Introduction
Previous research has indicated that intervertebral
disc degeneration (IVDD) is associated with biological alterations
of the intervertebral disc substrate (1). Research has also determined that a
large number of inflammatory substances and enzyme systems exist in
degenerated intervertebral disc tissues, primarily consisting of
three types: Cytokines, inflammatory medium and protease, as well
as its inhibitors (2). The
involvement of such materials may lead to relevant inflammatory
responses and cause damage of intervertebral disc substrate. There
are numerous types of cytokines (CKs), among which, tumor necrosis
factor (TNF), interleukin (IL)-1 and IL-6 are the most important
factors associated with IVDD, and are also the major targets of
research (3). TNF-α, IL-1β and
IL-6 may affect the normal metabolism of intervertebral disc
substrate, which causes internal environment disorder of the
intervertebral disc, metabolite accumulation, increased cell
apoptosis, inflammatory reaction aggravation, increase of capillary
permeability, and intervertebral disc nutritional disorder, which
are closely associated with IVDD (4).
Any damage to the intervertebral disc, even minor
damage, will cause oxidative stress reaction (5). Nucleus pulposus is the first one
affected, which generates a large number of oxygen free radicals
(6). Under normal physiological
status, the generation and removal of free radicals occurs under a
dynamic balancing state (7).
External factors, such as multiple-level spinal fracture
accompanied by disc intervertebral injuries, may lead to a decrease
in the capacity of free radical generation and scavenging (6). The body will be subject to oxidative
stress, which leads to free radical accumulation, a rise in body
peroxidation levels, cytotoxicity generation and body injury
(8).
As age increases, the intervertebral disc exhibits
different degrees of aging and degeneration. IVDD mainly presents
as reduced numbers of intervertebral disc cells, hypofunction,
dehydration of polysaccharide, a decrease in the aggregation of
proteoglycans, collagen type and distribution changes,
intervertebral disc tension, and pressure weakening or loosing
(9,10). The histological alterations
eventually lead to changes of intervertebral disc biomechanics
(11). It has been observed that
decreasing of intervertebral disc active cells, decreasing of
extracellular matrix components and composition change are the
pathological bases for IVDD (11).
Excessive apoptosis of intervertebral disc cells is the direct
cause for intervertebral disc cells decreasing (11).
With the local inflammatory vascular response
stimulated by the fibrous ring damage, the cells at the
inflammatory site generate growth factors, which work on
intervertebral disc cells isolated by the circulatory system.
Through signal transduction, the differentiation and proliferation
of intervertebral disc cells and a large amount of extracellular
matrix synthesis are promoted (12). It may be the major reason for
intervertebral disc fibrosis and degeneration. The majority of
research on inflammatory reactions after disc intervertebral
injuries focus on the interaction between cytokines (13). However, there are limited studies
on the autoimmune response mechanism arising from intervertebral
disc tissue. The association between inflammation reactions and
immune mechanisms remains to be identified (14). The two may be under a
cause-and-effect association, as well as mutual promotion (14). At present, further studies on
signal transduction mechanisms underlying inflammation and
immunology are undergoing, with the purpose of detecting the
specific antigen proteins of nucleus pulposus tissue, to determine
the immune foundation for inflammatory cytokines or enzyme reaction
during IVDD, and to explore the signal transduction rules of immune
response (15). The research is of
great significance for studying the immunologic mechanism of
fibrous ring of intervertebral disk inflammatory reactions at the
molecular level. It can provide a theoretical basis for IVDD
prevention (14).
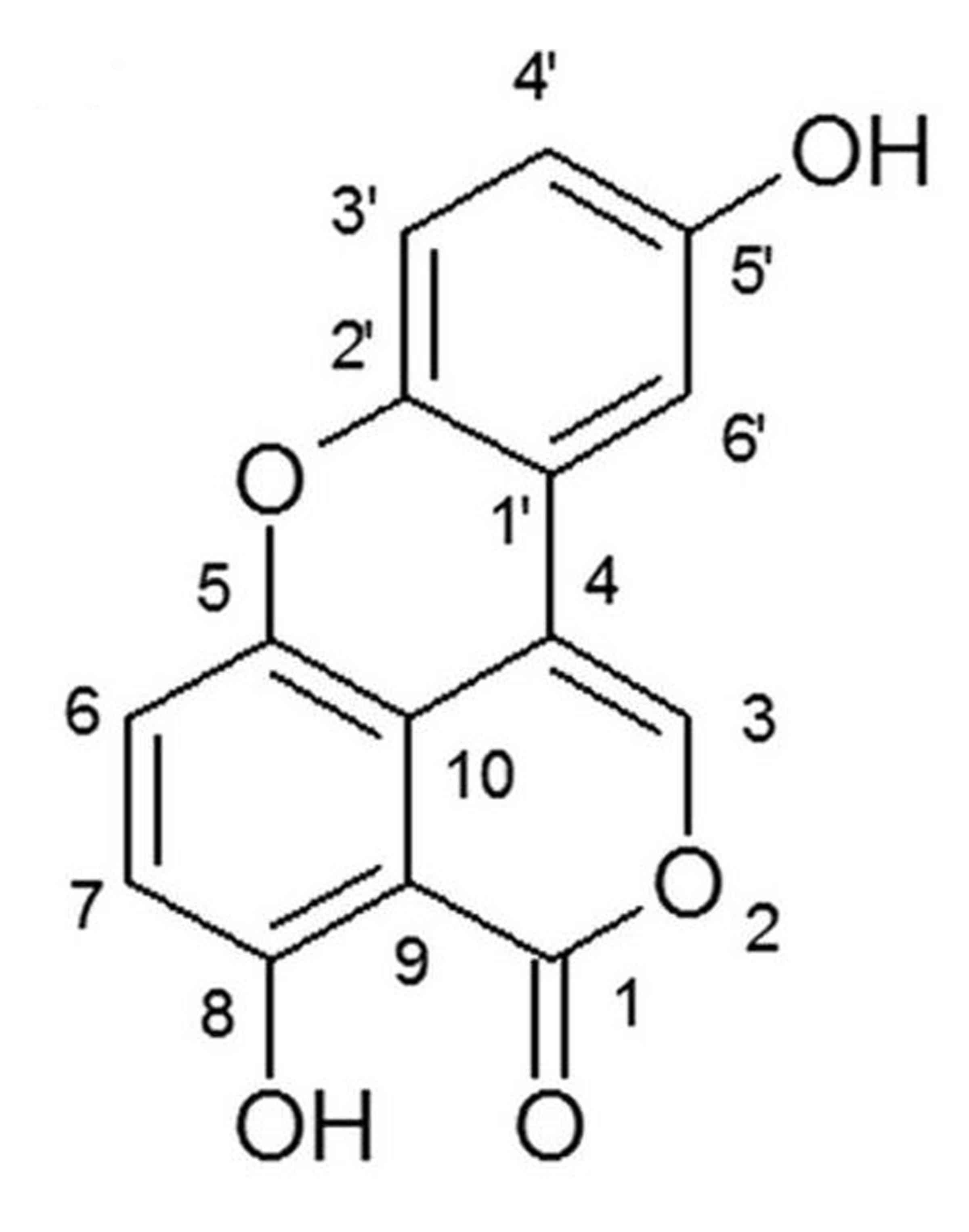
Sparstolonin B is an isocoumarin compound (Fig. 1) and is extracted from the tubers
of both Sparganium stoloniferum and Scirpus yagara
(15). Sparstolonin B is a novel
toll like receptor 4 (TLR4) antagonist derived from the traditional
Chinese medicine ‘SanLeng’ for the treatment of several
inflammatory diseases (16). The
present study evaluated the effect of Sparstolonin B in preventing
IVDD, and investigated the potential underlying mechanisms in
rats.
Materials and methods
Experimental design
Male Sprague-Dawley rats (age, 8–10 weeks; weight,
200–230 g; n=40) were recruited for this study, and were housed at
22–23°C and 55–60% humidity, 12-h light/dark cycle, free access to
food and water. Rats were anesthetized with 1% sodium pentobarbital
solution (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
All animals underwent a midline ventral longitudinal
incision to expose the L5/6 intervertebral disc. In experimental
rats, ~10 µg Fluoro-Gold neurotracer crystals (Fluorochrome, LLC,
Denver, CO, USA) were applied to the surface of the L5/6
intervertebral disc to label the dorsal root ganglion neurons
innervating the discs. Rats were randomly divided into four groups
(n=10 per group): Sham operation (Sham), IVDD model (model), 100
mg/kg Sparstolonin B (100-Spa B) and 200 mg/kg Sparstolonin B
(200-Spa B). The 100-Spa B and 200-Spa B group rats were
administered 100 mg/kg/once every three days or 200 mg/kg/once
every three days Sparstolonin B (Sigma-Aldrich; Merck KGaA.) for 4
weeks by gavage.
The present study was approved by the ethics
committee of West China Hospital (Chengdu, China).
Histological evaluation
After Sparstolonin B treatment, the rats were
sacrificed using <35 mg/kg pentobarbital sodium (Sigma-Aldrich;
Merck KGaA), and intervertebral discs or spinal motion segments
were harvested as described previously (17). L5-L6 segments were fixed with 10%
paraformaldehyde solution for 3–5 days and then fixed with
paraformaldehyde solution for 5 days at room temperature. Sections
were serial dewaxed, stained with haematoxylin at room temperature
for 15 min and rinsed with distilled water. The sections were
observed using a Digital Image Analyzer (Ni-E; Nikon Corporation,
Tokyo, Japan).
Evaluation of endplate
degeneration
L1/2 intervertebral discs were scanned using a
Siemens Micro-CT scanning system. Superior endplates were
re-established, and volume ratios of marrow contact channels in the
endplate and the condition of endplate nutritional supply were
evaluated to indicate the state of the endplate.
Determination of biological
factors
The T12/L1 and L1/2 intervertebral discs were
immediately lysed using lysis buffer (Cell Signaling Technology,
Inc., Danvers, MA, USA) on ice for 30–60 min and the protein
concentration was quantified by an Enhanced Bicinchoninic Acid
(BCA) Protein Assay kit (Beyotime Institute of Biotechnology,
Haimen, China). Total proteins (5 µg) were used to measure TNF-α
(cat. no. PT516), IL-1β (cat. no. PI303), IL-6 (cat. no. PI328),
malondialdehyde (cat. no. S0131), and superoxide dismutase (SOD;
cat. no. S0101) using ELISA assay kits (Beyotime Institute of
Biotechnology) at 450 nm. Total proteins (5 µg) were used to
measure caspase-3/9 activities using caspase-3/9 activities kits
(cat. no. C115; Beyotime Institute of Biotechnology) at 405 nm.
Western blot analysis
The T12/L1 and L1/2 intervertebral discs were
immediately lysed using lysis buffer on ice for 30–60 min and the
protein concentration was determined using an Enhanced BCA Protein
Assay kit. Total proteins (50 µg) were separated by 8–10% SDS-PAGE
at 100 V for 1.5 h and then transferred to nitrocellulose membranes
(EMD Millipore, Billerica, MA, USA). The membranes were blocked for
1 h with 5% non-fat dry milk at room temperature and incubated with
primary antibodies against TLR4 (cat. no. 14358; 1:2,000), myeloid
differentiation primary response protein 88 (MyD88; cat. no. 4283;
1:2,000), nuclear factor (NF)-κB (cat. no. 8242; 1:2,000), NAPDH
oxidase 2 (NOX2), phosphoinositide 3-kinase (PI3K; cat. no. 4249;
1:2,000), phosphorylated-protein kinase B (Akt; cat. no. 9614;
1:2,000) and GAPDH (cat. no. 5174; 1:5,000; all from Cell Signaling
Technology, Inc.) overnight at 4°C. After washing with PBS with
0.1% Tween 20, the membranes were incubated with anti-rabbit
horseradish peroxidase-conjugated secondary antibodies (cat. no.
7074; 1:5,000; Cell Signaling Technology, Inc.) for 1 h at room
temperature. Proteins were detected with an Enhanced
Chemiluminescence kit (Pierce; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and quantified by Image J version 3.0 software
(National Institutes of Health, Bethesda, MA, USA).
Statistical analysis
Data are expressed as the mean ± standard error
using SPSS version 19.0 software (IBM Corp., Armonk, NY, USA).
One-way analysis of variance by Tukey's post hoc test was used for
multiple group comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Sparstolonin B effects on IVDD
The present study used an IVDD model in vivo
to determine the histological score of disc degeneration and
endplate porosity of L2 superior endplates in lumbar IVDD after
treatment with Sparstolonin B. As presented in Fig. 2A, a significant increase of
histological score of disc degeneration was observed in the IVDD
model group compared with the sham group. Meanwhile, the inhibition
of endplate porosity of L2 superior endplates in lumbar IVDD was
markedly observed compared with the sham group (Fig. 2B). Treatment with Sparstolonin B
(100 and 200 mg/kg) significantly reversed these changes in IVDD
rats, compared with the IVDD model group (Fig. 2A and B).
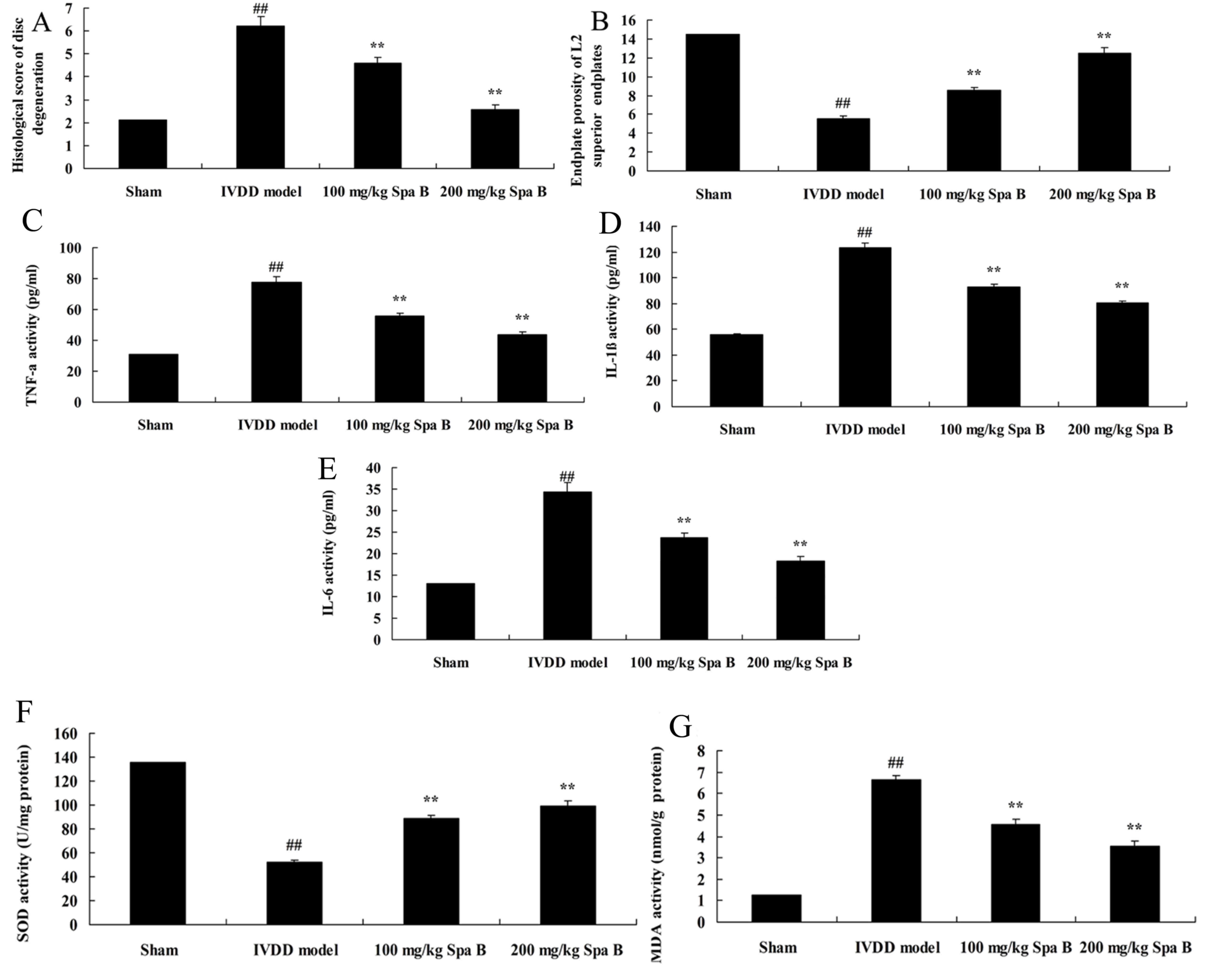 | Figure 2.Sparstolonin B effects on disc
degeneration, endplate porosity of L2 superior endplates,
inflammation and oxidative stress in IVDD. (A) Histological score
of disc degeneration. (B) Increased endplate porosity of L2
superior endplates. (C) TNF-α, (D) IL-1β, (E) IL-6, (F) SOD and (G)
MDA activities levels in an IVDD model. Data are presented as the
mean ± standard error. ##P<0.01 vs. sham group;
**P<0.01 vs. IVDD model group. Spa B, Sparstolonin B; IVDD,
intervertebral disc degeneration; IL, interleukin; SOD, superoxide
dismutase; TNF-α, tumor necrosis factor-α; MDA,
malondialdehyde. |
Sparstolonin B effects on inflammation
in IVDD
To investigate the protective effect of Sparstolonin
B on inflammation in IVDD, TNF-α, IL-1β and IL-6 levels were
measured by ELISA. There were significant increases of TNF-α
(Fig. 2C), IL-1β (Fig. 2D) and IL-6 (Fig. 2E) levels in the IVDD model group,
compared with the sham group. However, treatment with Sparstolonin
B (100 and 200 mg/kg) significantly reduced TNF-α, IL-1β and IL-6
content levels in IVDD rats, compared with IVDD model rats
(Fig. 2C-E).
Sparstolonin B effects on oxidative
stress in IVDD
To clarify the protective effect of Sparstolonin B
on oxidative stress in IVDD, MDA and SOD content levels were
measured by ELISA. Inhibition of SOD content (Fig. 2F) and induction of MDA content
(Fig. 2G) were markedly observed
compared with the sham group. Sparstolonin B treatment (100 and 200
mg/kg) significantly reversed the inhibition of SOD content and
induction of MDA content in IVDD rats, compared with the IVDD model
(Fig. 2F and G).
Sparstolonin B effects on caspase-3/9
activities in IVDD
To determine the protective effect of Sparstolonin B
on apoptosis in IVDD, caspase-3/9 activities were assessed by
ELISA. As presented in Fig. 3, the
caspase-3/9 activities of IVDD model rats were markedly higher
compared with the sham group. After treatment with 100 and 200
mg/kg Sparstolonin B, the induction of caspase-3/9 activities were
significantly inhibited, compared with the IVDD model (Fig. 3).
Sparstolonin B effects on TLR4, MyD88
and NF-κB protein expression in IVDD
The present study examined whether TLR4, MyD88 and
NF-κB were involved in the protection effect of Sparstolonin B on
IVDD. TLR4, MyD88 and NF-κB protein expression levels were measured
using western blot analysis. Western blot analysis demonstrated
that TLR4, MyD88 and NF-κB protein expression levels in the IVDD
model group were significantly increased, compared with sham group
(Fig. 4). Sparstolonin B (100 and
200 mg/kg) significantly suppressed TLR4, MyD88 and NF-κB protein
expression levels in IVDD rats, compared with IVDD model (Fig. 4).
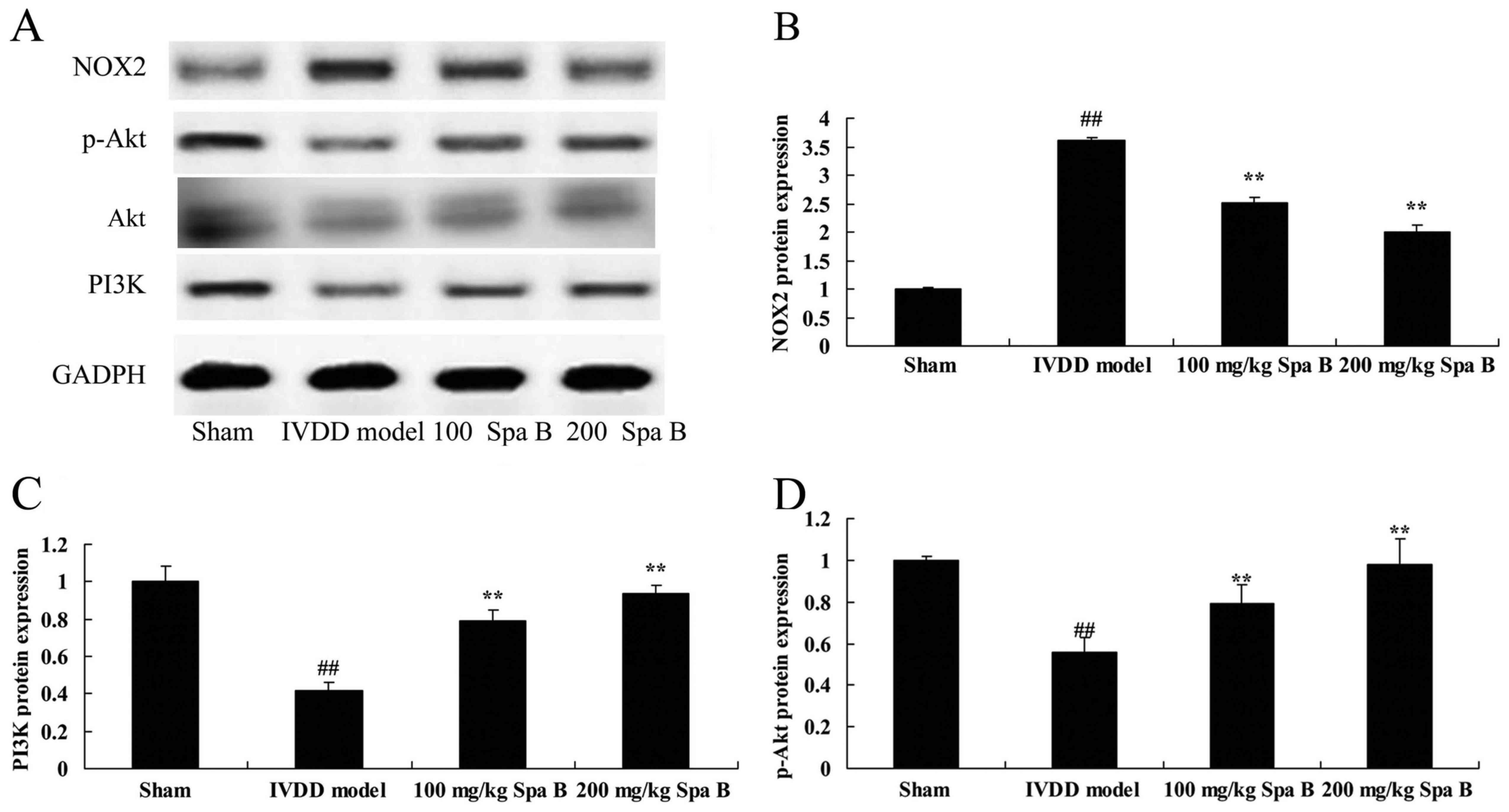
Sparstolonin B effects on NOX2, PI3K
and p-Akt protein expression in IVDD
The effect of Sparstolonin B on NOX2, PI3K and p-Akt
protein expression levels were examined (Fig. 5). Compared with sham operation
group, NOX2 protein expression in the IVDD model group was
increased (Fig. 5A and B).
Sparstolonin B (100 and 200 mg/kg) significantly suppressed NOX2
protein expression in IVDD rat, compared IVDD model group (Fig. 5A and B). Conversely, protein
expression levels of PI3K (Fig.
5C) and p-Akt (Fig. 5D) were
decreased in the IVDD model group compared with the sham-operated
group; however, both doses of Sparstolonin B significantly
ameliorated this effect.
Discussion
IVDD is a syndrome presenting intervertebral disc
deformation, accompanied by progressive fibrosis, which causes
corresponding lesions of adjacent joints and ligament, spinal
instability, or even compression of spinal cord, nerve root and
spinal artery, and other corresponding clinical symptoms and
physical signs (18). It is the
premise and basic pathological process for a series of spinal
degenerative diseases (19).
Generally, the intervertebral disc of humans will start
degenerating from 20 years old. It has been hypothesized that IVDD
is induced by a variety of factors, including aging, nutrition,
immune and trauma (19). In case
of multiple segmental spinal fracture and after spinal internal
fixation surgery, the degeneration rate of intervertebral disc will
increase. IVDD deteriorates gradually as age increases (20). However, its pathogenic mechanism is
still not clear. The present study demonstrated that Sparstolonin B
(100 and 200 mg/kg) significantly reversed these changes in IVDD
rats.
The activation of toll receptor signaling pathways
participates in the destruction of articular cartilage and synovial
membrane process; TLR4 is a member of toll receptor family
(21). It is mainly expressed in
various immune cells. A previous study has identified that TLR4 is
highly expressed in the articular cartilage and synovial membrane
of IVDD (21). The TLR4 signaling
pathway is closely associated with the pathogenetic mechanism of
IVDD (22). NF-κB is an essential
signal transduction molecule located in the downstream signaling
pathway of toll receptors (22).
Many in vivo cellular processes, such as inflammation,
immune response, cell apoptosis, tumor occurrence and metastasis,
are regulated by NF-κB. These results suggested that Sparstolonin B
(100 and 200 mg/kg) significantly reduced TNF-α, IL-1β and IL-6
content levels in IVDD rats through suppression of the TLR4/NF-κB
signaling pathway. It has been demonstrated that Sparstolonin B
protects mice against endotoxin shock by inhibiting the TLR2/4
signaling pathway (23).
Generally, it is believed that oxidative stress will
occur when the balance between the generation and scavenging of
oxygen free radicals is destroyed (21). The resulting damage is the primary
cause for cell aging. The cells will protect themselves through a
series of antioxidant system against free radicals (24). The extracellular antioxidant system
will participate in resistance and alleviating of oxidative damage
(25). Aging is a process affected
by multiple factors (25).
Oxidative stress is closely associated with aging, and aging is an
essential factor for IVDD (25).
Our previous study demonstrated that Sparstolonin B significantly
reversed the inhibition of superoxide dismutase content and
induction of malondialdehyde content in IVDD rats. It has been
demonstrated that Sparstolonin B attenuates early liver
inflammation via NADPH oxidase activation (16).
NADPH oxidase is detectable in neutrophile
granulocytes. Neutrophile granulocytes generate millimole levels of
O2 during phagocytosis. It also serves important effects
on host non-specific defense. The genovariation of the important
subunits of enzyme may lead to chronic guanulomatous disease
(characterized by recurrent episodes of lethal infection) (26). Among them, gp91phox is the basic
component of NADPH oxidase. Additionally, p47phox is the key
subunit for activity of NADPH oxidase. Under normal circumstances,
NADPH oxidase is under a dormant state in neutrophile granulocyte.
If appropriately stimulated, it will be activated rapidly (27). The cytoplasmic component p47phox is
then subject to phosphorylation and p67phox displacement (26). Eventually, it will be activated due
to the accumulation with cell membrane components, gp91phox and
p22phox. NADPH serves as the electron donor to catalyze oxygen to
O2 and further generate a reactive oxygen species
(26). The results of the present
study suggested that Sparstolonin B significantly suppressed NOX2
protein expression in IVDD rats. Furthermore, it also has been
detected that cell apoptosis may participate in pathophysiological
changes of intervertebral disc tissue degeneration (28). It has been indicated that cell
apoptosis serves important effects on IVDD process (29). Cell excessive apoptosis will lead
to a decrease of intervertebral disc activity cells (29). The subsequent decreasing of
extracellular matrix synthesis and composition change are the
pathology bases for IVDD (29).
Previous research has indicated that the oxidative stress arising
from reactive oxygen is an essential link causing cell apoptosis
(30,31).
The PI3K/Akt signaling pathway is involved and
activated substrates after acidification include serine or
threonine residues (such as Bcl-2-associated death promoter, NF-KB
and caspase-9) have biological functions including resistance to
contabescence and growth promotion (32). Studies have verified that the
PI3K/Akt transduction pathway is closely associated with cartilage
cell apoptosis (33). It has been
demonstrated that after adding PI3K inhibitor into rat bone
chondrocytes, the growth and differentiation velocity are decreased
significantly, and that the apoptotic cell ratio increases. The
difference in the number of apoptotic cells arising from
biomechanical changes is also increased through this pathway
(33). Consequently, the PI3K/Akt
transduction pathway is significant for the apoptosis of
chondrocytes (33). In the present
study, it was demonstrated that 100 and 200 mg/kg Sparstolonin B
significantly induced the PI3K/Akt signaling pathway in IVDD rats.
Liang et al (15) reported
that Sparstolonin B suppresses endothelial cell inflammation
through extracellular signal-regulated kinase 1/2 and the Akt
signaling pathway.
In conclusion, the results of the present study
demonstrated that Sparstolonin B prevents IVDD, and inhibits
IVDD-induced inflammation, oxidative stress and apoptosis through
TLR4/MyD88/NF-κB, NADPH oxidase activation and the PI3K/Akt
signaling pathway. Sparstolonin B may affect autophagy or other
mechanisms underlying IVDD, which require further study. Therefore,
Sparstolonin B has the potential to be used as a therapeutic agent
for IVDD in clinical applications.
References
|
1
|
Kang Q, Xiang Y, Li D, Liang J, Zhang X,
Zhou F, Qiao M, Nie Y, He Y, Cheng J, et al: MiR-124-3p attenuates
hyperphosphorylation of Tau protein-induced apoptosis via
caveolin-1-PI3K/Akt/GSK3β pathway in N2a/APP695swe cells.
Oncotarget. 8:24314–24326. 2017.PubMed/NCBI
|
|
2
|
Sun H, Wang P, Zhang Q, He X, Zai G, Wang
X, Ma M and Sun X: MicroRNA-21 expression is associated with the
clinical features of patients with gastric carcinoma and affects
the proliferation, invasion and migration of gastric cancer cells
by regulating Noxa. Mol Med Rep. 13:2701–2707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Yu J, Xu J, Zheng C, Li X and Du
J: The analysis of microRNA-34 family expression in human cancer
studies comparing cancer tissues with corresponding pericarcinous
tissues. Gene. 554:1–8. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao G, Xu L, Hui L and Zhao J: Level of
circulated microRNA-421 in gastric carcinoma and related
mechanisms. Int J Clin Exp Pathol. 8:14252–14256. 2015.PubMed/NCBI
|
|
5
|
Qin H, Chen GX, Liang MY, Rong J, Yao JP,
Liu H and Wu ZK: The altered expression profile of microRNAs in
cardiopulmonary bypass canine models and the effects of mir-499 on
myocardial ischemic reperfusion injury. J Transl Med. 11:1542013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomasovic A, Kurrle N, Wempe F, De-Zolt S,
Scheibe S, Koli K, Serchinger M, Schnütgen F, Sürün D, Sterner-Kock
A, et al: Ltbp4 regulates Pdgfrβ expression via TGFβ-dependent
modulation of Nrf2 transcription factor function. Matrix Biol.
59:109–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heinemann MK: Editor's Note: Expression of
transforming growth factor beta 1 in lung tissue during
cardiopulmonary bypass-induced lung injury in dogs by Wang et
al. (epub ahead of print). Thorac Cardiovasc Surg. 61:4572013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tutarel O, Dangwal S, Bretthauer J,
Westhoff-Bleck M, Roentgen P, Anker SD, Bauersachs J and Thum T:
Circulating miR-423_5p fails as a biomarker for systemic
ventricular function in adults after atrial repair for
transposition of the great arteries. Int J Cardiol. 167:63–66.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kurowska-Stolarska M, Hasoo MK, Welsh DJ,
Stewart L, McIntyre D, Morton BE, Johnstone S, Miller AM, Asquith
DL, Millar NL, et al: The role of microRNA-155/liver X receptor
pathway in experimental and idiopathic pulmonary fibrosis. J
Allergy Clin Immunol. 139:1946–1956. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He Y, Huang C, Sun X, Long XR, Lv XW and
Li J: MicroRNA-146a modulates TGF-beta1-induced hepatic stellate
cell proliferation by targeting SMAD4. Cell Signal. 24:1923–1930.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maitra SR, Bhaduri S, El-Maghrabi MR and
Shapiro MJ: Inhibition of matrix metalloproteinase on hepatic
transforming growth factor beta1 and caspase-3 activation in
hemorrhage. Acad Emerg Med. 12:797–803. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Q, Wang G, Yuan W, Wu J, Wang M and
Li C: The effects of phosphodiesterase-5 inhibitor sildenafil
against post-resuscitation myocardial and intestinal
microcirculatory dysfunction by attenuating apoptosis and
regulating microRNAs expression: Essential role of nitric oxide
syntheses signaling. J Transl Med. 13:1772015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu D, Talbot CC Jr, Liu Q, Jing ZC, Damico
RL, Tuder R, Barnes KC, Hassoun PM and Gao L: Identifying microRNAs
targeting Wnt/β-catenin pathway in end-stage idiopathic pulmonary
arterial hypertension. J Mol Med (Berl). 94:875–885. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan HY, Zhou CB, Chen JM, Liu XB, Wen SS,
Xu G and Zhuang J: MicroRNA-34a targets regulator of calcineurin 1
to modulate endothelial inflammation after fetal cardiac bypass in
goat placenta. Placenta. 51:49–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, van Mil A, Aguor EN, Siddiqi S,
Vrijsen K, Jaksani S, Metz C, Zhao J, Strijkers GJ, Doevendans PA
and Sluijter JP: MiR-155 inhibits cell migration of human
cardiomyocyte progenitor cells (hCMPCs) via targeting of MMP-16. J
Cell Mol Med. 16:2379–2386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Zhang M, Li X, Tang Z, Wang X,
Zhong M, Suo Q, Zhang Y and Lv K: Silencing microRNA-155 attenuates
cardiac injury and dysfunction in viral myocarditis via promotion
of M2 phenotype polarization of macrophages. Sci Rep. 6:226132016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liang X, Shen H, Shi WD, Ren S, Jiang W,
Liu H, Yang P, Sun ZY, Lin J and Yang HL: Effect of axial vertical
vibration on degeneration of lumbar intervertebral discs in
modified bipedal rats: An in-vivo study. Asian Pac J Trop Med.
10:714–717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Strube P, Pfitzner BM, Streitparth F,
Hartwig T and Putzier M: In vivo effects of bupivacaine and
gadobutrol on the intervertebral disc following discoblock and
discography: A histological analysis. Eur Radiol. 27:149–156. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vieira LA, De Marchi PL, dos Santos AA,
Christofolini DM, Barbosa CP, Fonseca FL, Bianco B and Rodrigues
LM: Analysis of FokI polymorphism of vitamin D receptor gene in
intervertebral disc degeneration. Genet Test Mol Biomarkers.
18:625–629. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rolving N, Oestergaard LG, Willert MV,
Christensen FB, Blumensaat F, Bünger C and Nielsen CV: Description
and design considerations of a randomized clinical trial
investigating the effect of a multidisciplinary
cognitive-behavioural intervention for patients undergoing lumbar
spinal fusion surgery. BMC Musculoskelet Disord. 15:622014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie YL, Chu JG, Jian XM, Dong JZ, Wang LP,
Li GX and Yang NB: Curcumin attenuates
lipopolysaccharide/d-galactosamine-induced acute liver injury by
activating Nrf2 nuclear translocation and inhibiting NF-kB
activation. Biomed Pharmacother. 91:70–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu S, Zhao N, Hui L, Song M, Miao ZW and
Jiang XJ: MicroRNA-124-3p inhibits the growth and metastasis of
nasopharyngeal carcinoma cells by targeting STAT3. Oncol Rep.
35:1385–1394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nataraj V, Batra A, Rastogi S, Khan SA,
Sharma MC, Vishnubhatla S and Bakhshi S: Developing a prognostic
model for patients with localized osteosarcoma treated with uniform
chemotherapy protocol without high dose methotrexate: A
single-center experience of 237 patients. J Surg Oncol.
112:662–668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu G, Kuang G, Jiang W, Jiang R and Jiang
D: Polydatin promotes apoptosis through upregulation the ratio of
Bax/Bcl-2 and inhibits proliferation by attenuating the β-catenin
signaling in human osteosarcoma cells. Am J Transl Res. 8:922–931.
2016.PubMed/NCBI
|
|
25
|
Li Z, Zhang J, Mulholland M and Zhang W:
mTOR activation protects liver from ischemia/reperfusion-induced
injury through NF-κB pathway. FASEB J. 31:3018–3026. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding Y, Wang Y, Chen J, Hu Y, Cao Z, Ren P
and Zhang Y: p21 overexpression sensitizes osteosarcoma U2OS cells
to cisplatin via evoking caspase-3 and Bax/Bcl-2 cascade. Tumour
Biol. 35:3119–3123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cao YH, Li DG, Xu B, Wang MQ, Zhen N, Man
LX, Zhang YY and Chi M: A microRNA-152 that targets the phosphatase
and tensin homolog to inhibit low oxygen induced-apoptosis in human
brain microvascular endothelial cells. Genet Mol Res. 15:2016.
View Article : Google Scholar
|
|
28
|
Chen J, Zhang L, Zhang Y, Zhang H, Du J,
Ding J, Guo Y, Jiang H and Shen X: Emodin targets the
beta-hydroxyacyl-acyl carrier protein dehydratase from Helicobacter
pylori: Enzymatic inhibition assay with crystal structural and
thermodynamic characterization. BMC Microbiol. 9:912009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Martynyuk L, Martynyuk L, Ruzhitska O and
Martynyuk O: Effect of the herbal combination Canephron N on
diabetic nephropathy in patients with diabetes mellitus: Results of
a comparative cohort study. J Altern Complement Med. 20:472–478.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Park SJ, Jin ML, An HK, Kim KS, Ko MJ, Kim
CM, Choi YW and Lee YC: Emodin induces neurite outgrowth through
PI3K/Akt/GSK-3β-mediated signaling pathways in Neuro2a cells.
Neurosci Lett. 588:101–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li H, Yang T, Zhou H, Du J, Zhu B and Sun
Z: Emodin combined with nanosilver inhibited sepsis by
anti-inflammatory protection. Front Pharmacol. 7:5362017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kohan DE, Heerspink Lambers HJ, Coll B,
Andress D, Brennan JJ, Kitzman DW, Correa-Rotter R, Makino H,
Perkovic V, Hou FF, et al: Predictors of atrasentan-associated
fluid retention and change in albuminuria in patients with diabetic
nephropathy. Clin J Am Soc Nephrol. 10:1568–1574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dwyer JP, Greco BA, Umanath K, Packham D,
Fox JW, Peterson R, Broome BR, Greene LE, Sika M and Lewis JB:
Pyridoxamine dihydrochloride in diabetic nephropathy
(PIONEER-CSG-17): Lessons learned from a pilot study. Nephron.
129:22–28. 2015. View Article : Google Scholar : PubMed/NCBI
|