Introduction
Renal cell carcinoma (RCC), a complex metabolic
disease that is associated with a number of different types of
cancer, occurs in the kidney. In recent years, RCC has become the
most common urological malignancy (1), accounting for ~2–3% of adult
malignancies and ~80–90% of adult kidney malignancies; thus, RCC
has a serious impact on public health. The current treatments
available for RCC include novel small molecular targeted drugs,
immune targeted therapy, adjuvant and neoadjuvant therapies and
biomarker research. The primary treatments applied for RCC include
tyrosine kinase inhibitors such as sunitinib and pazopanib, the
mTOR inhibitor temsirolimus, the monoclonal antibody inhibitor of
vascular endothelial growth factor (VEGF) bevacizumab and cytokine
treatments such as aldesleukin (2). The current principle of targeted RCC
therapy is based on the known molecular mechanisms of renal cancer
and aims to prevent the proliferation of tumor cells and inhibit
angiogenesis; Von Hippel-Lindau tumor suppressor and mTOR (3) are the widely used targets in clinical
practice. p53, a tumor suppressor protein, serves an important role
in apoptosis and the inhibition of angiogenesis; it is essential
for mouse double minute 2 proto-oncogene antagonist induced RCC
cell apoptosis (4). Recently, a
previous study demonstrated that co-treatment with curcumin and
temsirolimus activated the expression of p53, which induced RCC
cell apoptosis (5). In addition,
p53 crosslinking with transglutaminase 2 led to p53 depletion and
tumor survival (6); these findings
suggested that p53 may be a key regulator in RCC.
mTOR is known as a serine-threonine kinase and the
mTOR signaling pathway has been implicated in inflammatory,
metabolic, degenerative and proliferative activities, and in cancer
(7–10). It has been reported that mTOR is
activated and hyperactivated in RCC (11). In addition, mTOR mutations have
been observed in clear cell RCC (12). The results of these studies
indicated that mTOR may have an important role in the regulation of
RCC however, the molecular mechanism requires further
investigation. Therefore, the present study will further evaluate
the effect of mTOR in RCC.
Resveratrol (Trans-3,4′,5-trihydroxystilbene) is a
natural phytoalexin that is used to prevent human cardiovascular
diseases, and induce anti-inflammatory (13,14)
and anticancer (15) activities. A
previous study revealed that resveratrol is a promising therapy for
patients with chronic kidney disease (16). Liu et al (17) demonstrated that insulin- and
leucine-stimulated mTOR signaling was inhibited by resveratrol.
However, another previous study indicated that resveratrol may
negatively regulate mTOR via AMP-activated protein kinase (AMPK)
activation in sensory neurons (18). In RCC cells, resveratrol may
inhibit RCC cell proliferation and apoptosis via the angiotensin II
receptor type 1/VEGF signaling pathway (19). Whether resveratrol is able to
regulate RCC through the AMPK/mTOR signaling pathway requires
further study. In the present study, resveratrol-mediated cell
apoptosis in RCC through the p53 AMPK/mTOR signaling pathway is
investigated in order to provide a potential target for the
treatment of RCC.
Materials and methods
Cell culture
HK-2 and Ketr-3 cells were obtained from American
Type Culture Collection (Manassas, VA, USA). The cells were
maintained in Eagle's Minimum Essential Medium with 10% fetal
bovine serum (FBS) (Hangzhou Sijiqing Biological Engineering
Materials Co., Ltd., Hangzhou, China), and were cultured at 37°C in
a 5% humidified CO2 atmosphere until they reached 90%
confluence. Resveratrol was obtained from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany) and was dissolved in DMSO in order to create a
stock solution at a concentration of 100 mM; this was subsequently
diluted in the culture medium to the desired concentration (12.5,
25, 50 and 100 µM) for experiments. DMSO was used as the blank
control for all experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was extracted from Ketr cells after 48 h
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) after the cells were treated with resveratrol
(12.5, 25, 50 and 100 µM) for 72 h. The concentration of extracted
RNA was determined using a spectrophotometer at wavelength of 260
nm. Reverse transcription was performed using the
PrimeScript™ RT reagent kit with gDNA Eraser (Perfect
Real Time) according to the manufacturer's protocol (Takara
Biotechnology Co., Ltd., Dalian, China). The reactions were as
follows: 42°C for 60 min, followed by 95°C for 5 min. qPCR was
performed using the SYBR Premix Ex Taq™ II reaction
mixture (Takara Biotechnology Co., Ltd.). The thermocycling
conditions were as follows: 95°C for 30 sec for initial
denaturation, then 40 cycles of 95°C for 5 sec, 60°C for 30 sec and
72°C for 30 sec, followed by a final extension at 72°C for 5 min.
PCR reactions were carried out using the ABI Step One Plus
real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The primer sequences used were as follows: B-cell lymphoma 2
(Bcl-2) sense, 5′-ACCAAGCTGAGCGAGTGTC-3′ and antisense,
5′-ACAAAGATGGTCACGGTCTGCC-3′; Bcl-2-associated X (Bax) sense,
5′-ACCAAGCTGAGCGAGTGTC-3′ and antisense,
5′-ACAAAGATGGTCACGGTCTGCC-3′; p53 sense, 5′-GTTTCCGTCTGGGCTTCTTG-3′
and antisense, 5′-CCTGGGCATCCTTGAGTTCC-3′; light chain (LC)3 sense,
5′-TGTCCGACTTATTCGAGAGCAGCA-3′ and antisense,
5′-TTCACCAACAGGAAGAAGGCCTGA-3′; autophagy related (ATG)5 sense,
5′-TCGTACATATCCTGGCAGGTTCGC-3′ and antisense,
5′-GAAGTCGCAGGGGCGTTCCAT-3′ and ATG7 sense,
5′-CATTCCGCTATAGGCACCAT-3′ and antisense,
5′-CGGCAAAGGAGAGAACAAAG-3′. Relative gene expression was calculated
using the 2−ΔΔCq method (20), and the results were normalized to
those of β-actin (sense, 5′-AACCGCGAGAAGATGACCCAGATCATGTTT-3′ and
antisense, 5′-AGCAGCCGTGGCCATCTCTTGCTCGAAGTC-3′).
Western blot analysis
Ketr-3 cells were washed three times with cold PBS
and proteins were extracted using ice-cold radioimmunoprecipitation
buffer (containing 1% proteinase inhibitor PMSF; Beyotime Institute
of Biotechnology, Shanghai, China). Cells were centrifuged at
14,500 × g for 10 min at 4°C. A Bicinchoninic Acid Protein Assay
kit (Beyotime Institute of Biotechnology, Shanghai, China) was used
to determine total protein concentrations. Total proteins (15 µg)
were separated by 4–12% SDS-PAGE gel and then transferred onto a
nitrocellulose membrane (EMD Millipore, Billerica, MA, USA).
Membranes were blocked with 5% non-fat dry milk for 2 h at room
temperature. The membranes were then incubated with a diluted
primary antibody at 4°C overnight; Bax (D2E11) rabbit (cat. no.
5023; 1:1,000), Bcl-2 (cat. no. 15071; 1:1,000), p53 (7F5) rabbit
(cat. no. 2527, 1:1,000), phospho-AMPKα (Thr172) (40H9) rabbit mAb
(cat. no. 2535S, 1:1,000), AMPKα (D63G4) rabbit mAb (cat. no.
5832S, 1:1,000), phospho-mTOR (Ser2448) (D9C2) rabbit (cat. no.
5536, 1:1,000) mTOR (7C10) rabbit mAb (cat. no. 2983, 1:1,000) LC3B
(D11) XP® rabbit mAb (cat. no. 3868, 1:1,000) Atg5
(D5F5U) rabbit mAb (cat. no. 12994, 1:1,000) Atg7 (D12B11) rabbit
mAb (cat. no. 8558, 1:1,000) GAPDH (D16H11) XP rabbit mAb (cat. no.
5174S, 1: 5,000). All the primary antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Anti-β-actin
antibodies (cat. no. MAB-1501; 1:10,000) were obtained from EMD
Millipore. Anti-rabbit horseradish peroxidase-conjugated IgG
antibodies (cat. no. ADI-SAB-300; 1:2,000) were purchased from Enzo
Life Sciences, Inc. (Farmingdale, NY, USA) and incubated with the
membrane for 2 h at room temperature. The specific complexes were
visualized using the SuperSignal West Pico chemiluminescent
substrate (Shanghai Solarbio Science and Technology, Shanghai,
China). Densitometric analysis was performed to quantify the signal
intensity using ImageJ software (version 1.37; National Institutes
of Health, Bethesda, MD, USA).
MTT assay
Ketr-3 cell viability was measured using an MTT
assay. Briefly, 1×106 cells were seeded into 96-well
plates, and following 24 h at 37°C to allow for cell adhesion,
resveratrol was added at varying concentrations (12.5, 25, 50 and
100 µM) for 12, 24, 48 and 72 h. Control cultures were treated with
DMSO. A total of 20 µl of 5 mg/ml MTT solution was added to each
well and the plate was further incubated at 37°C for 4 h. The cells
were then washed with PBS and 150 µl DMSO was added to each well.
Absorbance was read at 570 nm using a spectrophotometer (EnSpire
2300 Multilabel Reader; PerkinElmer, Inc., Waltham, MA, USA).
Apoptosis assay
Tumor cells were digested with trypsin at 37°C for 5
min and inoculated in a 50 ml culture flask at 5×105/ml,
then 1×105 Ketr-3 cells in each group were seeded in
6-well plates and treated with various concentrations of
resveratrol (12.5, 25, 50 and 100 µM) for 24 h; control cells
treated with DMSO only. Cell apoptosis rate was detected using an
Annexin V-fluorescein isothiocyanate (FITC)/propidium iodide (PI)
Assay kit (BD Pharmingen; BD Biosciences, San Jose, CA, USA),
according to the manufacturer's protocol. A BD FACSCanto II flow
cytometer (BD Biosciences) was used to collect data for analysis
using FCSExpress software (version 3.0; De Novo Software, Glendale,
CA, USA).
Cell migration assay
Ketr-3 cells (1×105) after treatment with
resveratrol (12.5, 25, 50 and 100 µM) were seeded in the top
chamber of Transwell migration chambers (8–5 mm; BD Biosciences) in
serum-free medium, and the lower chamber contained 10% FBS.
Following culture for 24 h at 37°C, the cells in the bottom chamber
were stained with 0.1% crystal violet (Sigma-Aldrich; Merck KGaA)
at 37°C for 20 min. The cells were then evaluated by a light
microscopy (CK40; Olympus Corporation, Tokyo, Japan) at the
magnification at ×200. Images were captured, then the cells were
counted randomly in five fields and the average was calculated.
Statistical analysis
The results were analyzed using SPSS software
version 19.0 (IBM Corp., Armonk, NY, USA). Data are presented as
the mean ± standard deviation of at least three independent
experiments. An analysis of variance followed by a Tukey test was
performed to compare the differences between the different groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of resveratrol on Ketr-3 cell
viability
To investigate the effects of resveratrol on RCC
cells, Ketr-3 cells were treated with various concentrations (0,
12.5, 25, 50 and 100 µM) of resveratrol for 12, 24, 48 and 72 h.
Following treatment, cells were assayed by MTT and the results
revealed that the Ketr-3 cell proliferation was significantly
inhibited by resveratrol in a dose dependent manner (Fig. 1).
Resveratrol treatment inhibits Ketr-3
cell migration
To further evaluate the potential effect of
resveratrol, it's effect on Ketr-3 cell migration was determined by
measuring the number of migratory Ketr-3 cells following
resveratrol treatment. As shown in Fig. 2, the number of migrating Ketr-3
cells was significantly inhibited by resveratrol in a
dose-dependent manner.
Resveratrol induces apoptosis in
Ketr-3 cells
Previous studies have demonstrated that resveratrol
induces apoptosis in cancer cells (21–24).
The present study further confirmed the ability of resveratrol to
induce apoptosis in Ketr-3 cells. Annexin V-FITC/PI staining was
performed to detect the effect of resveratrol treatment on
apoptosis in cells. The results shown in Fig. 3 revealed that resveratrol treatment
significantly increased cell apoptosis. As Bcl-2 and Bax are
markers of apoptosis in RCC (25),
the present study detected their mRNA and protein expression.
Resveratrol treatment increased and decreased the expression of Bax
and Bcl-2, respectively, at both the mRNA and protein levels
(Fig. 4A and B). The results
indicated that resveratrol may serve as a tumor suppressor in
RCC.
Expression of p53 is downregulated in
Ketr-3 cells and upregulated by treatment with resveratrol
The resveratrol treatment significantly increased
the expression of p53 mRNA and protein in a dose-dependent manner
(Fig. 4C). However, when compared
with the normal kidney HK-2 cells, the expression of p53 was
significantly decreased in Ketr-3 cells (Fig. 5). These results indicated that p53
may serve an important role in resveratrol-induced Ketr-3 cell
apoptosis.
Resveratrol induces Ketr-3 cell
apoptosis through p53-mediated AMPK/mTOR signaling
Recently previous findings have indicated that AMPK
may serve a pivotal role in the control of the p53 signaling
pathway (26–28), however, it is unknown whether AMPK
is involved in resveratrol-mediated Ketr-3 cell apoptosis. The
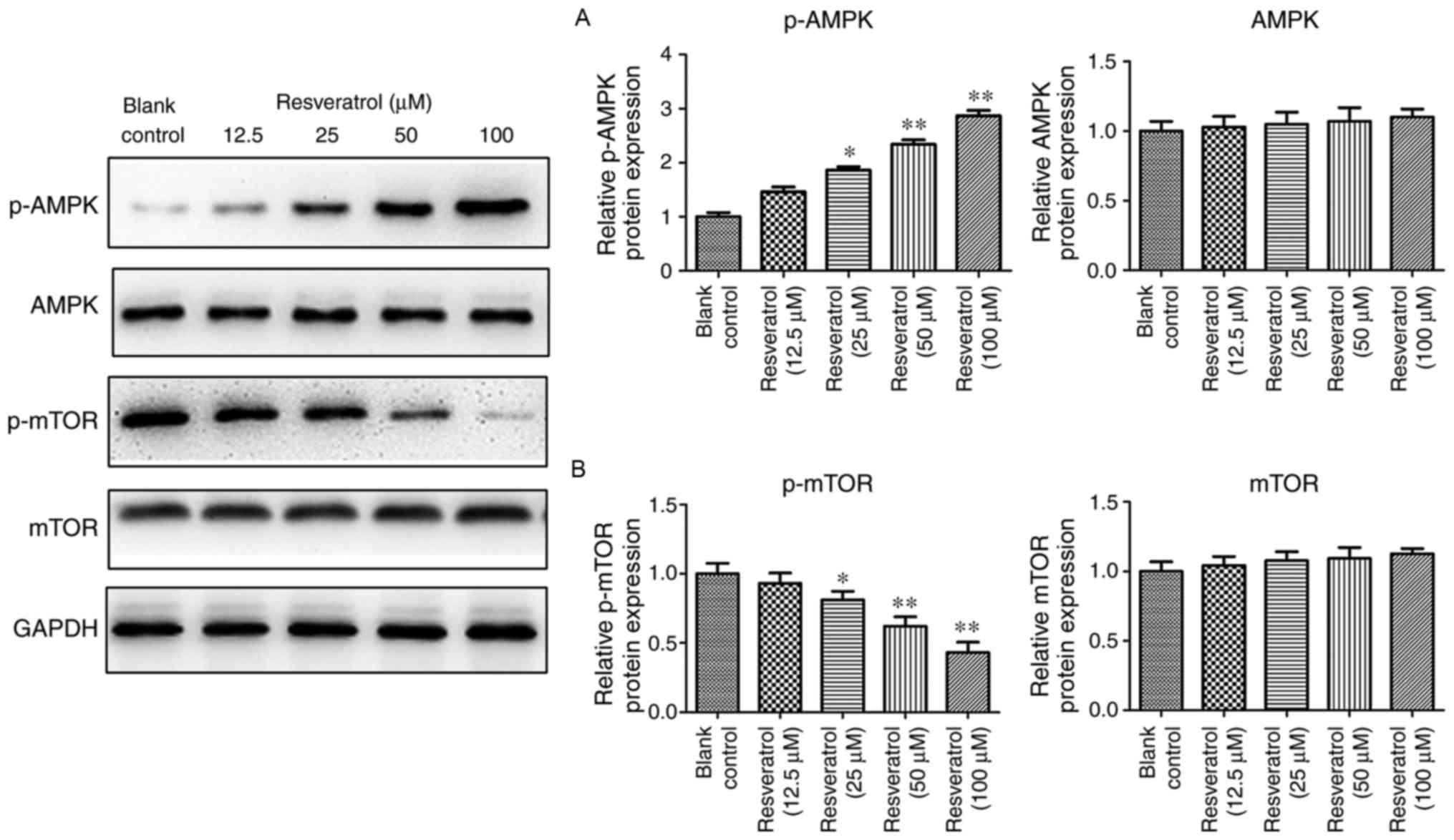
present study detected the expression of AMPK and demonstrated that
resveratrol treatment upregulated phosphorylated (p)-AMPK (Fig. 6A), which indicated that resveratrol
may activate p-AMPK and regulate downstream gene expression. As
mTOR is key in the p53 signaling pathway, the present study
detected the expression of mTOR and demonstrated that resveratrol
treatment downregulated p-mTOR, however, not the total expression
of mTOR (Fig. 6B). Thus,
resveratrol may induce Ketr-3 cell apoptosis via the p53-mediated
AMPK/mTOR signaling.
Resveratrol mediated apoptosis in
renal cell carcinoma cell potentially by promoting autophagy
Previous studies have revealed that the AMPK/mTOR
signaling pathway is important in autophagy, however, it is not
clear whether resveratrol regulates RCC though autophagy (17,18,29).
The present study detected the expression of the downstream genes
of the AMPK/mTOR-induced autophagy pathway. The results revealed
that the expression of LC3 (Fig.
7A), ATG5 (Fig. 7B) and ATG7
(Fig. 7C) were increased by
resveratrol. These results suggested that p53 may regulate
autophagy via AMPK/mTOR signaling, thereby promoting cell
apoptosis.
Discussion
Previous studies have demonstrated that resveratrol
acts as an anticancer factor in leukemia, and breast, stomach,
colon, prostate, ovarian and skin cancer (21,22,29–31).
The results of these studies all indicated that resveratrol could
effectively inhibit cancer cell activity, and that it could be used
for chemoprevention and anti-inflammation (23). In addition, resveratrol prevented
malignant tumor invasion by preventing kinase function (31,32).
In the present study, resveratrol induced RCC cell apoptosis, and
inhibited cell viability and migration, which is consistent with
the previous report (19). In
addition, the present study also demonstrated that resveratrol may
induce RCC apoptosis via the AMPK/mTOR autophagy pathway.
A previous study revealed that p53 was involved in
resveratrol-induced cancer cell apoptosis (24). Resveratrol treatment in human
breast cancer MCF-7 cells increased the expression of p53 and
Bax/Bcl-2, and resveratrol can be used as a neo-adjuvant in human
breast cancer (33). In addition,
it has been reported that resveratrol can inhibit Glioblastoma
multiforme growth by modulating p53 and protein kinase B (32). In RCC, p53 is a factor involved in
cell-cycle arrest and apoptosis; although resveratrol has been
reported to be associated with RCC, the specific role of p53 in
resveratrol anti-RCC remains unknown (34). In the present study, the expression
of p53, as well as Bax, was significantly upregulated in
resveratrol-induced RCC cell apoptosis. The results of the present
study are consistent with those of a previous study, which observed
transglutaminase 2-induced p53 mediated apoptosis in RCC (35).
The AMPK-targeting drug
5-Aminoimidazole-4-carboxamide riboside (26) and metformin may be used in clinical
oncology (36). AMPK is a key
regulator in cell growth and RCC tumorigenesis, and also when AMPK
activation inhibits RCC growth and survival (26). In RCC, 8-chloroadenosine activated
AMPK and inhibited mTOR pathway, and Silibinin, as a cancer
chemopreventive flavonoid, could induce an anti-metastatic effect
on RCC by targeting AMPK (37). In
the present study, the levels of p-AMPK were upregulated following
treatment with resveratrol; thus, AMPK may participate in the
apoptosis induced by resveratrol.
Autophagy regulation involves a variety of different
signaling pathways, of which, AMPK and mTOR are key regulators.
AMPK promotes and mTOR inhibits autophagy (38). In Zucker diabetic fatty rats,
liraglutide promotes autophagy by enhancing AMPK phosphorylation
and inhibiting mTOR phosphorylation (27). In addition, in cancer cells induced
by wild-type p53, docosahexaenoic acid attenuates autophagy by
increasing AMPK activation and decreasing the activity of mTOR
(28). The present study detected
the expression of the genes, LC3, ATG5 and ATG7, which are
associated with autophagy. The results demonstrated that
resveratrol upregulated their mRNA and protein levels, indicating
that resveratrol may be involved in autophagy regulation in RCC.
However, the role of resveratrol in the regulation of autophagy, in
RCC or other human cancers, has yet to be elucidated.
In conclusion, the present study demonstrated that
resveratrol treatment in RCC promoted apoptosis via p53-mediated
AMPK/mTOR autophagy signaling. These results indicate that
resveratrol may be a potential candidate for RCC treatment.
Acknowledgements
The present study was supported by the National
Natural Science Funds of China (grant no. 81202029).
References
|
1
|
Mitsunari K, Miyata Y, Watanabe SI, Asai
A, Yasuda T, Kanda S and Sakai H: Stromal expression of Fer
suppresses tumor progression in renal cell carcinoma and is a
predictor of survival. Oncol Lett. 13:834–840. 2017.PubMed/NCBI
|
|
2
|
Merza H and Bilusic M: Current management
strategy for metastatic renal cell carcinoma and future directions.
Curr Oncol Rep. 19:272017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho D, Signoretti S, Dabora S, Regan M,
Seeley A, Mariotti M, Youmans A, Polivy A, Mandato L, McDermott D,
et al: Potential histologic and molecular predictors of response to
temsirolimus in patients with advanced renal cell carcinoma. Clin
Genitourin Cancer. 5:379–385. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abolhasani M, Salarinejad S and Asgari M:
P53 and MDM2 Over-expression and five-year survival of kidney
cancer patients undergoing radical nephrectomy-iranian experience.
Asian Pac J Cancer Prev. 16:5043–5047. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu S, Yang Z, Fan Y, Guan B, Jia J, Gao Y,
Wang K, Wu K, Wang X, Zheng P, et al: Curcumin enhances
temsirolimus-induced apoptosis in human renal carcinoma cells
through upregulation of YAP/p53. Oncol Lett. 12:4999–5006.
2016.PubMed/NCBI
|
|
6
|
Kang JH, Lee JS, Hong D, Lee SH, Kim N,
Lee WK, Sung TW, Gong YD and Kim SY: Renal cell carcinoma escapes
death by p53 depletion through transglutaminase 2-chaperoned
autophagy. Cell Death Dis. 7:e21632016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Habib SL and Liang S: Hyperactivation of
Akt/mTOR and deficiency in tuberin increased the oxidative DNA
damage in kidney cancer patients with diabetes. Oncotarget.
5:2542–2550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lamming DW and Sabatini DM: A Central role
for mTOR in lipid homeostasis. Cell Metab. 18:465–469. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim YC and Guan KL: mTOR: A pharmacologic
target for autophagy regulation. J Clin Invest. 125:25–32. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu J, Pham CG, Albanese SK, Dong Y, Oyama
T, Lee CH, Rodrik-Outmezguine V, Yao Z, Han S, Chen D, et al:
Mechanistically distinct cancer-associated mTOR activation clusters
predict sensitivity to rapamycin. J Clin Invest. 126:3526–3540.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pal SK and Quinn DI: Differentiating mTOR
inhibitors in renal cell carcinoma. Cancer Treat Rev. 39:709–719.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garcia-Donas J, Rodriguez-Antona C and
Jonasch E: Molecular markers to predict response to therapy. Semin
Oncol. 40:444–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Conte E, Fagone E, Fruciano M, Gili E,
Iemmolo M and Vancheri C: Anti-inflammatory and antifibrotic
effects of resveratrol in the lung. Histol Histopathol. 30:523–529.
2015.PubMed/NCBI
|
|
14
|
Bo S, Ciccone G, Castiglione A, Gambino R,
De Michieli F, Villois P, Durazzo M, Cavallo-Perin P and Cassader
M: Anti-inflammatory and antioxidant effects of resveratrol in
healthy smokers a randomized, double-blind, placebo-controlled,
cross-over trial. Curr Med Chem. 20:1323–1331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wong JC and Fiscus RR: Resveratrol at
anti-angiogenesis/anticancer concentrations suppresses protein
kinase G signaling and decreases IAPs expression in HUVECs.
Anticancer Res. 35:273–281. 2015.PubMed/NCBI
|
|
16
|
Saldanha JF, Leal Vde O, Stenvinkel P,
Carraro-Eduardo JC and Mafra D: Resveratrol: Why is it a promising
therapy for chronic kidney disease patients? Oxid Med Cell Longev.
2013:9632172013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu M and Liu F: Resveratrol inhibits mTOR
signaling by targeting DEPTOR. Commun Integr Biol. 4:382–384. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tillu DV, Melemedjian OK, Asiedu MN, Qu N,
De Felice M, Dussor G and Price TJ: Resveratrol engages AMPK to
attenuate ERK and mTOR signaling in sensory neurons and inhibits
incision-induced acute and chronic pain. Mol Pain. 8:52012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Qiu M, Chen L, Liu L, Tan G and Liu
J: Resveratrol promotes regression of renal carcinoma cells via a
renin-angiotensin system suppression-dependent mechanism. Oncol
Lett. 13:613–620. 2017.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) methods. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kai L, Samuel SK and Levenson AS:
Resveratrol enhances p53 acetylation and apoptosis in prostate
cancer by inhibiting MTA1/NuRD complex. Int J Cancer.
126:1538–1548. 2010.PubMed/NCBI
|
|
22
|
Zhong LX, Zhang Y, Wu ML, Liu YN, Zhang P,
Chen XY, Kong QY, Liu J and Li H: Resveratrol and STAT inhibitor
enhance autophagy in ovarian cancer cells. Cell Death Discov.
2:150712016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Singh SK, Banerjee S, Acosta EP, Lillard
JW and Singh R: Resveratrol induces cell cycle arrest and apoptosis
with docetaxel in prostate cancer cells via a p53/ p21WAF1/CIP1 and
p27KIP1 pathway. Oncotarget. 8:17216–17228. 2017.PubMed/NCBI
|
|
24
|
Heiss EH, Schilder YD and Dirsch VM: Cvs.
J Biol Chem. 282:26759–26766. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saker Z, Tsintsadze O, Jiqia I, Managadze
L and Chkhotua A: Importance of apoptosis markers (MDM2, BCL-2 and
BAX) in conventional renal cell carcinoma. Georgian Med News.
27–33. 2015.PubMed/NCBI
|
|
26
|
Woodard J, Joshi S, Viollet B, Hay N and
Platanias LC: AMPK as a therapeutic target in renal cell carcinoma.
Cancer Biol Ther. 10:1168–1177. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Y, Ling Y, Yang L, Cheng Y, Yang P,
Song X, Tang H, Zhong Y, Tang L, He S, et al: Liraglutide relieves
myocardial damage by promoting autophagy via AMPK-mTOR signaling
pathway in zucker diabetic fatty rat. Mol Cell Endocrinol.
448:98–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jing K, Song KS, Shin S, Kim N, Jeong S,
Oh HR, Park JH, Seo KS, Heo JY, Han J, et al: Docosahexaenoic acid
induces autophagy through p53/AMPK/mTOR signaling and promotes
apoptosis in human cancer cells harboring wild-type p53. Autophagy.
7:1348–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Park SY, Chae SY, Park JO, Lee KJ and Park
G: Gold-conjugated resveratrol nanoparticles attenuate the invasion
and MMP-9 and COX-2 expression in breast cancer cells. Oncol Rep.
35:3248–3256. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jing X, Cheng W, Wang S, Li P and He L:
Resveratrol induces cell cycle arrest in human gastric cancer
MGC803 cells via the PTEN-regulated PI3K/Akt signaling pathway.
Oncol Rep. 35:472–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu B, Zhou Z, Zhou W, Liu J, Zhang Q, Xia
J, Liu J, Chen N, Li M and Zhu R: Resveratrol inhibits
proliferation in human colorectal carcinoma cells by inducing
G1/S-phase cell cycle arrest and apoptosis through
caspase/cyclin-CDK pathways. Mol Med Rep. 10:1697–1702. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Clark PA, Bhattacharya S, Elmayan A,
Darjatmoko SR, Thuro BA, Yan MB, van Ginkel PR, Polans AS and Kuo
JS: Resveratrol targeting of AKT and p53 in glioblastoma and
glioblastoma stem-like cells to suppress growth and infiltration. J
Neurosurg. 126:1448–1460. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ku BM, Kim DS, Kim KH, Yoo BC, Kim SH,
Gong YD and Kim SY: Transglutaminase 2 inhibition found to induce
p53 mediated apoptosis in renal cell carcinoma. FASEB J.
27:3487–3495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shi T, Liou LS, Sadhukhan P, Duan ZH,
Novick AC, Hissong JG, Almasan A and DiDonato JA: Effects of
resveratrol on gene expression in renal cell carcinoma. Cancer Biol
Ther. 3:882–888. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mondal A and Bennett LL: Resveratrol
enhances the efficacy of sorafenib mediated apoptosis in human
breast cancer MCF7 cells through ROS, cell cycle inhibition,
caspase 3 and PARP cleavage. Biomed Pharmacother. 84:1906–1914.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ben Sahra I, Le Marchand-Brustel Y, Tanti
JF and Bost F: Metformin in cancer therapy: A new perspective for
an old antidiabetic drug? Mol Cancer Ther. 9:1092–1099. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li F, Ma Z, Guan Z, Chen Y, Wu K, Guo P,
Wang X, He D and Zeng J: Autophagy induction by silibinin
positively contributes to its anti-metastatic capacity via
AMPK/mTOR pathway in renal cell carcinoma. Int J Mol Sci.
16:8415–8429. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Das G, Shravage BV and Baehrecke EH:
Regulation and function of autophagy during cell survival and cell
death. Cold Spring Harb Perspect Biol. 4:pii: a008813. 2012.
View Article : Google Scholar : PubMed/NCBI
|