Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most
common type of lymphoma, representing 25% of all
lymphoproliferative disorders (1).
Despite its aggressive disease course, ~50–70% of patients may
experience benefits with R-CHOP (rituximab plus cyclophosphamide,
doxorubicin, vincristine and prednisone) chemotherapy (1). However, there remain certain patients
with relapse or disease which is refractory to R-CHOP, ultimately
with only ~10% achieving a cure, requiring aggressive salvage
chemotherapy and transplantation (2). Therefore, novel therapeutic drugs are
being developed to improve the outcomes of this disease.
Quinacrine [QC;
6-chloro-9-(diethylamino-1-methylbutypamino)-2-methoxyacridine] is
a 9-aminoacridine derivative clinically used as an antimalarial
drug, which has additionally been observed to have anti-cancer
activity (3,4). A number of studies have suggested
that the anti-cancer activity of QC is not associated with its
DNA-binding ability, and is mediated via the suppression of
survival signaling in cancer cells (3). Simultaneous activation of cellular
tumor antigen p53 (p53) and suppression of the
phosphatidylinositol-3 kinase/RAC-α serine/threonine-protein
kinase/protein kinase mTOR and nuclear factor (NF)-κB pathways
serve an important role in the anti-cancer activity of QC (3,5,6).
Additionally, in human colon carcinoma cell lines, QC has been
demonstrated to promote tumor necrosis factor ligand superfamily
member 10, oxaliplatin and 5-fluorouracil cytotoxicity by inducing
NF-κB inactivation (6,7). QC is a chemosensitizer which is able
to enhance chemotherapeutic drug-induced apoptosis in cancer cells
(8–11). However, the effect of QC on DLBCL
cells has not been reported.
The present study investigated the effects of QC on
proliferation and apoptosis in DLBCL cell lines and clarified the
possible target molecules of QC in DLBCL cells in vitro.
Materials and methods
Reagents
QC was obtained from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany; cat. no. Q3251) and dissolved in PBS as a 10 mM
stock solution. Dilutions to the required concentrations were made
in RPMI-1640 medium. Rabbit polyclonal to RNA-binding protein
Musashi homolog 2 (MSI2; cat. no. ab50829) antibody was purchased
from Abcam (Cambridge, UK); rabbit monoclonal protein numb homolog
(Numb; cat. no. 2761S), Myc proto-oncogene protein (c-Myc; cat. no.
5605), β-actin (cat. no. 8457S) antibody, rabbit polyclonal
poly-ADP ribose polymerase 1 (PARP) antibody (cat. no. 9542S),
rabbit monoclonal cyclin-dependent kinase (CDK)6 (cat. no. D4S8S),
rabbit monoclonal CDK4 (cat. no. D9G3E) and rabbit polyclonal
caspase-3 antibody (cat. no. 9665S) were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA).
Cell culture
DLBCL cell lines OCI-Ly01 and SU-DHL-8 were
purchased from the American Type Culture Collection (Manassas, VA,
USA) and passaged for <6 months following receipt or
resuscitation from stocks, and were maintained in RPMI-1640 medium
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) supplemented
with 10% fetal bovine serum (AusGeneX, Molendinar, Queensland,
Australia), 4 mM L-glutamine (Sigma-Aldrich; Merck KGaA), 100 U/ml
penicillin (Hyclone; GE Healthcare Life Sciences) and 100 U/ml
streptomycin (Hyclone; GE Healthcare Life Sciences). All cell
cultures were performed at 37°C in a humidified atmosphere with 5%
CO2.
Cell viability analysis
The cell viability of DLBCL cell lines was measured
using the MTS method (CellTiter 96®Aqueous One Solution;
cat. no. 207284; Promega Corporation, Madison, WI, USA). A total of
2×104 cells/well were incubated in quadruplicate in a
96-well microculture plate, in the presence of different
concentrations of QC in a final volume of 0.1 ml for 48 h at 37°C.
Subsequently, each well was treated with MTS (20 µl MTS/100 µl) for
4 h, and the absorption values at 590 nm were determined using an
automatic ELISA plate reader (iMark; Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Values were normalized to untreated (control)
samples.
Cell cycle analysis
Cells (1.0×105/ml) were treated with 0,
1, 1.5 and 2 µmol/l QC for 48 h, and subsequently fixed with 100%
cold ethanol at −20°C for 48 h, followed by staining with a Cell
Cycle Staining kit [propidium iodide (PI); MultiSciences Biotech
Co., Ltd., Hangzhou, China; cat. no. CCS012] in the presence of
RNase for 15 min at room temperature. Cell-cycle distribution was
assessed using a FACScan instrument (BD FACSCanto™ II; BD
Biosciences, Franklin Lakes, NJ, USA). Data were analyzed using
FlowJo 7.6.1 software (FlowJo LLC, Ashland, OR, USA).
Analysis of apoptosis
Cells (1.0×105/ml) were treated with 0,
0.8, 1.6 and 3.2 µmol/l QC for 24 h. Staining was performed using
annexin V-fluorescein isothiocyanate (Multisciences Biotech Co.,
Ltd.; cat. no. 4100546) in conjunction with PI, according to the
manufacturer's protocol, and was assessed using a FACScan
instrument (BD FACSCanto™ II; BD Biosciences). Data were analyzed
using BD FACSDiva software version 3.3.11. Apoptosis was validated
via PARP cleavage and analyzed through western blotting.
Protein extraction and western blot
analysis
Cells were lysed using SDS buffer (BBI Solutions,
Cardiff, UK) containing proteinase inhibitors (phenylmethylsulfonyl
fluoride). Cell extracts containing 50 µg of proteins, determined
by the bicinchoninic acid method, were separated by SDS-PAGE on a
12% gel, and transferred onto polyvinylidene difluoride membranes
(Bio-Rad Laboratories, Inc.). The membrane was blocked in 5% nonfat
milk (Shanghai Bright Diary Group Co., Ltd, Shanghai, China) at
room temperature for 2 h and incubated with specific antibodies
(1:1,000) overnight at 4°C. Primary antibodies were detected by
incubating the membrane in anti-rabbit IgG, HRP-linked antibody
(cat. no. 7074; Cell Signaling Technology, Inc.) for 2 h at room
temperature, using enhanced chemiluminescence (PerkinElmer, Inc.,
Waltham, MA, USA). Densitometry quantification of immunoblot
analyses was performed using Image Lab software (version 5.2.1;
Bio-Rad Laboratories, Inc.).
Statistical analysis
All statistical analyses were performed using SPSS
17.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean
± standard deviation. The statistical significance of the
differences observed between experimental groups was determined
using one-way analysis of variance and a post hoc LSD test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
QC inhibits the growth of SU-DHL-8 and
OCI-Ly01 cells
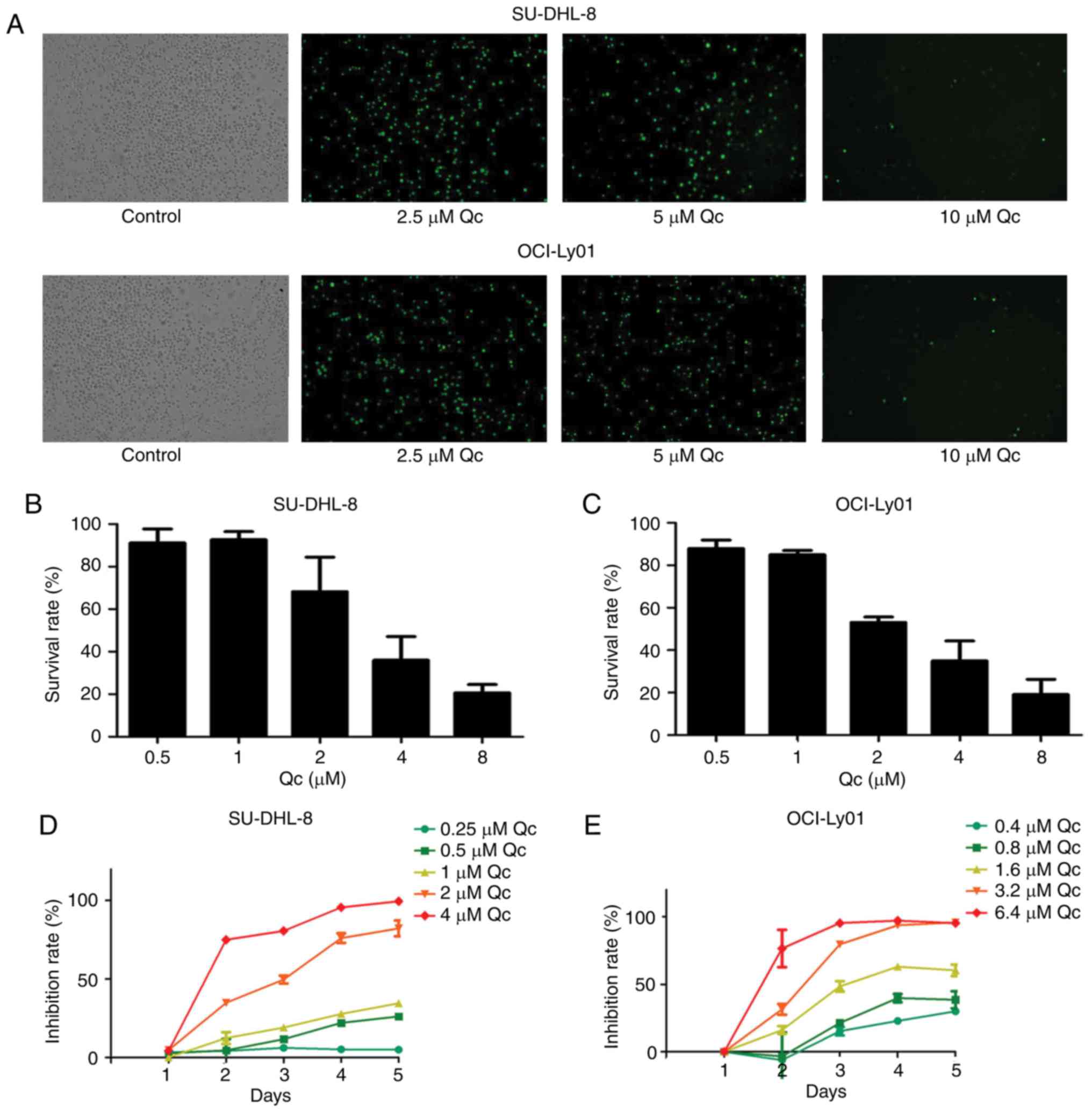
The present study investigated whether QC leads to
the inhibition of DLBCL cell growth. The two DLBCL cell lines
(OCI-Ly01 and SU-DHL-8) were cultured with varying concentrations
of QC (0, 2.5, 5 and 10 µM) for 24 h, and it was observed that the
cells exhibited green fluorescence, and that the fluorescence
intensity gradually weakened with the increase in QC concentration
(Fig. 1A). Cell viability was
assessed by MTS assay. As the dose of QC increased from 1 to 8 µM,
cell growth inhibition increased in a dose-dependent manner in the
two DLBCL cell lines (Fig. 1B and
C). The half-maximal inhibitory concentrations
(IC50) for SU-DHL-8 and OCI-Ly01 were 2 and 1.8 µM,
respectively. The two cell lines were treated with a variety of
different concentrations of QC for 96 h, and cell growth was
inhibited in a dose- and time-dependent manner (Fig. 1D and E).
QC arrests the cell cycle of SU-DHL-8
and OCI-Ly01 cells at the G0/G1 phase
In order to understand whether the growth inhibitory
effect of QC contributed to cell cycle arrest, the effects of QC on
the cell cycle were evaluated. It was observed that QC (2 µM)
induced apparent G0/G1 phase arrest in OCI-Ly01 compared with
control cells (P=0.00022; Fig. 2A and
B). QC (1, 1.5 and 2 µM) was able to decrease the protein
expression of CDK4 compared with control cells (P=0.002,
P<0.001, P<0.001), and consistent results were observed with
CDK6 (P<0.001) in OCI-Ly01 cells (Fig. 2C and D).
Consistent results were also observed in SU-DHL-8
cells. QC (1.5 and 2 µM) induced apparent G0/G1 phase arrest
compared with the control cells (P=0.002, P=0.0002)
(Fig. 3A and B), the protein
expression of CDK4 decreased following treatment with QC (1, 1.5
and 2 µM) compared with control cells (P<0.001, respectively)
and the same was observed for CDK6 (P<0.001) (Fig. 3C and 3D).
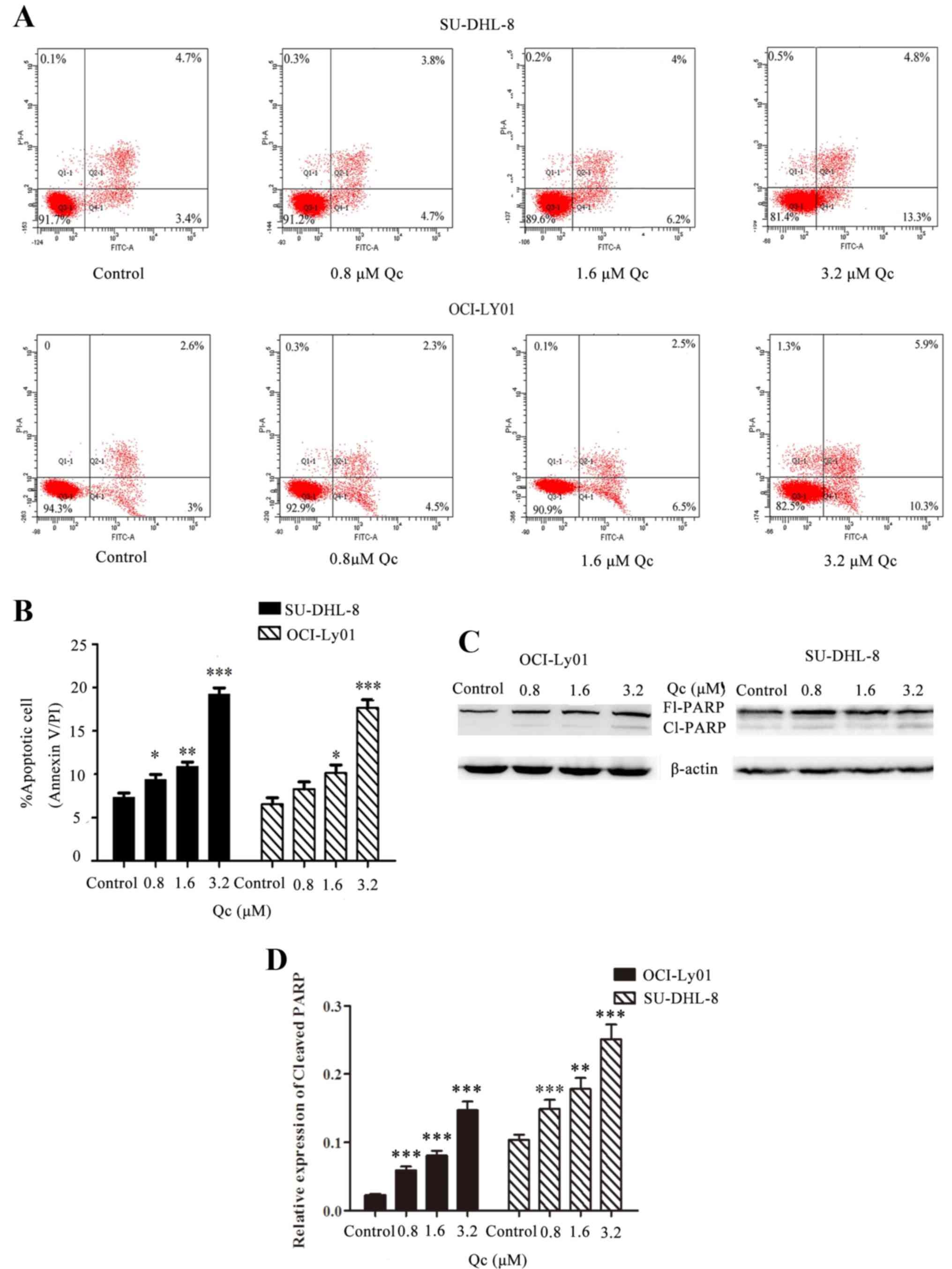
QC induces apoptosis of SU-DHL-8 and
OCI-Ly01 cell lines
In order to study the induction of apoptosis,
SU-DHL-8 and OCI-Ly01 cells were treated with four different QC
concentrations, 0, 0.8, 1.6 and 3.2 µM, for 24 h. Compared with the
control group, the percentages of apoptotic cells of groups treated
with QC increased significantly in a dose-dependent manner in
SU-DHL-8 and OCI-Ly01 cells (Fig. 4A
and B). In addition, the expression levels of cleaved PARP
protein increased in groups treated with QC compared with control
cells in the OCI-Ly01 cell line (P<0.001), and the same
results were noted in the SU-DHL-8 cell line (P<0.001, P=0.007
and P<0.001, respectively) (Fig. 4C
and D).
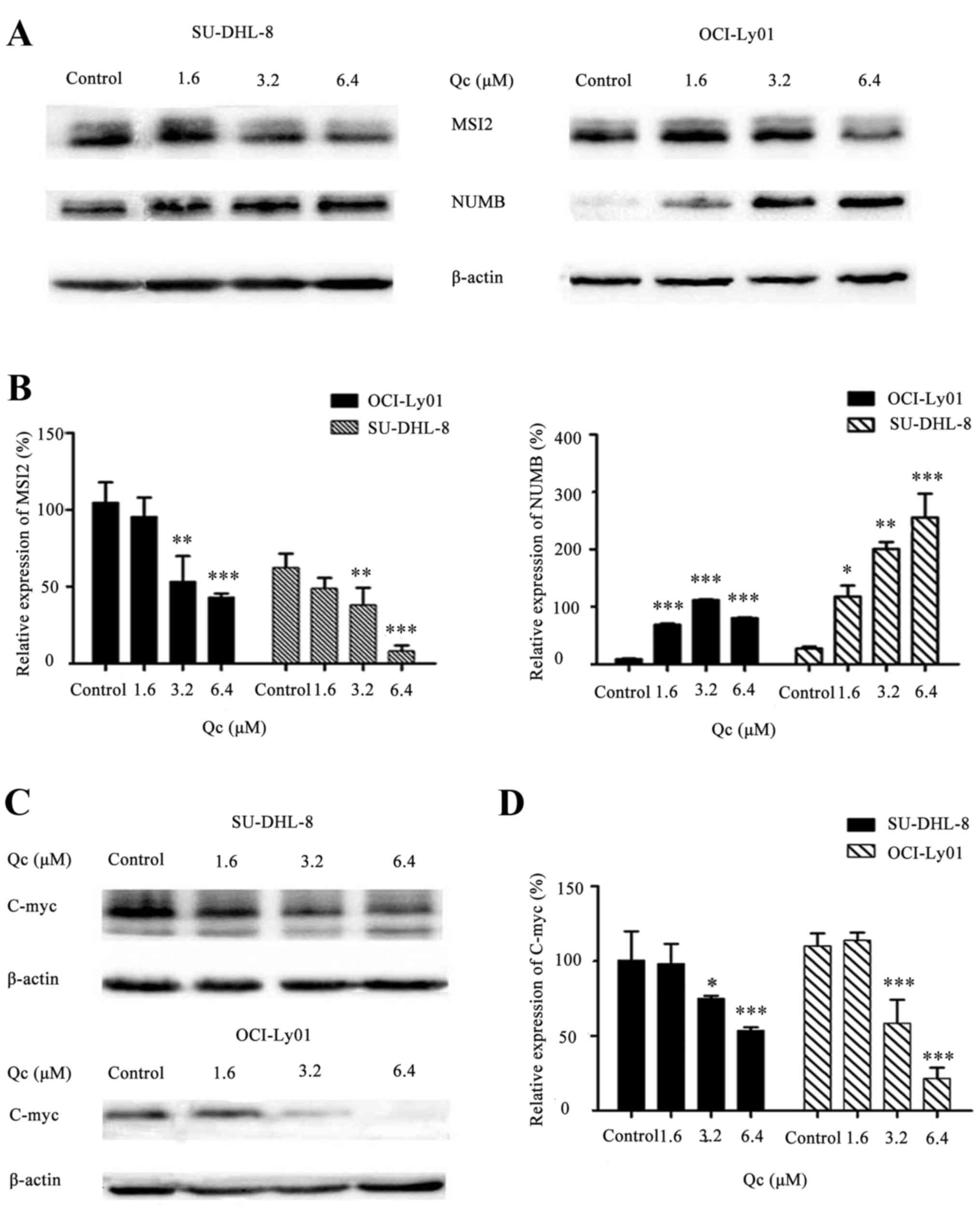
QC regulates the MSI2-NUMB-c-Myc
signaling pathway
It was previously reported that MSI2 served an
important role in hematopoietic stem cell function and cell fate
determination (12). MSI2 inhibits
the expression of Numb, which is an evolutionarily well-conserved
protein (13,14). Therefore, the present study
examined whether QC may affect the MSI2-Numb signaling pathway.
SU-DHL-8 and OCI-Ly01 constitutively expressed MSI2 and Numb. When
SU-DHL-8 and OCI-Ly01 were treated with 0, 1.6, 3.2 and 6.4 µM QC
for 24 h, the expression of MSI2 decreased, whereas that of Numb
increased (Fig. 5A and B),
suggesting that the inhibition of MSI2 may lead to the accumulation
of Numb protein. Previous studies have demonstrated that c-Myc was
regulated by Numb (15).
Therefore, the present study assessed the expression level of c-Myc
by western blotting. As hypothesized, QC decreased c-Myc expression
with the increase of drug concentration in SU-DHL-8 and OCI-Ly01
cells (Fig. 5C and D).
Discussion
QC is a well-known antimalarial drug, and its
anticancer effects have been demonstrated (16–19).
It has been demonstrated that QC may induce apoptosis and arrest
the cell cycle at the S phase via inhibition of topoisomerase
activity, and induction of p53 and CDK inhibitor 1 in breast cancer
and colon cancer cells (4,20), and inhibition of NF-κB and
Wnt-T-cell factor signaling via the adenomatous polyposis coli gene
in breast cancer (21). However,
to the best of our knowledge, the inhibition of QC in DLBCL cells
has been not reported. The present study investigated the
antiproliferative potential of QC in SU-DHL-8 and OCI-Ly01 cells.
It was observed that QC inhibited cell growth. The IC50
of QC in SU-DHL-8 and OCI-Ly01 cells was 1.8 and 2 µM,
respectively, approximately equal to the IC50 of QC in
other cancer cells, including non-small cell lung cancer (22), breast cancer (4) and leukemia K562 cells (23). The increased protein expression of
cleaved PARP following treatment with QC indicated that QC was able
to induce cellular apoptosis in OCI-Ly01 and SU-DHL-8 cell lines,
which was consistent with previous reports (4,5,11).
QC additionally induced cell cycle arrest at the G0/G1 phase and
apoptosis in human DLBCL cell lines SU-DHL-8 and OCI-Ly01 in a
dose-dependent manner, and the decreased expression of CDK6 and
CDK4 confirmed G0/G1 cell cycle arrest. However, previous reports
indicated that QC induced cell cycle arrest at the G1/S and G2/M
(22), and sub-G1 and S phases
(24), which is not consistent
with the results of the present study. A possible reason for this
is that the molecular mechanism of QC is heterogeneous in different
types of cells.
The Musashi proteins are RNA-binding proteins which
are encoded by two translational regulatory genes, MSI1 and MSI2,
located on chromosomes 12 and 17 (25,26).
They distinctively regulate transcriptional events and act as cell
cycle regulators (27). The
Musashi gene family is highly expressed in stem cells (28,29).
MSI1 and MSI2 expression has been associated with an unfavorable
prognosis in several types of tumor, including glioma, pediatric
brain tumor, breast cancer and colorectal cancer (30,31).
In previous studies, high MSI2 expression indicated a poor
prognosis and facilitated risk and treatment stratification in
adult and pediatric patients with B-cell acute lymphoblastic
leukemia (32,33), indicating that MSI2 was able to
serve an important role in B-cell neoplasm. In the present study,
MSI2 expression was detected in SU-DHL-8 and OCI-Ly01 cells, which
further supported this hypothesis. Notably, the results of the
present study demonstrated that QC was able to downregulate MSI2
expression and upregulate Numb expression. Previous studies
demonstrated that MSI2 was able to inhibit Numb mRNA translation,
promoting the development and progression of pancreatic cancer
(34).
In addition, the knockdown of Numb has been
demonstrated to increase c-Myc expression (15). c-Myc is the master transcription
factor of cell proliferation and is involved in numerous
hematological and solid types of cancer (35), including DLBCL (36). In the present study, it was
observed that QC induced cell cycle arrest at the G0/G1 phase.
Consistent with the results of the present study, downregulation of
MSI2 and c-Myc has been observed to induce cell cycle arrest at the
G0/G1 phase (37) and Numb
overexpression may inhibit cell cycle progression at the G0/G1
phase (38,39). Additionally, CDK4 and CDK6 are two
classical cell cycle-associated proteins which are involved in cell
cycle transformation between the G0/G1 and S phases. It was
observed that QC decreased CDK4 and CDK6 expression in the OCI-Ly01
and SU-DHL-8 cell lines, consistent with the observed G0/G1 cell
cycle arrest. Therefore, it was hypothesized that QC may inhibit
MSI2 expression, increase NUMB expression, suppress c-Myc
expression, and decrease CDK4 and CDK6 expression.
In conclusion, the results of the present study
indicated that QC may inhibit the growth of DLBCL cells, possibly
via the MSI2-NUMB signaling pathway, and is a potential drug for
the treatment of DLBCL. However, further studies in vivo are
required to confirm the clinical effects of QC in DLBCL.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant nos. LY17H160005 and
LY14H080001), the Project from Traditional Chinese Medicine
Administration of Zhejiang Province (grant no. 2015ZZ018) and the
Natural Science Foundation of Ningbo (grant no. 2014A610217).
References
|
1
|
Coiffier B, Thieblemont C, Van Den Neste
E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M,
Sebban C, et al: Long-term outcome of patients in the LNH-98.5
trial, the first randomized study comparing rituximab-CHOP to
standard CHOP chemotherapy in DLBCL patients: A study by the Groupe
d'Etudes des Lymphomes de l'Adulte. Blood. 116:2040–2045. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Friedberg JW: Relapsed/refractory diffuse
large B-cell lymphoma. Hematology Am Soc Hematol Educ Program.
2011:498–505. 2011.PubMed/NCBI
|
|
3
|
Ehsanian R, Van Waes C and Feller SM:
Beyond DNA binding-a review of the potential mechanisms mediating
quinacrine's therapeutic activities in parasitic infections,
inflammation, and cancers. Cell Commun Signal. 9:132011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Preet R, Mohapatra P, Mohanty S, Sahu SK,
Choudhuri T, Wyatt MD and Kundu CN: Quinacrine has anticancer
activity in breast cancer cells through inhibition of topoisomerase
activity. Int J Cancer. 130:1660–1670. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang W, Ho WC, Dicker DT, MacKinnon C,
Winkler JD, Marmorstein R and El-Deiry WS: Acridine derivatives
activate p53 and induce tumor cell death through Bax. Cancer Biol
Ther. 4:893–898. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gallant JN, Allen JE, Smith CD, Dicker DT,
Wang W, Dolloff NG, Navaraj A and El-Deiry WS: Quinacrine
synergizes with 5-fluorouracil and other therapies in colorectal
cancer. Cancer Biol Ther. 12:239–251. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jani TS, DeVecchio J, Mazumdar T, Agyeman
A and Houghton JA: Inhibition of NF-kappaB signaling by quinacrine
is cytotoxic to human colon carcinoma cell lines and is synergistic
in combination with tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL) or oxaliplatin. J Biol Chem.
285:19162–19172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Friedman J, Nottingham L, Duggal P, Pernas
FG, Yan B, Yang XP, Chen Z and Van Waes C: Deficient TP53
expression, function, and cisplatin sensitivity are restored by
quinacrine in head and neck cancer. Clin Cancer Res. 13:6568–6578.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Bi Q, Dong L, Li X, Ge X, Zhang X,
Fu J, Wu D and Li S: Quinacrine enhances cisplatin-induced
cytotoxicity in four cancer cell lines. Chemotherapy. 56:127–134.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Gallant JN, Katz SI, Dolloff NG,
Smith CD, Abdulghani J, Allen JE, Dicker DT, Hong B, Navaraj A and
El-Deiry WS: Quinacrine sensitizes hepatocellular carcinoma cells
to TRAIL and chemotherapeutic agents. Cancer Biol Ther. 12:229–238.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu X, Wang Y, Wang H, Wang Q, Wang L, Miao
J, Cui F and Wang J: Quinacrine inhibits cell growth and induces
apoptosis in human gastric cancer cell line SGC-7901. Curr Ther Res
Clin Exp. 73:52–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park SM, Deering RP, Lu Y, Tivnan P,
Lianoglou S, Al-Shahrour F, Ebert BL, Hacohen N, Leslie C, Daley
GQ, et al: Musashi-2 controls cell fate, lineage bias, and TGF-β
signaling in HSCs. J Exp Med. 211:71–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhong W, Feder JN, Jiang MM, Jan LY and
Jan YN: Asymmetric localization of a mammalian numb homolog during
mouse cortical neurogenesis. Neuron. 17:43–53. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guo M, Jan LY and Jan YN: Control of
daughter cell fates during asymmetric division: Interaction of Numb
and Notch. Neuron. 17:27–41. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu Y, Xu W, Ji J, Feng D, Sourbier C, Yang
Y, Qu J, Zeng Z, Wang C, Chang X, et al: Alternative splicing of
the cell fate determinant Numb in hepatocellular carcinoma.
Hepatology. 62:1122–1131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gurova K: New hopes from old drugs:
Revisiting DNA-binding small molecules as anticancer agents. Future
Oncol. 5:1685–1704. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neznanov N, Gorbachev AV, Neznanova L,
Komarov AP, Gurova KV, Gasparian AV, Banerjee AK, Almasan A,
Fairchild RL and Gudkov AV: Anti-malaria drug blocks proteotoxic
stress response: Anti-cancer implications. Cell Cycle. 8:3960–3970.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo C, Gasparian AV, Zhuang Z, Bosykh DA,
Komar AA, Gudkov AV and Gurova KV: 9-Aminoacridine-based anticancer
drugs target the PI3K/AKT/mTOR, NF-kappaB and p53 pathways.
Oncogene. 28:1151–1161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gurova KV, Hill JE, Guo C, Prokvolit A,
Burdelya LG, Samoylova E, Khodyakova AV, Ganapathi R, Ganapathi M,
Tararova ND, et al: Small molecules that reactivate p53 in renal
cell carcinoma reveal a NF-kappaB-dependent mechanism of p53
suppression in tumors. Proc Natl Acad Sci USA. 102:17448–17453.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohapatra P, Preet R, Das D, Satapathy SR,
Choudhuri T, Wyatt MD and Kundu CN: Quinacrine-mediated autophagy
and apoptosis in colon cancer cells is through a p53- and
p21-dependent mechanism. Oncol Res. 20:81–91. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Preet R, Mohapatra P, Das D, Satapathy SR,
Choudhuri T, Wyatt MD and Kundu CN: Lycopene synergistically
enhances quinacrine action to inhibit Wnt-TCF signaling in breast
cancer cells through APC. Carcinogenesis. 34:277–286. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dermawan JK, Gurova K, Pink J, Dowlati A,
De S, Narla G, Sharma N and Stark GR: Quinacrine overcomes
resistance to erlotinib by inhibiting FACT, NF-κB, and cell-cycle
progression in non-small cell lung cancer. Mol Cancer Ther.
13:2203–2214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Changchien JJ, Chen YJ, Huang CH, Cheng
TL, Lin SR and Chang LS: Quinacrine induces apoptosis in human
leukemia K562 cells via p38 MAPK-elicited BCL2 down-regulation and
suppression of ERK/c-Jun-mediated BCL2L1 expression. Toxicol Appl
Pharmacol. 284:33–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Preet R, Siddharth S, Satapathy SR, Das S,
Nayak A, Das D, Wyatt MD and Kundu CN: Chk1 inhibitor synergizes
quinacrine mediated apoptosis in breast cancer cells by
compromising the base excision repair cascade. Biochem Pharmacol.
105:23–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barbouti A, Höglund M, Johansson B, Lassen
C, Nilsson PG, Hagemeijer A, Mitelman F and Fioretos T: A novel
gene, MSI2, encoding a putative RNA-binding protein is recurrently
rearranged at disease progression of chronic myeloid leukemia and
forms a fusion gene with HOXA9 as a result of the cryptic
t(7;17)(p15;q23). Cancer Res. 63:1202–1206. 2003.PubMed/NCBI
|
|
26
|
Kawahara H, Imai T, Imataka H, Tsujimoto
M, Matsumoto K and Okano H: Neural RNA-binding protein Musashi1
inhibits translation initiation by competing with eIF4G for PABP. J
Cell Biol. 181:639–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Imai T, Tokunaga A, Yoshida T, Hashimoto
M, Mikoshiba K, Weinmaster G, Nakafuku M and Okano H: The neural
RNA-binding protein Musashi1 translationally regulates mammalian
numb gene expression by interacting with its mRNA. Mol Cell Biol.
21:3888–3900. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kong DS, Kim MH, Park WY, Suh YL, Lee JI,
Park K, Kim JH and Nam DH: The progression of gliomas is associated
with cancer stem cell phenotype. Oncol Rep. 19:639–643.
2008.PubMed/NCBI
|
|
29
|
Siddall NA, McLaughlin EA, Marriner NL and
Hime GR: The RNA-binding protein Musashi is required intrinsically
to maintain stem cell identity. Proc Natl Acad Sci USA.
103:8402–8407. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang XY, Yin Y, Yuan H, Sakamaki T, Okano
H and Glazer RI: Musashi1 modulates mammary progenitor cell
expansion through proliferin-mediated activation of the Wnt and
Notch pathways. Mol Cell Biol. 28:3589–3599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sureban SM, May R, George RJ, Dieckgraefe
BK, McLeod HL, Ramalingam S, Bishnupuri KS, Natarajan G, Anant S
and Houchen CW: Knockdown of RNA binding protein musashi-1 leads to
tumor regression in vivo. Gastroenterology. 134:1448–1458. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mu Q, Wang Y, Chen B, Qian W, Meng H, Tong
H, Chen F, Ma Q, Ni W, Chen S and Jin J: High expression of
Musashi-2 indicates poor prognosis in adult B-cell acute
lymphoblastic leukemia. Leuk Res. 37:922–927. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Aly RM and Ghazy HF: Prognostic
significance of MSI2 predicts unfavorable outcome in adult B-acute
lymphoblastic leukemia. Int J Lab Hematol. 37:272–278. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sheng W, Dong M, Chen C, Li Y, Liu Q and
Dong Q: Musashi2 promotes the development and progression of
pancreatic cancer by down-regulating Numb protein. Oncotarget.
8:14359–14373. 2017.PubMed/NCBI
|
|
35
|
Wierstra I and Alves J: The c-myc
promoter: Still MysterY and challenge. Adv Cancer Res. 99:113–333.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Valera A, López-Guillermo A,
Cardesa-Salzmann T, Climent F, González-Barca E, Mercadal S,
Espinosa I, Novelli S, Briones J, Mate JL, et al: MYC protein
expression and genetic alterations have prognostic impact in
patients with diffuse large B-cell lymphoma treated with
immunochemotherapy. Haematologica. 98:1554–1562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han Y, Ye A, Zhang Y, Cai Z, Wang W, Sun
L, Jiang S, Wu J, Yu K and Zhang S: Musashi-2 silencing exerts
potent activity against acute myeloid leukemia and enhances
chemosensitivity to daunorubicin. PLoS One. 10:e01364842015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sima J, Zhang B, Yu Y, Sima X and Mao Y:
Overexpression of Numb suppresses growth, migration, and invasion
of human clear cell renal cell carcinoma cells. Tumour Biol.
36:2885–2892. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Schweinfest CW, Fujiwara S, Lau LF and
Papas TS: c-myc can induce expression of G0/G1 transition genes.
Mol Cell Biol. 8:3080–3087. 1988. View Article : Google Scholar : PubMed/NCBI
|