Introduction
Despite considerable advances in the pharmacotherapy
of epilepsy, ~1/3 of epilepsy patients refractory to antiepileptic
drugs (AEDs) do not become seizure-free (1). Therefore, seizures, which increase
the risk of death from epilepsy, still pose a major health problem.
Although many drugs have different mechanisms of action, a patient
who is resistant to one major AED is commonly refractory to other
AEDs as well. Unfortunately, the mechanisms of antiepileptic drug
resistance remain unclear. However, a recently identified
consequence of seizure activity that limits pharmacotherapy with
antiepileptic drugs is the overexpression of multidrug
transporters, such as P-glycoprotein (P-gp) and the multidrug
resistance-associated protein (MRP).
Almost all insults to the brain, including prolonged
seizures, result in reactive gliosis, which is characterized by
severe morphological and biochemical changes of activated
astrocytes (2). Astrocytes, the
most abundant glial cell type in the brain, provide metabolic
substrates and neurotropic factors for neurons, control
extracellular pH, potassium and glutamate levels, and participate
in the formation and preservation of the blood-brain barrier (BBB).
In addition, glial pathology is a universal feature of focal
epilepsy (3). However, gliosis,
where astrocytes can undergo morphological changes, become
hypertrophied and increase in number, is not simply a response to
neuronal degeneration. For instance, hypertrophy of astrocytes has
been observed during the process of epileptogenesis before the
development of seizures and in the absence of other pathological
changes.
Tumor necrosis factor (TNF)-α is known to stimulate
the proliferation of astrocytes; therefore, it may play a role in
reactive gliosis following brain injury (4). In addition, nuclear factor (NF)-κB
activity, which can be stimulated by TNF, has been confirmed in
glial cells in neuronal plasticity and neurodegenerative disorders
(5). Thus, several animal studies
have demonstrated the involvement of TNF-α and NF-κB in seizure
activity. One study indicates that inflammatory cytokines and
related genes are involved in spontaneously recurring seizures when
expressed in the hippocampus (6).
NF-κB is a transcription factor that regulates the expression of a
wide variety of genes involved in cellular events such as
inflammation, immune response, proliferation, apoptosis and
multidrug resistance (7–9). P-gp (ABCB1) and multidrug
resistance-associated protein 1 (Mrp1 or ABCC1) are two
ATP-dependent transmembrane glycoproteins involved in multidrug
resistance. Initially, these glycoproteins appeared to participate
in the multidrug resistance of tumor cells found in many tissues
(10–12). P-gp has been characterized in the
endothelial cells of brain capillaries, astrocyte foot processes
associated with capillaries (13),
and in primary cultures of rat astrocytes (14,15).
The expression of Mrp1 has been identified in parenchyma and
isolated capillaries in addition to primary rat astrocyte and
neuron cultures (16–18). Interestingly, the expression of
various transporters can influence the intracellular concentrations
of naturally occurring compounds and pharmacological agents in
astrocytes and microglia (16).
Currently, it is not clear whether the heightened
expression of P-gp, also known as Mdr1, in the endothelium and
parenchyma is a consequence of epilepsy, uncontrolled seizures and
chronic AED treatment, or if the constitutive expression is present
before the onset of epilepsy. Although a number of in vitro
studies separately report that seizures induce gliosis as well as
the expression of MDR or MRPs (3,19),
little is known abort the relationship between the two. The authors
hypothesized that reactive astrocytes may increase the expression
of P-gp and Mrp1 through TNF-α and NF-κB signaling based on a
series of observations. First, during the continued occurrence of
seizures astrocytes repeatedly undergo accrementition. Moreover,
hypertrophy of astrocytes during the process of epileptogenesis has
been observed before the development of seizures (20). Secondly, MDR1 and MRPs are
overexpressed in drug refractory epileptic tissue, and
MDR1-mediated drug secretion was increased in human reactive
astrocytes compared with ‘normal’ astrocytes (9,21).
Third, the expression of MDR and MRPs is increased in primary
cultures of rat astrocytes. Specifically, Gaillard et al
(22) demonstrated increased P-gp
expression in astrocytes in an in vitro model. Fourth, TNF-α
is a major cytokine released during seizures that stimulates
astrocyte proliferation and plays a role in reactive gliosis
following seizures (23). Rizzi
et al (24) demonstrated
that both glial cell activation and cytokine expression, which
included TNF-α, were increased in the hippocampus 4 and 24 h
following kainic acid-induced status epilepticus. In the central
nervous system, TNF-α is primarily produced by astrocytes and
serves a role in cell reaction, a pathogenic mechanism of epilepsy
(25). In addition, NF-κB, a
downstream target of TNF-α, can regulate the expression of MDR and
MRPs (26).
In the present study, the authors characterized the
association between activated astrocytes and the expression of
multidrug resistance genes. Cultured astrocytes were exposed to
TNF-α for differing periods of time to achieve degrees of
hyperplasia in reactive astrocytes. At each time point, the
expression of MDR or MRPs was characterized by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting assays. The authors demonstrated that TNF-α and
NF-κB may increase the expression of P-gp and Mrp1 in reactivate
astrocytes in a complex, time-dependent manner. Therefore, P-gp and
Mrp1 are novel targets of a TNF-α and NF-κB-dependent signaling
pathway in activated astrocytes.
Materials and methods
Preparation and treatment of primary
rat astrocyte cultures
Primary astrocytes were prepared from newborn
Sprague-Dawley rat cerebral cortices (supplied by the University
Laboratory Animal Services Centre, The Medical College of Xi'an
Jiaotong University, Xi'an, China) and cultured as described
previously (26). The brain was
obtained from neonatal rats under sterile conditions. Following
removal of the pia mater and vessels, the cortex was collected,
minced mechanically on a 250-µm nylon-mesh filter (Nippon Rikagaku
Kikai Co., Ltd., Tokyo, Japan), digested in 0.25% trypsin for 30
min at 37°C, incubated with complete medium (DMEM/F12) containing
20% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) to stop digestion, and then filtered and isolated
by centrifugation for 5 min at 4,000 × g at 4°C. Following
differential adherence for 60 min, the cell suspension was
transferred to a poly-L-lysine-coated 75 cm2 culture
flask. Cells at 2×105/cm2 were maintained at
37°C in a humid atmosphere with 5% CO2. The medium was
changed once every 3 days. When the cells reached 70–80% confluency
(~9–14 days), microglia and oligodendrocytes were removed from the
astroglial bed layer following an 18 h incubation on a swing bed
(960 × g) at 37°C. The adherent astrocytes were then removed with
0.25% trypsin + EDTA and replaced for experimental use. The
astrocyte purity of cultures prepared in this manner was >98% as
defined by staining with anti-glial fibrillary acidic protein
(GFAP) antibody (Santa Cruz Biotechnology, Inc., Dallas, TX,
USA).
MTT assay for cell viability
Following three passages, TNF-α was added to
astrocytes at a final concentration of 2 ng/ml and incubated for 2,
24 or 48 h. The degree of proliferation and cell viability
following TNF-α stimulation was assessed by colorimetic MTT assay.
Confluent cells were cultured in 96-well plates at 5,000 cells/well
and exposed to TNF-α for 2, 24, or 48 h. The media was discarded
and the cells were washed twice with PBS before serum-free media
was added back to each well. Following a 1 h incubation at 37°C,
MTT 0.5 g/l was added to each well and allowed to incubate for an
additional 2 h. The media was then aspirated from the plate, and
200 µl DMSO was added to dissolve any formazan crystals.
Proliferation and cell viability was determined based on the
measured optical density at 490 nm using a microplate reader.
RT-qPCR analysis
Total cellular RNA was isolated from treated cells
or control samples using TRIzol Reagent (Gibco; Thermo Fisher
Scientific, Inc.). Briefly, at the designated time, cells were
lysed in 1 ml TRIzol reagent. RNA was extracted from each sample by
adding 0.2 ml chloroform, mixing by inversion, and shaking
vigorously for 15 sec. The mixture was centrifuged, and the top
aqueous phase was collected. The RNA was then precipitated
overnight with 0.5 ml isopropanol at 20°C and isolated by
centrifugation 8,000 × g for 20 min at 4°C the following day. The
RNA pellet was washed, dried and dissolved in RNase-free water. The
RNA concentration was determined by spectrophotometry at 260 nm.
The purity was estimated by the ratio of A260/A280 nm, which ranged
from 1.7 to 2.0.
RT-qPCR analysis for Mdr1, Mrp1, NF-κB and β-actin
expression was performed according to a modified protocol (17), with specific primers for rat Mdr1
(sense primer, 5′-TCCAGCGGCAGAACAGCAAC-3′; antisense primer,
5′-GAGCAGCGTCATTGGCAAGC-3′; 231 bp), rat Mrp1 (sense primer,
5′-CAGCAGCACAGCAGCACAG-3′; antisense primer, 5-AGG CGA CGG GAG GCA
AAG-3′; 368 bp), rat NF-κB (sense primer, 5-CAC CAA AGA CCC ACC TCA
CC-3; antisense primer, 5′-CGCACTGTCACCTGGAAGC-3; 267 bp), and
control β-actin (sense primer, 5′-CTATCGGCAATGAGCGGTTCC-3′;
antisense primer, 5′-TGTGTTGGCATAGAGGTCTTTACG-3′; 146 bp). PCR
amplification of cDNA was achieved with one cycle at 95°C for 5
min, followed by 35 cycles at 94°C for 30 sec, 56°C for 30 sec,
72°C for 30 sec used by RevertAid™ kit (Fermentas;
Thermo Fisher Scientific, Inc.). The RT-qPCR products were
visualized by electrophoresis in 2% agarose gel containing 0.5
µg/ml ethidium bromide. Analysis of RT-qPCR products was conducted
by scanning densitometry with Bio-Rad ChemiDoc XRS Gel
Documentation system and quantified using Bio-Rad Quantity One 1-D
analysis software (Bio-Rad Laboratories, Inc.).
Western blot analysis
Sample preparation and western blotting was carried
out as previously described (27).
Protein samples were separated by SDS-PAGE, and the primary
antibodies used were anti-P-gp, anti-Mrp1, anti-Gfap (Santa Cruz
Biotechnology, Inc.). The membranes were blocked for 1 h at 4°C in
TBS [15 mM TrisHCl, 150 mM NaCl, (pH 7.6)] containing 0.05% (v/v)
Tween-20 (TBS-T) and 5% (m/v) dry skim milk powder. Following six
washes (5 min each) with TBS-T, membranes were incubated with P-gp
(cat. no. ABIN4904743), Mrp1 (cat. no. ABIN1584832; 1:250 in 5%
milk), or β-actin antibody (cat. no. ABIN1742508; 1:1,000 in 5%
milk) overnight at 4°C. Following a second wash, the membranes were
incubated for 2 h in the presence of anti-rabbit (cat. no. A0545;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) horseradish
peroxidase-conjugated secondary antibodies (1:1,000) in 5% milk at
room temperature. Protein bands were visualized using an enhanced
chemiluminescence (EMD Millipore, Billerica, MA, USA) western
blotting analysis system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) and analyzed by Image J software version 1.37 (Media
Cybernetics, Inc., National Institutes of Health Bethesda, MD,
USA).
Cell immunohistochemical staining
(IHC)
The treated cells were fixed with 4%
paraformaldehyde and permeabilized using 0.2% Triton X-100. Then
the fixed cells were incubated with P-gp, and Mrp1 primary antibody
followed by streptavidin peroxidase-conjugated secondary antibody
(SABC method) (28). Using
previously reported immunohistochemical techniques (28), the staining was visualized by using
diaminobenzidine and counterstained with hematoxylin, ten
independent high-magnification fields (magnification, ×400) were
evaluated for each section using a laser scanning confocal
microscope (TCS2 SP5; Leica Microsystems GmbH, Wetzlar,
Germany).
Statistical analysis
Data are expressed as the mean ± standard deviation
of three separate experiments. SPSS software, 16.0 (SPSS, Inc.,
Chicago, IL, USA) was used for analysis. Statistical analysis was
performed using an analysis of variance followed by the
Bonferroni/Dunn test. P<0.05 was considered to indicate a
statistically significant difference.
Results
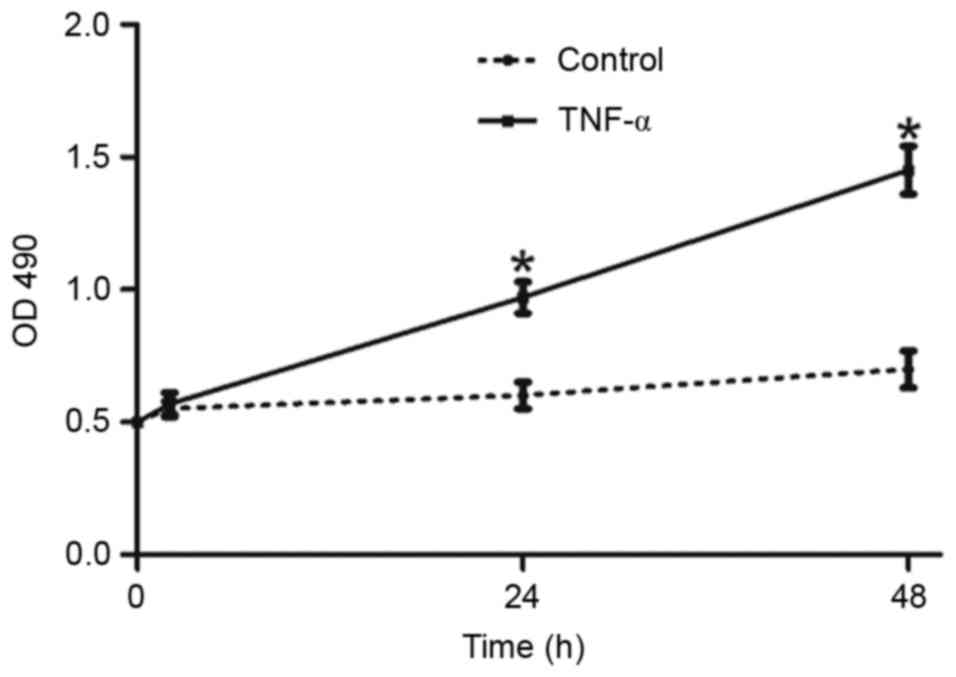
TNF-α induces proliferation and
viability in cultured astrocytes
The effect of TNF-α on proliferation and cell
viability is presented in Fig. 1.
Astrocytes were exposed to TNF for 0, 2, 24 or 48 h, the authors
demonstrated that TNF-α significantly increased the proliferation
and cell viability at each time point.
Reactivate astrocytes have increased
P-gp and Mrp1 mRNA expression
The level of P-gp mRNA increased over time in
primary astrocytes following TNF-α exposure (Fig. 2A). While P-gp mRNA expression was
detectable at each time point, the levels differed dramatically. At
2 h following TNF-α exposure, there was a sharp increase in P-gp
expression, which peaked around 24 h before it began to decline.
Compared with β-actin, the expression level was affected in a
time-dependent manner (P<0.05). The timing of induced P-gp
expression correlated with astrocyte proliferation, indicating that
the two events may be related.
In addition, the level of MRP mRNA in primary
astrocytes increased following exposure to TNF-α (Fig. 2B). Similar to P-gp, Mrp1 mRNA was
detectable at each time point. However, 2 h following stimulation,
Mrp1 expression decreased slightly. Expression then increased 24 h
following TNF-α exposure before dropping back to a steady state
level by 48 h. Again, the Mrp1 expression level changed in a
time-dependent manner (P<0.05) that correlated with astrocyte
proliferation. Therefore, Mrp1 expression and astrocyte
proliferation may be related.
Consistent with a previous study, the authors noted
that untreated astrocytes cultured in vitro expressed high
amounts of Mrp1 but little P-gp (28). Surprisingly, at the mRNA level, it
was reported that 2 h following TNF-α treatment had a greater
effect on P-gp expression than Mrp1. Specifically, the expression
of P-gp increased two-fold at this early time point, while there
was no change in Mrp1 expression. At 24 h, P-gp expression
increased seven-fold, whereas Mrp1 only increased three-fold. While
the expression of both genes began to decrease following 24 h, P-gp
expression was still three-fold higher at 48 h, but Mrp1 expression
had reduced back to steady state levels. Therefore, changes in P-gp
mRNA expression were more dramatic than Mrp1 changes in astrocytes
stimulated with TNF-α. This may be a characteristic controlling
some aspects of the multidrug resistance phenomenon.
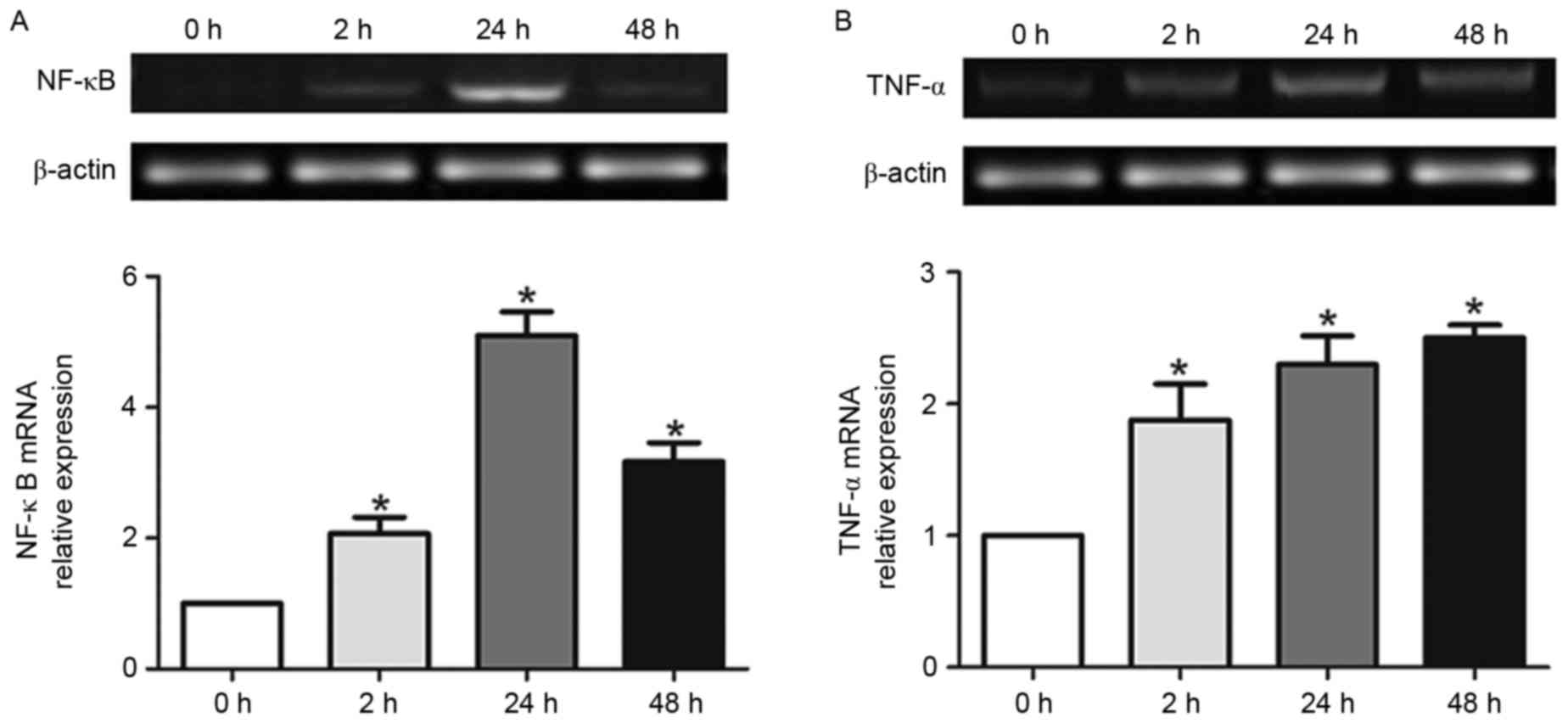
TNF-α induced P-gp and Mrp1 expression
may rely on NF-κB expression
The results above demonstrated that the expression
of P-gp and Mrp1 is induced in response to TNF-α stimulation;
however, other factors that regulate this response remained to be
determined. Therefore, the mRNA expression of candidate regulators
NF-κB and TNF-α were characterized in astrocytes following exposure
to TNF-α. There was a significant increase in NF-κB expression
following 2 h of TNF-α exposure (Fig.
3A). The expression of NF-κB continued to increase through 24 h
before tapering at ~48 h. These differences were statistically
significant (P<0.05), which indicated that reactive astrocytes
induced NF-κB expression in a time-dependent manner.
Interestingly, TNF-α exposure induces its own
expression in primary astrocytes (Fig.
3B). In contrast with P-gp, Mrp1, and NF-κB, the expression of
TNF-α was induced over time but did not diminish by 48 h. This may
be the result of a continuous feed-forward loop in which TNF-α
expression induces further TNF-α expression.
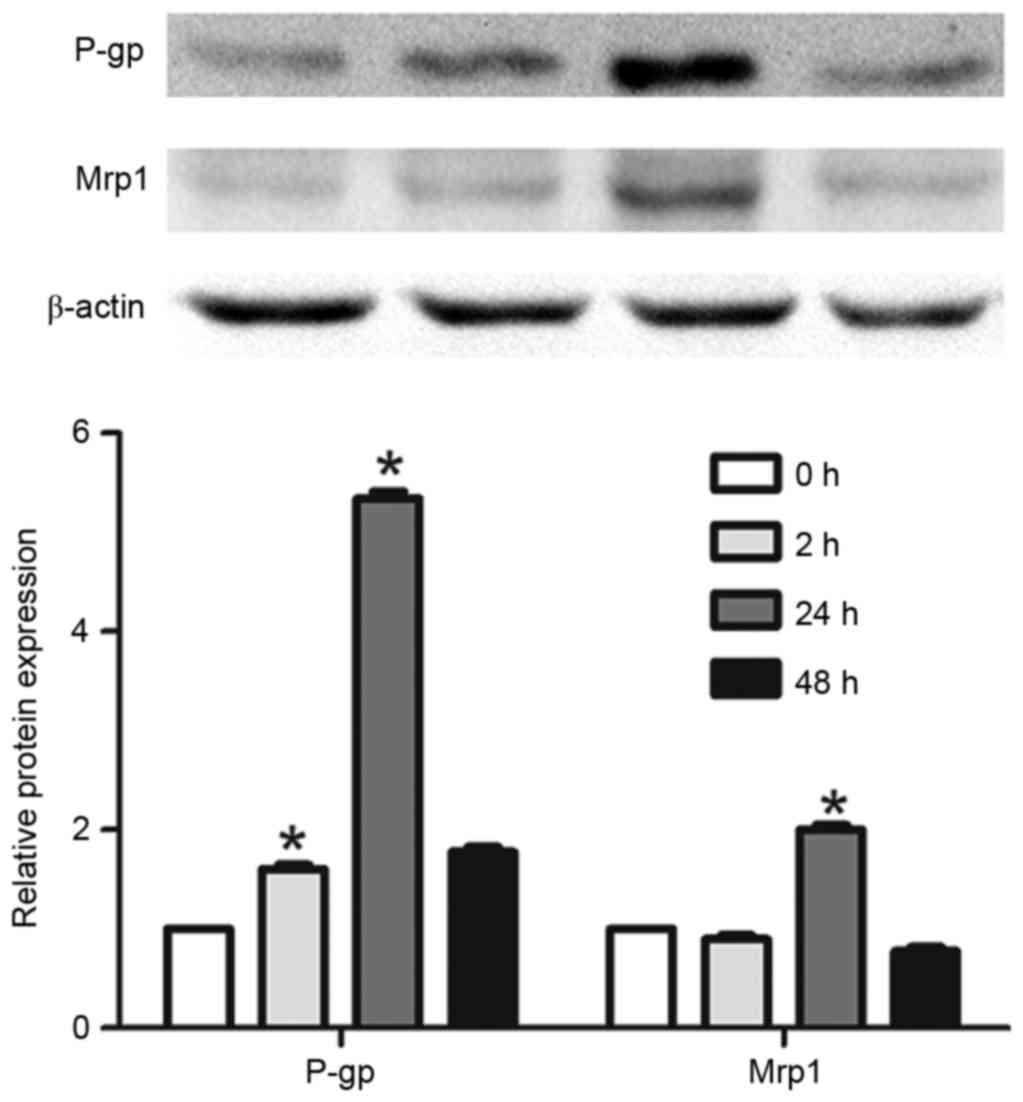
Verification of results by western
blot analysis
To confirm these results, the protein expression of
P-gp and Mrp1 was determined by western blotting. It was difficult
to detect P-gp protein expression in untreated astrocytes, but
expression increased following TNF-α exposure (Fig. 4). Mrp1 protein expression was also
difficult to detect regardless of TNF-α exposure. Although
expression was hard to detect, it was clear that the protein
expression of P-gp and Mrp1 increased and peaked following 24 h of
TNF-α exposure. Moreover, the authors performed IHC to confirm the
similar results with the western blot (Fig. 5). This was in accordance with the
trend of mRNA expression and astrocyte proliferation, which
indicated that reactive astrocytes may control proliferation and
multidrug resistance in a similar manner.
Discussion
The current study was undertaken to compare cell
proliferation with the expression of P-gp and Mrp1 in reactive
astrocytes. To the best of the authors' knowledge, the present
study provides the first evidence that TNF-α-induced reactive
astrocytes express P-gp and Mrp1, which may be dependent on induced
NF-κB expression.
There is growing evidence that astrocytes function
in many ways to support the function of the central nervous system.
They appear to be intimate partners of adjacent neurons, providing
them with nutrients and neurotropic factors as well as aiding in
signal transmission by ions and neurotransmitters (29). It is well known that astrocytes
undergo accrementition during the occurrence of continued seizures.
TNF-α is a major cytokine released during seizures, and a regulator
of reactive gliosis following seizures. Interestingly, a report by
Dvoriantchikova (30) demonstrated
that TNF-α initiated the activation in astrocytes. Furthermore, Cui
et al (31) demonstrated
that TNF-α increased astrocyte proliferation, which was confirmed
in the present study. From the results presented in Fig. 1, it may be concluded that TNF-α
induces reactive astrocytes to proliferate as soon as 2 h following
stimulation, and cell proliferation peaks by 24 h. However, the
proliferation effect subsided by 48 h post-treatment. It is
possible that long exposures to TNF-α caused the astrocytes secrete
nitric oxide and other factors related to apoptosis, which may
explain why the rate of proliferation reduced. In addition,
exposure to TNF-α increased cellular production of TNF-α, which may
result in a positive feed-forward loop.
Brain permeability of xenobiotics, including drugs,
is not only highly regulated at the brain barriers [i.e.,
blood-brain barrier (BBB) and blood-CSF barrier], but can also be
regulated by brain parenchymal cells. For instance, astroglia can
control the uptake of xenobiotics and, thus, the resulting cerebral
effects (32). Therefore, the
activity of membrane transporters in astroglia may be of
significance in the treatment of various clinical conditions,
including epilepsy. Several families of cell membrane proteins can
modulate drug transport in the CNS. In particular, members of the
ATP-binding cassette superfamily of transporters, such as P-gp and
MRPs, can serve a major role in the effect of drugs in the brain.
Although the functional expression and localization of these
transporters has been extensively examined at the brain barriers,
the expression of these transporters in parenchymal cells is poorly
defined. For example, the role of P-gp in astrocytes has not been
clearly elucidated; although, it has been hypothesized that P-gp
might limit CNS uptake of P-gp substrates due to expression in
astrocyte foot processes of the brain microvasculature. In addition
to astrocyte foot processes, P-gp is also expressed in astrocyte
processes next to the endothelial cells of brain vessels,
suggesting that the P-gp expression in astrocytes, rather than
endothelial cells, maintains the BBB-function (13). The localization of Mrp1 was
restricted to certain membrane areas in astrocytes, especially in
the foot-processes, which suggests a possible role in physiological
transport. The current data confirms a previous study demonstrating
that P-gp and Mrp1 mRNAs are expressed in primary cultures of rat
astrocytes (17). Furthermore, it
was confirmed that the expression of Mrp1 is higher than P-gp in
rat astrocytes under basal conditions (13,33).
Epileptic patient samples have increased P-gp
staining in capillary endothelium and astrocytes. In addition,
ten-fold increases in P-gp mRNA expression have been detected in
patients with medically intractable epilepsy (9). Recent findings have demonstrated that
TNF-α is produced both by microglia and astrocytes in adult rodent
brain in various neuropathological conditions, including seizures
(33). This cytokine, together
with other proinflammatory molecules, appears to contribute to the
mechanisms mediating excitotoxic and/or apoptotic neuronal cell
death (34). However, astrocytes
undergo accrementition during continued seizures, and the authors
observed cellular proliferation in vitro, rather than
apoptosis. In addition, the expression of both P-gp and Mrp1
correlated with the status of astrocyte cell proliferation and
activation (Figs. 2 and 3). In some pathological conditions, such
as epilepsy, astrocytes are reactive and release a large amount of
cytokines. Therefore, ‘second-line defense’ mechanisms in the
perivascular glia might be induced and be responsible for poor
responsiveness to medication (35,36).
In addition, the change in P-gp expression in activated astrocytes
was much larger than Mrp1. Under common conditions, the authors
hypothesize that Mrp1 serves a major physiological transport
function. Under pathological conditions, activated astrocytes
proliferate and MDR expression is induced, which may be a principal
factor in multidrug resistance.
P-gp and Mrp1 expression are induced in many
circumstances, such as in tumor tissues, ischemia and epileptic
attack. TNF-α and other cytokines are also produced in many of
those circumstances. Many in vivo experiments have
emphasized that TNF-α exposure induces P-gp and Mrp1 expressions.
In addition, several animal studies have demonstrated a
relationship between TNF-α and NF-κB in seizure activity (6,10).
NF-κB is a target of TNF-α signaling (37), its activation induced P-gp
expression in kidney proximal tubule cells (38). As presented in Fig. 3A, TNF-α induced a significant
increase of NF-κB expression in astrocytes as early as 2 h
following exposure, which may explain a mechanism by which
downstream genes (MDR, Mrp1) are expressed. Notably, TNF-α can
induce both anti-apoptotic and pro-apoptotic pathways. Activation
of the NF-κB pathway results in activation of genes that mediate
anti-apoptotic or protective functions. It is possible that
induction of P-gp protects the cell against apoptosis. In support
of this hypothesis, a direct association between P-gp
overexpression and apoptosis was established by Johnstone, Cretney
and Smyth (39), who observed that
a T-cell leukemia cell line transduced with a retroviral construct
containing human MDR1 was more resistant to TNF-α-induced apoptosis
than control cells (39).
Therefore, these possibilities should be addressed in future
experiments.
In conclusion, to investigate the association
between proliferation and P-gp or Mrp1 expression in reactive
astrocytes, the authors stimulated rat astrocytes with TNF-α for 2,
24 or 48 h to activate them. The expression of P-gp or Mrp1 was
observed using RT-qPCR or western blotting, and identified that
reactivate astrocytes increased the expression of P-gp and Mrp1,
possibly through TNF-α and NF-κB signaling. Although activated
astrocytes expressed P-gp and Mrp1, the alteration in MDR
expression was much larger than Mrp1. The authors suggested that,
under normal conditions, Mrp1 serves a major physiological
transport function in astrocytes. However, under pathological
conditions, P-gp expression may be a principal agent in multidrug
resistance phenomenon. These are the first findings to confirm that
activated astrocyte can induce P-gp and Mrp1 expression in
vitro. Furthermore, NF-κB may be the TNF-α target that
regulates this phenomenon. The current findings offer novel
insights into the cellular mechanisms that underlie of refractory
epilepsy and suggest that blocking cytokine signaling may reverse
multidrug resistance.
References
|
1
|
Löscher W and Schmidt D: New horizons in
the development of antiepileptic drugs: The search for new targets.
Epilepsy Res. 60:77–159. 2004.PubMed/NCBI
|
|
2
|
Ravizza T, Gagliardi B, Noé F, Boer K,
Aronica E and Vezzani A: Innate and adaptive immunity during
epileptogenesis and spontaneous seizures: Evidence from
experimental models and human temporal lobe epilepsy. Neurobiol
Dis. 29:142–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khurgel M and Ivy GO: Astrocytes in
kindling: Relevance to epileptogenesis. Epilepsy Res. 26:163–175.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Balasingam V, Tejada-Berges T, Wright E,
Bouckova R and Yong VW: Reactive astrogliosis in the neonatal mouse
brain and its modulation by cytokines. J Neurosci. 14:846–856.
1994.PubMed/NCBI
|
|
5
|
Mattson MP and Camandola S: NF-kappaB in
neuronal plasticity and neurodegenerative disorders. J Clin Invest.
107:247–254. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Simoni MG, Perego C, Ravizza T, Moneta
D, Conti M, Marchesi F, De Luigi A, Garattini S and Vezzani A:
Inflammatory cytokines and related genes are induced in the rat
hippocampus by limbic status epilepticus. Eur J Neurosci.
12:2623–2633. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen F, Castranova V and Shi X: New
insights into the role of nuclear factor-kappaB in cell growth
regulation. Am J Pathol. 159:387–397. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jobin C and Sartor RB: The I kappa
B/NF-kappa B system: A key determinant of mucosalinflammation and
protection. Am J Physiol Cell Physiol. 278:C451–C462.
2000.PubMed/NCBI
|
|
9
|
Sisodiya SM, Lin WR, Harding BN, Squier MV
and Thom M: Drug resistance in epilepsy: Expression of drug
resistance proteins in common causes of refractory epilepsy. Brain.
125:22–31. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cole SP, Bhardwaj G, Gerlach JH, Mackie
JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM and
Deeley RG: Overexpression of a transporter gene in a
multidrug-resistant human lung cancer cell line. Science.
258:1650–1654. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Juliano RL and Ling V: A surface
glycoprotein modulating drug permeability in Chinese hamster ovary
cell mutants. Biochim Biophys Acta. 455:152–162. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Callaghan R, Crowley E, Potter S and Kerr
ID: P-glycoprotein: So many ways to turn it on. J Clin Pharmacol.
48:365–378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Golden PL and Pardridge WM: P-Glycoprotein
on astrocyte foot processes of unfixed isolated human brain
capillaries. Brain Res. 819:143–146. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Declèves X, Regina A, Laplanche JL, Roux
F, Boval B, Launay JM and Scherrmann JM: Functional expression of
P-glycoprotein and multidrug resistance-associated protein (Mrp1)
in primary cultures of rat astrocytes. J Neurosci Res. 60:594–601.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pardridge WM, Golden PL, Kang YS and
Bickel U: Brain microvascular and astrocyte localization of
P-glycoprotein. J Neurochem. 68:1278–1285. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mercier C, Declèves X, Masseguin C,
Fragner P, Tardy M, Roux F, Gabrion J and Scherrmann JM:
P-glycoprotein (ABCB1) but not multidrug resistance-associated
protein 1 (ABCC1) is induced by doxorubicin in primary cultures of
rat astrocytes. J Neurochem. 87:820–830. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Regina A, Koman A, Piciotti M, El Hafny B,
Center MS, Bergmann R, Couraud PO and Roux F: Mrp1 multidrug
resistance-associated protein and P-glycoprotein expression in rat
brain microvessel endothelial cells. J Neurochem. 71:705–715. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hirrlinger J, König J and Dringen R:
Expression of mRNAs of multidrug resistance proteins (Mrps) in
cultured rat astrocytes, oligodendrocytes, microglial cells and
neurones. J Neurochem. 82:716–719. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brandt C, Bethmann K, Gastens AM and
Löscher W: The multidrug transporter hypothesis of drug resistance
in epilepsy: Proof-of-principle in a rat model of temporal lobe
epilepsy. Neurobiol Dis. 24:202–211. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wetherington J, Serrano G and Dingledine
R: Astrocytes in the epileptic brain. Neuron. 58:168–178. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marchi N, Hallene KL, Kight KM, Cucullo L,
Moddel G, Bingaman W, Dini G, Vezzani A and Janigro D: Significance
of MDR1 and multiple drug resistance in refractory human epileptic
brain. BMC Med. 2:372004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaillard PJ, van der Sandt IC, Voorwinden
LH, Vu D, Nielsen JL, de Boer AG and Breimer DD: Astrocytes
increase the functional expression of P-glycoprotein in an in vitro
model of the blood-brain barrier. Pharm Res. 17:1198–1205. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
de Bock F, Dornand J and Rondouin G:
Release of TNF alpha in the rat hippocampus following epileptic
seizures and excitotoxic neuronal damage. Neuroreport. 7:1125–1129.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Rizzi M, Perego C, Aliprandi M, Richichi
C, Ravizza T, Colella D, Velískŏvá J, Moshé SL, De Simoni MG and
Vezzani A: Glia activation and cytokine increase in rat hippocampus
by kainic acid-induced status epilepticus during postnatal
development. Neurobiol Dis. 14:494–503. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vezzani A, Moneta D, Richichi C, Aliprandi
M, Burrows SJ, Ravizza T, Perego C and De Simoni MG: Functional
role of inflammatory cytokines and antiinflammatory molecules in
seizures and epileptogenesis. Epilepsia. 43 Suppl 5:S30–S35. 2002.
View Article : Google Scholar
|
|
26
|
Bharti AC and Aggarwal BB: Nuclear
factor-kappa B and cancer: Its role in prevention and therapy.
Biochem Pharmacol. 64:883–888. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarkar D, Lebedeva IV, Emdad L, Kang DC,
Baldwin AS Jr and Fisher PB: Human polynucleotide phosphorylase
(hPNPaseold-35): A potential link between aging and inflammation.
Cancer Res. 64:7473–7478. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Spiegl-Kreinecker S, Buchroithner J,
Elbling L, Steiner E, Wurm G, Bodenteich A, Fischer J, Micksche M
and Berger W: Expression and functional activity of the
ABC-transporter proteins P-glycoprotein and multidrug-resistance
protein 1 in human brain tumor cells and astrocytes. J Neurooncol.
57:27–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Travis J: Glia: The brain's other cells.
Science. 266:970–972. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dvoriantchikova G and Ivanov D: Tumor
necrosis factor-alpha mediates activation of NF-κB and JNK
signaling cascades in retinal ganglion cells and astrocytes in
opposite ways. Eur J Neurosci. 40:3171–3178. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui M, Huang Y, Tian C, Zhao Y and Zheng
J: FOXO3a inhibits TNF-α- and IL-1β-induced astrocyte
proliferation: Implication for reactive astrogliosis. Glia.
59:641–654. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Faijerson J, Tinsley RB, Apricó K,
Thorsell A, Nodin C, Nilsson M, Blomstrand F and Eriksson PS:
Reactive astrogliosis induces astrocytic differentiation of adult
neural stem/progenitor cells in vitro. J Neurosci Res.
84:1415–1424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Golden PL and Pardridge WM: Brain
microvascular P-glycoprotein and a revised model of multidrug
resistance in brain. Cell Mol Neurobiol. 20:165–181. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Allan SM and Rothwell NJ: Cytokines and
acute neurodegeneration. Nat Rev Neurosci. 2:734–744. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abbott NJ, Khan EU, Rollinson CM, Reichel
A, Janigro D, Dombrowski SM, Dobbie MS and Begley DJ: Drug
resistance in epilepsy: The role of the blood-brain barrier.
Novartis Found Symp. 243(38–53): 180–185. 2002.
|
|
36
|
Löscher W and Potschka H: Role of
multidrug transporters in pharmacoresistance to antiepileptic
drugs. J Pharmacol Exp Ther. 301:7–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fernandes A, Barateiro A, Falcão AS, Silva
SL, Vaz AR, Brito MA, Silva RF and Brites D: Astrocyte reactivity
to unconjugated bilirubin requires TNF-α and IL-1β receptor
signaling pathways. Glia. 59:14–25. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Thévenod F, Friedmann JM, Katsen AD and
Hauser IA: Up-regulation of multidrug resistance P-glycoprotein via
nuclear factor-kappaB activation protects kidney proximal tubule
cells from cadmium- and reactive oxygen species-induced apoptosis.
J Biol Chem. 275:1887–1896. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Johnstone RW, Cretney E and Smyth MJ:
P-glycoprotein protects leukemia cells against caspase-dependent,
but not caspase-independent, cell death. Blood. 93:1075–1085.
1999.PubMed/NCBI
|