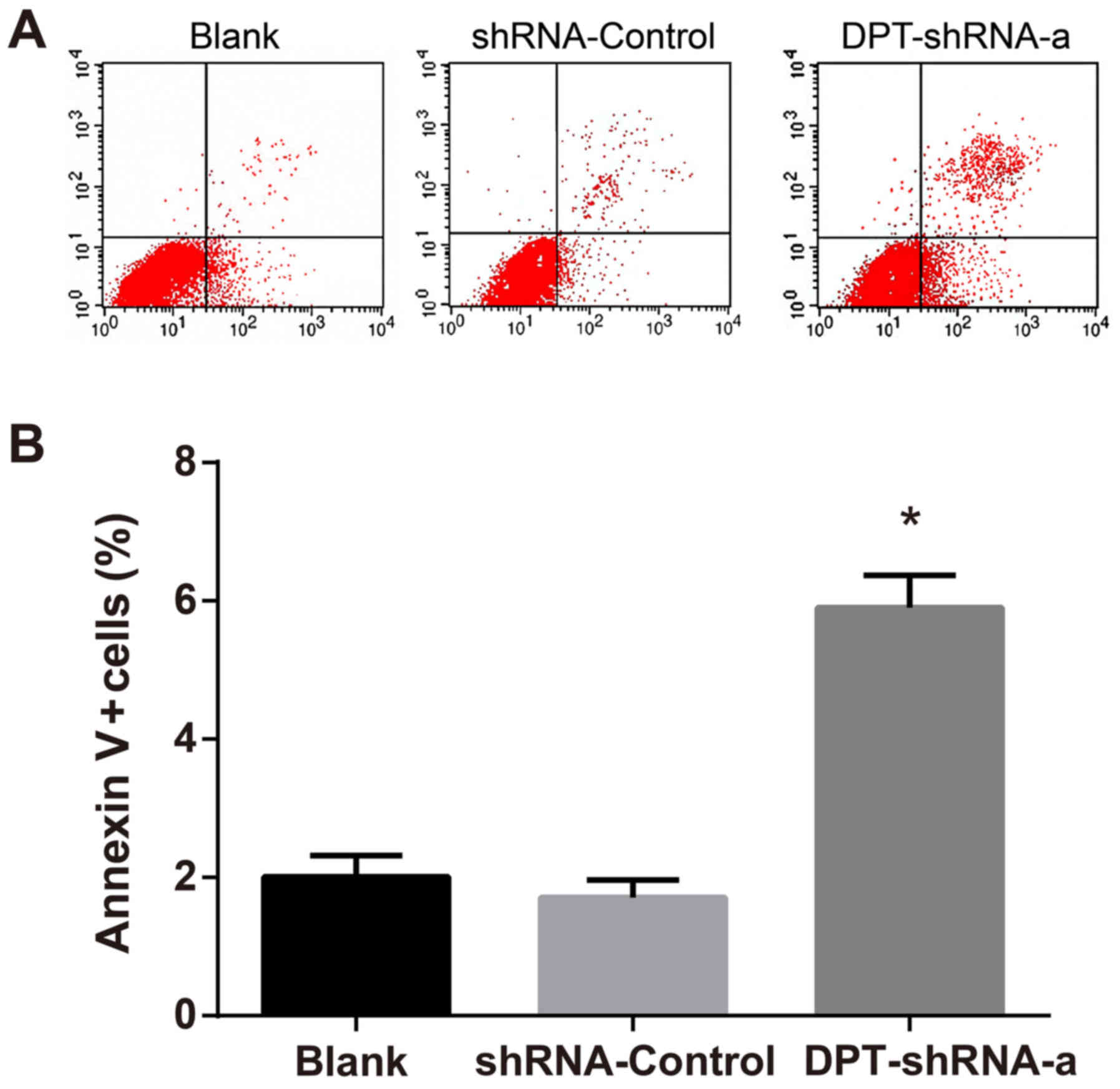
After double staining with Annexin V and PI, flow
cytometry measurement was carried out to detect the percentage of
Annexin V+/PI- cells, which was used as the apoptotic rate and was
compared among different groups. The results revealed that the
apoptotic rate in the DPT-shRNA-a group was significantly higher
than that in the blank group and the shRNA-control group (both
P<0.05), but there was no significant difference between the
shRNA-control group and the blank group (P>0.05) (Fig. 4).
Discussion
It has been shown that current treatment of
osteosarcoma by surgical operation and adjuvant chemotherapy cannot
achieve satisfactory outcomes (5,19,20).
Multiple examples are given to demonstrate the potential positive
role of gene therapy in the treatment of osteosarcoma (7,21,22),
indicating that the search of osteosarcoma target on genetic level
may be of great significance for a better treatment of
osteosarcoma. In this study, the effect of DPT gene
silencing on the apoptosis and proliferation of osteosarcoma MG-63
cells was studied, and the results may provide some insights for
the development of new DPT-targeting inhibitors with
clinical efficacy in the treatment of osteosarcoma.
Initially, in this study, DPT gene silencing
was found to reduce the proliferation of MG-63 cells. DPT is
an acidic protein that promotes the formation of collagen fibril
and reduces the diameter of newly formed collagen fibril (23,24).
It is reported that DPT in mammals may promote the adhesion
of fibroblasts through an integrated receptor channel function as
well as accelerating the synthesis of collagen fibrils (25). DPT binds to decorin, a small
leucine-rich proteoglycan, which can be involved in tumor stroma
formation, normal tissue development and differentiation (26). Besides, Decorin may mediate the
function of proteins involved in extracellular-matrix formation
(27,28). Study conducted by Catherino et
al also suggested that altered DPT expression could
interfere with decorin activity and cause abnormal
extracellular-matrix formation (16). Furthermore, RNA interference, a
well-known technique for silencing gene expression, can cause the
inhibition of proliferation of tumor cells (29). We can therefore speculate that
DPT gene silencing could reduce the proliferation of MG-63
cells. The further mechanism should be further investigated.
Besides, in this study, it was also shown that
DPT gene silencing can slow down the cell cycle progression
and promote apoptosis. The ability of cell adhesion and invasion is
the key to cancer metastasis, and as a proteoglycan binding to
other proteins, DPT can enhance the adhesion between
fibroblasts and keratinocytes by accelerating the formation of
collagen and fibronectin fibrils as well as by promoting the
cell-matrix interaction (13,30).
When DPT is active, it can improve wound healing by altering
the extracellular environment; whereas when DPT is not
active, it cannot induce early osteogenesis (30–32),
suggesting that cell regeneration is relatively slow when
DPT is inactive. DPT is a downstream target of
vitamin D receptor during the differentiation of pluripotent
stromal cells into osteoblasts and may function as growth factors,
i.e., DPT produced by tumor cells may up-regulate growth
factor expression in stromal cells (18). Therefore, it can be deduced that
DPT silencing will reduce the expression of cell growth
factors, thereby slowing the cell cycle progression. Apoptosis is
an important mechanism to eliminate malignant cells and cancer
cells can block the apoptotic pathway, thus avoiding apoptosis
(33). Takeuchi et al
(18) showed that DPT
receptors are located in the tumor matrix of the rats and will
promote the occurrence and progression of certain cancers,
suggesting that DPT is not conducive to apoptosis.
Therefore, it can be postulated that DPT silencing may
reinstate the apoptosis mechanism and promote cell death.
In conclusion, shRNA plasmids that can effectively
silence the expression of DPT gene were successfully
constructed in our study. The results showed that DPT gene
silencing could effectively reduce the proliferation of MG-63
cells, slow down the cell cycle progression and promote cell
apoptosis. This provides some insights for the development of new
inhibitors targeting DPT in the treatment of osteosarcoma.
However, the discovery reported in this paper is still preliminary.
Therefore, further studies are required to investigate the
potential application of relevant drugs as well as their role in
the prognosis of osteosarcoma.
Acknowledgements
The present study was supported by Guangxi Zhuang
Autonomous Region Natural Science Foundation (grant no.
2014jjAA40654), The authors would like to acknowledge the helpful
comments on this paper received from our reviewers.
References
|
1
|
Clark JC, Dass CR and Choong PF: A review
of clinical and molecular prognostic factors in osteosarcoma. J
Cancer Res Clin Oncol. 134:281–297. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu H, Li W, Zhang M, Zhu S, Zhang D and
Wang X: Inhibitory roles of miR-320 in osteosarcoma via regulating
E2F1. J Cancer Res Ther. 12:68–71. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Qureshi A, Ahmad Z, Azam M and Idrees R:
Epidemiological data for common bone sarcomas. Asian Pac J Cancer
Prev. 11:393–395. 2010.PubMed/NCBI
|
|
4
|
Baldauf C, Jeschke A, Kanbach V,
Catala-Lehnen P, Baumhoer D, Gerull H, Buhs S, Amling M, Nollau P,
Harroch S and Schinke T: The protein tyrosine phosphatase Rptpζ
suppresses osteosarcoma development in Trp53-heterozygous mice.
PLoS One. 10:e01377452015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fromigué O, Haÿ E, Modrowski D, Bouvet S,
Jacquel A, Auberger P and Marie PJ: RhoA GTPase inactivation by
statins induces osteosarcoma cell apoptosis by inhibiting
p42/p44-MAPKs-Bcl-2 signaling independently of BMP-2 and cell
differentiation. Cell Death Differ. 13:1845–1856. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kansara M, Teng MW, Smyth MJ and Thomas
DM: Translational biology of osteosarcoma. Nat Rev Cancer.
14:722–735. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kansara M, Tsang M, Kodjabachian L, Sims
NA, Trivett MK, Ehrich M, Dobrovic A, Slavin J, Choong PF, Simmons
PJ, et al: Wnt inhibitory factor 1 is epigenetically silenced in
human osteosarcoma, and targeted disruption accelerates
osteosarcomagenesis in mice. J Clin Invest. 119:837–851. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kuijjer ML, Rydbeck H, Kresse SH, Buddingh
EP, Lid AB, Roelofs H, Bürger H, Myklebost O, Hogendoorn PC,
Meza-Zepeda LA and Cleton-Jansen AM: Identification of osteosarcoma
driver genes by integrative analysis of copy number and gene
expression data. Genes Chromosomes Cancer. 51:696–706. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sadikovic B, Yoshimoto M, Chilton-MacNeill
S, Thorner P, Squire JA and Zielenska M: Identification of
interactive networks of gene expression associated with
osteosarcoma oncogenesis by integrated molecular profiling. Hum Mol
Genet. 18:1962–1975. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Okamoto O, Hozumi K, Katagiri F, Takahashi
N, Sumiyoshi H, Matsuo N, Yoshioka H, Nomizu M and Fujiwara S:
Dermatopontin promotes epidermal keratinocyte adhesion via
alpha3beta1 integrin and a proteoglycan receptor. Biochemistry.
49:147–155. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Markway BD, Cho H, Anderson DE, Holden P,
Ravi V, Little CB and Johnstone B: Reoxygenation enhances tumour
necrosis factor alpha-induced degradation of the extracellular
matrix produced by chondrogenic cells. Eur Cell Mater. 31:425–439.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu H, Liu S, Zhang G, Kwong LN, Zhu Y,
Miller JP, Hu Y, Zhong W, Zeng J, Wu L, et al: Oncogenic
BRAF-mediated melanoma cell invasion. Cell Rep. 15:2012–2024. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamatoji M, Kasamatsu A, Kouzu Y, Koike H,
Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H and Uzawa K:
Dermatopontin: A potential predictor for metastasis of human oral
cancer. Int J Cancer. 130:2903–2911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu Y, Feng MX, Yu J, Ma MZ, Liu XJ, Li J,
Yang XM, Wang YH, Zhang YL, Ao JP, et al: DNA methylation-mediated
silencing of matricellular protein dermatopontin promotes
hepatocellular carcinoma metastasis by α3β1 integrin-Rho GTPase
signaling. Oncotarget. 5:6701–6715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kuroda K, Okamoto O and Shinkai H:
Dermatopontin expression is decreased in hypertrophic scar and
systemic sclerosis skin fibroblasts and is regulated by
transforming growth factor-beta1, interleukin-4, and matrix
collagen. J Invest Dermatol. 112:706–710. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Catherino WH, Leppert PC, Stenmark MH,
Payson M, Potlog-Nahari C, Nieman LK and Segars JH: Reduced
dermatopontin expression is a molecular link between uterine
leiomyomas and keloids. Genes Chromosomes Cancer. 40:204–217. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russell SB, Russell JD, Trupin KM, Gayden
AE, Opalenik SR, Nanney LB, Broquist AH, Raju L and Williams SM:
Epigenetically altered wound healing in keloid fibroblasts. J
Invest Dermatol. 130:2489–2496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Takeuchi T, Suzuki M, Kumagai J, Kamijo T,
Sakai M and Kitamura T: Extracellular matrix dermatopontin
modulates prostate cell growth in vivo. J Endocrinol. 190:351–361.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bacci G, Bertoni F, Longhi A, Ferrari S,
Forni C, Biagini R, Bacchini P, Donati D, Manfrini M, Bernini G and
Lari S: Neoadjuvant chemotherapy for high-grade central
osteosarcoma of the extremity. Histologic response to preoperative
chemotherapy correlates with histologic subtype of the tumor.
Cancer. 97:3068–3075. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Allison DC, Carney SC, Ahlmann ER,
Hendifar A, Chawla S, Fedenko A, Angeles C and Menendez LR: A
meta-analysis of osteosarcoma outcomes in the modern medical era.
Sarcoma. 2012:7048722012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xin M, Qiao Z, Li J, Liu J, Song S, Zhao
X, Miao P, Tang T, Wang L, Liu W, et al: miR-22 inhibits tumor
growth and metastasis by targeting ATP citrate lyase: Evidence in
osteosarcoma, prostate cancer, cervical cancer and lung cancer.
Oncotarget. 7:44252–44265. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng S, Zhang X, Huang N, Qiu Q, Jin Y
and Jiang D: Down-regulation of S100A9 inhibits osteosarcoma cell
growth through inactivating MAPK and NF-κB signaling pathways. BMC
Cancer. 16:2532016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neame PJ, Choi HU and Rosenberg LC: The
isolation and primary structure of a 22-kDa extracellular matrix
protein from bovine skin. J Biol Chem. 264:5474–5479.
1989.PubMed/NCBI
|
|
24
|
Tracy LE, Minasian RA and Caterson EJ:
Extracellular matrix and dermal fibroblast function in the healing
wound. Adv Wound Care (New Rochelle). 5:119–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kato A, Okamoto O, Ishikawa K, Sumiyoshi
H, Matsuo N, Yoshioka H, Nomizu M, Shimada T and Fujiwara S:
Dermatopontin interacts with fibronectin, promotes fibronectin
fibril formation, and enhances cell adhesion. J Biol Chem.
286:14861–14869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Riquelme C, Larrain J, Schonherr E,
Henriquez JP, Kresse H and Brandan E: Antisense inhibition of
decorin expression in myoblasts decreases cell responsiveness to
transforming growth factor beta and accelerates skeletal muscle
differentiation. J Biol Chem. 276:3589–3596. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dudás J, Kovalszky I, Gallai M, Nagy JO,
Schaff Z, Knittel T, Mehde M, Neubauer K, Szalay F and Ramadori G:
Expression of decorin, transforming growth factor-beta 1, tissue
inhibitor metalloproteinase 1 and 2, and type IV collagenases in
chronic hepatitis. Am J Clin Pathol. 115:725–735. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Costacurta A, Priante G, D'Angelo A,
Chieco-Bianchi L and Cantaro S: Decorin transfection in human
mesangial cells downregulates genes playing a role in the
progression of fibrosis. J Clin Lab Anal. 16:178–186. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang S, Yang JH, Guo CK and Cai PC: Gene
silencing of TKTL1 by RNAi inhibits cell proliferation in human
hepatoma cells. Cancer Lett. 253:108–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Krishnaswamy VR and Korrapati PS: Role of
dermatopontin in re-epithelialization: Implications on keratinocyte
migration and proliferation. Sci Rep. 4:73852014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kato A, Okamoto O, Wu W, Matsuo N, Kumai
J, Yamada Y, Katagiri F, Nomizu M and Fujiwara S: Identification of
fibronectin binding sites in dermatopontin and their biological
function. J Dermatol Sci. 76:51–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Behnam K, Murray SS and Brochmann EJ: BMP
stimulation of alkaline phosphatase activity in pluripotent mouse
C2C12 cells is inhibited by dermatopontin, one of the most abundant
low molecular weight proteins in demineralized bone matrix. Connect
Tissue Res. 47:271–277. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fernald K and Kurokawa M: Evading
apoptosis in cancer. Trends Cell Biol. 23:620–633. 2013. View Article : Google Scholar : PubMed/NCBI
|