Introduction
Therapies for hepatocellular carcinomas (HCCs)
remain a global problem. Until now, HCCs have been difficult to
treat using traditional methods, including surgery and internal
medicine. Gene therapy, a modern molecular therapeutic strategy,
has a promising future in the treatment of HCCs (l,2). However, the
low targeting specificity and the poor transduction efficiency of
the therapeutic gene caused by the lack of tumor selectivity are
still the main challenges in the gene therapy field. The use of
stem cells as vehicles that effectively carry the therapeutic gene
and facilitate tropism to the tumor has attracted substantial
interest (3,4).
Bone marrow-derived mesenchymal stem cells (MSCs)
differentiate into cells of multiple lineages, including
osteocytes, adipocytes, myocytes, neurocytes and hepatocytes
(5–9). To date, MSCs have been widely used in
tissue engineering and regenerative medicine. As reported in recent
studies, MSCs exhibit tropism to liver tumors. These stem cells
could be recruited to and specifically target the tumor to
participate in the formation of the tumor stroma in response to
signals sent from the tumor microenvironment (10,11).
This tumor-targeting feature makes MSCs a potential candidate for
delivering therapeutic genes in gene therapy strategies. MSCs
appear to be more advantageous than other cell types due to their
abundance, accessibility and ease of genetic manipulation.
The main issue in cell-based therapeutic strategies
is how to monitor the fate and migration of cells after injection.
A noninvasive method has been developed to track and quantify the
transplanted cells by labeling the cells in vitro with
superparamagnetic iron oxide (SPIO) nanoparticles, followed by
magnetic resonance imaging (MRI) in vivo (12–16).
Our previous study investigated the feasibility of MRI of stem
cells that had been transplanted in an acute injury liver model and
observed highly specific tropism of magnetically labeled MSCs to
the injured liver tissue that was efficiently monitored using MRI
(16). To date, there has been no
report concerning the in vivo tracking of the tropism of
MSCs to liver tumors with MRI. Therefore, the aim of the present
study was to evaluate the homing capacity of MSCs to liver tumors
and to delineate the spatial and temporal distributions of MSCs in
liver tumors after transplantation using a clinical 1.5-T MRI
system.
Materials and methods
Animals
New Zealand white rabbits (n=10) were used in this
study; 2 were used to harvest MSCs, and 8 were used to establish
the tumor model. The animals were provided by the Medical
Experimental Animal Center of the Third Military Medical University
(Chongqing, China) and were maintained under conventional
conditions in the Laboratory Animal Center of Chongqing Medical
University (Chongqing, China). All the animal experiments were
approved by the Ethics Committee of Chongqing Medical University
(Chongqing, China) and were performed in strict accordance with the
recommendations in the Guide for the Care and Use of Laboratory
Animals of Chongqing Medical University. All surgeries were
performed under anesthesia with intraperitoneal sodium
pentobarbital (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China) at a dose of 30 mg/kg, and all efforts were made to minimize
suffering.
Cell harvest and culture
The MSCs were harvested from the long tubular bones
of donor rabbits using Caplan's method (17). Briefly, New Zealand white rabbits
(150–200 g) were euthanized by cervical dislocation, and the femurs
and tibias were isolated. Bone marrow was flushed out from the long
bones with Dulbecco's modified Eagle's medium (DMEM; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany) supplemented with 10% fetal bovine
serum (FBS; Sigma-Aldrich; Merck KGaA), antibiotics (50 µg/ml
streptomycin and 50 IU penicillin/ml) and heparin (10,000 U/ml;
Sigma-Aldrich; Merck KGaA). Subsequently, the bone marrow
suspension was centrifuged at 251.78 × g at room temperature for 10
min to isolate the MSC cells. These cells were transferred into a
25-cm2 plastic flat-bottom flask and cultured with DMEM
supplemented with 10% FBS and antibiotics. A total of 24 h later,
the MSCs adhered to the flask bottom and formed cell colonies. The
non-adherent cells were removed from the culture media by repeated
washes with phosphate-buffered saline (PBS, pH 7.4) and a
subsequent medium change. After reaching 80–90% confluence, the
cells were collected using 0.25% trypsin (Sigma-Aldrich; Merck
KGaA) and passaged at a ratio of 1:3. After 3 passages, the cells
were harvested for further experiments.
Cell labeling
The MSCs were colabeled with SPIO particles and
4′,6-diamidino-2-phenylindole (DAPI; Shanghai Bioengineering Co.,
Shanghai, China) according to a previously developed protocol
(16). Briefly, the SPIO particles
(Feridex; 50 µg/ml; Advanced Magnetics, Cambridge, MA, USA) were
mixed with the transfection agent poly-L-lysine (PLL; Shanghai
Bioengineering Co., Shanghai, China) in culture media to obtain a
Feridex-PLL complex (the final concentrations were 25 µg/ml for
Feridex and 0.75 µg/ml for PLL). Following this, the culture medium
containing the Feridex-PLL complex was supplemented with DAPI at a
final concentration of 50 µg/ml. Finally, the culture media
containing both the Feridex-PLL complex and DAPI were incubated
with the cells at room temperature. After a 24-h staining, the
excess Feridex-PLL and DAPI were removed by repeated washes with
PBS, and the colabeled MSCs were harvested for the subsequent
studies. Prussian blue staining was performed at room temperature
for 30 min to assess the magnetic labeling efficiency of the
labeled cells using a previously reported protocol (16).
Assessment of cell viability
Cell viability was assessed using the trypan blue
dye exclusion method. At day 1 and 1, 2, 3 and 4 weeks after
labeling, the cells were exposed to trypan blue dye at room
temperature for 5 min. Subsequently, the treated cells were
observed using a BH2 light microscope (Olympus Corporation, Tokyo,
Japan). The non-labeled cells were treated in the same manner as
the control. The ratio of the non-stained, viable cells to the
total cells (non-stained, viable cells and the stained, nonviable
cells) was calculated and defined as the trypan blue resistance
rate. The observer was blinded to the labeling procedures to ensure
the accuracy of the test.
Animal model and cell
transplantation
Adult New Zealand white rabbits of mixed genders,
weighing 3.0–3.5 kg, were used to establish the liver tumor model.
The tumor implantation procedures are described below. First, the
animals were anesthetized with an intravenous injection of 30 mg/kg
body weight sodium pentobarbital (Shanghai Sino-West Pharmaceutical
Co., Shanghai, China). Second, a midline laparotomy was performed
to expose the liver and a 1-mm3 VX2 tumor fragment was
inserted into the subcapsule of the right-medial lobe of the rabbit
liver. Next, the liver capsule was manually compressed for ~2 min,
and the abdomen was sutured in two layers. The implanted tumor
blocks were allowed to grow in the livers for 1 week to develop
into solitary lesions with a diameter of ~1 cm, which were detected
using MRI. New Zealand white rabbits with tumors (n=8) were
randomly divided into two groups. In the labeled cells group (n=4),
rabbits were intravenously injected with 1×106
magnetically labeled MSCs resuspended in 1 ml DMEM via the marginal
ear vein, and the rabbits in the control group (n=4) received the
same quantity of non-labeled MSCs.
In vivo MRI
The rabbits in the experimental and control groups
were subjected to MRI immediately before and at 3, 7 and 14 days
after cells transplantation. MRI was performed using a clinical
1.5-T MRI system (Signa Excite HD, General Electric, Milwaukee, WI,
USA) with a kneel coil. A T2*-weighted gradient-echo sequence was
applied to obtain MR images. The imaging acquisition parameters
were: TR:TE=25/12, with a flip angle of 15; field of view: 180×150
mm; matrix size: 256×256; section thickness: 2 mm; number of
acquisitions: 2. The signal patterns of the tumors and the adjacent
hepatic parenchyma were observed and compared.
Postmortem analysis
Histological analyses were performed immediately
after the MRI examination. One experimental rabbit and one control
rabbit were sacrificed by an intraperitoneal injection of an
overdose of pentobarbital sodium at each time point. By midline
laparotomy, the liver tumors were extracted from the abdominal
cavities of rabbits. The liver specimens were then prepared by
freezing, embedding in paraffin, and sectioning into 5-µm-thick
slices. Subsequently, Prussian blue staining (at 4°C for 30 min)
and fluorescent observations were alternatively performed on
adjacent slices using our previously described protocol (16). The aim was to identify DAPI and
Prussian blue double-positive cells. The kidneys, spleens, lungs,
hearts, brains and muscles were also subjected to the same
histological analyses to assess the distribution of the cells
throughout the rabbits using a BX53 fluorescence microscope
(Olympus Corporation).
Statistical analysis
All data are expressed as the mean ± standard
deviation, and an independent sample t-test was performed to
compare the viability of the labeled and non-labeled cells at
various time points. The statistical analyses were performed using
SPSS version 18 (IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
In vitro observations of the labeled
MSCs
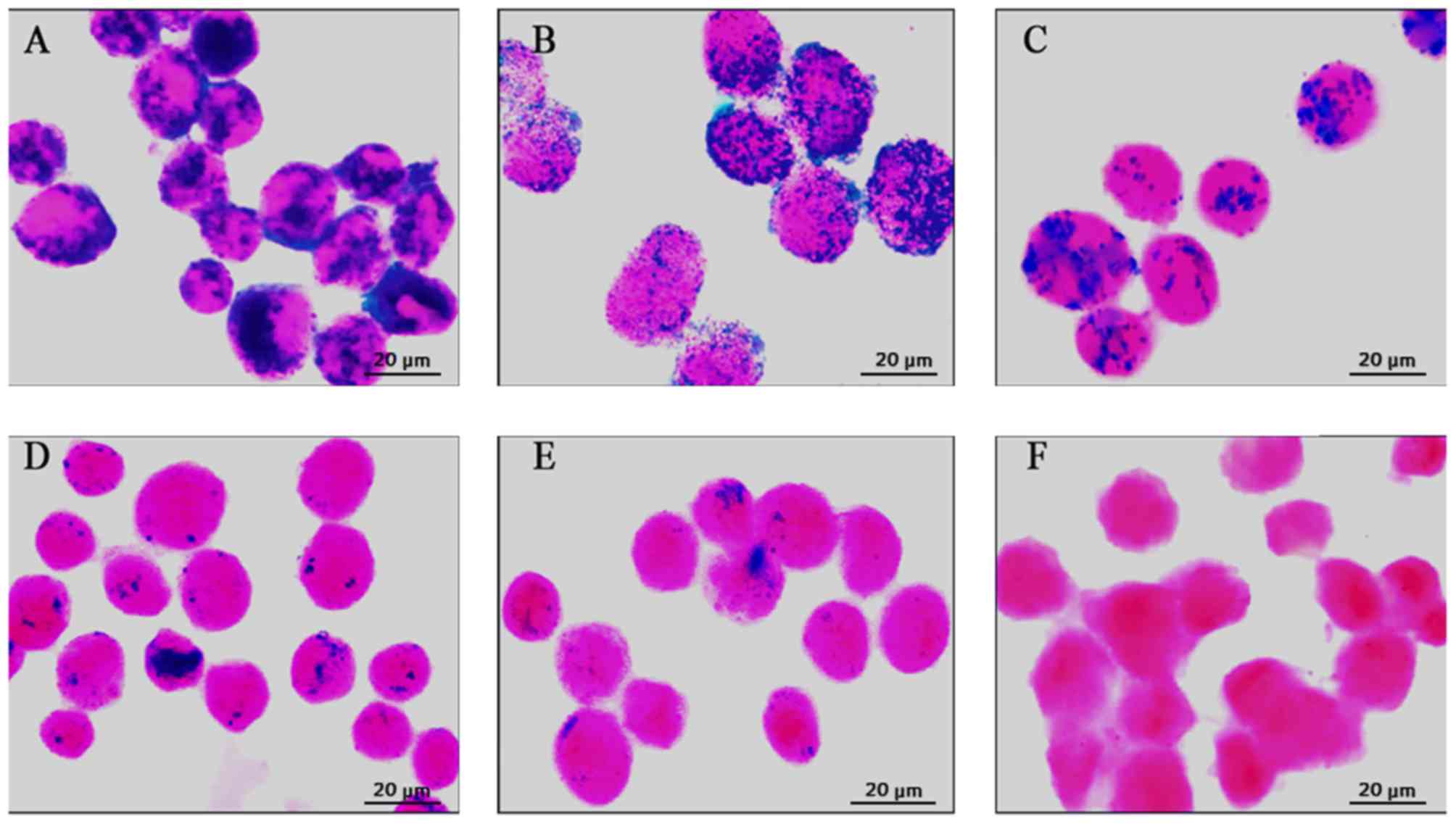
At 1 day after labeling, numerous Prussian
blue-stained iron particles were detected in the cytoplasm of MSCs
(Fig. 1A). Thereafter, the
intracellular Prussian blue-stained iron particles faded as the
cells proliferated (Fig. 1B-D). At
4 weeks after labeling, only a small number of Prussian
blue-stained iron particles were observed (Fig. 1E). In contrast, no Prussian
blue-stained particles were observed in the unlabeled cells
(Fig. 1F).
Cell viability, which is presented as the trypan
blue resistance rate, was not significantly different between the
labeled and non-labeled MSCs at 1 day and 1, 2, 3 and 4 weeks after
labeling (Fig. 2).
In vivo MRI of transplanted MSCs
Prior to injection of the labeled MSCs, the liver
tumor exhibited hyperintensity in the T2*-weighted MR images
(Fig. 3A). At 3 days after cell
injection, MRI showed heterogeneous hypointensity in the tumor,
with an unclear demarcation (Fig.
3B). At 7 days after cell injection, the tumor exhibited an
isointense MRI signal, whereas a hypointense ring was detected at
the border of the tumor (Fig. 3C).
At 14 days after injection, the MRI signal recovered the
hyperintensity to the level observed prior to the cell transplant
(Fig. 3D). In contrast, the
control rabbits infused with unlabeled cells did not exhibit any
changes in the tumor or liver signals within the 2 weeks of
follow-up examinations with MRI (data not shown).
Postmortem analysis
The histological findings, including the Prussian
blue staining and fluorescence imaging, primarily revealed labeled
MSCs in the liver tumors, rather than the non-tumor liver tissue
and other organs. Thus, the MSCs targeted the liver tumor with a
high specificity. According to the Prussian blue staining, numerous
Prussian blue-stained iron particles were detected throughout the
tumor at 3 days after cell injection, compared with a few Prussian
blue-stained iron particles in the adjacent liver tissue (Fig. 4A). However, at 7 days, the Prussian
blue-stained iron particles were mainly located at the border of
the tumor (Fig. 4B). At 14 days
after cell injection, the iron particles remained at the border of
the tumor, although the number was obviously decreased (Fig. 4C). This distribution paralleled the
distribution observed in the MR images and reflected MSC migration
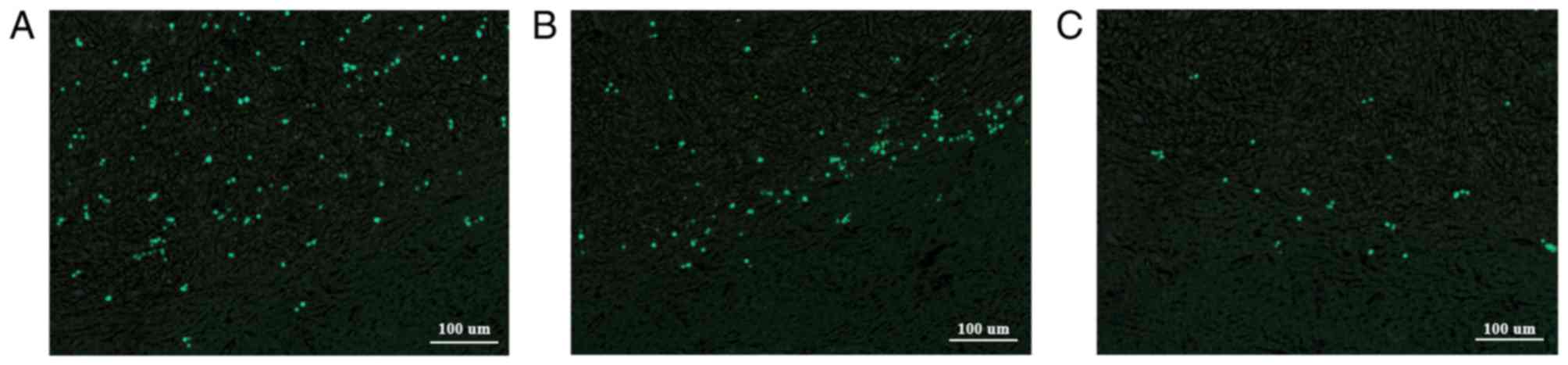
with active tumor growth. Fluorescence microscopy of adjacent
sections revealed that the bright, DAPI-stained and fluorescent
nuclei matched the distribution of the Prussian blue-stained iron
particles (Fig. 5). Therefore, the
iron particles remained in the originally labeled cells.
Discussion
As demonstrated in the present study, systemically
administered MSCs migrate to hepatic tumors with high specificity,
similar to the results observed in our previous study investigating
MSC tropism to acutely injured rat livers (16). After homing to the tumor bed, the
MSCs scattered throughout the tumor at an early stage and gradually
migrated to the border of the tumor at a later stage. The
recruitment and distribution of the magnetically labeled MSCs in
the liver tumor could be tracked in vivo for 1 week using a
1.5-T clinical MRI scanner. These attributes would provide a
short-term in vivo monitoring strategy for a tumor-targeted
therapy.
Homing of MSCs toward experimental tumors has been
reported in several animal models, including brain glioma (18), breast cancer (19), lung cancer (20), colon cancer (21) and liver cancer (22). Based on these studies, chemokines
released from the tumors are likely to be involved in the mechanism
by which MSCs home to tumors. These chemokines would attract stem
cells in a manner similar to the mechanism by which the
inflammatory factors recruit leukocytes (23). However, the specific chemokines and
their contributions to the homing and migration of MSCs have not
been well delineated. In contrast to the definite protective effect
of leukocytes, the real function of MSCs homing to tumors remains
unclear. MSCs were previously demonstrated to promote tumor growth
and metastasis by enhancing the tumor neovasculature (24–26),
whereas in recent studies, the MSCs did not appreciably contribute
to tumor development (27).
Furthermore, in other studies, MSCs suppressed tumor progression by
modifying and reducing tumor vessels (28,29).
These contradictory findings may be attributed to the differences
in experimental systems or animal models used. In our study, the
distribution of systemically transplanted MSCs in liver tumors
varied temporally and spatially with the development of the tumor.
At an early stage of tumor growth, the MSCs mainly infiltrated the
tumor bed and were scattered throughout the tumor. However, at a
later stage, most of the labeled stem cells were detected between
the tumor and the surrounding normal parenchyma. Based on these
observations, the stem cells migrate in the tumor during tumor
growth. Stem cell migration seemed to be associated with the tumor
neovasculature, which spread throughout the entire tumor at an
early stage and extended to the surrounding region during tumor
growth and infringement. In a previous study, Wu et al
(18) observed the same
distribution of transplanted stem cells in gliomas and verified
that the stem cells were incorporated into the tumor vessels.
Unfortunately, the present study did not investigate the stem cell
outcomes because the aim was to visualize the stem cells using MRI;
in addition, the stem cells were not visible after 7 days
post-transplantation. In this short time period, it is impossible
for the stem cells to have been incorporated into the tumor
vasculature or differentiated into vascular-related cell lines. A
further study should be performed to elucidate the mechanism by
which the stem cells migrate in the liver tumor.
Because MSCs have a tumor-homing property and
potential applications in tumor gene therapy as a delivery vehicle,
the grafted MSCs must be assessed in vivo using a
noninvasive method. Recent studies have confirmed the feasibility
of using MRI to track the stem cells pre-labeled with iron
particles prior to transplantation (30–32).
In liver cancer, histochemical methods and fluorescence imaging
have been used to verify the tumor homing of stem cells (33–36).
However, the spatial and temporal distribution of grafted stem
cells in the tumor has not been determined. In the present study,
homing of the magnetically labeled stem cells to the tumor was
observed as a dark signal on the T2*WI images, due to the
incorporation of the iron oxide nanoparticles within the cytoplasm.
In addition, the dark signal was distributed throughout the tumor
at an earlier stage, but it was mainly located at the border of the
tumor at a later stage. This pattern of change in the MRI signals
paralleled the results of the Prussian blue staining. At 14 days
after MSC transplantation, a small number of Prussian blue-positive
cells were still observed at the periphery of the liver tumor,
whereas no signal change was detected with MRI. Thus, the 1.5-T MRI
scanner used in the present study was not sensitive enough to trace
the magnetically labeled stem cells due to the reduced number of
cells and the dilution of the intracellular iron particles.
Therefore, a more effective visualization of the transplanted stem
cells at higher magnetic field strengths would be expected.
One problem associated with the SPIO-based in
vivo MRI tracking of stem cells is determining whether the MRI
signal void is generated by the iron particles in the pre-labeled
cells. Based on previous studies, the iron particles may be
released from dead cells or may be taken up by macrophages, which
could lead to misinterpretations of the MRI findings (37,38).
In the present study, stem cells were colabeled with SPIO particles
and DAPI, and iron was co-localized with the fluorescent marker
DAPI in the histological analyses. This result excluded the
possibility that the MRI signal was generated by the iron released
from dead stem cells. On the other hand, both the MRI and
histological findings demonstrated varied distribution patterns of
cells at the different time points, which is consistent with the
migratory feature of stem cells other than of phagocytes. If the
released iron was taken up by phagocytes in the tumor, the change
in the signal would be persistent. However, in the present study,
the change in the MRI signal was not detected at 7 days after cell
transplantation, indicating that most of the iron in the stem cells
had been cleared from the tumors and was not retained in the
phagocytes.
Currently, SPIOs are the most widely used contrast
agents for labeling and tracking diverse cells, due to their high
relaxivity (39–41). However, long-term tracking of the
transplanted cells is not possible using this direct labeling
approach due to the continuous reduction of the contrast agent in
the cells as they divide (42–44),
which is also the main limitation of this study. In the present
study, the MRI tracking period was only 1 week, which is
insufficient to monitor the fate of the transplanted stem cells. To
circumvent this problem, it is urgent that a genetic MRI reporter
system be used. Prior to transplantation, the cells are transfected
with a constitutively expressed reporter gene and subsequently
produce MRI contrast within the cells (45). In such a manner, the transplanted
stem cells could be persistently traced in vivo.
In conclusion, the present study demonstrated that
systemically administered MSCs can migrate to liver tumors with
high specificity. The distribution patterns of the stem cells in
the tumors indicated that the stem cells possess a migratory
property with active tumor growth. These results indicated that the
targeting and distribution of the magnetically labeled stem cells
in liver tumor can be tracked in vivo using a clinical 1.5-T
MRI scanner. However, the duration of the MRI tracking is only 1
week because the intracellular iron is diluted as the cells
proliferate. Thus, a genetic MRI reporter system must be developed
for long-term longitudinal in vivo tracking of stem cells
used as a therapeutic strategy.
Acknowledgements
This study was supported by a grant from the
National Natural Science Foundation of China (81171387). The
manuscript has been modified by American Journal Experts editing
service.
Glossary
Abbreviations
Abbreviations:
|
MSCs
|
mesenchymal stem cells
|
|
MRI
|
magnetic resonance imaging
|
|
SPIO
|
superparamagnetic iron oxide
|
|
DAPI
|
4′,6-diamidino-2-phenylindole
|
|
HCCs
|
hepatocellular carcinomas
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
PBS
|
phosphate-buffered saline
|
|
PLL
|
poly-L-lysine
|
References
|
1
|
Badawy AA, El-Hindawi A, Hammam O, Moussa
M, Gabal S and Said N: Impact of epidermal growth factor receptor
and transforming growth factor-α on hepatitis C virus-induced
hepatocarcinogenesis. APMIS. 123:823–831. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marquardt JU, Galle PR and Teufel A:
Molecular diagnosis and therapy of hepatocellular carcinoma (HCC):
An emerging field for advanced technologies. J Hepatol. 56:267–275.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prieto J, Fernandez-Ruiz V, Kawa MP,
Sarobe P and Qian C: Cells as vehicles for therapeutic genes to
treat liver diseases. Gene Ther. 15:765–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Choi SA, Lee YE, Kwak PA, Lee JY, Kim SS,
Lee SJ, Phi JH, Wang KC, Song J, Song SH, et al: Clinically
applicable human adipose tissue-derived mesenchymal stem cells
delivering therapeutic genes to brainstem gliomas. Cancer Gene
Ther. 22:302–311. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heino TJ and Hentunen TA: Differentiation
of osteoblasts and osteocytes from mesenchymal stem cells. Curr
Stem Cell Res Ther. 3:131–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gou S, Wang C, Liu T, Wu H, Xiong J, Zhou
F and Zhao G: Spontaneous differentiation of murine bone
marrow-derived mesenchymal stem cells into adipocytes without
malignant transformation after long-term culture. Cells Tissues
Organs. 191:185–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quevedo HC, Hatzistergos KE, Oskouei BN,
Feigenbaum GS, Rodriguez JE, Valdes D, Pattany PM, Zambrano JP, Hu
Q, McNiece I, et al: Allogeneic mesenchymal stem cells restore
cardiac function in chronic ischemic cardiomyopathy via trilineage
differentiating capacity. Proc Nat Acad Sci. 106:14022–14027. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Q, Cheng G, Wang Z, Zhan S, Xiong B
and Zhao X: Bone marrow-derived mesenchymal stem cells
differentiate into nerve-like cells in vitro after transfection
with brain-derived neurotrophic factor gene. In Vitro Cell Dev Biol
Anim. 51:319–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ke Z, Mao X, Li S, Wang R, Wang L and Zhao
G: Dynamic expression characteristics of Notch signal in bone
marrow-derived mesenchymal stem cells during the process of
differentiation into hepatocytes. 45:95–100. 2013.
|
|
10
|
Abd-Allah SH, Shalaby SM, El-Shal AS,
Elkader EA, Hussein S, Emam E, Mazen NF, El Kateb M and Atfy M:
Effect of bone marrow-derived mesenchymal stromal cells on
hepatoma. Cytotherapy. 16:1197–1206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bayo J, Marrodán M, Aquino JB, Silva M,
García MG and Mazzolini G: The therapeutic potential of bone
marrow-derived mesenchymal stromal cells on hepatocellular
carcinoma. Liver International. 34:330–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hua P, Wang YY, Liu LB, Liu JL, Liu JY,
Yang YQ and Yang SR: In vivo magnetic resonance imaging tracking of
transplanted superparamagnetic iron oxide-labeled bone marrow
mesenchymal stem cells in rats with myocardial infarction. Mol Med
Rep. 11:113–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Markides H, Kehoe O, Morris RH and El Haj
AJ: Whole body tracking of superparamagnetic iron oxide
nanoparticle-labelled cells-a rheumatoid arthritis mouse model.
Stem Cell Res Ther. 4:1262013. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jackson JS, Golding JP, Chapon C, Jones WA
and Bhakoo KK: Homing of stem cells to sites of inflammatory brain
injury after intracerebral and intravenous administration: A
longitudinal imaging study. Stem Cell Res Ther. 1:172010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei MQ, Wen DD, Wang XY, Huan Y, Yang Y,
Xu J, Cheng K and Zheng MW: Experimental study of endothelial
progenitor cells labeled with superparamagnetic iron oxide in
vitro. Mol Med Rep. 11:3814–3819. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cai J, Zhang X, Wang X, Li C and Liu G: In
vivo MR imaging of magnetically labeled mesenchymal stem cells
transplanted into rat liver through hepatic arterial injection.
Contrast Media Mol Imaging. 3:61–66. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wakitani S, Saito T and Caplan AI:
Myogenic cells derived from rat bone marrow mesenchymal stem cells
exposed to 5-azacytidine. Muscle Nerve. 18:1417–1426. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wu X, Hu J, Zhou L, Mao Y, Yang B, Gao L,
Xie R, Xu F, Zhang D, Liu J and Zhu J: In vivo tracking of
superparamagnetic iron oxide nanoparticle-labeled mesenchymal stem
cell tropism to malignant gliomas using magnetic resonance imaging.
J Neurosurg. 108:320–329. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao D, Najbauer J, Annala AJ, Garcia E,
Metz MZ, Gutova M, Polewski MD, Gilchrist M, Glackin CA, Kim SU and
Aboody KS: Human neural stem cell tropism to metastatic breast
cancer. Stem Cells. 30:314–325. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xin H, Kanehira M, Mizuguchi H, Hayakawa
T, Kikuchi T, Nukiwa T and Saijo Y: Targeted delivery of CX3CL1 to
multiple lung tumors by mesenchymal stem cells. Stem Cells.
25:1618–1626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yi BR, Park MA, Lee HR, Kang N, Choi KJ,
Kim SU and Choi KC: Suppression of the growth of human colorectal
cancer cells by therapeutic stem cells expressing cytosine
deaminase and interferon-β via their tumor-tropic effect in
cellular and xenograft mouse models. Mol Oncol. 7:543–554. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yan C, Yang M, Li Z, Li S, Hu X, Fan D,
Zhang Y, Wang J and Xiong D: Suppression of orthotopically
implanted hepatocarcinoma in mice by umbilical cord-derived
mesenchymal stem cells with sTRAIL gene expression driven by AFP
promoter. Biomaterials. 35:3035–3043. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fox JM, Chamberlain G, Ashton BA and
Middleton J: Recent advances into the understanding of mesenchymal
stem cell trafficking. Br J Haematol. 137:491–502. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang T, Lee Y, Rui Y, Cheng T, Jiang X
and Li G: Bone marrow-derived mesenchymal stem cells promote growth
and angiogenesis of breast and prostate tumors. Stem Cell Res Ther.
4:702013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki K, Sun R, Origuchi M, Kanehira M,
Takahata T, Itoh J, Umezawa A, Kijima H, Fukuda S and Saijo Y:
Mesenchymal stromal cells promote tumor growth through the
enhancement of neovascularization. Mol Med. 17:579–587. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hou Y, Ryu CH, Jun JA, Kim SM, Jeong CH
and Jeun S-S: IL-8 enhances the angiogenic potential of human bone
marrow mesenchymal stem cells by increasing vascular endothelial
growth factor. Cell BiolInt. 38:1050–1059. 2014.
|
|
27
|
Usha L, Rao G, Christopherson K and Xu X:
Mesenchymal stem cells develop tumor tropism but do not accelerate
breast cancer tumorigenesis in a somatic mouse breast cancer model.
PLoS One. 8:e678952013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kéramidas M, de Fraipont F, Karageorgis A,
Moisan A, Persoons V, Richard MJ, Coll JL and Rome C: The dual
effect of mscs on tumour growth and tumour angiogenesis. Stem Cell
Res Ther. 4:412013. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gomes C: The dual role of mesenchymal stem
cells in tumor progression. Stem Cell Res Ther. 4:422013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Azzabi F, Rottmar M, Jovaisaite V, Rudin
M, Sulser T, Boss A and Eberli D: Viability, differentiation
capacity, and detectability of super-paramagnetic iron
oxide-labeled muscle precursor cells for magnetic-resonance
imaging. Tissue Eng Part C Methods. 21:182–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Y-X, Xuan S, Port M and Idee J-M:
Recent advances in superparamagnetic iron oxide nanoparticles for
cellular imaging and targeted therapy research. Curr Pharm Des.
19:6575–6593. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang Y, Zhang J, Qian Y, Dong S, Huang H,
Boada FE, Fu FH and Wang JH: Superparamagnetic iron oxide is
suitable to label tendon stem cells and track them in vivo with MR
imaging. Ann Biomed Eng. 41:2109–2119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Niess H, Bao Q, Conrad C, Zischek C,
Notohamiprodjo M, Schwab F, Schwarz B, Huss R, Jauch KW, Nelson PJ
and Bruns CJ: Selective targeting of genetically engineered
mesenchymal stem cells to tumor stroma microenvironments using
tissue-specific suicide gene expression suppresses growth of
hepatocellular carcinoma. Ann Surg. 254:767–774. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gong P, Wang Y, Wang Y, Jin S, Luo H,
Zhang J, Bao H and Wang Z: Effect of bone marrow mesenchymal stem
cells on hepatocellular carcinoma in microcirculation. Tumor Biol.
34:2161–2168. 2013. View Article : Google Scholar
|
|
35
|
Belmar-Lopez C, Mendoza G, Oberg D, Burnet
J, Simon C, Cervello I, Iglesias M, Ramirez JC, Lopez-Larrubia P,
Quintanilla M and Martin-Duque P: Tissue-derived mesenchymal
stromal cells used as vehicles for anti-tumor therapy exert
different in vivo effects on migration capacity and tumor growth.
BMC Medicine. 11:1392013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klopp AH, Spaeth EL, Dembinski JL,
Woodward WA, Munshi A, Meyn RE, Cox JD, Andreeff M and Marini FC:
Tumor irradiation increases the recruitment of circulating
mesenchymal stem cells into the tumor microenvironment. Cancer Res.
67:11687–11695. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Terrovitis J, Stuber M, Youssef A, Preece
S, Leppo M, Kizana E, Schär M, Gerstenblith G, Weiss RG, Marbán E
and Abraham MR: Magnetic resonance imaging overestimates
ferumoxide-labeled stem cell survival after transplantation in the
heart. Circulation. 117:1555–1562. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Amsalem Y, Mardor Y, Feinberg MS, Landa N,
Miller L, Daniels D, Ocherashvilli A, Holbova R, Yosef O, Barbash
IM and Leor J: Iron-oxide labeling and outcome of transplanted
mesenchymal stem cells in the infarcted myocardium. Circulation.
116:38–45. 2007. View Article : Google Scholar
|
|
39
|
Cohen ME, Muja N, Fainstein N, Bulte JWM
and Ben-Hur T: Conserved fate and function of ferumoxides-labeled
neural precursor cells in vitro and in vivo. J Neurosci Res.
88:936–944. 2009.
|
|
40
|
Barczewska M, Wojtkiewicz J, Habich A,
Janowski M, Adamiak Z, Holak P, Matyjasik H, Bulte JW, Maksymowicz
W and Walczak P: MR monitoring of minimally invasive delivery of
mesenchymal stem cells into the porcine intervertebral disc. PLoS
One. 8:e746582013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
England TJ, Bath PMW, Abaei M, Auer D and
Jones DRE: Hematopoietic stem cell (CD34+) uptake of
superparamagnetic iron oxide is enhanced by but not dependent on a
transfection agent. Cytotherapy. 15:384–390. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lee J-H, Jung MJ, Hwang YH, Lee YJ, Lee S,
Lee DY and Shin H: Heparin-coated superparamagnetic iron oxide for
in vivo MR imaging of human MSCs. Biomaterials. 33:4861–4871. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Farrell E, Wielopolski P, Pavljasevic P,
van Tiel S, Jahr H, Verhaar J, Weinans H, Krestin G, O'Brien FJ,
van Osch G and Bernsen M: Effects of iron oxide incorporation for
long term cell tracking on MSC differentiation in vitro and in
vivo. Biochem Biophys Res Commun. 369:1076–1081. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Arbab AS, Bashaw LA, Miller BR, Jordan EK,
Bulte JWM and Frank JA: Intracytoplasmic tagging of cells with
ferumoxides and transfection agent for cellular magnetic resonance
imaging after cell transplantation: Methods and techniques.
Transplantation. 76:1123–1130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
He X, Cai J, Liu B, Zhong Y and Qin Y:
Cellular magnetic resonance imaging contrast generated by the
ferritin heavy chain genetic reporter under the control of a Tet-On
switch. Stem Cell Res Ther. 6:2072015. View Article : Google Scholar : PubMed/NCBI
|