Introduction
Prostate cancer is one of the most common types of
malignant tumor in Europe and the United States (1,2).
Prostate cancer is the second most common non-skin cancer in men
and is the fifth leading cause of cancer-associated mortality in
men worldwide. A total of ~14% (1,22,000) of men diagnosed with
prostate cancer worldwide in 2008 were in the Asia Pacific region,
with 32% in Japan, 28% in China and 15% in Australia (3). It was recently reported that the
incidence and mortality of prostate cancer in the majority of Asian
countries gradually increased between 2012 and 2016 (4). It is established that prostate cancer
is associated with genetic factors, diet, infection and hormonal
factors. At present, the molecular mechanisms of growth and
migration in human prostate cancer have not been completely
elucidated.
Protein arginine methyltransferase 6 (PRMT6) is a
type I arginine methyltransferase that is primarily expressed in
the nucleus and has functions in the regulation of transcription
and the cell cycle, and DNA repair (5). PRMT6 has also been demonstrated to
act as a coactivator in estrogen, progesterone and glucocorticoid
receptor transcription. Furthermore, El-Andaloussi et al
(6) reported that PRMT6 had a key
role in DNA base excision repair regulation as it forms a complex
with methylated DNA polymerase β. Several studies have indicated
that the expression of PRMT6 was usually observed in various types
of tumor cells, including non-small cell lung cancer (7), hepatocellular carcinoma (8), breast cancer (9) and prostate cancer (10). In addition, studies have reported
that PRMT6 knockdown inhibits cell growth and the cell cycle in
lung cancer and U2OS human osteosarcoma cells (11,12).
Phalke et al (13) reported
that PRMT6 exhibited an oncogenic function by directly binding to
and inhibiting the promoter of p21, which stimulated cell growth
and protected the cell cycle from senescence in breast cancer
cells. Although certain studies have reported that the expression
of PRMT6 may be associated with the motility and invasion of tumor
cells (10,14), the molecular mechanisms of PRMT6 in
the regulation of cell growth and migration have not been
completely elucidated.
It has been reported that Ganoderma lucidum
(G. lucidum) exhibited preventive and therapeutic effects in
cancer (15), chronic bronchitis
(16), bronchial asthma (17) and hepatitis (18). Polysaccharides, which consist of
glucose, mannose, galactose, xylose, fucose and arabinose, are one
of the most important active components of G. lucidum
(19). Several in vitro and
in vivo studies have demonstrated that polysaccharides
extracted from G. lucidum (GLP) exhibited significant
effects on tumorigenesis, oxidative stress, inflammation and
immunoregulation (20,21). Xu et al (22) reported that GLP affected the
function of T lymphocytes, B lymphocytes, macrophages and natural
killer cells. Although certain studies have reported that GLP
exhibited potential antiproliferative, pro-apoptotic and inhibitory
effects on migration in several cancer cell lines, including colon
cancer (23,24), hepatocellular carcinoma (25), acute myeloid leukemia (26,27)
and breast cancer (28,29), it is not established whether GLP is
effective in regulating the growth and migration of prostate cancer
cells. Therefore, the present study aimed to investigate the effect
of GLP on the growth and migration of human prostate cancer cells,
and to investigate the underlying molecular mechanism.
Materials and methods
Isolation and analysis of GLP
G. lucidum was provided by the College of
Food Science of Shenyang Agricultural University (Shenyang, China).
GLP was extracted from G. lucidum as described previously
(30). To obtain a crude
polysaccharide sample, fermentation broth of G. lucidum was
concentrated and precipitated by 90% alcohol. Identification and
quantification of the monosaccharides was performed using a gas
chromatography (GC) analyzer (Beckman Coulter, Inc., Brea, CA,
USA). According to the method described by He et al
(31), 5 mg dry GLP was hydrolyzed
in 2 M trifluoroacetic acid at 120°C for 5 h. The hydrolysate was
reduced by NaBH4 and was acetylated using acetic
anhydride. The acetylated monosaccharides of GLP were added into a
GC analyzer and GLP was analyzed by gas chromatography, which
determined that GLP was composed of arabinose, galactose, glucose
and xylose in an approximate molar ratio of 4:2:10:1.
Cell culture and GLP treatment
LNCaP prostate cancer cells were purchased from
ScienCell Research Laboratories, Inc., (San Diego, California,
USA), and were cultured according to supplier instructions. Cells
in culture bottle were washed three times with PBS and were
cultured in RPMI-1640 (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), which was supplemented with 10% fetal calf serum (Thermo
Fisher Scientific, Inc.), 2 mM glutamine, 100 U/ml penicillin and
100 mg/ml streptomycin. A total of
1.2×106−1.8×107 cells/well were subsequently
treated with 5 and 20 µg/ml GLP and the control cells were treated
with 0.01 M PBS (1XPBS) l at 37°C for 72 h. The concentrations of
GLP employed were selected based on a previous study (32). All cells were cultured at 37°C, 5%
CO2 and 100% humidity.
Plasmids and small interfering (si)RNA
transfection
The PRMT6 expression plasmid pVAX1 (4 µg) acted as a
control plasmid purchased from Thermo Fisher Scientific, Inc.,
PRMT6 siRNA (50 mM) and siR-Ribo™ (50 mM) acted as
negative control were purchased from ScienCell Research
Laboratories, Inc., which were transfected into LNCaP cells using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol at 37°C for 4 h. The
following siRNA sequences were employed: PRMT6 siRNA,
5′-GAGCAAGACACGGACGUUU-3′. (GenBank, accession number BC073866).
Cells following 4 h transfection were subsequently treated with 5
and 20 µg/ml GLP and the control cells were treated with 0.01 M PBS
(1XPBS) at 37°C for 72 h.
Cell growth curve, cell clones and
cycle analysis
For cell growth curve analysis, 5×104
cells were plated in each well of 12 well plates in triplicate.
Cells were maintained in culture with 10% fetal calf serum at 37°C,
5% CO2 and 100% humidity for 12 h, and then cells were
transfected with PRMT6 overexpression plasmid or PRMT6 siRNA for 4
h. Following transfection, 5×104 cells were treated with
5 or 20 µg/ml GLP and control cells were treated with 0.01 M PBS
(1XPBS) at 37°C for 0, 24, 48, 72, 96 and 120 h and were
immediately counted by a Coulter counter (Beckman Coulter, Inc.)
through an optical microscope (Olympus Corporation, Tokyo, Japan)
following staining with 0.4% trypan blue solution for 10 min at
room temperature. A total of 5×104 cells/well were
plated in 6-well plates in triplicate. Cells were maintained in
culture with 10% fetal calf serum at 37°C, 5% CO2 and
100% humidity for 12 h, and then cells were transfected with the
PRMT6 overexpression plasmid or PRMT6 siRNA for 4 h. Following
transfection cells were treated with 5 or 20 µg/ml GLP and with
0.01 M PBS (1XPBS) as control at 37°C for 120 h, and then were
fixed using 4% paraformaldehyde for 15 min at room temperature and
stained with 0.4% trypan blue solution for 10 min at room
temperature. Images were taken with an optical microscope (Olympus
Corporation) magnification, ×100 and gray value analysis was using
by imagepro version 6.0 (Media Cybernetics, Inc.) of five fields of
view. For cell cycle analysis, 2×105 cells were fixed
70% pre-cooled ethanol, stored at 4°C overnight and then stained
with PBS containing 5 mg/ml RNase, 0.1% Triton X-100 and 20 mg/ml
propidium iodide in the dark at room temperature for 30 min and
analyzed using a flow cytometer (Attune NxT; Thermo Fisher
Scientific, Inc.). The amount of DNA in G1, S and G2/M phases was
analyzed using ModFit 161 LT version 3.0 software (Verity Software
House, Inc., Topsham, ME, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) and RT was performed
using ABScript II cDNA First-Strand Synthesis kit (ABclonal Biotech
Co., Ltd., Wuhan, China) according to the manufacturer's protocol.
cDNA was quantified by qPCR using SYBR Premix Ex Taq II (Takara
Bio, Inc., Otsu, Japan) on a Mx3000P qPCR system (Agilent, Santa
Clara, CA, USA). The reaction was as follows: cDNA 2 µl, ddH2O 6.4
µl, upstream and downstream primers 0.8 µl, SYBR Premix Ex TaqTMII1
10 and 20 µl total reaction system. Amplification conditions:
annealing 56°C, 30 sec; extending 72°C, 30 sec; 45 cycles. The
experiment was repeated 3 times. Gene expression levels were
calculated using Stratagene Mx3000P software (version Mx3005P;
Shanghai PuDi Biotechnology Co., Ltd., Shanghai, China). The
relative amount of PCR products generated from each primer set was
determined on the basis of the threshold cycle (Ct) number using
the 2−ΔΔCq method (33). GAPDH was used as control to
normalize the amount of cDNA used. Relative
expression=2[[Ct(control) gene X-Ct (treatment) gene
X]-[Ct(control) 36B4-Ct(treatment) 36B4]]. The following primers
were used: PRMT6, 5′-AGACACGGACGTTTCAGGAG-3′ (forward) and
5′-CCACTTTGTAGCGCAGCG-3′ (reverse); p21, 5′-TGAGCCGCGACTGTGATG-3′
(forward) and 5′-GTCTCGGTGACAAAGTCGAAGTT-3′ (reverse);
cyclin-dependent kinase 2 (CDK2), 5′-ATGATGACGATGAGGGTGTGCCAA-3′
(forward) and 5′-GGTCACCATTGCAGCTGTCGAAAT-3′ (reverse); focal
adhesion kinase (FAK), 5′-AGATGTACATCAAGGCATTTA-3′ (forward) and
5′-AATGCCTTGATGTACATCT-3′ (reverse); SRC,
5′-GCCTGTTCACCCGTACTCTGCC-3′ (forward) and
5′-GGTAACTGCCGATCATAAGGT-3′ (reverse); and GAPDH,
5′-AGAAGCTGGGGTCATTTG-3′ (forward) and 5′-AGGGGCCATCCACAGTCTTC-3′
(reverse).
Western blot analysis
Western blot analysis was performed as described
previously (34). The cell protein
was extracted by radioimmunoprecipitation lysate kit (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China). The
protein concentration was determined by bicinchoninic assay protein
concentration kit (Vazyme, Piscataway, NJ, USA). Briefly, 20 µg
protein/lane was loaded and resolved by 10% SDS-PAGE. Protein was
subsequently transferred to polyvinylidene difluoride membranes.
Following blocking with 5% bovine serum albumin (Thermo Fisher
Scientific, Inc.) for 1 h at room temperature, primary antibodies
against PRMT6 (1:500; cat. no. sc-271744; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA), p21 (1:500; cat. no. sc-136020; Santa Cruz
Biotechnology, Inc.), CDK2 (1:1,000; cat. no. sc-70829; Santa Cruz
Biotechnology, Inc.), FAK (1:200; cat. no. sc-271195; Santa Cruz
Biotechnology, Inc.), SRC (1:1,000; cat. no. sc-32789; Santa Cruz
Biotechnology, Inc.) and GAPDH (1:5,000; cat. no. sc-66163; Santa
Cruz Biotechnology, Inc.) were incubated with the membranes at 4°C.
After 12 h incubation, the blot was washed and incubated with goat
anti-mouse immunoglobulin (Ig) G-horse radish peroxidase (HRP;
1:2,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) and goat
anti-rabbit IgG-HRP (1:2,000; cat. no. sc-2004; Santa Cruz
Biotechnology, Inc.) for 2.5 h at room temperature. Proteins were
visualized using a Clarity Western enhanced chemiluminescence
Substrate (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and a
Tanon 5200 Full automatic chemiluminescence image analysis system
(Tanon Science and Technology Co., Ltd., Shanghai, China).
Transwell assay
Briefly, 5×105 cells were treated with 5
or 20 µg/ml GLP, or control treatment, for 24 h at 37°C and were
seeded into the RPMI-1640 medium in the upper well of a Boyden
chamber at a concentration of 5×104 cells per well. The
lower compartment was filled with 1 ml RPMI-1640 (Thermo Fisher
Scientific, Inc.), which was supplemented with 20% fetal calf serum
(Thermo Fisher Scientific, Inc.). Following incubation at 37°C for
48 h, migratory cells on the lower surface of the filter were fixed
with 95% ethanol for 10 min at room temperature, stained with 0.4%
trypan blue solution for 10 min at room temperature and imaged
using an optical microscope (Olympus Corporation) and an AxioCam
HRc CCD camera (Olympus Corporation; magnification, ×400
times).
Scratch assay
Scratch assay was performed as described in a
previous study (35). Briefly,
1×108 cells were plated into 6 well plates in triplicate
at subconfluence and cultured for 24 h at 37°C. Confluent cells
were treated either 5 or 20 µg/ml GLP, or PBS for the control
treatment for 24 h at 37°C prior to cell scraping using 1 ml
pipette tips. Cells were then washed with growth medium and
continually cultured in RPMI-1640 (Thermo Fisher Scientific, Inc.),
which was supplemented with 10% fetal calf serum (Thermo Fisher
Scientific, Inc.), 2 mM glutamine, 100 U/ml penicillin and 100
mg/ml streptomycin for 48 h at 37°C, and during this time, cells
were also treated either 5 or 20 µg/ml GLP, or PBS for the control
treatment. Cell migration was photographed in ten regions at 0 and
48 h.
Statistical analysis
Data are presented as the mean ± standard deviation,
and were analyzed by analysis of variance followed by
Student-Newman-Keuls post-hoc test, and Chi-squared test using SPSS
software (version 20.0; IBM Corp., Armonk, NY, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
GLP inhibits cell growth in LNCaP
cells
The effects of different GLP concentrations on cell
growth were determined using growth curve analyses and
morphological observation. The results demonstrated that the growth
ratio of cells was significantly decreased in either 5 or 20 µg/ml
GLP groups compared with the control group following incubation for
72 h (Fig. 1A). Furthermore,
morphological results also revealed that GLP (5 and 20 µg/ml)
significantly inhibited cell growth compared with the control group
(Fig. 1B-C).
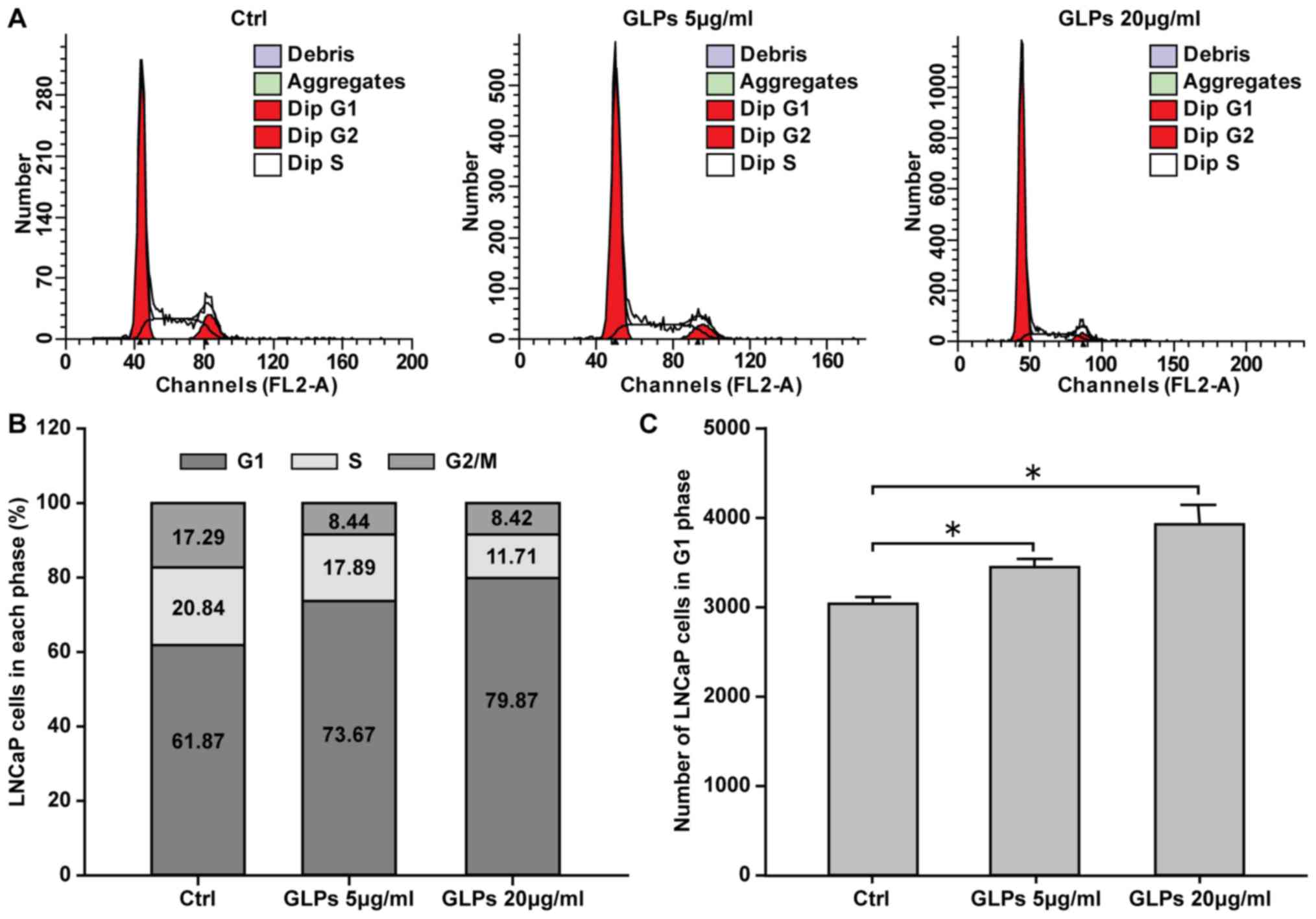
GLP induces cell cycle arrest in LNCaP
cells
Flow cytometry was performed to investigate the
effect of GLP on the cell cycle. The results demonstrated that 5
and 20 µg/ml GLP significantly induced cell cycle arrest, with an
increased number of cells in the G1 phase, of LNCaP cells in a
dose-dependent manner, compared with control cells (Fig. 2).
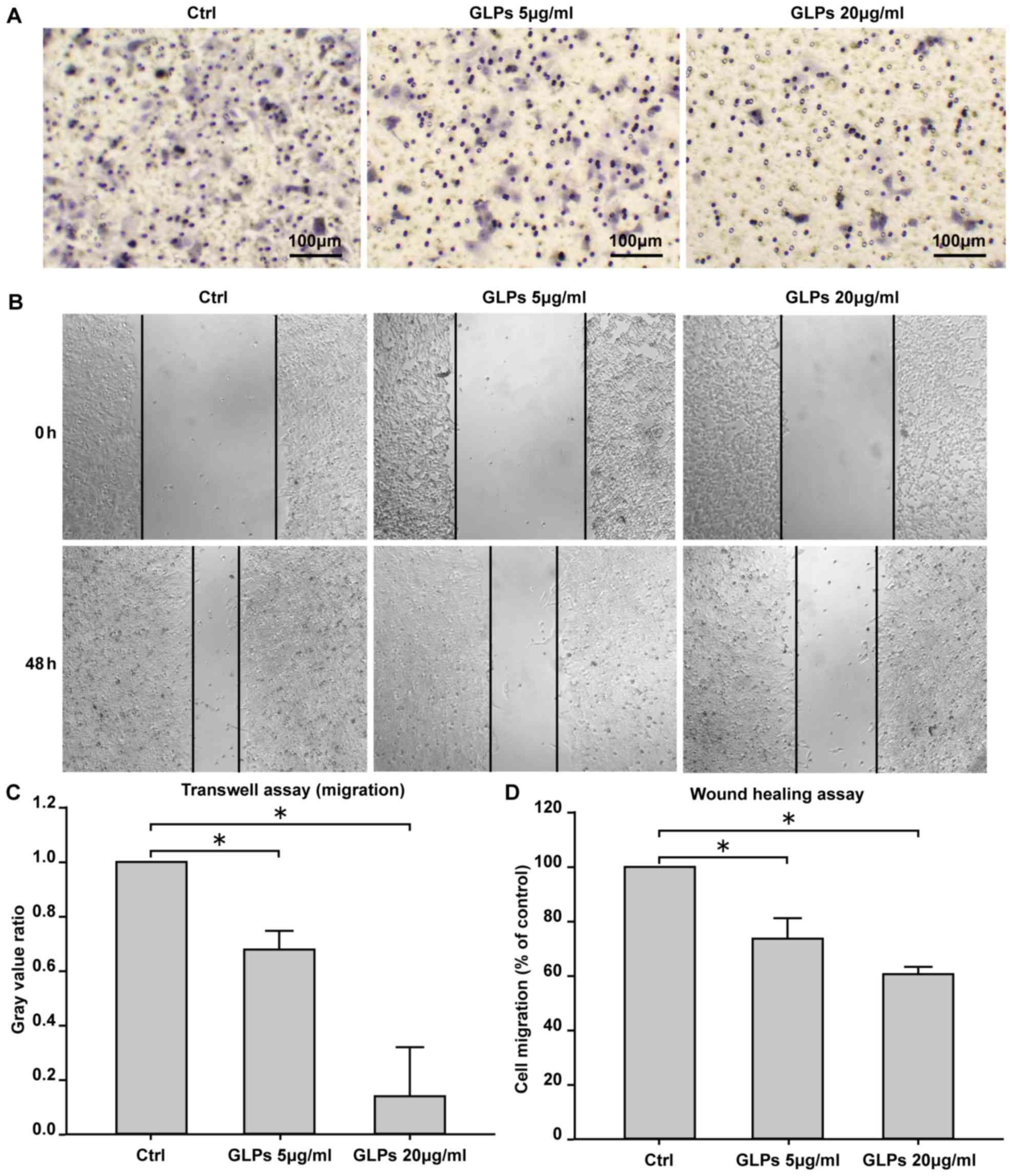
GLP inhibits cell migration in LNCaP
cells
Transwell and scratch assays were performed to
investigate the effect of GLP on the migration of LNCaP cells. The
results demonstrated that 5 and 20 µg/ml GLP significantly
inhibited cell migration compared with the control group (Fig. 3).
Effect of GLP on the PRMT6 signaling
pathway and migration-associated proteins in LNCaP cells
To determine the effect of GLP on the PRMT6
signaling pathway and migration-associated proteins in LNCaP cells,
the expression of PRMT6, p21, CDK2, FAK and FRC were determined by
RT-qPCR and western blot analysis. The results demonstrated that 5
and 20 µg/ml GLP decreased the protein expression of PRMT6 and
CDK2, and increased p21 expression (Fig. 4A). In addition, the protein
expression of migration-associated proteins FAK and FRC were
decreased in GLP groups compared with the control group (Fig. 4A). Results for mRNA expression
demonstrated that PRMT6, CDK2, FAK and FRC levels were
significantly reduced at 5 and 20 µg/ml GLP, while p21 levels were
significantly increased, compared with the control group (Fig. 4B-F).
 | Figure 4.Effect of GLP on the PRMT6 signaling
pathway in LNCaP cells. The mRNA and protein expression of PRMT6,
p21, CDK2, FAK and SRC were determined by RT-qPCR and western blot
analysis, respectively. (A) Expression of PRMT6, p21, CDK2, FAK and
SRC protein was assessed by western blot analysis. RT-qPCR was
performed to investigate the mRNA expression of (B) PRMT6, (C) p21,
(D) CDK2, (E) FAK and (F) SRC. Data are presented as the mean ±
standard deviation. *P<0.05 vs. Ctrl. GLP, Ganoderma
lucidum polysaccharide; PRMT6, protein arginine
methyltransferase 6; CDK2, cyclin-dependent kinase 2; FAK, focal
adhesion kinase; RT-qPCR, reverse transcription-quantitative
polymerase chain reaction; Ctrl, control; SRC, Steroid receptor
coactivator. |
GLP inhibits the migration of LNCaP
cells via the PRMT6 signaling pathway
To determine whether GLP regulates the migration of
LNCaP cells via the PRMT6 signaling pathway, cells were transfected
with either a PRMT6 overexpression plasmid or PRMT6 siRNA, and were
subsequently treated with 5 or 20 µg/ml GLP. The results
demonstrated that overexpression of PRMT6 increased the expression
of PRMT6, CDK2, FAK and FRC, and decreased p21 expression, compared
with the vector-transfected control. By contrast, PRMT6 knockdown
decreased PRMT6, CDK2, FAK and FRC expression, and increased p21
expression compared with the vector-transfected control. The
results also demonstrated that GLP treatment inhibited PRMT6, CDK2,
FAK and FRC expression, and promoted p21 expression in cells
transfected with the PRMT6 overexpression plasmid, whereas PRMT6
knockdown by siRNA inhibited the effect of GLP on CDK2, p21, FAK
and FRC expression (Fig. 5). In
addition, Transwell results confirmed that GLP significantly
inhibited the migration of cells transfected the PRMT6
overexpression plasmid, and results for PRMT6 siRNA-transfected
cells indicated that PRMT6 siRNA prevented the effects of GLP on
cell migration (Fig. 6).
 | Figure 5.GLP inhibited the PRMT6 signaling
pathway in LNCaP cells. LNCaP cells were transfected with either a
PRMT6 overexpression plasmid or PRMT6 siRNA, and were subsequently
treated with 5 or 20 µg/ml GLP. The protein expression of PRMT6,
p21, CDK2, FAK and SRC was determined by western blot analysis in
cells transfected with (A) PRMT6 overexpression plasmid and (B)
PRMT6 siRNA. Reverse transcription-quantitative polymerase chain
reaction was used to investigate mRNA expression. PRMT6 mRNA
expression in cells transfected with (C) PRMT6 overexpression
plasmid and (D) PRMT6 siRNA. p21 mRNA expression in cells
transfected with (E) PRMT6 overexpression plasmid and (F) PRMT6
siRNA. CDK2 mRNA expression in cells transfected with (G) PRMT6
overexpression plasmid and (H) PRMT6 siRNA. FAK mRNA expression in
cells transfected with (I) PRMT6 overexpression plasmid and (J)
PRMT6 siRNA. SRC mRNA expression in cells transfected with (K)
PRMT6 overexpression plasmid and (L) PRMT6 siRNA. Data are
presented as the mean ± standard deviation. *P<0.05 vs. Ctrl.
#P<0.05 vs. 0 µg/ml GLP + over-PRMT6/si PRMT6. GLP,
Ganoderma lucidum polysaccharide; PRMT6, protein arginine
methyltransferase 6; siRNA, small interfering RNA; CDK2,
cyclin-dependent kinase 2; FAK, focal adhesion kinase; Ctrl,
control; SRC, steroid receptor coactivator. |
Discussion
In recent years, the morbidity and mortality
associated with prostate cancer has been increasing in China. The
present study demonstrated that GLP inhibited the cell growth, cell
cycle and cell migration, decreased PRMT6, CDK2, FAK and FRC
expression, and increased p21 expression in LNCaP cells.
Furthermore, the results indicated that GLP significantly inhibited
cell migration and altered CDK2, FAK, FRC and p21 expression in
cells transfected with a PRMT6 overexpression plasmid. By contrast,
PRMT6 knockdown by siRNA reduced the effect of GLP on cell
migration and CDK2, FAK, FRC and p21 expression.
Similar results were reported in a study by Ghafar
et al (25), which
demonstrated that GLP significantly inhibited the growth of
hematoma cells and eliminated regulatory T cell suppression of T
cell proliferation. Li et al (36) also reported that GLP decreased the
adhesion of PC-3 M human prostate carcinoma cells to umbilical cord
vascular endothelial cells. Furthermore, Liang et al
(23) demonstrated that GLP
exhibited potential antitumor activity by inhibiting migration and
inducing apoptosis in human colon cancer cells. In addition, a
previous study reported that Ganoderma atrum polysaccharide
ameliorated the antitumor effect of cyclophosphamide, which was
mediated via the induction of apoptosis and immune system
activation in sarcoma 180-bearing mice (37). Collectively, the results of the
current study indicated that GLP exhibited antitumor activity,
which may partially be mediated by inhibiting the growth and
migration of LNCaP cells.
PRMT6 expression is reported to be increased in
various types of tumor cells, and it may participate in cell cycle
regulation in tumor cells (38–41).
p21 is an important downstream gene of PRMT6 that is involved in
the development of numerous tumor types, including osteosarcoma
(42,43), liver cancer (44) and prostatic cancer (45–47).
In addition, p21 functions in the promotion G1 cell cycle arrest
(48,49). CDK2 has important roles in several
tumor types by modulating the migration and motility of cancer
cells (50,51). FAK and FRC are key markers of tumor
cell migration, which are closely associated with the development
of certain tumor types, including hepatocellular carcinoma
(52), breast cancer (53) and U87-MG glioma (54). In the current study, the results
demonstrated that overexpression of PRMT6 significantly increased
expression of PRMT6, CDK2, FAK and FRC, and decreased p21
expression. By contrast, PRMT6 knockdown significantly decreased
PRMT6, CDK2, FAK and FRC expression, and increased p21 expression
in LNCaP cells. A similar report indicated that PRMT6 knockdown
significantly increased the expression of p21 and induced cell
cycle arrest in breast cancer cells (13). In addition, Wang et al
(55) demonstrated that PRMT6
overexpression reduced cell cycle arrest at G1 phase and decreased
the intensity of p16-CDK4 association in A549 human lung
adenocarcinoma cells. A previous study demonstrated that PRMT6
overexpression significantly decreased the cell growth and colony
forming ability of MCF7 breast cancer cells compared with controls
(14). It has also been reported
that PRMT6 promoted the proliferation of U2OS human osteosarcoma
cells and inhibited cell senescence by suppressing p21 expression
(40,56). In conclusion, these results
indicate that PRMT6 knockdown may inhibit cell migration by
upregulating the expression of p21 and downregulating CDK2
expression in LNCaP cells.
However, it has been reported that GLP significantly
inhibited the proliferation of S180 tumor-bearing mice by
macrophage activation and improved immune system functions via the
toll-like receptor 4-mediated nuclear factor-κB signaling pathway
(57). The mitogen-activated
protein kinase (MAPK) signaling pathway was also reported to be
activated in GLP-induced RAW264.7 cells (58). In addition, a study by Liang et
al (23) demonstrated that GLP
inhibited migration and apoptosis by activating a
Fas/caspase-dependent signaling pathway in human colon cancer
cells. The results of the present study demonstrated that GLP
significantly inhibited the migration of LNCaP cells transfected
with a PRMT6 overexpression plasmid, whereas PRMT6 knockdown
reduced the effect of GLP on cell migration, indicating that GLP
may inhibit the migration of cells via the PRMT6 signaling pathway.
A similar result was reported by Wu et al (59), which demonstrated that GLP
inhibited the migration of MDA-MB-231 breast cancer cells,
primarily by activating the FAK-Src signaling pathway. In addition,
Yang et al (26) reported
that GLP induced cell cycle arrest and apoptosis by blocking the
extracellular signal-regulated kinase/MAPK pathway, and activating
p38 and c-Jun N-terminal kinase MAPK pathways in HL-60 acute
leukemia cells. WEES-G6, a triterpene-enriched extract from G.
lucidum, was reported to inhibit the growth of Huh-7 human
hepatoma cells, and activated JNK and p38 MAPK pathways in
hepatocellular carcinoma (60).
In conclusion, the results of the present study
indicated that GLP significantly inhibited the growth, cell cycle
and migration of LNCaP cells. In addition, GLP inhibited the
migration of cells transfected with a PRMT6 overexpression plasmid,
whereas PRMT6 knockdown reduced the effect of GLP on cell
migration, indicating that GLP may inhibit LNCaP cell migration via
the PRMT6 signaling pathway. Therefore, it is suggested that GLP
may act as a tumor suppressor have potential as a treatment for
prostate cancer. The results of the present study provide both the
preliminary theoretical and experimental basis for the
investigation of GLP as a therapeutic agent.
Acknowledgements
The present study was supported by the Headmaster
Foundation of Liaoning Medical University-Special Fund on
Construction of Clinical Medicine (grant no. XZJJ20140233), the
Science Technology Project in Liaoning Province (grant no.
20141173) and the National Natural Science Foundation of China
(grant no. 11171042).
References
|
1
|
Hashim D, Boffetta P, La Vecchia C, Rota
M, Bertuccio P, Malvezzi M and Negri E: The global decrease in
cancer mortality: Trends and disparities. Ann Oncol. 27:926–933.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stegeman S, Amankwah E, Klein K, O'Mara
TA, Kim D, Lin HY, Permuth-Wey J, Sellers TA, Srinivasan S, Eeles
R, et al: A large-scale analysis of genetic variants within
putative miRNA binding sites in prostate cancer. Cancer Discov.
5:368–379. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baade PD, Youlden DR, Cramb SM, Dunn J and
Gardiner RA: Epidemiology of prostate cancer in the Asia-Pacific
region. Prostate Int. 1:47–58. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Monn MF, Tatem AJ and Cheng L: Prevalence
and management of prostate cancer among East Asian men: Current
trends and future perspectives. Urol Oncol. 34(58): e1–e9.
2016.
|
|
5
|
Luo M, Li Y, Guo H, Lin S, Chen J, Ma Q,
Gu Y, Jiang Z and Gui Y: Protein Arginine Methyltransferase 6
Involved in Germ Cell Viability during Spermatogenesis and
Down-Regulated by the Androgen Receptor. Int J Mol Sci.
16:29467–29481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Andaloussi N, Valovka T, Toueille M,
Steinacher R, Focke F, Gehrig P, Covic M, Hassa PO, Schär P,
Hübscher U and Hottiger MO: Arginine methylation regulates DNA
polymerase beta. Mol Cell. 22:51–62. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ma WL, Wang L, Liu LX and Wang XL: Effect
of phosphorylation and methylation on the function of the p16INK4a
protein in non-small cell lung cancer A549 cells. Oncol Lett.
10:2277–2282. 2015.PubMed/NCBI
|
|
8
|
Meerzaman DM, Yan C, Chen QR, Edmonson MN,
Schaefer CF, Clifford RJ, Dunn BK, Dong L, Finney RP, Cultraro CM,
et al: Genome-wide transcriptional sequencing identifies novel
mutations in metabolic genes in human hepatocellular carcinoma.
Cancer Genomics Proteomics. 11:1–12. 2014.PubMed/NCBI
|
|
9
|
Dowhan DH, Harrison MJ, Eriksson NA,
Bailey P, Pearen MA, Fuller PJ, Funder JW, Simpson ER, Leedman PJ,
Tilley WD, et al: Protein arginine methyltransferase 6-dependent
gene expression and splicing: Association with breast cancer
outcomes. Endocr Relat Cancer. 19:509–526. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vieira FQ, Costa-Pinheiro P,
Ramalho-Carvalho J, Pereira A, Menezes FD, Antunes L, Carneiro I,
Oliveira J, Henrique R and Jerónimo C: Deregulated expression of
selected histone methylases and demethylases in prostate carcinoma.
Endocr Relat Cancer. 21:51–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kleinschmidt MA, de Graaf P, van Teeffelen
HA and Timmers HT: Cell cycle regulation by the PRMT6 arginine
methyltransferase through repression of cyclin-dependent kinase
inhibitors. PLoS One. 7:e414462012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshimatsu M, Toyokawa G, Hayami S, Unoki
M, Tsunoda T, Field HI, Kelly JD, Neal DE, Maehara Y, Ponder BA, et
al: Dysregulation of PRMT1 and PRMT6, Type I arginine
methyltransferases, is involved in various types of human cancers.
Int J Cancer. 128:562–573. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phalke S, Mzoughi S, Bezzi M, Jennifer N,
Mok WC, Low DH, Thike AA, Kuznetsov VA, Tan PH, Voorhoeve PM and
Guccione E: p53-Independent regulation of p21Waf1/Cip1 expression
and senescence by PRMT6. Nucleic Acids Res. 40:9534–9542. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim NH, Kim SN, Seo DW, Han JW and Kim YK:
PRMT6 overexpression upregulates TSP-1 and downregulates MMPs: Its
implication in motility and invasion. Biochem Biophys Res Commun.
432:60–65. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gill BS, Navgeet and Kumar S: Ganoderma
lucidum targeting lung cancer signaling: A review. Tumour Biol.
39:10104283177074372017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Batra P, Sharma AK and Khajuria R: Probing
Lingzhi or Reishi medicinal mushroom Ganoderma lucidum
(higher Basidiomycetes): A bitter mushroom with amazing health
benefits. Int J Med Mushrooms. 15:127–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu C, Yang N, Song Y, Wang L, Zi J, Zhang
S, Dunkin D, Busse P, Weir D, Tversky J, et al: Ganoderic acid C1
isolated from the anti-asthma formula, ASHMI™ suppresses TNF-α
production by mouse macrophages and peripheral blood mononuclear
cells from asthma patients. Int Immunopharmacol. 27:224–231. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiu HF, Fu HY, Lu YY, Han YC, Shen YC,
Venkatakrishnan K, Golovinskaia O and Wang CK: Triterpenoids and
polysaccharide peptides-enriched Ganoderma lucidum: A
randomized, double-blind placebo-controlled crossover study of its
antioxidation and hepatoprotective efficacy in healthy volunteers.
Pharm Biol. 55:1041–1046. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Soccol CR, Bissoqui LY, Rodrigues C, Rubel
R, Sella SR, Leifa F, de Souza Vandenberghe LP and Soccol VT:
Pharmacological properties of biocompounds from spores of the
lingzhi or reishi medicinal mushroom ganoderma lucidum
(Agaricomycetes): A Review. Int J Med Mushrooms. 18:757–767. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gokce EC, Kahveci R, Atanur OM, Gürer B,
Aksoy N, Gokce A, Sargon MF, Cemil B, Erdogan B and Kahveci O:
Neuroprotective effects of Ganoderma lucidum polysaccharides
against traumatic spinal cord injury in rats. Injury. 46:2146–2155.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu YJ, Du JL, Cao LP, Jia R, Shen YJ,
Zhao CY, Xu P and Yin GJ: Anti-inflammatory and hepatoprotective
effects of Ganoderma lucidum polysaccharides on carbon
tetrachloride-induced hepatocyte damage in common carp (Cyprinus
carpio L.). Int Immunopharmacol. 25:112–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu Z, Chen X, Zhong Z, Chen L and Wang Y:
Ganoderma lucidum polysaccharides: Immunomodulation and
potential anti-tumor activities. Am J Chin Med. 39:15–27. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liang ZE, Yi YJ, Guo YT, Wang RC, Hu QL
and Xiong XY: Inhibition of migration and induction of apoptosis in
LoVo human colon cancer cells by polysaccharides from Ganoderma
lucidum. Mol Med Rep. 12:7629–7636. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liang Z, Guo YT, Yi YJ, Wang RC, Hu QL and
Xiong XY: Ganoderma lucidum polysaccharides target a
Fas/caspase dependent pathway to induce apoptosis in human colon
cancer cells. Asian Pac J Cancer Prev. 15:3981–3996. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li A, Shuai X, Jia Z, Li H, Liang X, Su D
and Guo W: Ganoderma lucidum polysaccharide extract inhibits
hepatocellular carcinoma growth by downregulating regulatory T
cells accumulation and function by inducing microRNA-125b. J Transl
Med. 13:1002015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang G, Yang L, Zhuang Y, Qian X and Shen
Y: Ganoderma lucidum polysaccharide exerts anti-tumor
activity via MAPK pathways in HL-60 acute leukemia cells. J Recept
Signal Transduct Res. 36:6–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chui CH, Wong RS, Cheng GY, Lau FY, Kok
SH, Cheng CH, Cheung F, Tang WK, Teo IT, Chan AS and Tang JC:
Antiproliferative ability of a combination regimen of crocodile egg
extract, wild radix ginseng and natural Ganoderma lucidum on
acute myelogenous leukemia. Oncol Rep. 16:1313–1316.
2006.PubMed/NCBI
|
|
28
|
Zhang Y: Ganoderma lucidum (Reishi)
suppresses proliferation and migration of breast cancer cells via
inhibiting Wnt/β-catenin signaling. Biochem Biophys Res Commun.
488:679–684. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Smina TP, Nitha B, Devasagayam TP and
Janardhanan KK: Ganoderma lucidum total triterpenes induce
apoptosis in MCF-7 cells and attenuate DMBA induced mammary and
skin carcinomas in experimental animals. Mutat Res. 813:45–51.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang JH, Ailati A and Mao J: Purification
and Structural Identification of a Bioactive Polysaccharide
Fraction from Ganoderma lucidum. Food Sci. 32:301–304.
2011.
|
|
31
|
He Y, Ye M, Du Z, Wang H, Wu Y and Yang L:
Purification, characterization and promoting effect on wound
healing of an exopolysaccharide from Lachnum YM405. Carbohydr
Polym. 105:169–176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shang D, Li Y, Wang C, Wang X, Yu Z and Fu
X: A novel polysaccharide from Se-enriched Ganoderma lucidum
induces apoptosis of human breast cancer cells. Oncol Rep.
25:267–272. 2011.PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
Relative Gene Expression Data Using RealTime Quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SY, Seo M, Kim Y, Lee YI, Oh JM, Cho
EA, Kang JS and Juhnn YS: Stimulatory heterotrimeric GTP-binding
protein inhibits hydrogen peroxide-induced apoptosis by repressing
BAK induction in SH-SY5Y human neuroblastoma cells. J Biol Chem.
283:1350–1361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jing H, Liaw L, Friesel R, Vary C, Hua S
and Yang X: Suppression of Spry4 enhances cancer stem cell
properties of human MDA-MB-231 breast carcinoma cells. Cancer Cell
Int. 16:192016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li YB, Wang R, Wu HL, Li YH, Zhong LJ, Yu
HM and Li XJ: Serum amyloid A mediates the inhibitory effect of
Ganoderma lucidum polysaccharides on tumor cell adhesion to
endothelial cells. Oncol Rep. 20:549–556. 2008.PubMed/NCBI
|
|
37
|
Li W, Nie S, Chen Y, Wang Y, Li C and Xie
M: Enhancement of cyclophosphamide-induced antitumor effect by a
novel polysaccharide from Ganoderma atrum in sarcoma
180-bearing mice. J Agric Food Chem. 59:3707–3716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim C, Lim Y, Yoo BC, Won NH, Kim S and
Kim G: Regulation of post-translational protein arginine
methylation during HeLa cell cycle. Biochim Biophys Acta.
1800:977–985. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stein C, Nötzold RR, Riedl S, Bouchard C
and Bauer UM: The arginine methyltransferase PRMT6 cooperates with
polycomb proteins in regulating HOXA Gene Expression. PLoS One.
11:e01488922016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stein C, Riedl S, Ruthnick D, Nötzold RR
and Bauer UM: The arginine methyltransferase PRMT6 regulates cell
proliferation and senescence through transcriptional repression of
tumor suppressor genes. Nucleic Acids Res. 40:9522–9533. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Neault M, Mallette FA, Vogel G,
Michaud-Levesque J and Richard S: Ablation of PRMT6 reveals a role
as a negative transcriptional regulator of the p53 tumor
suppressor. Nucleic Acids Res. 40:9513–9521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lv H, Gao G, Zhang L and Sun Y: Pololike
kinase 3 inhibits osteosarcoma cell proliferation and tumorigenesis
via cooperative interaction with p21. Mol Med Rep. 12:6789–6796.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wan D, Jiang C, Hua X, Wang T and Chai Y:
Cell cycle arrest and apoptosis induced by aspidin PB through the
p53/p21 and mitochondria-dependent pathways in human osteosarcoma
cells. Anticancer Drugs. 26:931–941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang J, Lei ZJ, Guo Y, Wang T, Qin ZY,
Xiao HL, Fan LL, Chen DF, Bian XW, Liu J and Wang B:
miRNA-regulated delivery of lincRNA-p21 suppresses β-catenin
signaling and tumorigenicity of colorectal cancer stem cells.
Oncotarget. 6:37852–37870. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Al-Azayzih A, Gao F and Somanath PR: P21
activated kinase-1 mediates transforming growth factor β1-induced
prostate cancer cell epithelial to mesenchymal transition. Biochim
Biophys Acta. 1853:1229–1239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Di Giacomo V, Di Valerio V, Rapino M,
Bosco D, Cacciatore I, Ciulla M, Marrazzo A, Fiorito J, Di Stefano
A and Cataldi A: MRJF4, a novel histone deacetylase inhibitor,
induces p21 mediated autophagy in PC3 prostate cancer cells. Cell
Mol Biol (Noisy-le-grand). 61:17–23. 2015.PubMed/NCBI
|
|
47
|
Isin M, Uysaler E, özgur E, Koseoglu H,
Sanli ö, Yucel öB, Gezer U and Dalay N: Exosomal lncRNA-p21 levels
may help to distinguish prostate cancer from benign disease. Front
Genet. 6:1682015.PubMed/NCBI
|
|
48
|
Kim SH, Hwang KA, Shim SM and Choi KC:
Growth and migration of LNCaP prostate cancer cells are promoted by
triclosan and benzophenone-1 via an androgen receptor signaling
pathway. Environ Toxicol Pharmacol. 39:568–576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin HP, Lin CY, Huo C, Hsiao PH, Su LC,
Jiang SS, Chan TM, Chang CH, Chen LT, Kung HJ, et al: Caffeic acid
phenethyl ester induced cell cycle arrest and growth inhibition in
androgen-independent prostate cancer cells via regulation of Skp2,
p53, p21Cip1 and p27Kip1. Oncotarget. 6:6684–6707. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wei K, Ye Z, Li Z, Dang Y, Chen X, Huang
N, Bao C, Gan T, Yang L and Chen G: An immunohistochemical study of
cyclin-dependent kinase 5 (CDK5) expression in non-small cell lung
cancer (NSCLC) and small cell lung cancer (SCLC): A possible
prognostic biomarker. World J Surg Oncol. 14:342016. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bisht S, Nolting J, Schutte U, Haarmann J,
Jain P, Shah D, Brossart P, Flaherty P and Feldmann G:
Cyclin-Dependent Kinase 5 (CDK5) controls melanoma cell motility,
invasiveness and metastatic spread-identification of a promising
novel therapeutic target. Transl Oncol. 8:295–307. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen JS, Li HS, Huang JQ, Dong SH, Huang
ZJ, Yi W, Zhan GF, Feng JT, Sun JC and Huang XH: MicroRNA-379-5p
inhibits tumor invasion and metastasis by targeting FAK/AKT
signaling in hepatocellular carcinoma. Cancer Lett. 375:73–83.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sanchez Castillo R, Gomez R and Salazar
Perez E: Bisphenol A Induces Migration through a GPER-, FAK-, Src-
and ERK2-Dependent Pathway in MDA-MB-231 Breast Cancer Cells. Chem
Res Toxicol. 29:285–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li S, Li C, Ryu HH, Lim SH, Jang WY and
Jung S: Bacitracin inhibits the migration of U87-MG glioma cells
via interferences of the integrin outside-in signaling pathway. J
Korean Neurosurg Soc. 59:106–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang X, Huang Y, Zhao J, Zhang Y, Lu J and
Huang B: Suppression of PRMT6-mediated arginine methylation of p16
protein potentiates its ability to arrest A549 cell proliferation.
Int J Biochem Cell Biol. 44:2333–2341. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Michaud-Levesque J and Richard S:
Thrombospondin-1 is a transcriptional repression target of PRMT6. J
Biol Chem. 284:21338–21346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang S, Nie S, Huang D, Huang J, Wang Y
and Xie M: Polysaccharide from Ganoderma atrum evokes
antitumor activity via Toll-like receptor 4-mediated NF-κB and
mitogen-activated protein kinase signaling pathways. J Agric Food
Chem. 61:3676–3682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yu Q, Nie SP, Wang JQ, Yin PF, Li WJ and
Xie MY: Polysaccharide from Ganoderma atrum induces tumor
necrosis factor-α secretion via phosphoinositide 3-kinase/Akt,
mitogen-activated protein kinase and nuclear factor-κB signaling
pathways in RAW264.7 cells. Int Immunopharmacol. 14:362–368. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wu GS, Song YL, Yin ZQ, Guo JJ, Wang SP,
Zhao WW, Chen XP, Zhang QW, Lu JJ and Wang YT: Ganoderiol
A-enriched extract suppresses migration and adhesion of MDA-MB-231
cells by inhibiting FAK-SRC-paxillin cascade pathway. PLoS One.
8:e766202013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin SB, Li CH, Lee SS and Kan LS:
Triterpene-enriched extracts from Ganoderma lucidum inhibit
growth of hepatoma cells via suppressing protein kinase C,
activating mitogen-activated protein kinases and G2-phase cell
cycle arrest. Life Sci. 72:2381–2390. 2003. View Article : Google Scholar : PubMed/NCBI
|