Introduction
Osteoporosis, a condition characterized by a
reduction in bone mass and strength, is associated with increased
risks for fracture. It has become an overwhelming public health
problem worldwide, particularly in postmenopausal women (1). The average bone loss during the five
years around the menopause or perimenopause reaches 15%, and
postmenopausal women with low bone density are very likely to be
predisposed to fractures (2,3).
Currently, pharmacological interventions for osteoporosis are
classified into antiresorptive agents that prevent bone resorption,
such as hormone replacement therapy, bisphosphonates and denosumab,
and anabolic agents, which help with formation of new bones,
including strontium and teriparatide. However, the efficacy of
certain drugs is limited by perceived intolerance and long-term
adverse events (4–6). Mechanical strain is known as the
elementary physiological factor that regulates bone formation and
regeneration, as well as maintaining the integrity of bone
structure and function. Evidence indicated that physical exercise
may improve skeletal resistance to bone fracture, and delay the
progress of osteoporosis by enhancing bone mass and strength
(7,8). Therefore, physical activity may be
used as a non-invasive intervention in osteoporosis prevention and
treatment. However, little is known about the specific mechanism
that regulates bone remodeling in osteoporosis.
Bone mesenchymal stem cells (BMSCs) are
force-sensitive cells capable of detecting, transducing and
responding to an extracellular stimulus, and thus differentiate
into multiple cell lineages (9,10).
Evidence indicates that the osteogenic ability of BMSCs is key in
bone remodeling. The alterations in BMSCs associated with estrogen
reduction may result in the attenuated regenerative ability of
bone, which consequently results in osteoporosis. Additionally,
BMSCs are proposed to be of great importance in the response of
bone to mechanical stimulation (11–13).
However, few studies focused on the signaling pathway involved in
bio-mechanical transduction of BMSCs from ovariectomized rats (OVX
BMSCs) in vitro. Thus, such studies regarding the effect of
mechanical strain on OVX BMSCs may elucidate the mechanism of bone
remodeling in osteoporosis.
It is well known that the signal transduction
initiated by external chemical or mechanical stimulation is
important in regulating bone development (14). The phosphatidylinositol 3-kinase
(PI3K)/Akt signaling pathway is one of the most common signaling
pathways that has been identified to be implicated in BMSC
proliferation and differentiation by modulating the transcriptional
activity of downstream genes (15). In addition, there is substantial
evidence that the PI3K/Akt signaling pathway is essential for human
and murine MSC osteogenesis in vitro (16,17).
However, whether the PI3K/Akt signaling pathways is involved in the
response of OVX BMSCs to mechanical strain has not, to the best of
our knowledge, been thoroughly investigated. Therefore, by adopting
an FX-4000T™ Tension Plus™ system, the mechanical environment of
BMSCs in vivo was mimicked in the present study.
Furthermore, the effect of continuous mechanical strain (CMS) on
osteogenic differentiation of OVX BMSCs, and the involvement and
function of the PI3K/Akt signaling pathway in biomechanical signal
transduction were investigated.
Materials and methods
Animals and cell culture
The current study was conducted in accordance with
the regional Ethics Committee guidelines. Sixty female
Sprague-Dawley rats (age, 6 weeks), weighing an average of 200 g,
were obtained from Shanghai SLAC Experimental Animal Center
(Shanghai, China). The animals underwent surgical ovariectomy
according to FDA guidelines (18).
The rats were then housed separately in a temperature-controlled
room at 21°C with relative humidity at 60% under a 12-h light/dark
cycle. Then, 12 weeks after ovariectomy, all rats were sacrificed.
The humeri and tibiae were isolated from the OVX rats. The bone
marrow was flushed out using Dulbecco's modified Eagle's essential
medium (HyClone; GE Healthcare Life Sciences, Logan, UT, USA),
supplemented with 100 U/ml penicillin, and 100 µg/ml streptomycin
(Hyclone; GE Healthcare Life Sciences). Non-adherent cells were
removed by replacing the medium after 72 h and it was subsequently
refreshed every 3 days. On reaching 70–80% confluence, the cells
were trypsinized with 10% trypsin-EDTA (Hyclone; GE Healthcare Life
Sciences) and passaged. OVX BMSCs from passages 2 to 5 were used
during the experiments.
Application of CMS
CMS of 10% elongation at a frequency of 1 Hz was
applied using an FX-4000T™ Flexercell Tension Plus™ unit (Flexcell
International Corp., Burlington, NC, USA). BMSCs were plated on
Flexcell 6-well silicone rubber plates at a density of
2×104/cm2. After 24–48 h incubation, the
cells had attached and reached ~80% confluence. The BMSCs were then
subjected to CMS for 48 h.
Alkaline phosphatase (ALP) staining
and relative ALP activity detection
The presence of ALP in the cell layers was assessed
according to the manufacturer's protocol of the
5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium
Alkaline Phosphatase Color Development kit (cat. no. C3206;
Beyotime Institute of Biotechnology, Haimen, China) and described
as follows. The OVX BMSCs were rinsed with phosphate-buffered
saline (PBS) three times and fixed with 4% paraformaldehyde for 15
min. Coloration was then assessed and observed with a digital
camera (Eclipse TS100; Nikon Corporation, Tokyo, Japan). The
relative ALP activity was detected according to the manufacturer's
protocol with the Alkaline Phosphatase Assay kit (Beyotime
Institute of Biotechnology). After exposing to CMS for 24 and 48 h,
samples from all groups were washed twice with double-distilled
water and lysed via sonification. Cell lysates were incubated with
p-nitrophenol phosphate (Beyotime Institute of Biotechnology) at
37°C for 1 h. The enzymatic reaction was stopped using 1 M sodium
hydroxide and absorbance was measured at a wavelength of 405
nm.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of the cells was isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to the manufacturer's recommended protocol. The RNA
concentrations were determined using a NanoDrop spectrophotometer
(Thermo Fisher Scientific, Inc.) and cDNA was synthesized using a
cDNA Synthesis Reverse Transcription kit (cat. no. RR037A; Takara
Biotechnology Co., Ltd., Dalian, China). qPCR was performed using a
Light-Cycler system with SYBR Premix Ex Taq™ (RR420A, Takara
Biotechnology Co., Ltd.), according to the manufacturer's protocol.
The conditions of the qPCR were as follows: Denaturation at 95°C
for 10 sec, and 50 cycles of 95°C for 10 sec and 60°C for 30 sec,
with a final dissociation stage (95°C for 5 min) to complete the
amplification procedure. β-actin served as an internal control. The
data were analyzed using comparative Cq (2−ΔΔCq) method
and expressed as a fold-change respective to the control (19). Each sample was analyzed in
triplicate. The primer sequences used in the current study are
presented in Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer sequences for target genes. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer sequences for target genes.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| β-actin |
CACCCGCGAGTACAACCTTC |
CCCATACCCACCATCACACC |
| Alkaline
phosphatase |
TATGTCTGGAACCGCACTGAAC |
CACTAGCAAGAAGAAGCCTTTGG |
| Type I collagen |
CAGGCTGGTGTGATGGGATT |
CCAAGGTCTCCAGGAACACC |
| Runt related
transcription factor 2 |
ATCCAGCCACCTTCACTTACACC |
GGGACCATTGGGAACTGATAGG |
Western blotting
The cells were lysed on ice for 30 min in SDS lysis
buffer (Beyotime Institute of Biotechnology) supplemented with
protease inhibitors. For western blot analysis, 20 µg sample was
resolved on a 10% SDS-PAGE gel and electro-transferred onto
nitrocellulose membranes with a constant voltage of 90 V and
duration of 70 min (Whatman, GE Healthcare Life Sciences). The
following primary antibodies were used: Anti-runt related
transcription factor 2 (Runx2; cat. no. 12256; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA); anti-Akt (cat. no.
ab8805; 1:1,000; Abcam, Cambridge, MA, USA) and anti-p-Akt (cat.
no. ab38449; 1:1,000; Abcam). For the normalization of protein
loading, a GAPDH antibody (cat. no. 5174; Cell Signaling
Technology, Inc.) was used at a dilution of 1:2,000. Horseradish
peroxidase-conjugated secondary antibodies were used at a dilution
of 1:5,000 (cat. no. ab6721; Abcam). The antigen-antibody complexes
were visualized using an Enhanced Chemiluminescence Detection
system (EMD Millipore, Billerica, MA), according to the
manufacturer's protocols. Protein band intensities on the scanned
films were compared to their respective controls using Alpha Image
software.
Inhibition of the PI3K/Akt signaling
pathway
In order to assess the role of the PI3K/Akt
signaling pathway in the strain-induced differentiation of OVX
BMSCs, the selective inhibitor, LY294002 was used. Preliminary
experiments indicated that the optimum concentration of LY294002
was 10 µM. Cells were pre-treated with inhibitors for 1 h prior to
application of the strain stimulus, and they were present during
the entire strain application.
Immunofluorescence analysis
Subsequent to mechanical loading, cells were fixed
with 4% paraformaldehyde for 10 min, then washed with PBS and
incubated in 0.1% Triton X-100 for 15 min at room temperature and
then blocked with 5% bovine serum albumin for 1h at room
temperature. The prepared samples were incubated overnight at 4°C
with rabbit monoclonal anti-phosphorylated (p)-Akt (Ser473;
dilution, 1:300) or rabbit monoclonal anti-Akt (dilution, 1:300)
that were obtained from Cell Signaling Technology, Inc., and
detected with Alexa 594 conjugate (dilution, 1:200; Thermo Fisher
Scientific, Inc.) at room temperature for 2 h. Nuclei were labeled
with 1 mg/ml Hoechst for 10 min at room temperature (Roche
Diagnostics, Basel, Switzerland). Slides were examined under an
Olympus IX71 fluorescent microscope. At least three overview images
were obtained from three independent experiments.
Statistical analysis
All experiments were performed a minimum of three
times and data are expressed as means ± standard deviation.
Differences between two groups were identified using unpaired
t-tests. Significant differences between the non-load and multiple
stretch groups were determined using a one-way analysis of variance
followed by the Least Significant Difference post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effect of CMS on osteogenic
differentiation of OVX BMSCs
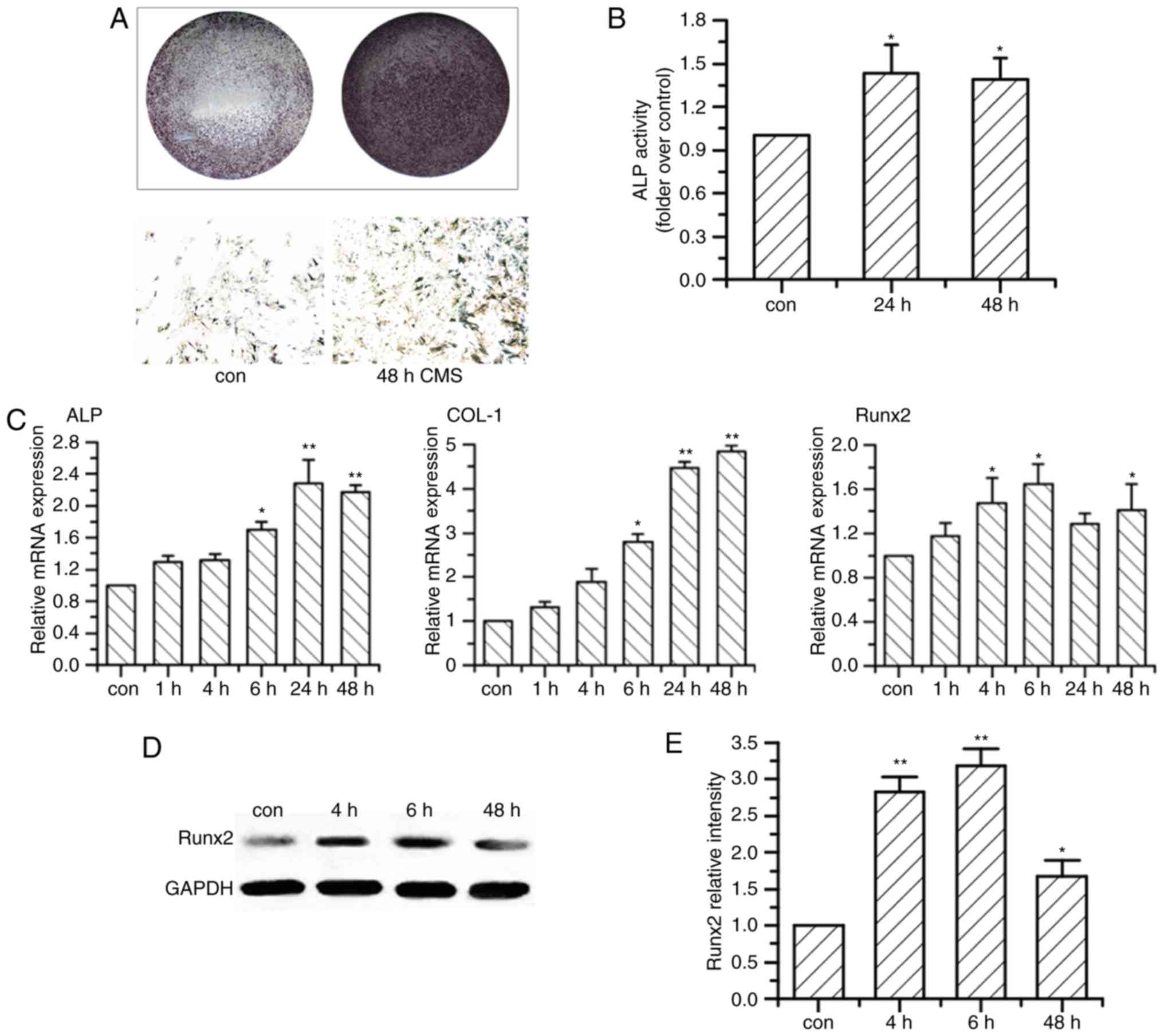
After exposure to CMS, OVX BMSCs demonstrated higher
ALP activity and deeper staining at 24 and 48 h when compared with
the non-loaded OVX BMSC group (Fig. 1A
and B). CMS upregulated the mRNA expression levels of
osteogenesis-associated markers of OVX BMSCs, ALP, type I collagen
(COL I) and Runx2, as they began to increase significantly at 4 or
6 h after exposure to CMS, and reached to a peak value at 24 or 48
h (Fig. 1C). Additionally, the
protein expression level of Runx2 was elevated in a time-dependent
manner in OVX BMSCs compared with the non-loaded group, with a
significant increase at 4 and 6 h (Fig. 1D and E).
Effects of CMS on induction of the
PI3K/Akt signaling pathway
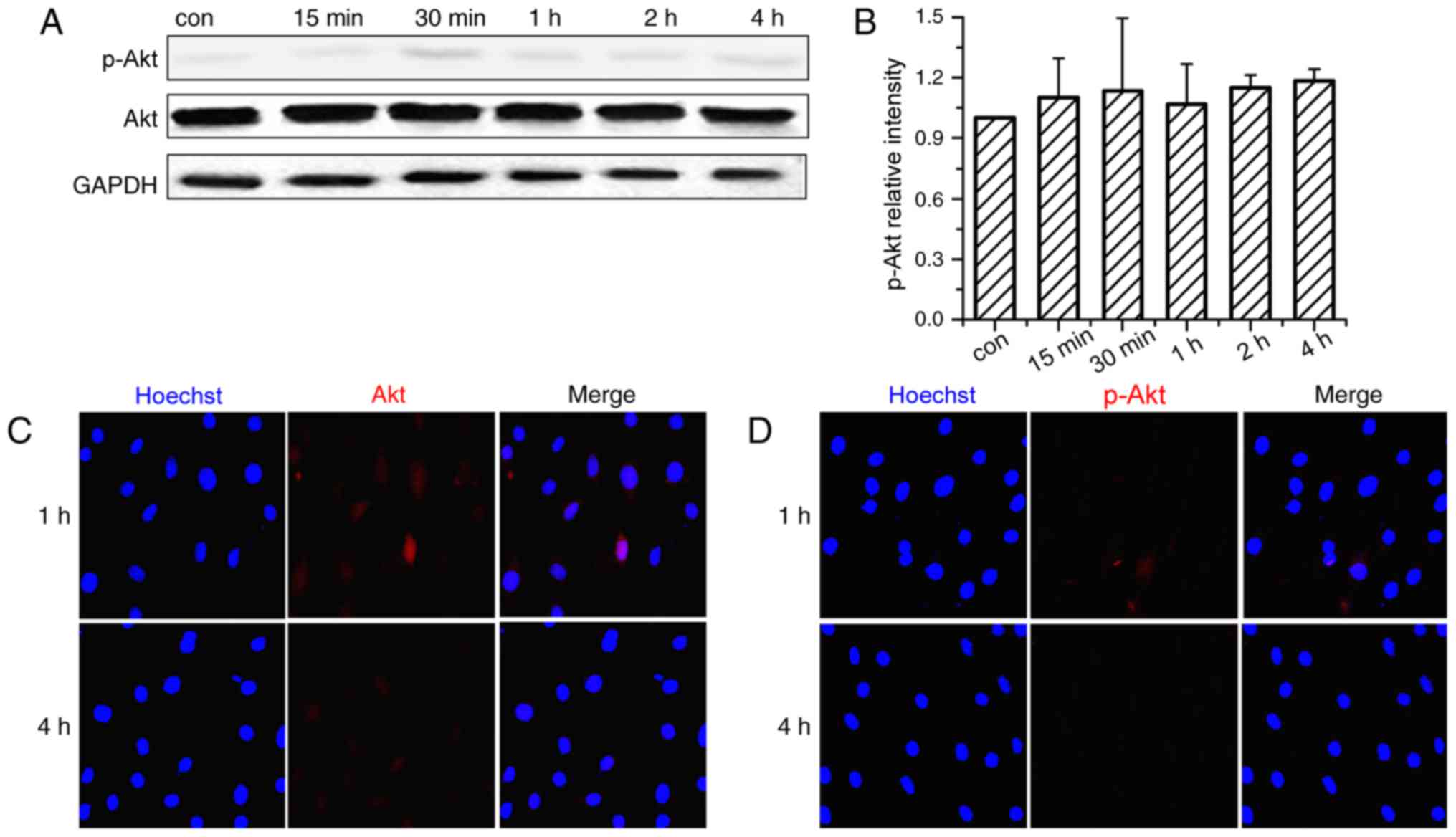
The activation time course of the PI3K/Akt signaling
pathway was investigated in OVX BMSCs subjected to CMS. As
demonstrated in Fig. 2A and B, Akt
was significantly phosphorylated soon after the onset of
stimulation and peaked at 15 min. The phosphorylation level
subsequently declined gradually, but remained higher than the
non-loaded group at 30 min. After 1 h of loading, the levels of
p-Akt returned almost to baseline or were lower than the control
group. The cellular localization of Akt and p-Akt was also examined
by immunofluorescence analysis (Fig.
2C and D) and the nuclei were co-stained by Hoechst. Following
CMS stimulation, Akt staining was performed with Akt antibodies,
and observed in the cytoplasm and the nucleus at 1 and 4 h. p-Akt
staining was more strongly expressed in the nucleus than in the
cytoplasm at 1 h, and became markedly weaker at 4 h.
Effects of a PI3K/Akt inhibitor on
CMS-induced OVX BMSCs
The above-mentioned findings indicate the activation
of the PI3K/Akt signaling pathway by CMS. After demonstrating that
PI3K/Akt may be involved in the mechanotransduction of CMS, its
function in CMS-induced osteogenesis of OVX BMSCs was further
investigated in the current study via pharmacological inhibition.
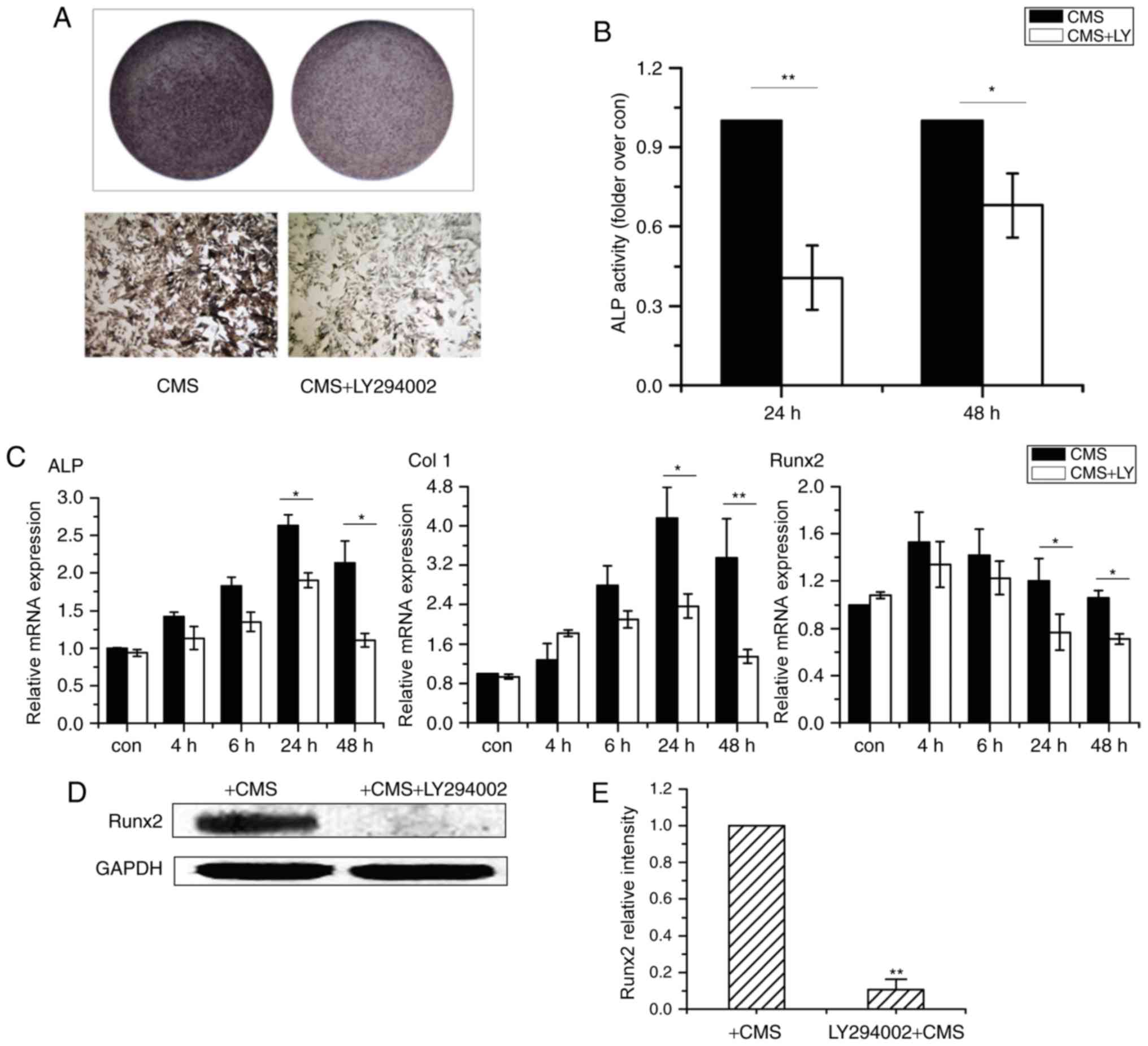
LY294002 was used to block the activation of p-Akt in OVX BMSCs.
Fig. 3A and B demonstrated that
pre-treatment with Akt-specific inhibitor significantly blocked the
phosphorylation of Akt and had no cytotoxic effect on the cells
(data not shown). Immunofluorescence analysis of p-Akt co-confirmed
that the activation of p-Akt was blocked by LY294002 treatment as
p-Akt were predominantly stained in the cytoplasm (Fig. 3C and D).
Effects of a PI3K/Akt inhibitor on
osteogenic differentiation of OVX BMSCs
Subsequently, CMS-induced osteogenesis of OVX BMSCs
was assessed using a PI3K/Akt inhibitor. Pretreatment with LY294002
inhibited CMS-stimulated ALP activity (Fig. 4A and B). Furthermore, the
CMS-induced mRNA expression levels of ALP, Col I and Runx2 were
significantly repressed at 24 and 48 h (Fig. 4C). Similarly, as presented in
Fig. 4D and E, the CMS-induced
Runx2 protein expression level was attenuated by LY294002. These
results indicate that the PI3K/Akt signaling pathway is responsible
for the CMS-induced osteogenesis of OVX BMSCs.
Discussion
Recent studies have demonstrated that mechanical
stimuli are essential for the differentiation of stem cells into
different lineages. Lack of mechanical stress significantly
attenuates the differentiating capability of BMSCs into
osteoblasts, which may lead to disuse osteoporosis (20,21).
Characterized by decreased bone strength, osteoporosis is a chronic
disease that easily predisposes individuals to fractures (22). As BMSCs are the progenitor cells of
osteoblast cells, they are crucial in bone remodeling (23,24).
The current study was designed to evaluate the effects and specific
underlying mechanism of CMS on the osteogenic differentiation of
OVX BMSCs, with the aim of improving treatment strategies for
osteoporosis.
BMSCs from osteoporosis patients exhibited longer
population doubling duration. In addition, ovariectomy alters the
synthesis of mineralized matrix and gene expression markers
associated with osteogenic differentiation in BMSCs, and thus
results in the reduction of the osteogenic potential (25,26).
Although our previous studies indicated that the ability of
osteogenic lineage commitment of OVX BMSCs was weaker than sham
BMSCs under the exposure of intermittent mechanical strain; the
current study demonstrated that OVX BMSCs exposed to CMS underwent
osteoblastic differentiation when compared with non-loaded OVX
BMSCs (27). ALP activity and
expression levels serve as indicators of osteoblastic activity.
Extracellular matrix molecules, such as COL I, are considered to be
of great importance in osteoblast proliferation and
differentiation. Additionally, Runx2 has been shown to be
significant in regulating osteogenic differentiation (28). In the current study, the mRNA
expression levels of ALP, COL I and Runx2 were enhanced in OVX
BMSCs. Furthermore, OVX BMSCs subjected to CMS demonstrated higher
ALP activities and deeper staining at 24 and 48 h when compared
with the non-loaded OVX BMSC group. In addition, the protein
expression level of Runx2 was increased at 4 and 6 h. These results
demonstrated that OVX BMSCs underwent osteoblastic differentiation
due to CMS.
The PI3K/Akt signaling pathway is key in the
physiology and pathophysiology of various types of cell, exerting
profound effects on processes, including proliferation, migration,
metabolism and differentiation (29). In the current study, Akt was
phosphorylated under the stimulation of CMS, with phosphorylation
levels peaking at 15 min and then gradually declining; however, the
level remained greater than that of the unloaded group. Meanwhile,
as indicated by immunostaining, OVX BMSCs subjected to CMS
demonstrated greater accumulation of p-Akt in the nucleus,
indicating that mechanical strain enhances phosphorylation and
nuclear translocation of the Akt protein. After confirming the
activation of Akt, the OVX BMSCs were pre-treated with an inhibitor
of the Akt signaling pathway (LY294002) to determine whether their
strain-induced osteogenic commitment was dependent on Akt
activation. Following treatment with LY294002, the strain-induced
gene expression of osteogenic markers and Runx2 protein expression
decreased significantly. Previous studies demonstrated that Akt was
particularly important in bone formation and was activated early in
the transcriptional activation of osteogenesis (15,30,31).
Substantial evidence indicated that PI3K/Akt signaling was required
for murine osteogenesis in vitro, including mouse embryonic
fibroblasts, murine BMSCs, and in the mouse MSC line, C3H10T1/2
(17). Nuclear translocation of
activated Akt may lead to the phosphorylation of key transcription
factors, which in turn affects the levels of certain proliferation
or differentiation-associated genes (32). Additionally, Akt is the
mechanically activated kinase responsible for numerous other
interventions. For example, the PI3K/Akt signaling pathway
participates in matrix metalloproteinase-2 expression by 10%
mechanical stretch in vascular smooth muscle cells and by 18% in
human aortic smooth muscle cells (33,34).
Furthermore, ultrasound stimulation promotes bone formation in
osteoblasts via the integrin/protein tyrosine kinase 2/PI3K/Akt and
extracellular-signal-regulated kinase signaling pathway (35). Studies also indicated that
mammalian target of rapamycin complex 2 was required for mechanical
activation of Akt and that mechanical inhibition of glycogen
synthase kinase was dependent on Akt activation (36). However, as Akt is a pleiotropic
signaling molecule with downstream targets that are differentially
regulated depending upon the nature of the activating input,
further studies investigating the downstream targets of
strain-induced osteogenic commitment on Akt activation are
required.
In conclusion, continuous short-term mechanical
strain induced the early differentiation of OVX BMSCs towards an
osteogenic phenotype, and CMS may activate the PI3K/Akt signaling
pathway during osteoblastic differentiation. The present study may
provide a promising strategy for regulating strain-induced bone
remodeling in osteoporosis, however, further research is required
regarding the downstream target and the in vivo
conditions.
Acknowledgements
The present study was supported in part by grants
from the National Natural Science Foundation of China (NSFC) (grant
nos. 81371121, 11342005, 30901698, 10972142 and 81570950), the
‘Chen Xing’ Project from Shanghai Jiaotong University, and Shanghai
Summit and Plateau Disciplines.
References
|
1
|
Diab DL and Watts NB: Postmenopausal
osteoporosis. Curr Opin Endocrinol Diabetes Obes. 20:501–509. 2013.
View Article : Google Scholar
|
|
2
|
Andreopoulou P and Bockman RS: Management
of postmenopausal osteoporosis. Annu Rev Med. 66:329–342. 2015.
View Article : Google Scholar
|
|
3
|
Kemmler W, Bebenek M, Kohl M and von
Stengel S: Exercise and fractures in postmenopausal women. Final
results of the controlled Erlangen fitness and osteoporosis
prevention study (EFOPS). Osteoporos Int. 26:2491–2499. 2015.
View Article : Google Scholar
|
|
4
|
Choi HJ: New antiresorptive therapies for
postmenopausal osteoporosis. J Menopausal Med. 21:1–11. 2015.
View Article : Google Scholar :
|
|
5
|
Iwamoto J, Takeda T and Sato Y: Efficacy
and safety of alendronate and risedronate for postmenopausal
osteoporosis. Curr Med Res Opin. 22:919–928. 2006. View Article : Google Scholar
|
|
6
|
Appelman-Dijkstra NM and Papapoulos SE:
Modulating bone resorption and bone formation in opposite
directions in the treatment of postmenopausal osteoporosis. Drugs.
75:1049–1058. 2015. View Article : Google Scholar :
|
|
7
|
Ehrlich PJ and Lanyon LE: Mechanical
strain and bone cell function: A review. Osteoporos Int.
13:688–700. 2002. View Article : Google Scholar
|
|
8
|
Massafra U, Integlia D, Broccoli S and
Migliore A: Mixed treatment comparison to rank antiresorptive
agents in preventing new non vertebral fractures in postmenopausal
osteoporosis. Value Health. 18:A6362015. View Article : Google Scholar
|
|
9
|
Weyts FA, Bosmans B, Niesing R, van
Leeuwen JP and Weinans H: Mechanical control of human osteoblast
apoptosis and proliferation in relation to differentiation. Calcif
Tissue Int. 72:505–512. 2003. View Article : Google Scholar
|
|
10
|
Jagodzinski M, Drescher M, Zeichen J,
Hankemeier S, Krettek C, Bosch U and van Griensven M: Effects of
cyclic longitudinal mechanical strain and dexamethasone on
osteogenic differentiation of human bone marrow stromal cells. Eur
Cell Mater. 7:35–41; discussion 41. 2004. View Article : Google Scholar
|
|
11
|
Liedert A, Kaspar D, Blakytny R, Claes L
and Ignatius A: Signal transduction pathways involved in
mechanotransduction in bone cells. Biochem Biophys Res Commun.
349:1–5. 2006. View Article : Google Scholar
|
|
12
|
Mauney JR, Sjostorm S, Blumberg J, Horan
R, O'Leary JP, Vunjak-Novakovic G, Volloch V and Kaplan DL:
Mechanical stimulation promotes osteogenic differentiation of human
bone marrow stromal cells on 3-D partially demineralized bone
scaffolds in vitro. Calcif Tissue Int. 74:458–468. 2004. View Article : Google Scholar
|
|
13
|
Gao Y, Li JH, Han LC, Ma YQ, Hu J, Qu D
and Xu YC: Osteoblastic differentiation and gene expression profile
change in rat bone marrow mesenchymal stem cells after a single
period of mechanical strain. Hua Xi Kou Qiang Yi Xue Za Zhi.
27:213–216. 2009.
|
|
14
|
Thompson WR, Rubin CT and Rubin J:
Mechanical regulation of signaling pathways in bone. Gene.
503:179–193. 2012. View Article : Google Scholar :
|
|
15
|
Baker N, Sohn J and Tuan RS: Promotion of
human mesenchymal stem cell osteogenesis by PI3-kinase/Akt
signaling, and the influence of caveolin-1/cholesterol homeostasis.
Stem Cell Res Ther. 6:2382015. View Article : Google Scholar :
|
|
16
|
Ghosh-Choudhury N, Abboud SL, Nishimura R,
Celeste A, Mahimainathan L and Choudhury GG: Requirement of
BMP-2-induced phosphatidylinositol 3-kinase and Akt
serine/threonine kinase in osteoblast differentiation and
Smad-dependent BMP-2 gene transcription. J Biol Chem.
277:33361–33368. 2002. View Article : Google Scholar
|
|
17
|
Mukherjee A, Wilson EM and Rotwein P:
Selective signaling by Akt2 promotes bone morphogenetic protein
2-mediated osteoblast differentiation. Mol Cell Biol. 30:1018–1027.
2010. View Article : Google Scholar
|
|
18
|
Thompson DD, Simmons HA, Pirie CM and Ke
HZ: FDA Guidelines and animal models for osteoporosis. Bone.
17:125S–133S. 1995. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen T: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2−ΔΔCt method. Methods. 25:402–408. 2001. View Article : Google Scholar
|
|
20
|
Marie PJ, Jones D, Vico L, Zallone A,
Hinsenkamp M and Cancedda R: Osteobiology, strain, and
microgravity: Part I. Studies at the cellular level. Calcif Tissue
Int. 67:2–9. 2000. View Article : Google Scholar
|
|
21
|
Li R, Liang L, Dou Y, Huang Z, Mo H, Wang
Y and Yu B: Mechanical strain regulates osteogenic and adipogenic
differentiation of bone marrow mesenchymal stem cells. Biomed Res
Int. 2015:8732512015.
|
|
22
|
Modder UI, Roforth MM, Hoey K, McCready
LK, Peterson JM, Monroe DG, Oursler MJ and Khosla S: Effects of
estrogen on osteoprogenitor cells and cytokines/bone-regulatory
factors in postmenopausal women. Bone. 49:202–207. 2011. View Article : Google Scholar :
|
|
23
|
Yamazaki S, Mizumoto T, Nasu A, Horii T,
Otomo K, Denno H, Takebayashi T, Miyamoto K and Horiuchi T:
Regulation of osteogenetic differentiation of mesenchymal stem
cells by two axial rotational culture. J Artif Organs. 14:310–317.
2011. View Article : Google Scholar
|
|
24
|
Koike M, Shimokawa H, Kanno Z, Ohya K and
Soma K: Effects of mechanical strain on proliferation and
differentiation of bone marrow stromal cell line ST2. J Bone Miner
Metab. 23:219–225. 2005. View Article : Google Scholar
|
|
25
|
Boeloni JN, de MON, Silva JF, Correa CR,
Bertollo CM, Hell RC, de MPM, Goes AM and Serakides R: Osteogenic
differentiation of bone marrow mesenchymal stem cells of
ovariectomized and non-ovariectomized female rats with thyroid
dysfunction. Pathol Res Pract. 209:44–51. 2013. View Article : Google Scholar
|
|
26
|
Varkey M, Kucharski C, Doschak MR, Winn
SR, Brochmann EJ, Murray S, Matyas JR, Zernicke RF and Uludag H:
Osteogenic response of bone marrow stromal cells from normal and
ovariectomized rats treated with a low dose of basic fibroblast
growth factor. Tissue Eng. 13:809–817. 2007. View Article : Google Scholar
|
|
27
|
Wu Y, Zhang P, Dai Q, Yang X, Fu R, Jiang
L and Fang B: Effect of mechanical stretch on the proliferation and
differentiation of BMSCs from ovariectomized rats. Mol Cell
Biochem. 382:273–282. 2013. View Article : Google Scholar
|
|
28
|
Zhang P, Dai Q, Ouyang N, Yang X, Wang J,
Zhou S, He N, Fang B and Jiang L: Mechanical strain promotes
osteogenesis of BMSCs from ovariectomized rats via the ERK1/2 but
not p38 or JNK-MAPK signaling pathways. Curr Mol Med. 15:780–789.
2015. View Article : Google Scholar
|
|
29
|
Ping C, Lin Z, Jiming D, Jin Z, Ying L,
Shigang D, Hongtao Y, Yongwei H and Jiahong D: The phosphoinositide
3-kinase/Akt-signal pathway mediates proliferation and secretory
function of hepatic sinusoidal endothelial cells in rats after
partial hepatectomy. Biochem Biophys Res Commun. 342:887–893. 2006.
View Article : Google Scholar
|
|
30
|
Tsai KS, Kao SY, Wang CY, Wang YJ, Wang JP
and Hung SC: Type I collagen promotes proliferation and
osteogenesis of human mesenchymal stem cells via activation of ERK
and Akt pathways. J Biomed Mater Res A. 94:673–682. 2010.
|
|
31
|
Ling L, Dombrowski C, Foong KM, Haupt LM,
Stein GS, Nurcombe V, van Wijnen AJ and Cool SM: Synergism between
Wnt3a and heparin enhances osteogenesis via a phosphoinositide
3-kinase/Akt/RUNX2 pathway. J Biol Chem. 285:26233–26244. 2010.
View Article : Google Scholar :
|
|
32
|
Das M, Bouchey DM, Moore MJ, Hopkins DC,
Nemenoff RA and Stenmark KR: Hypoxia-induced proliferative response
of vascular adventitial fibroblasts is dependent on g
protein-mediated activation of mitogen-activated protein kinases. J
Biol Chem. 276:15631–15640. 2001. View Article : Google Scholar
|
|
33
|
Seo KW, Lee SJ, Kim YH, Bae JU, Park SY,
Bae SS and Kim CD: Mechanical stretch increases MMP-2 production in
vascular smooth muscle cells via activation of PDGFR-beta/Akt
signaling pathway. PLoS One. 8:e704372013. View Article : Google Scholar :
|
|
34
|
Liu X, Huang X, Chen L, Zhang Y, Li M,
Wang L, Ge C, Wang H and Zhang M: Mechanical stretch promotes
matrix metalloproteinase-2 and prolyl-4-hydroxylase alpha1
production in human aortic smooth muscle cells via Akt-p38 MAPK-JNK
signaling. Int J Biochem Cell Biol. 62:15–23. 2015. View Article : Google Scholar
|
|
35
|
Tang CH, Yang RS, Huang TH, Lu DY, Chuang
WJ, Huang TF and Fu WM: Ultrasound stimulates cyclooxygenase-2
expression and increases bone formation through integrin, focal
adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in
osteoblasts. Mol Pharmacol. 69:2047–2057. 2006. View Article : Google Scholar
|
|
36
|
Case N, Thomas J, Sen B, Styner M, Xie Z,
Galior K and Rubin J: Mechanical regulation of glycogen synthase
kinase 3beta (GSK3beta) in mesenchymal stem cells is dependent on
Akt protein serine 473 phosphorylation via mTORC2 protein. J Biol
Chem. 286:39450–39456. 2011. View Article : Google Scholar :
|