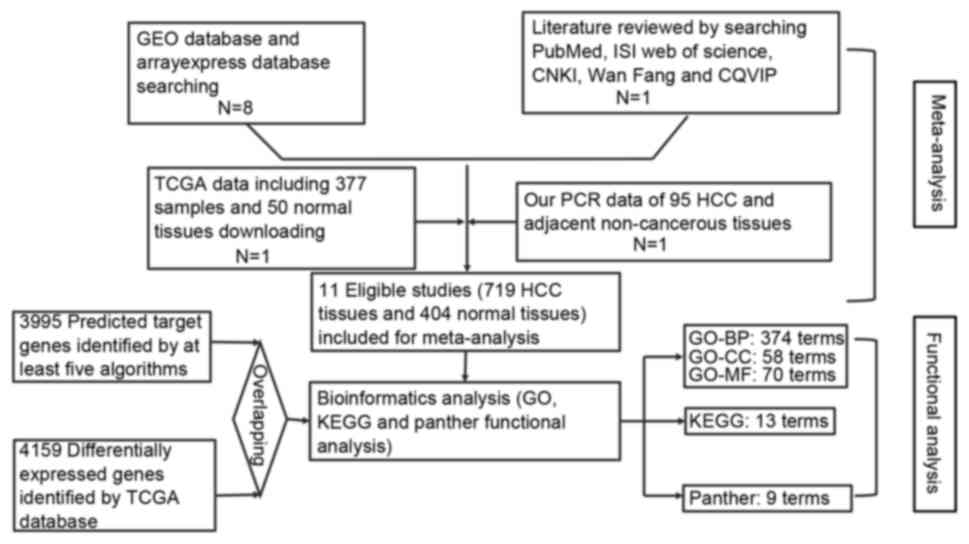
Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and lethal malignancies in the world with extremely poor
prognosis. HCC is also the second top cause of cancer-related death
and its incidence ranked fifth in diagnosed cancers globally
(1). Moreover, HCC is highly
prevalent in many Asian countries owing to its high endemicity of
hepatitis B virus (HBV) infection, especially in China (2–4).
Multiple treatment possibilities are available for HCC, such as
curative resection, liver transplantation, radiofrequency ablation,
systemic targeted agents like sorafenib, whereas the management of
advanced HCC still presents as a therapeutic challenge over the
years (5–9). Despite these developments in surgery
and other strategies, the prognosis of HCC patients is still
unsatisfactory due to the non-curable stages at the time of
diagnosis (10–14). Therefore, early diagnosis of HCC is
expected and the molecular pathogenesis of HCC needs to be better
elucidated.
MicroRNAs (miRNAs) are a class of highly conserved,
endogenous, small non-coding RNA molecules of 18–23 nucleotides in
length, which act as post-transcriptional regulators by binding to
3′-untranslated regions (3′-UTR) of mRNA for target genes (15–19).
In recent years, researchers have focused on the importance of
miRNAs in carcinogenesis (20–22).
A large amount of evidence has suggested that miRNAs may act as
oncogenes or tumor suppressors by adapting pathophysiological
progressions (23–27). In the new era of cancer therapy,
miRNAs are anticipated for early diagnosis and treatment of
patients in order to prolong their survival time (28–31).
miR-133a-3p, which belongs to the miR-133 family,
was first experimentally characterized in mice (32). In the human genome, miR-133a-3p is
a multicopy gene, with two known copies: miR-133a-1 and miR-133a-2,
located on chromosomes 18, 20 respectively. To date, multiple
functional roles of miR-133a-3p have been elucidated, such as
regulating myoblast proliferation and differentiation (33), inhibiting embryonic cardiomyocyte
proliferation (34) and avoiding
genetic cardiac hypertrophy (35).
Furthermore, it has been reported that miR-133a-3p is among the
most frequently downregulated miRNAs in various types of human
malignancies, which suggested that miR-133a-3p may serve a critical
part in tumor progression of various malignancies, including
non-small cell lung cancer (36),
ovarian cancer (37), colorectal
cancer (38), bladder cancer
(39), breast cancer (40) and prostate cancer (41). Nevertheless, there are rare reports
focusing on the role of miR-133a-3p in HCC. The results of
miR-133a-3p profile detection in HCC compared to non-tumor liver
tissues by microarray technologies, reverse
transcription-quantitative polymerase chain reaction and high
throughput technologies are contradictory. Moreover, previous
individual conclusion may not be reliable due to the small sample
size. Further explorations of the clinical role and the molecular
mechanism of miR-133a-3p in HCC are urgently needed.
Hence, the authors investigated the expression of
miR-133a-3p in tissues of HCC patients to illuminate its value as a
diagnostic biomarker based on data from Gene Expression Omnibus
(GEO), ArrayExpress, The Cancer Genome Atlas (TCGA) and literature.
In addition, the authors studied these data sets with an integrated
bioinformatics framework to explore how miR-133a-3p prospectively
performs its function in HCC. The pipeline of the present study is
charted in Fig. 1.
Materials and methods
Dataset sources
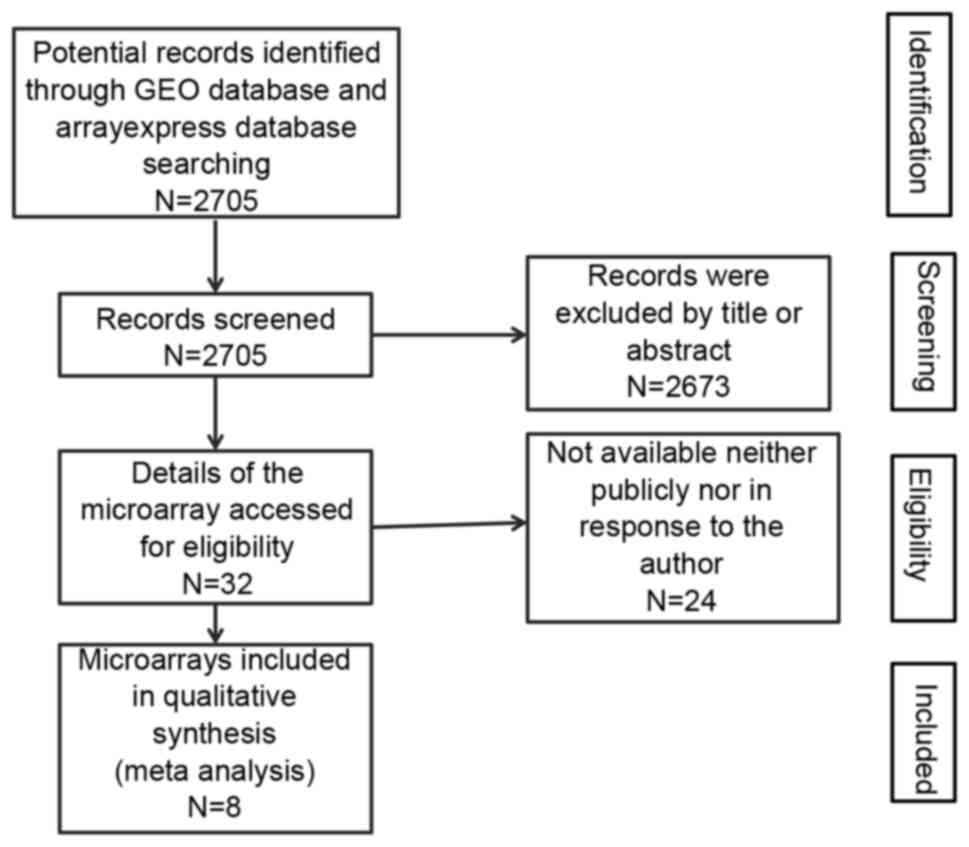
GEO and ArrayExpress database searching
Firstly, the microarrays related to HCC were
obtained from GEO database (www.ncbi.nlm.gov/geo) and ArrayExpress (www.ebi.ac.uk/arrayexpress). The search terms
included ‘malignant’ or ‘cancer’ or ‘tumor’ or ‘tumour’ or
‘neoplasm or ‘carcinoma’ and ‘hepatocellular or liver or hepatic or
HCC’. Then, microarrays that contained miR-133a-3p probe were
screened for further analysis (Fig.
2).
TCGA data including 377 samples and 50 normal
tissues downloading
The publicly available miRNA-seq and RNA-seq data of
the miRNA and mRNA level of Liver hepatocellular carcinoma (LIHC)
samples were obtained directly from the TCGA data portal
(https://tcga-data.nci.nih.gov/tcga/tcgaHome2.jsp)
via bulk download mode [LIHC (cancer type), miRNASeqV2 or RNASeqV2
(data type), level 3 (data level), All (preservation) and 1.12.0
(data version)] on October 16, 2016. The data were sequenced based
on Illumina Genome Analyzer miRNA or RNA Sequencing platform. Gene
expression data from miRNASeqV2 or RNASeqV2 results were quantified
by Expectation-Maximization (RSEM) (42,43)
with the ‘rsem.gene.normalized _results’ file type. Extracted data
were applied with no further transformation, except by rounding off
values to integers. These downloaded data included a total of 377
HCC samples and 50 normal liver samples. The data of miR-133a were
extracted and analyzed. The differentially expressed genes (DEGs)
between the HCC and the normal liver control samples were
identified by calculating the level of fold change (FC) in HCC vs.
normal liver tissue with R. The genes with a FC value >2 or
<0.5 and with P<0.05 were analyzed with Student's t-test were
selected as DEGs in the present study.
Literature reviewing
Studies published before October 16, 2016 that
reported the relationship between miR-133a-3p expression and HCC
were retrieved from PubMed, Web of Science, EMBASE, Wiley Online
Library, Cochrane Central Register of Controlled Trials, Science
Direct, Google Scholar, Ovid, LILACS, Chinese CNKI, Chong Qing VIP,
Wan Fang and China Biology Medicine disc. The searching terms were
as follows: (‘malignant’ or ‘cancer’ or ‘tumor’ or ‘tumour’ or
‘neoplasm’ or ‘carcinoma’) and (‘hepatocellular or liver or hepatic
or HCC’) and (miR-133a or miRNA-133a or microRNA-133a or miR133a or
miRNA133a or microRNA133a or ‘miR 133a’ or ‘miRNA 133a’ or
‘microRNA 133a’ or miR-133a-3p or miRNA-133a-3p or microRNA-133a-3p
or miR133a-3p or miRNA133a-3p or microRNA133a-3p or ‘miR 133a-3p’
or ‘miRNA 133a-3p’ or ‘microRNA 133a-3p’).
Study identification
Studies including microarrays or publications were
considered eligible if they met following criteria: i) Study on
human HCC tissues; ii) miR-133a-3p expression in tissues was
measured and analyzed; iii) literatures were published in Chinese
or English; iv) when data of the same patients were published in
more than one article, only the latest publication was included;
and v) as to microarrays, both HCC patients and normal samples were
included in each dataset, and each group contained more than two
samples.
Studies were excluded under the following criteria:
i) Letters, case reports, reviews, or conference reports were
excluded; ii) the required data could not be extracted or
calculated from the original article; iii) the article was not
found in full or had been published repeatedly; and iv) in
vitro studies.
Data extraction
All the eligible studies were carefully reviewed and
then the information was extracted from the inclusive studies
independently by two investigators (Hai-Wei Liang and Xia Yang).
Disagreements were negotiated by a third investigator (Gang Chen)
and agreements were reached by discussion. With the included
criteria, the following characteristics were obtained from eligible
articles: First author's name or series (prefix, GSE) accession
number, year of publication, country, number of patients and means
and standard deviations of miR-133a-3p expression level.
Prediction of targeted genes of miR-133a-3p
An online prediction software miRWalk, which
included 12 silico databases [such as TargetScan (www.targetscan.org), miRanda (www.miRnada.org) and PicTar (pictar.mdc-berlin.de)] was utilized to acquire the
potential target mRNAs of miR-133a-3p. A total of 3,995 target
genes were predicted initially, and only those genes appearing in
more than five prediction software programs were essential for the
present study.
Functional analyses
To further explore the biological processes and
signal pathways in which miR-133a-3p could be involved and also to
provide a theoretical foundation for future studies, functional
analyses were performed subsequently. Gene Ontology (GO) analysis
was conducted, since it demonstrates the predominant functions of
the target genes from three aspects: Biological process, molecular
function, as well as cellular component. Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway analysis (www.genome.jp/kegg) was used to excavate remarkable
pathways associated with target genes. All GO, KEGG pathway and
panther analyses were performed to gain a further insight into the
function of these potential target genes of miR-133a-3p in HCC by
database for Annotation, Visualization and Integrated Discovery
(DAVID). P<0.01 for GO terms and P<0.05 for pathways were
considered statistically significant.
Protein- protein interactions (PPIs) network
construction
The potential target genes in the top terms of KEGG
analysis were employed to construct PPIs network by STRING 10.0
(http://string-db.org/), a Search Tool for the
Retrieval of Interacting Genes/Proteins, with the minimum required
interaction score 0.4 (44). The
related data were downloaded from Tables/Exports module and the
complete PPI network was drawn, then hub genes were identified. The
proteins interactions information in STRING was derived from four
sources, i.e., i) literature-reported protein interactions; ii)
high-throughput experiments; iii) genome analysis and prediction;
and iv) co-expression studies. The protein product of a gene serves
as a node in the PPI network, and the connectivity degree denotes
the interplayed protein numbers of the specific protein.
Statistical analysis
As for data obtained from the TCGA database, the
miR-133a expression values were presented in a way as means ±
standard deviation. Receiver operating characteristic (ROC) curves
were performed to judge the diagnostic value of miR-133a in HCC.
The area under the curve (AUC) and P-value were calculated.
Student's t-test was applied to verify the difference between two
corresponding groups of various clinical features. Box-plots were
drawn when significant difference existed in two compared ones. All
above analyses were carried out via SPSS software (version 22.0;
IBM, SPSS, Armonk, NY, USA). With respect to meta-analysis,
standardized mean difference (SMD) and 95% confidence interval (CI)
were counted for pooled values. Sensitivity analysis was conducted
to assess the stability of included studies. Cochran's Q and
I2 test were used to evaluate heterogeneity, and
P<0.1 indicated significant heterogeneity. Publication bias was
estimated by the Egger's and Begg's test. Meta-analysis was
conducted using STATA 12.0 (StataCorp LLC, College Station, TX,
USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
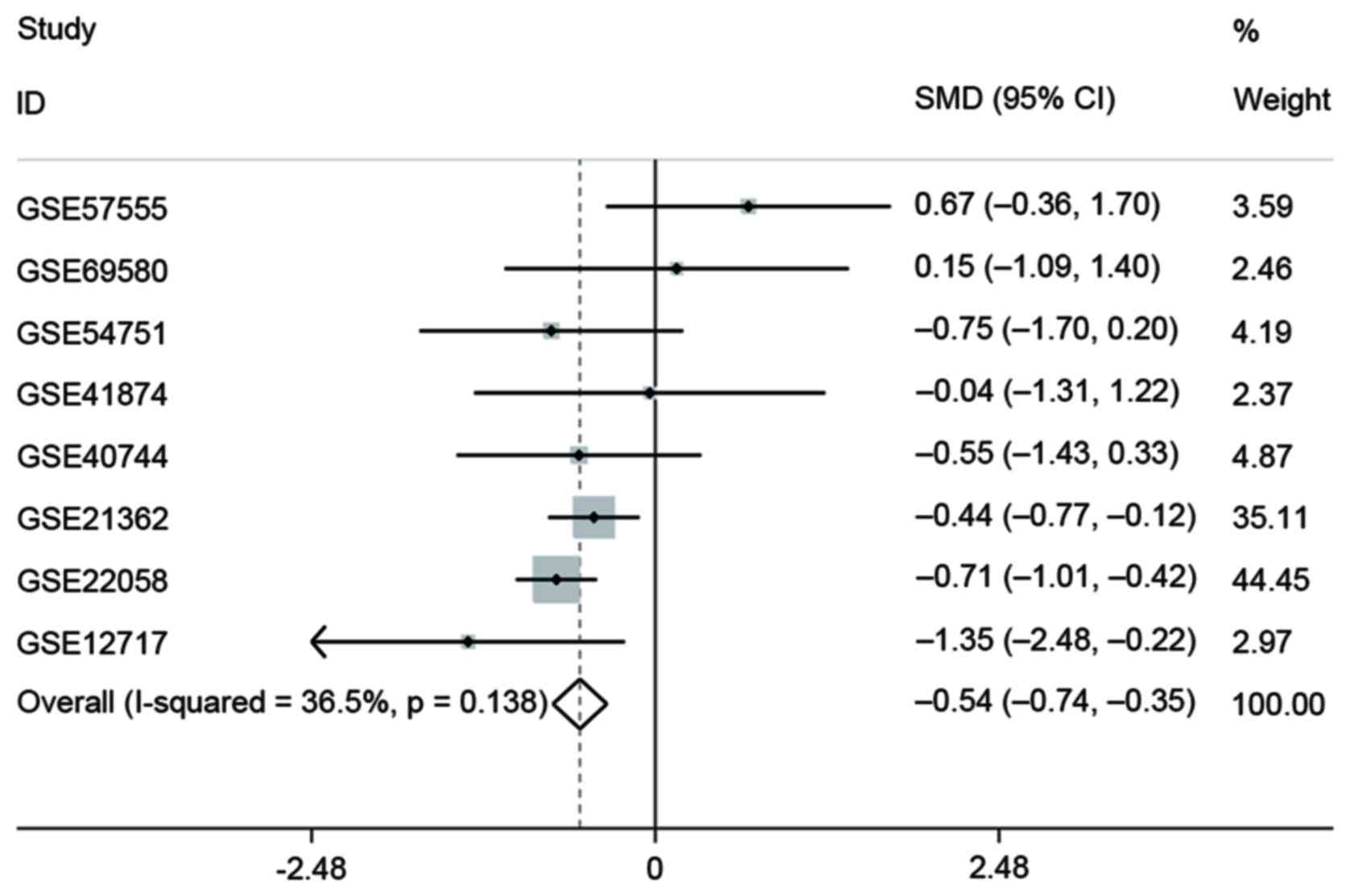
Signatures of GEO datasets and
meta-analysis
The authors first searched GEO datasets to assess
the expression of miR-133a-3p between HCC and non-cancer tissues.
miR-133a-3p levels in eight datasets GSE57555 (Japan), GSE69580
(Taiwan), GSE54751 (USA), GSE41874 (Japan), GSE40744 (USA),
GSE21362 (Japan), GSE22058 (USA) and GSE12717 (USA) which included
217 HCC and 219 normal tissues are presented in Fig. 3. One dataset about recurrence
(GSE64989, Germany) and two for invasiveness (GSE67138; GSE67139,
USA) were also presented. Then meta-analysis containing eight
microarrays was conducted to evaluate the diagnostic value of
miR-133a-3p in HCC. SMD with 95% CIs from involved datasets were
pooled, and default fixed-effects model was first used. The pooled
results revealed that noticeable difference was observed between
tumor and normal groups (SMD=−0.54; 95% CI, −0.74 to −0.35;
P<0.001; Fig. 4). No
significant heterogeneity was found (I2=36.5%;
P=0.138).
 | Figure 3.Box-plots presenting miR-133a-3p
expression in 11 evaluable microarray chips. (A) miR-133a-3p
expression in HCC tissues, CCC tissues and adjacent non-cancerous
tissues, P=0.2111. (B) miR-133a-3p expression in HCC tissues and
normal liver tissues, P=0.8557. (C) miR-133a-3p expression in HCC
and adjacent non-tumor part, P=0.1264. (D) miR-133a-3p expression
in primary hepatocellular carcinoma tissues, metastatic
hepatocellular carcinoma tissues and normal tissues, P=0.9510. (E)
miR-133a-3p expression in HCC tissues, HCC-CIR, CIR, ALF, NLA and
none liver cancer donors, P=0.0285. (F) miR-133a-3p expression in
HCC tissues and non-tumor tissues, P=0.0049. (G) miR-133a-3p
expression in HCC tissues and human healthy liver tissues,
P<0.001. (H) miR-133a-3p expression in HCC and normal tissues,
P=0.0203. (I) miR-133a-3p expression in recurrent HCC tissues and
non-recurrent HCC tissues, P=0.4130. (J) miR-133a-3p expression in
tumor vascular invasion tissues and tumor vascular invasiveness
tissues, P=0.0072. (K) miR-133a-3p expression in tumor vascular
invasion tissues and tumor vascular invasiveness tissues,
P<0.001. *P<0.05, **P<0.01, ***P<0.001 as indicated.
miR, microRNA; HCC, hepatocellular carcinoma; CCC,
cholangiocarcinoma; HCC-CIR, HCC surrounding non-tumorous tissue
affected by cirrhosis; CIR, HCV-associated cirrhosis without HCC;
ALF, HBV-associated acute liver failure; NLA, surrounding normal
liver of liver angioma. |
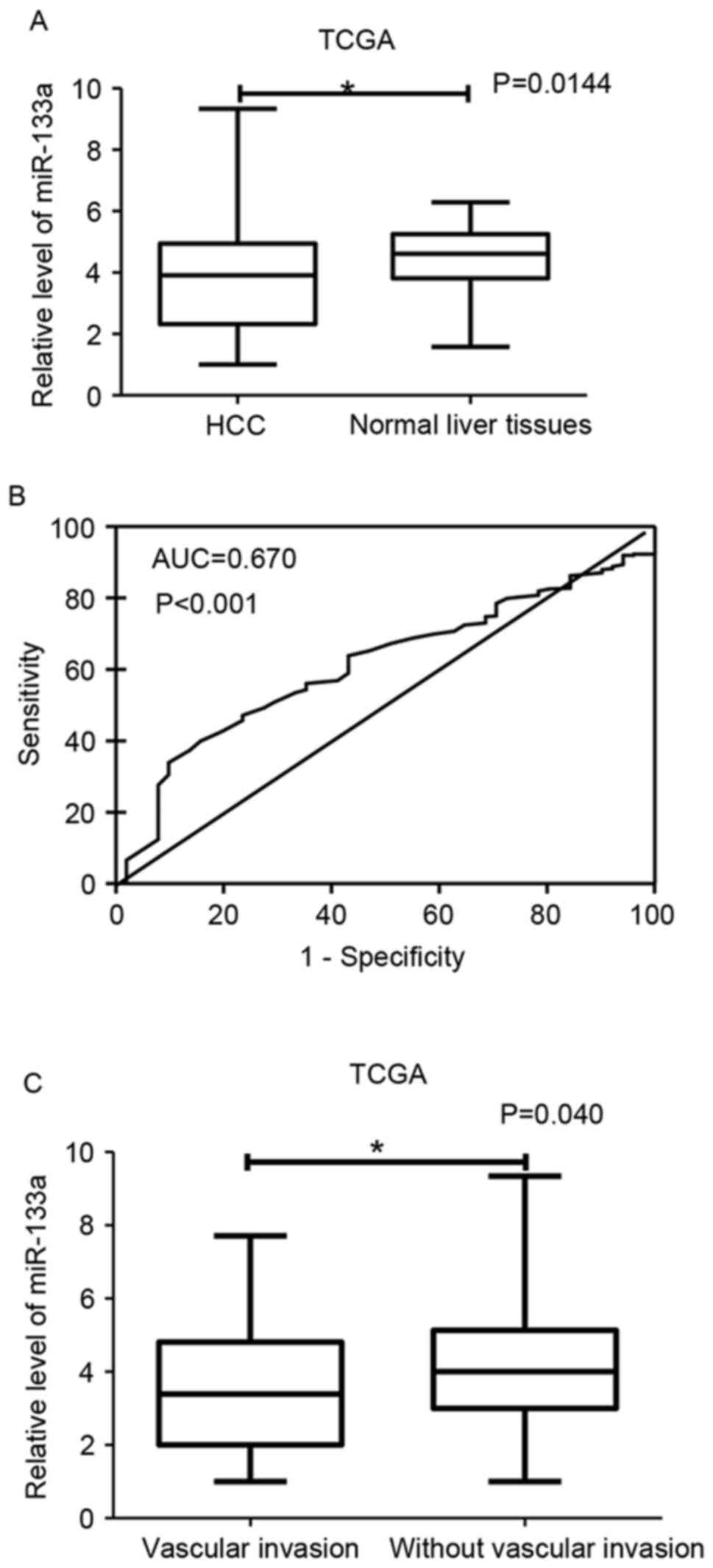
Clinical role of miR-133a in HCC
analyzed with TCGA data
In order to gain a larger number samples and
strengthen the reliability of our results, the authors subsequently
searched and downloaded TCGA data for further analysis. Before
adding to meta-analysis, they calculated the clinical significance
of miR-133a-1 in HCC with SPSS 20.0, since no data of the mature
miRNA, e.g., miR-133a-3p-1 were available for HCC in TCGA. The
results demonstrated that level of miR-133a-1 in HCC was 3.48±1.93,
which was reduced markedly when compared to that in non-cancerous
liver tissues (4.44±1.12, P<0.001; Fig. 5A). The AUC value of low expression
miR-133a-1 for HCC diagnosis was 0.670 (P<0.001; Fig. 5B). Regarding the difference of
miR-133a-1 expression between two corresponding groups of various
clinical features, the authors revealed that miR-133a-1 expression
in vascular invasion group (3.32±1.82) demonstrated a significant
reduced pattern compared to non-invasiveness group (3.77±1.75,
P=0.04; Fig. 5C). The relationship
between miR-133a-1 and vascular invasion was confirmed by Spearman
correlation test (r=−0.127; P=0.029). The data of miR-133a-2 was
not sufficient for analysis in TCGA database (data not shown).
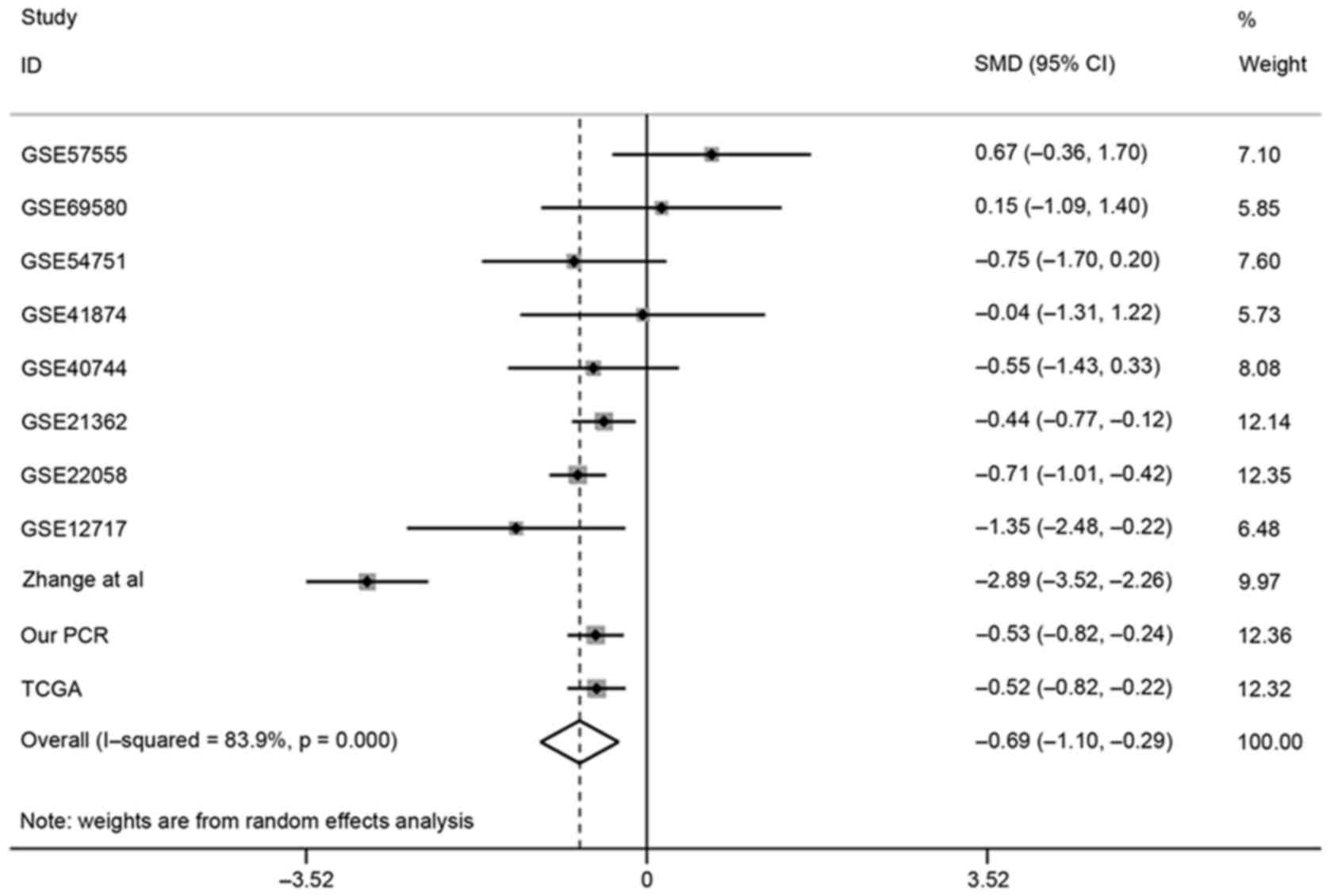
Meta-analysis with data from GEO,
TCGA, PubMed and PCR in house
Since only one publication from the literature
search was achieved for the comprehensive meta-analysis, the data
derived from TCGA, PubMed, literature (38) and the PCR data in house based on 95
HCC and matched adjacent normal tissue (data on file) were finally
merged in the present meta-analysis. In total, 719 HCC tissues and
404 normal tissues were involved. The number of HCC patients ranged
from 5 to 367 per dataset. The involved datasets signatures were
showed in Table I. The result
generated by fixed-effects model indicated that significant
heterogeneity existed among individual datasets
(I2=83.9%, P<0.001; Fig.
6). Thus, a random-effects model was selected to evaluate the
pooled SMD with 95% CI. The combined SMD suggested that remarkable
difference was noted between HCC group and normal control group
(SMD=−0.69; 95% CI, −1.10 to −0.29; P=0.001; Fig. 7). In other words, miR-133a-3p
expression in HCC patients was remarkably lower than that in
non-cancerous controls.
 | Table I.Characteristics of studies included
in the meta-analysis. |
Table I.
Characteristics of studies included
in the meta-analysis.
|
|
|
| Age (years) | Sex | n | Mean ± standard
deviation |
|---|
|
|
|
|
|
|
|
|
|---|
| Study | Year
(publication) | Country | <60 | ≥60 | Male | Female | HCC | Normal control | HCC | Normal |
|---|
| GSE57555 | 2015 | Japan | 6 | 15 | 18 | 3 | 5 | 16 |
−0.042±0.006 |
−0.053±0.018 |
| GSE69580 | 2015 | Taiwan | – | – | – | – | 5 | 5 |
1.854±1.106 |
1.697±0.937 |
| GSE54751 | 2014 | USA | 6 | 14 | 10 | 10 | 13 | 7 |
0.001±0.001 |
0.005±0.009 |
| GSE41874 | 2013 | Japan | 9 | 0 | 6 | 3 | 6 | 4 |
1.032±0.206 |
1.043±0.346 |
| GSE40744 | 2013 | USA | 16 | 5 | 15 | 6 | 9 | 12 |
1.741±0.171 |
1.862±0.247 |
| GSE21362 | 2011 | Japan | – | – | – | – | 73 | 73 |
2.632±1.514 |
3.316±1.571 |
| GSE22058 | 2010 | USA | – | – | – | – | 96 | 96 |
−0.746±0.369 |
−0.530±0.216 |
| GSE12717 | 2008 | USA | 16 | 0 | 4 | 12 | 10 | 6 |
6.475±1.395 |
7.997±0.219 |
| Zhang et
al | 2015 | China | – | – | 24 | 16 | 40 | 40 |
0.510±0.110 |
1.050±0.240 |
| PCR | 2016 | China | – | – | 75 | 20 | 95 | 95 |
3.285±2.379 |
4.497±2.195 |
| TCGA | – | – | 172 | 204 | 255 | 122 | 367 | 50 |
3.484±1.928 |
4.444±1.116 |
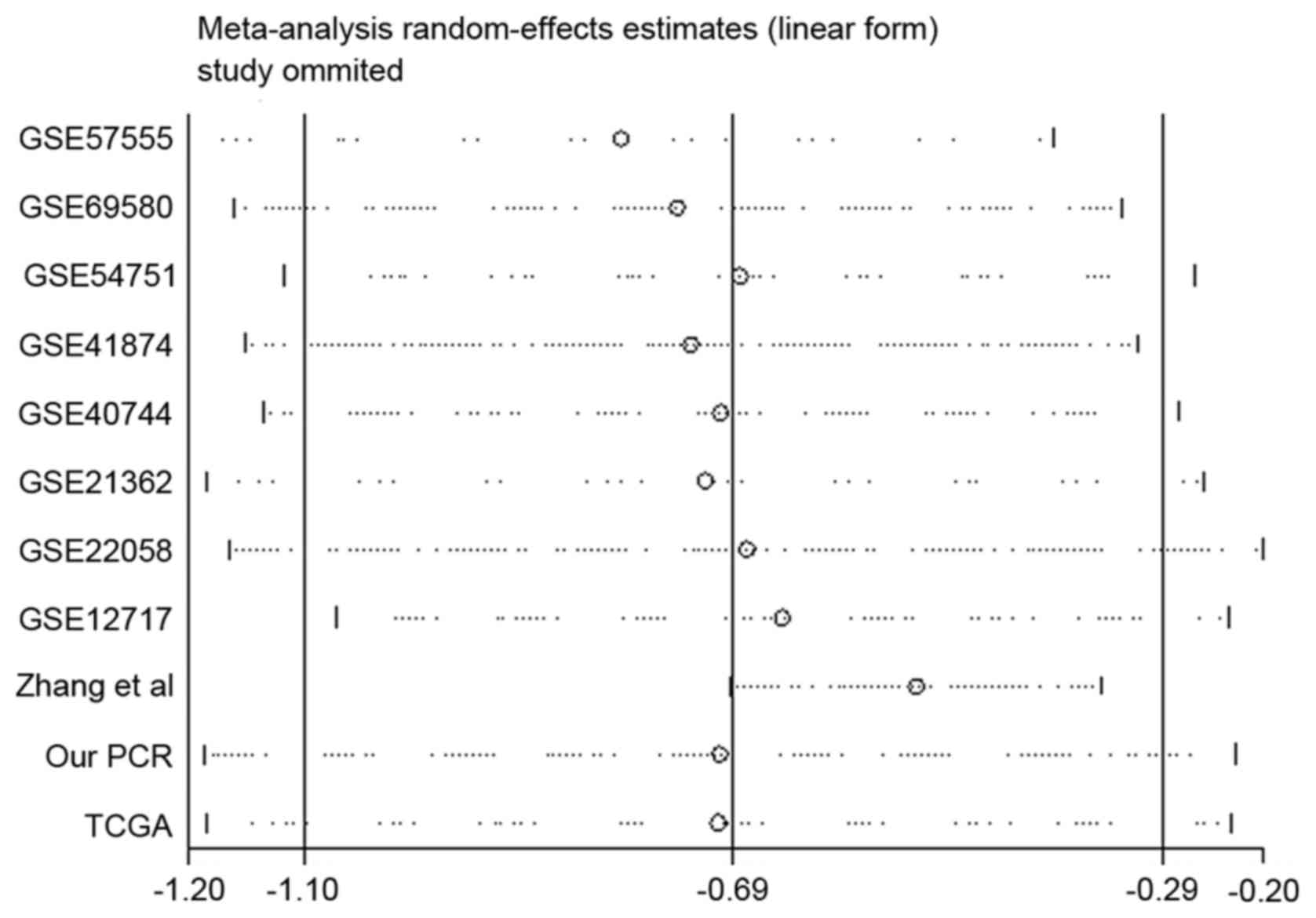
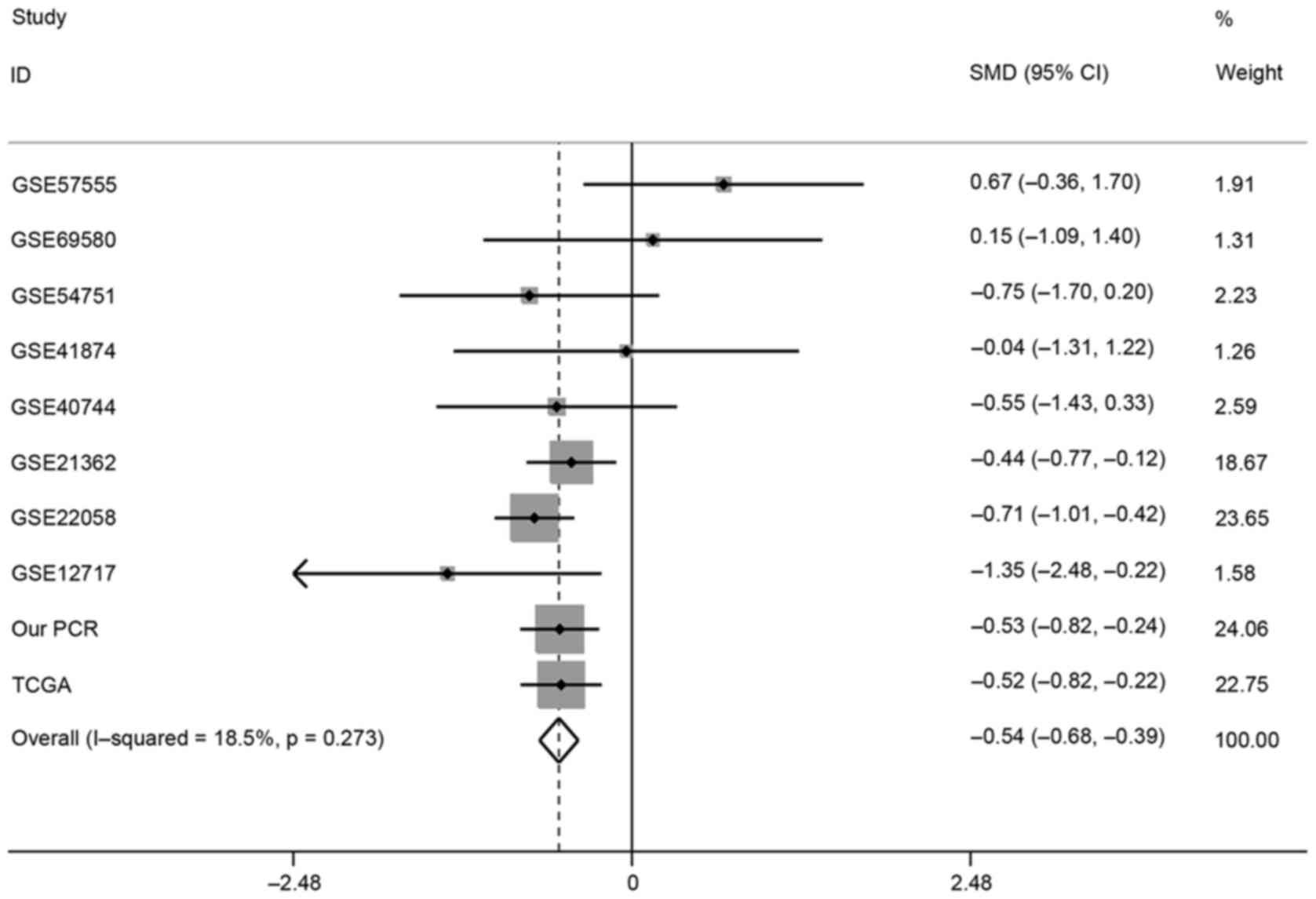
Sensitivity analysis
To identify the reason why notable heterogeneity
occurred in meta-analysis, the authors conducted a sensitivity
analysis (Fig. 6). According to
the results, dataset from Zhang et al (38) reported obvious deviation from the
estimate. Heterogeneity was found significantly reduced following
omission of the dataset of Zhang et al (I2=18.5%;
P=0.273), revealing heterogeneity may be perplexed by the present
study. The combined SMD with 95% CI after removal of data from
Zhang et al (SMD=−0.54, 95% CI, −0.68 to −0.39, P<0.001)
is presented in Fig. 8.
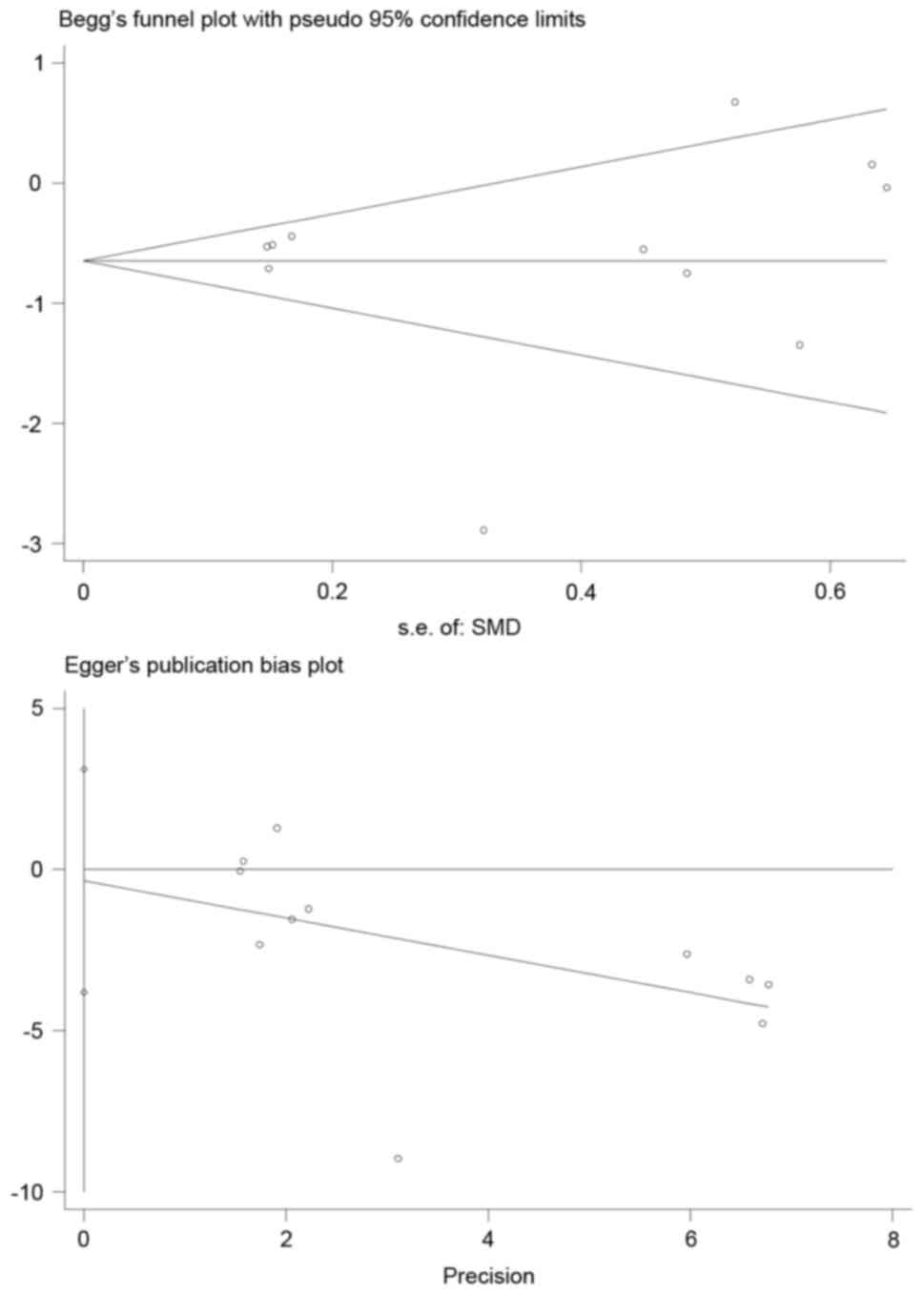
Publication bias
The authors performed both Begg's funnel plots and
Egger's tests for all datasets to evaluate the publication bias.
The results generated a Begg's test score of P=0.755>0.05
(Fig. 9) and an Egger's test score
of P=0.827>0.05, which suggested the absence of publication bias
in the present meta-analysis (Fig.
9).
Targets prediction and functional
enrichment
Both DEGs from TCGA and target genes of miR-133a-3p
predicted from 12 prediction algorithms were obtained. In total,
4,159 DEGs were retrieved from TCGA. In order to increase the
reliability, 3,995 potential target genes were identified by at
least five prediction algorithms. Then genes from both approaches
(DEGs from TCGA and target genes predicted) were overlapped and 828
putative targets were selected for further functional analyses.
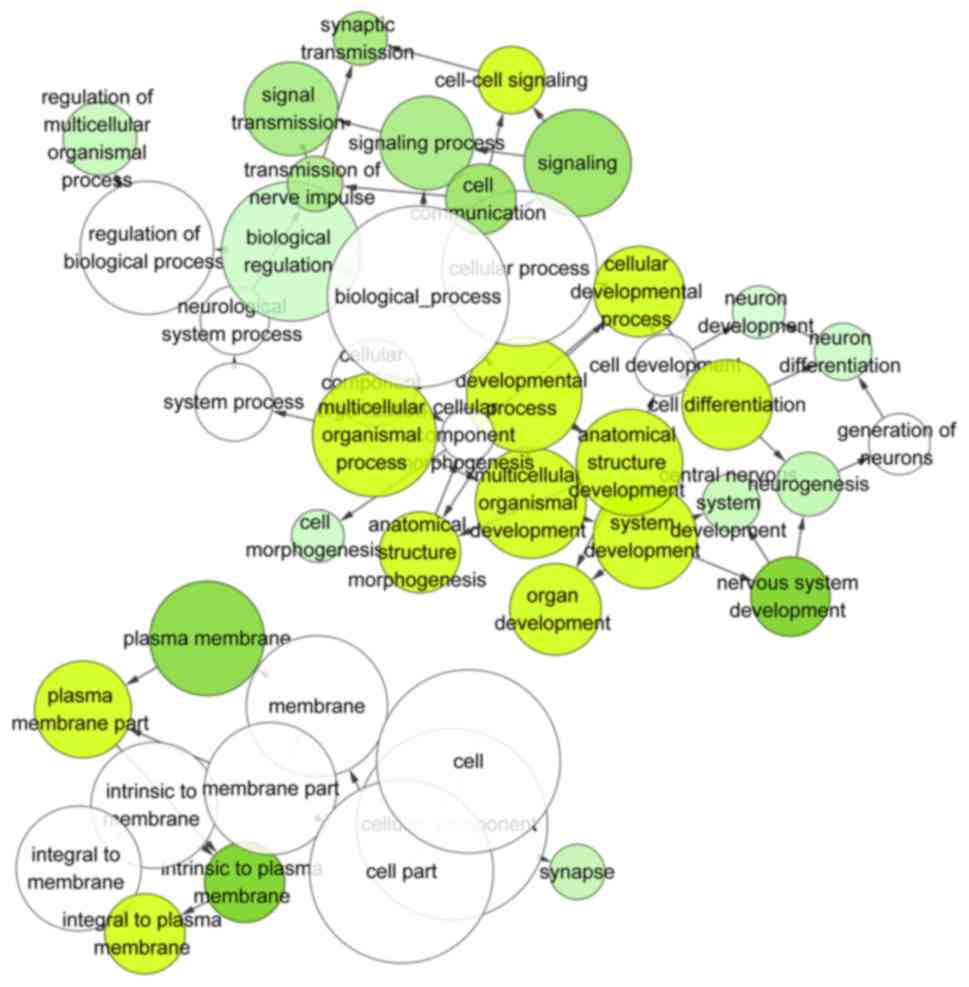
In the three categories of GO pathway analyses,
target genes were statistically enriched in biological process
(BP), in which 372 terms were identified and FDR were <0.05 in
21 terms. The top enriched BP term was cell-cell signaling (GO:
0007267), which contained 70 genes, and the FDR reached
4.02×10−9. Besides, the top term of cellular components
(CC) and molecular functions (MF) were the plasma membrane part
(GO: 0044459; FDR=2.42×10−10) and calcium ion binding
(GO: 0005509; FDR=0.014), respectively. The top 10 terms of each
category are listed in Table II,
and the GO pathway map is presented in Fig. 10.
 | Table II.GO functional annotation for most
significantly related targets of miR133a-3p. |
Table II.
GO functional annotation for most
significantly related targets of miR133a-3p.
| GO ID | GO term | Count (%) | P-value | Benjamini | FDR | Genesa |
|---|
| Biological
process |
|
GO:0007267 | Cell-cell
signaling | 70 (0.9) |
2.24×10−12 | 6.42E-09 | 4.02E-09 | SYT1, PDGFA,
SLC6A2, SLC6A4, EFNA3, LPAR3, ILDR2, GABBR2, ENPEP, GJA5 |
|
GO:0007268 | Synaptic
transmission | 40 (0.5) |
4.78×10−9 | 6.83E-06 | 8.56E-06 | PPFIA3, SYT1,
RAB3A, SLC6A2, OPRK1, SLC6A4, LPAR3, OXTR, GABBR2, DFNB31 |
|
GO:0019226 | Transmission of
nerve | 43 (0.6) |
1.63×10−8 | 1.55E-05 | 2.91E-05 | PPFIA3, SYT1,
RAB3A, SLC6A2, OPRK1, SLC6A4, LPAR3, OXTR, GABBR2, DFNB31 |
|
GO:0048666 | Neuron
development | 41 (0.6) |
5.71×10−8 | 4.08E-05 | 1.02E-04 | RAB3A, CDK5R1,
PAX2, CXCL12, EPHB1, EPHB2, TGFB2, DFNB31, LINGO1, BDNF |
|
GO:0030182 | Neuron
differentiation | 47 (0.6) |
1.99×10−7 | 1.14E-04 | 3.57E-04 | RAB3A, CDK5R1,
NNAT, BRSK1, PAX2, CXCL12, EPHB1, TGFB2, EPHB2, DFNB31 |
|
GO:0000902 | Cell
morphogenesis | 41 (0.6) |
2.17×10−7 | 1.04E-04 | 3.90E-04 | RAB3A, CDK5R1,
TFCP2L1, BRSK1, SOX6, SOX9, PAX2, CXCL12, EPHB1, EPHB2 |
|
GO:0031175 | Neuron
projection | 33 (0.4) |
3.37×10−7 | 1.38E-04 | 6.04E-04 | RAB3A, CDK5R1,
PAX2, CXCL12, EPHB1, EPHB2, LINGO1, BDNF, PVRL1, ANK3 |
|
GO:0000904 | Cell
morphogenesis | 32 (0.4) |
3.62×10−7 | 1.29E-04 | 6.48E-04 | RAB3A, CDK5R1,
SOX9, PAX2, CXCL12, EPHB1, EPHB2, TGFB2, BDNF, PVRL1 |
|
GO:0030030 | Cell
projection | 41 (0.6) |
5.20×10−7 | 1.65E-04 | 9.32E-04 | RAB3A, CDK5R1,
CROCC, PDGFA, LPAR3, PAX2, CXCL12, EPHB1, EPHB2, DFNB31 |
|
GO:0048667 | Cell morphogenesis
involved | 28 (0.4) |
1.48×10−6 | 4.23E-04 | 0.002647 | RAB3A, CDK5R1,
PAX2, CXCL12, EPHB1, EPHB2, BDNF, PVRL1, ANK3, BAI1 |
| Cellular
component |
|
GO:0044459 | Plasma membrane
part | 176 (2.4) |
1.74×10−13 | 6.66E-11 | 2.42E-10 | SYT1, ENAH, ADORA3,
SGMS2, SLC6A20, SLC6A2, SYT2, EFNA3, SYT3, SLC6A4 |
|
GO:0005887 | Integral to
plasma | 108 (1.5) |
2.56×10−11 | 4.88E-09 | 3.54E-08 | ADORA3, SGMS2,
SLC6A20, SLC6A2, SLC6A4, EFNA3, SLC7A8, CSPG4, LPAR3, ENPEP |
|
GO:0031226 | Intrinsic to
plasma | 109 (1.5) |
4.46×10−11 | 5.68E-09 | 6.18E-08 | ADORA3, SGMS2,
SLC6A20, SLC6A2, SLC6A4, EFNA3, SLC7A8, CSPG4, LPAR3, ENPEP |
|
GO:0005886 | Plasma
membrane | 239 (3.2) |
7.37×10−8 | 7.04E-06 | 1.02E-04 | SYT1, ADORA3, GLDN,
SGMS2, SYT2, EFNA3, SYT3, LPAR3, SYT9, SYP |
|
GO:0045202 | Synapse | 40 (0.5) |
7.75×10−7 | 5.92E-05 | 0.001073 | RAB3A, SYT1,
CDK5R1, ENAH, RAB3B, LZTS1, RAB3C, SYT2, SYT3, SYT9 |
|
GO:0016021 | Integral to
membrane | 299 (4.0) |
3.61×10−5 | 0.002295 | 0.049989 | CYP3A4, SYT1,
ADORA3, SGMS2, GLDN, SYT2, SLC9A2, EFNA3, SYT3, SYT9 |
|
GO:0034702 | Ion channel
complex | 25 (0.3) |
3.75×10−5 | 0.002043 | 0.051895 | KCNA3, CACNB3,
KCNJ14, KCNJ13, TTYH3, KCNQ4, BEST1, TTYH2, KCNE1, CHRFAM7A |
|
GO:0031224 | Intrinsic to
membrane | 307 (4.1) |
5.18×10−5 | 0.002469 | 0.071712 | CYP3A4, SYT1,
ADORA3, SGMS2, GLDN, SYT2, SLC9A2, SYT3, EFNA3, SYT9 |
|
GO:0030054 | Cell junction | 46 (0.6) |
5.59×10−5 | 0.002369 | 0.077386 | SYT1, ENAH, LZTS1,
SYT2, SYT3, FERMT1, SYT9, OXTR, BRSK1, GABBR2 |
|
GO:0043005 | Neuron
projection | 34 (0.5) |
7.87×10−5 | 0.003003 | 0.109018 | SYT1, CDK5R1,
LZTS1, TACR1, GABBR2, KLC2, TGFB2, DFNB31, PCSK1, KCNQ4 |
| Molecular
function |
|
GO:0005509 | Calcium ion
binding | 73 (1.0) |
9.04×10−6 | 0.007564 | 0.013963 | SYT1, LDLR, MASP1,
SYT2, MMP9, SYT3, SYT9, ENPEP, PCDHGA4, MMP3 |
|
GO:0004935 | Adrenoceptor
activity | 6 (0.1) |
4.55×10−5 | 0.018938 | 0.070301 | ADRB1, HTR4,
ADRA1B, ADRA1A, ADRA2B, ADRA1D |
|
GO:0005003 | Ephrin receptor
activity | 7 (0.1) |
8.10×10−5 | 0.022426 | 0.125062 | EPHA7, EFNB3,
EFNA3, EPHA10, EFNA4, EPHB1, EPHB2 |
|
GO:0022832 | Voltage-gated
channel activity | 23 (0.3) |
1.16×10−4 | 0.024163 | 0.179776 | HCN2, KCND3,
CACNA1I, CACNG4, KCNA3, KCNJ10, CACNB3, KCNK13, KCNJ14, KCTD7 |
|
GO:0005244 | Voltage-gatedion
channel activity | 23 (0.3) |
1.16×10−4 | 0.024163 | 0.179776 | HCN2, KCND3,
CACNA1I, CACNG4, KCNA3, KCNJ10, CACNB3, KCNK13, KCNJ14, KCTD7 |
|
GO:0022843 | Voltage-gatedcation
channel activity | 19 (0.3) |
1.71×10−4 | 0.02837 | 0.264299 | HCN2, KCND3,
CACNA1I, CACNG4, KCNA3, KCNJ10, CACNB3, KCNJ14, KCTD7, KCNJ13 |
|
GO:0005261 | Cation channel
activity | 28 (0.4) |
2.23×10−4 | 0.030778 | 0.344365 | KCNA3, KCNJ10,
CACNB3, KCNK13, KCNJ14, KCNK10, KCNJ13, KCNQ4, GPM6A, KCNE |
|
GO:0015267 | Channel
activity | 37 (0.5) |
2.31×10−4 | 0.027299 | 0.355692 | KCNA3, KCNJ10,
CACNB3, KCNK13, GJA5, KCNJ14, KCNK10, KCNJ13, MIP, TTYH3 |
|
GO:0022803 | Passive
transmembrane transporter activity | 37 (0.5) |
2.41×10−4 | 0.024983 | 0.371552 | KCNA3, KCNJ10,
CACNB3, KCNK13, GJA5, KCNJ14, KCNK10, KCNJ13, MIP, TTYH3 |
|
GO:0022838 | Substrate specific
channel activity | 36 (0.5) |
2.48×10−4 | 0.022846 | 0.3818 | KCNA3, KCNJ10,
CACNB3, KCNK13, KCNJ14, KCNK10, KCNJ13, MIP, TTYH3, KCNQ4 |
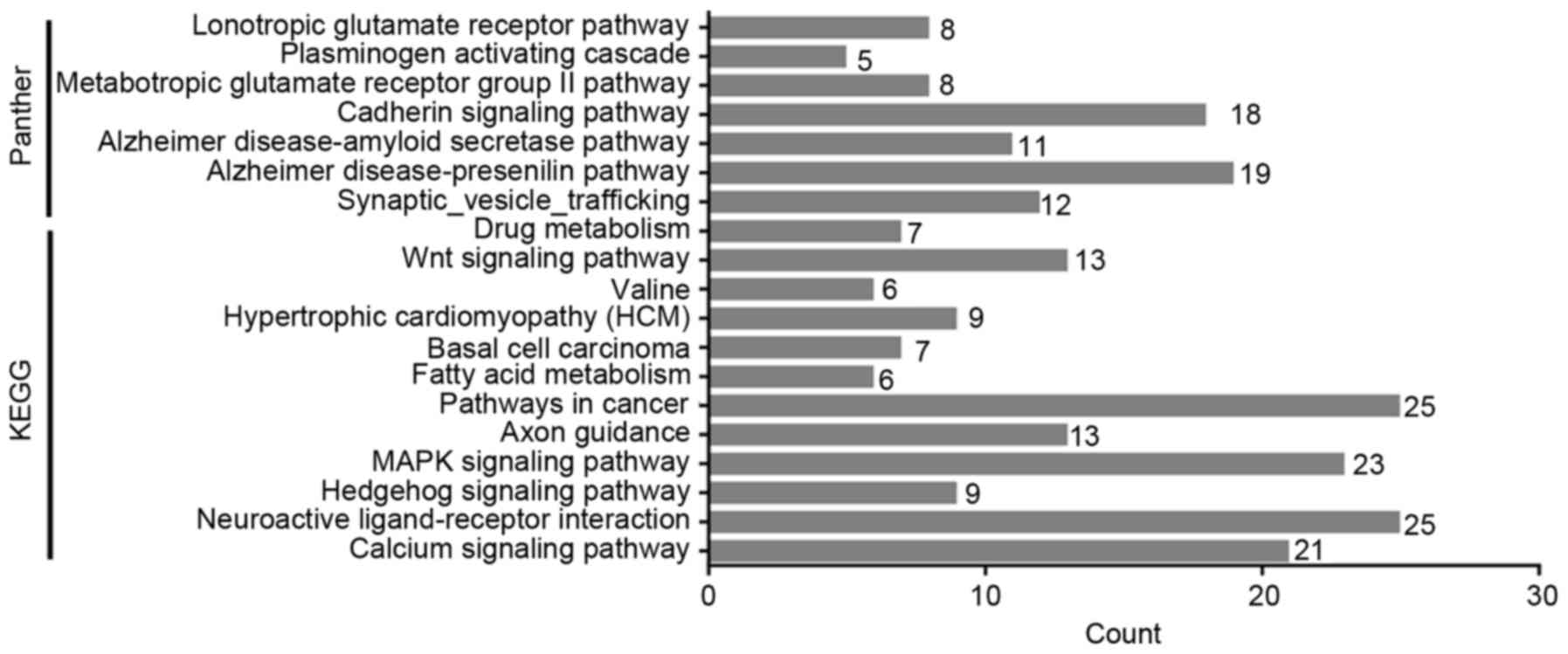
The KEGG pathways were also identified, many of
which were related to signaling pathway, such as the calcium
signaling pathway (hsa04020; P=2.94×10−4), neuroactive
ligand-receptor interaction (hsa04080; P=0.001) and the Hedgehog
signaling pathway (hsa04340; P=0.005). The target genes were
significantly enriched in PANTHER terms of
Synaptic-vesicle-trafficking (P05734; P=4.32×10−5), the
Alzheimer disease-presenilin pathway (P00004, P=0.002), the
Alzheimer disease-amyloid secretase pathway (P00003; P=0.016). All
KEGG and PANTHER pathway terms are listed in Table III and Fig. 11.
 | Table III.KEGG and PANTHER functional
annotation for most significantly related targets of
miR133a-3p. |
Table III.
KEGG and PANTHER functional
annotation for most significantly related targets of
miR133a-3p.
| KEGG ID | KEGG term | Count (%) | P-value | Benjamini | FDR | Genesa |
|---|
| hsa04020 | Calcium signaling
pathway | 21 (0.3) | 2.94E-04 | 0.044902 | 0.353432 | PTGER3, NOS1,
TACR1, CACNA1I, HTR4, OXTR, PTGFR, PRKCB, ADRB1, CAMK4 |
| hsa04080 | Neuroactive
ligand-receptor interaction | 25 (0.3) | 0.001306 | 0.096932 | 1.559243 | AVPR2, ADORA3,
TACR1, OPRK1, PTH1R, LPAR3, OXTR, GABBR2, GALR2,ADRA2B |
| hsa04340 | Hedgehog signaling
pathway | 9
(0.1) | 0.005185 | 0.236846 | 6.058102 | WNT10B, WNT4,
WNT7B, HHIP, LRP2, BMP7, BMP8B, ZIC2, WNT2B |
| hsa04010 | MAPK signaling
pathway | 23 (0.3) | 0.009768 | 0.318076 | 11.13201 | PDGFA, MAP2K3,
CACNA1I, FGF23, CACNG4, CACNB3, ACVR1C, TGFB2, CDC25B, PRKCB |
| hsa04360 | Axon guidance | 13 (0.2) | 0.022042 | 0.501133 | 23.50711 | EPHA7, SEMA6C,
EFNB3, LIMK2, PAK3, LIMK1, EFNA3, NFATC4, EFNA4, CXCL12 |
| hsa05200 | Pathways in
cancer | 25 (0.3) | 0.027097 | 0.51044 | 28.12737 | E2F1, APC2, PDGFA,
MMP9, ARNT2, FOXO1, TGFB2, ACVR1C, WNT4, CDKN2B |
| hsa00071 | Fatty acid
metabolism | 6
(0.1) | 0.042643 | 0.621365 | 40.78121 | ACADSB, ACADS,
ALDH1B1, ADH6, ADH1B, ACSL6 |
| hsa05217 | Basal cell
carcinoma | 7
(0.1) | 0.048982 | 0.624439 | 45.32723 | DVL2, WNT10B, WNT4,
WNT7B, APC2, HHIP, WNT2B |
| hsa05410 | Hypertrophic
cardiomyopathy (HCM) | 9
(0.1) | 0.052995 | 0.610863 | 48.03755 | ACE, CACNG4,
ITGA10, CACNB3, ITGA3, PRKAA2, TPM2, CACNA1C, TGFB2 |
| hsa00280 | Valine, leucine and
isoleucine degradation | 6
(0.1) | 0.060294 | 0.620966 | 52.65274 | BCAT1, ALDH6A1,
ACADSB, ACADS, ALDH1B1, AOX1 |
| hsa04310 | Wnt signaling
pathway | 13 (0.2) | 0.06213 | 0.597346 | 53.75325 | DVL2, WNT10B, WNT4,
WNT7B, NKD1, SFRP1, APC2, SFRP4, CAMK2B, NFATC4 |
| hsa00982 | Drug
metabolism | 7
(0.1) | 0.078798 | 0.655957 | 62.7222 | CYP3A4, GSTM2,
FMO2, AOX1, ADH6, ADH1B, UGT2B10 |
| P05734 | Synaptic vesicle
trafficking | 12 (0.2) | 4.32E-05 | 0.004006 | 0.047245 | SYP, RAB3A, SYT1,
RAB3B, STX1A, RAB3C, SYT2, SYT3, VAMP1, SNAP25 |
| P00004 | Alzheimer
disease-presenilin pathway | 19 (0.3) | 0.001606 | 0.072016 | 1.744225 | DVL2, WNT10B,
TRPC3, LDLR, MMP9, CDH3, MMP14, CTNNA3, WNT2B, PCSK |
| P00003 | Alzheimer
disease-amyloid secretase pathway | 11 (0.1) | 0.015543 | 0.384681 | 15.75932 | MAPK12, CHRM3,
MAPK13, BACE2, CACNB3, KLC2, CHRFAM7A, CACNA1C, PCSK5, PRKCB |
| P00012 | Cadherin signaling
pathway | 18 (0.2) | 0.021774 | 0.4006 | 21.41572 | WNT10B, PCDHGC5,
PCDH9, CDH3, PCDH17, PCDHGA4, PCDHGB2, PCDHGA2, CTNNA3 |
| P00040 | Metabotropic
glutamate receptor group II pathway | 8
(0.1) | 0.046646 | 0.588732 | 40.72335 | BDNF, GNAO1,
CACNA1E, CACNB3, VAMP1, SNAP25, CACNA1A |
| P00050 | Plasminogen
activating cascade | 5
(0.1) | 0.048863 | 0.539986 | 42.2145 | MMP9, SERPINE1,
MMP3, PLG |
| P00037 | Ionotropic
glutamate receptor pathway | 8
(0.1) | 0.065277 | 0.592151 | 52.24159 | CACNG4, CACNA1E,
CAMK2B, VAMP1, CAMK2A, SNAP25, CACNA1A |
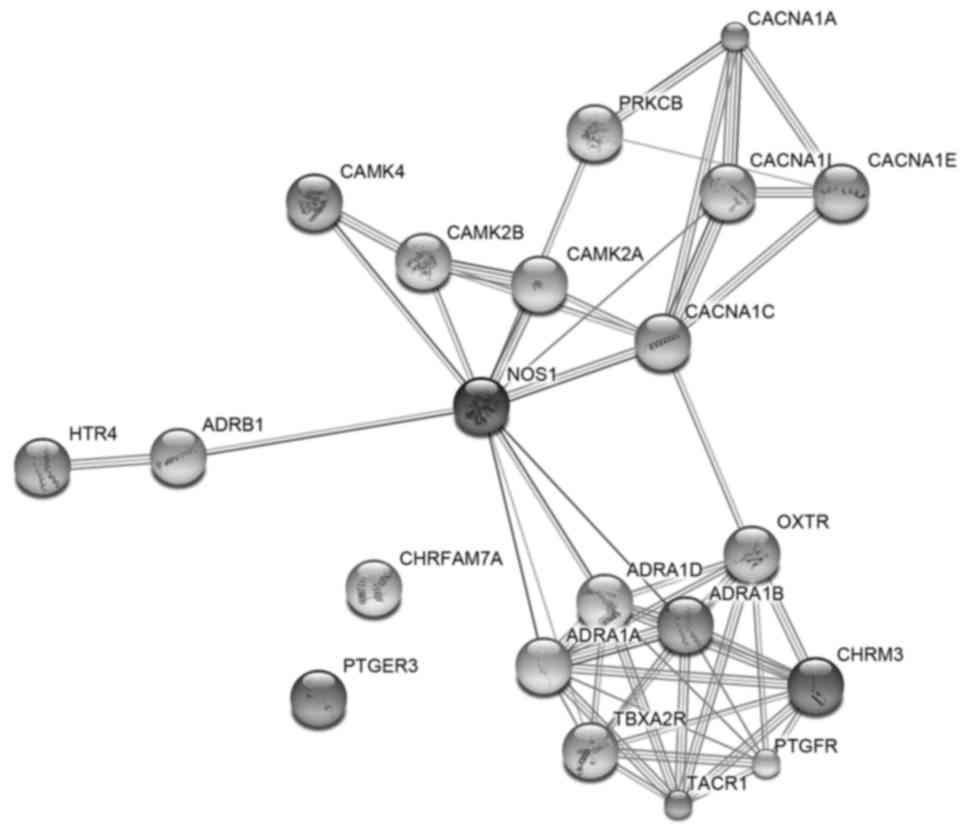
PPIs network of calcium signaling
pathway
Proteins rarely act alone as their functions tend to
be regulated. PPIs refer to lasting or ephemeral physical contacts
of high specificity which was established between two or more
protein molecules as a result of biochemical events steered by
electrostatic forces including the hydrophobic effect. Commonly
they refer to physical contacts with molecular associations between
chains that occur in a cell or in a living organism in a specific
biomolecular context. Thus, the authors decided to further analyze
the most significant pathway of KEGG by constructing PPIs network.
So ‘Calcium signaling pathway’ with 21 genes was included for the
PPI analysis. As a result, a total of five key genes with the
number of more than eight edges indicating higher connectivity
degree were identified, including NOS1, ADRA1A, ADRA1B, ADRA1D and
TBXA2R. PPIs network were presented in Fig. 12.
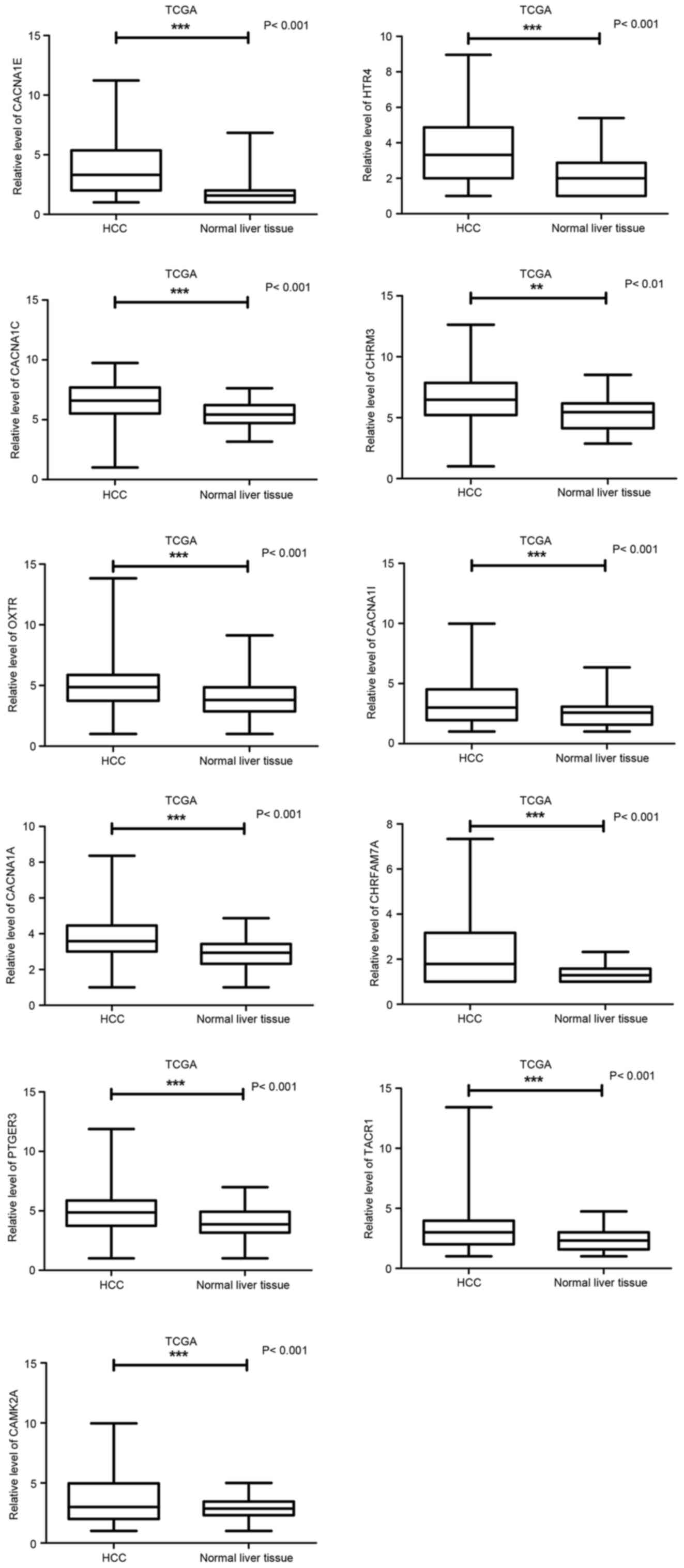
Validation of some potential target
gene expression based on TCGA dataset
Among all the significant pathways in the present
study, the Calcium signaling pathway (KEGG ID: hsa04020) was
selected for validation. There were 21 genes involved in the
pathway. The authors then analyzed the relative expression (on a
log2 scale) of these 21 predicted genes of miR-133a-3p by using
TCGA HCC dataset. Eight genes (NOS1, PRKCB, ADRA1A, ADRB1, TBXA2R,
CAMK4, ADRA1B, CAMK2B) were downregulated and they could not be
real targets of miR-133a-3p due to same trend of the expression in
HCC (Table IV). It was
interesting that 11 among the rest 13 genes presenting extremely
significant overexpressed pattern in liver cancers as compared to
that in the adjacent liver tissues (P<0.01; Table IV; Fig. 13A-K), such as CACNA1E, HTR4,
CACNA1C, CHRM3, OXTR, CACNA1I, CACNA1A, CHRFAM7A, PTGER3, TACR1,
CAMK2A.
 | Table IV.Expressions of 21 genes based on The
Cancer Genome Atlas dataset (374 HCC tissues and 50 normal liver
tissues). |
Table IV.
Expressions of 21 genes based on The
Cancer Genome Atlas dataset (374 HCC tissues and 50 normal liver
tissues).
| Gene name | HCC tissue (Mean ±
SD) | Normal liver tissue
(Mean ± SD) | t | P-value | FC |
|---|
| CACNA1E |
3.86±2.16 |
1.81±1.33 | −5.97 |
3.64×10−6a | 2.05 |
| HTR4 |
3.52±1.89 |
2.13±1.14 | −5.82 |
5.36×10−7a | 1.4 |
| CACNA1C |
6.5±1.65 |
5.38±1.03 | −6.62 |
2.81×10−9a | 1.12 |
| CHRM3 |
6.3±2.12 |
5.23±1.4 | −4.7 |
1.02×10−5a | 1.07 |
| OXTR |
4.91±1.79 |
3.86±1.66 | −3.83 |
1.47×10−4a | 1.04 |
| CACNA1I |
3.51±2.18 |
2.51±1.24 | −4.03 |
1.60×10−4a | 1.00 |
| CACNA1A |
3.73±1.3 |
2.85±0.82 | −6.44 |
7.71×10−9a | 0.88 |
| CHRFAM7A |
2.25±1.35 |
1.38±0.48 | −4.22 |
7.54×10−4a | 0.86 |
| PTGER3 |
4.88±1.73 |
4.03±1.42 | −3.23 |
1.35×10−3a | 0.84 |
| TACR1 |
3.21±1.7 |
2.38±0.91 | −4.65 |
1.46×10−5a | 0.84 |
| CAMK2A |
3.6±2.19 |
2.82±1.03 | −3.94 |
1.34×10−4a | 0.78 |
| ADRA1D |
3.61±2.26 |
3.27±1.74 | −0.72 |
4.69×10−1 | 0.34 |
| PTGFR |
5.92±3.04 |
5.6±1.21 | −1.37 |
1.74×10−1 | 0.32 |
| NOS1 |
2.64±1.84 |
2.97±0.98 | 1.66 |
9.98×10−2 | −0.33 |
| PRKCB |
6.67±1.65 |
7.78±1.43 | 4.56 |
6.62×10−6a | −1.12 |
| ADRA1A |
5.78±2.62 |
6.95±4.59 | 1.76 |
8.35×10−2 | −1.17 |
| ADRB1 |
3.71±1.76 |
5.03±1.49 | 5.71 |
2.37×10−7a | −1.33 |
| TBXA2R |
6.87±1.09 |
8.32±1.17 | 8.28 |
1.46×10−11a | −1.45 |
| CAMK4 |
5.25±1.44 |
7±1.09 | 10.17 |
1.09×10−15a | −1.74 |
| ADRA1B |
6.55±1.86 |
8.47±0.74 | 13.47 |
8.19×10−28a | −1.92 |
| CAMK2B |
5.32±2.63 |
8.55±0.89 | 17.13 |
3.33×10−41a | −3.23 |
Discussion
As the investigation surrounding miRNA function is
currently very significant in research, more and more miRNAs have
been reported to exert functions in HCC and development (45–54).
Downregulated miR-133a-3p has been identified in several types of
cancers, such as bladder (55),
colorectal (15), osteosarcoma
(56–58), non-small-cell lung (36,59)
and esophageal cancers (60).
However, the expression of miR-133a-3p in HCC tissues remains
controversial. Most pilot studies proposed that miR-133a-3p may
play as a tumor suppressor in HCC, while several studies present
inconsistency (GSE57555, GSE69580). How miR-133a-3p exerts its
function is still unclear. Comparing the data collected from GEO
datasets, TCGA and literature, the authors found that the uneven
sample size from different studies may contribute to the
contradictory results. In this meta-analysis, miR-133a-3p
expression was pooled from eight microarray datasets from GEO and
the other three studies from literature, TCGA and the authors' own
PCR data. The result generated by fixed-effects model indicated
that significant heterogeneity was present among individual
datasets (I2=83.9%; P<0.001; Fig. 6). The significant heterogeneity may
result from the following reasons: Firstly, different laboratories
differed broadly as a result of the inter-platform alterations. The
eight included GEO datasets utilized various platforms of
microarray by different research teams; thus, there existed
significant heterogeneity. Secondly, the samples were from
different countries, which indicated that different races may lead
to the significant heterogeneity. The eight GEO datasets included
four datasets from USA (GSE40744, GSE54751, GSE22058 and GSE12717),
three datasets from Japan (GSE57555, GSE41874 and GSE21362) and one
dataset (GSE69580) from Taiwan, while data of the literature and
the authors' own PCR were from mainland China. In addition, two
ethnicities, Asian and Caucasian, were pooled in this
meta-analysis. Furthermore, different techniques to detect the
expression of miR-133a-3p may also result in the significant
heterogeneity. In the present meta-analysis, the eight microarray
datasets from GEO, the studies from literature and the authors' own
PCR data used different means to detect the expression of
miR-133a-3p. The studies from the literature and our own data both
used PCR to detect the expression of miR-133a-3p, while the eight
GEO datasets used difference microarray/RNA-seq platforms. When the
data from Zhang et al (38)
was excluded, the heterogeneity changed from 83.9 to 18.5%, which
suggested that heterogeneity may be perplexed by this dataset.
However, their result indicated that miR-133a-3p could function as
a tumor suppressor in HCC, which was consistent with our combined
effect of miR-133a-3p. A total of 40 pairs of HCC tissue samples of
the study of Zhang et al (38) were collected from the First
Hospital of Jilin University (Changchun, China). Compared to other
case-control studies, the authors hypothesize that the limited
sample sizes could be the main reason for the current
heterogeneity. Wang et al (61) investigated 10 primary HCC samples
as well as their matched normal adjacent hepatic tissues, and
demonstrated that miR-133a-3p level was reduced in HCC. However,
this study was not included into this meta-analysis, for the means
and standard deviation or original data could not be collected.
Furthermore, Wang et al (61) also stated that overexpression of
miR-133a-3p suppressed cell growth, migration and invasion, whereas
the overexpression of miR-133a-3p enhanced apoptosis in HCC cells.
Though, some evidence reported that miR-133a-3p level was reduced
in HCC tissue, the non-invasive detection is more valuable for
clinic; therefore, the miR-133a-3p level in the serum of HCC
patients presents more clinical practice significance. Lin et
al (62) compared 108 patients
with HCC and 149 matched controls also indicated that miR-133a
notably expressed in the serum of HCC. In their study, the authors
identified a miRNA classifier (Cmi) containing seven
differentially expressed miRNAs (miR-29a, miR-29c, miR-133a,
miR-143, miR-145, miR-192, and miR-505) was a potential biomarker
for HCC. It could identify small-size, early-stage and
α-fetoprotein-negative HCC in patients at risk. The diagnosis value
of seven-miRNA combination achieved high performance (AUC>0.800
and accuracy>80%). However, Lin et al (62) indicated that miR-133a level in the
serum of HCC increased when compared with the control patients with
chronic hepatitis B, which was inconsistent with the authors'
present result. The different expression trend of miR-133a-3p
between tissue and serum might because of the different patient
samples and detection methods. For example, the matched controls of
this study included 51 healthy controls, 51 patients with chronic
hepatitis B, and 47 with HBV-induced liver cirrhosis; miRNAs level
were detected by reverse transcription-quantitative PCR. Thus, more
explorations are required to fig. out the miR-133a-3p level in the
serum of HCC patients.
Even though eight microarray gene sets did not show
noticeable heterogeneity in the meta-analysis, two of them
(GSE57555 and GSE69580) reported that miR-133a-3p expression was
higher in HCC than in normal tissues. One (GSE41874) demonstrated
no significant difference between HCC and normal tissues. Having
analyzed the three microarrays (GSE57555, GSE69580 and GSE41874),
the authors noticed that the number of normal samples (n=16) was
obviously larger than that of HCC tissues (n=5) in GSE57555, while
both tumor and normal samples were small in GSE69580 (n=5 for HCC,
n=5 for control) and GSE41874 (n=6 for HCC, n=4 for control). It
should be noted that, CIs intervals showed the same trend, which
provides more reliable evidence that miR-133a-3p serves a
suppressive role in HCC. Furthermore, miR-133a-3p could also be a
diagnostic marker for HCC.
In the present study, the authors observed that
miR-133a-1 expression in vascular invasion group (3.32±1.82)
revealed a significantly reduced pattern compared to the
non-invasiveness group (3.77±1.75; P=0.04; Fig. 5) from the TCGA data. Similarly, in
one GEO dataset (GSE67138), the expression of miR-133a-3p in the
vascular invasion group also showed significant reduced pattern
compared to without vascular invasion group (P=0.0072; Fig. 3J). It was indicated that decreased
expression of miR-133a-3p was closely correlated with vascular
invasion. Meanwhile, the present functional analyses indicated that
several target genes of miR-133a-3p were both involved in the
calcium signaling pathway and the mitogen-associated protein kinase
(MAPK) signaling pathway; from which the calcium signaling pathway
may involve in the vascular invasion of HCC (63), and the MAPK signaling pathway also
has been reported to promote cell growth and invasion in HCC
(64). Thus, miR-133a-3p may exert
its effect of vascular invasion by targeting CACNA1A, CACNA1C,
CACNA1E, CACNA1I and PRKCB in the calcium signaling pathway and the
MAPK signaling pathway.
The mature miRNA microRNA-133a-3p (miR-133a-3p,
previously named miR-133a) can be generated from miR-133a-1 and
miR-133a-2 (the precursor-miRNA, pre-miRNA). miR-133a-1 and
miR-133a-2 are located on different chromosomes and have different
sequences, with diverse functions in the process of transcription
(65). Theoretically, miRNAs are
transcribed as long primary-miRNA (pri-miRNA) transcripts that are
cleaved in the nucleus by the DROSHA (Drosha) enzyme to liberate
the precursor-miRNA (pre-miRNA) hairpin. The pre-miRNA is
subsequently exported from the nucleus and further processed by the
enzyme DICER1 (Dicer) in the cytoplasm to produce mature miRNAs.
miR-133a is encoded by two separate genes in humans (miR-133a-1 and
miR-133a-2). miR-133a-1 is located on chromosome 18 (MI0000450),
and miR-133a-2 is located on chromosome 20 (MI0000451). The
processing of the precursor transcripts by Dicer generates mature
miRNAs: miR-133a-3p.
The different sequences of miR-133a-1, miR-133a-2
and miR-133a-3p are as follows:
>hsa-mir-133a-1 MI0000450
ACA AUG CUU UGC UAG AGC UGG UAA AAU GGA ACC AAA UCG
CCU CUU CAA UGG AUU UGG UCC CCU UCA ACC AGC UGU AGC UAU GCA
UUGA
>hsa-mir-133a-2 MI0000451
GGG AGC CAA AUG CUU UGC UAG AGC UGG UAA AAU GGA ACC
AAA UCG ACU GUC CAA UGG AUU UGG UCC CCU UCA ACC AGC UGU AGC UGU GCA
UUG AUG GCG CCG
>hsa-miR-133a-3p MIMAT0000427
UUU GGU CCC CUU CAA CCA GCUG
miR-133a-1, miR-133a-2 and miR-133a-3p have
different sequences and different targets bind to their promoter
region in the transcription or translation process. Based on the
understanding of their diverse functions, the authors intend to
understand disease progression more precisely, and this may provide
new insights for disease treatment.
The molecular mechanism of miR-133a-3p in HCC is of
great importance to understand the carcinogenesis of HCC. Previous
studies have reported that miR-133a-3p may target fascin
actin-bundling protein 1 (FSCN1), insulin-like growth factor 1
receptor (IGF-1R), epidermal growth factor receptor, transgelin 2,
matrix metallopeptidase-9 (MMP-9), ATPase copper transporting β,
death associated protein kinase 2, SRY-box 4 and serum response
factor, to exert its function in different kinds of tumors.
Furthermore, FSCN1, IGF-1R and MMP-9 have been described as targets
of miR-133a-3p and influenced cell proliferation, colony formation,
migration and invasion in HCC cells. Thus, the authors deduce that
miR-133a-3p may target multiple mRNAs in different signaling
pathways of HCC. To get the whole picture of miR-133a-39 related
potential target networks, they conducted targets prediction and
functional pathway analyses. A total of 12 target prediction tools
were used for collection of the potential targets genes. In order
to minimize false positives, the potential target genes need to be
predicted in at least five in silico tools out of 12.
Subsequently, predicted targets were overlapped with DEGs from
TCGA, which are representative of key genes in the tumorigenesis of
HCC, to eventually obtain final target genes of miR-133a-3p in HCC.
Then functional analysis was conducted to investigate potential
molecular pathway of miR-133a-3p, which will contribute to the
understanding of molecular mechanisms of the tumorigenesis and
progression of HCC. The possible multiple pathways suggested that
miR-133a-3p can potentially regulate many of the required steps in
HCC development, from variations in cell morphogenesis to channel
activity, as well as various signaling pathways. The molecular
functions of these pathways may initiate a new trial on HCC
pathogenesis. These pathways could be a prospective source of novel
treatment targets and markers. Five key genes NOS1, ADRA1A, ADRA1B,
ADRA1D and TBXA2R were obtained from PPIs network of the calcium
signaling pathway, which was the most significantly enriched
pathway achieved from KEGG, are potentially targeted by miR-133a-3p
in HCC. However, these five hub genes have not yet been researched
in HCC; thus further experimental validation is needed.
Some limitations should be noted in the present
study. Firstly, significant heterogeneity was observed when all
data were pooled together in the meta-analysis. Subgroup analyses
have not been designed due to the small number of included studies.
Other confounding introduced by RNA extraction or individual
background may also disturb the result of the meta-analysis.
Secondly, the small sample size limited the robustness of the
meta-analysis conclusion. Further studies entailing large sample
size should be designed to confirm miR-133a-3p expression levels in
HCC. Thirdly, the potential target genes and pathways were only
predicted by bioinformatics without experimental verification. The
authors' group plans to perform in vitro and in vivo
experiments to validate these in silico findings in the
future.
To better explore the possibility of the target
candidates from simple prediction, the authors further inspected
the gene expression data from TCGA dataset and confirmed the
overexpression of 13 of 21 genes described in the calcium signaling
pathway (KEGG ID: hsa04020), which was the most significant KEGG
term. Especially, six genes were dramatically upregulated in liver
tumor tissues, giving an FC of >1. These genes may have more
possibility to be the real targets of miR-133a-3p in HCC due to
their noteworthy overexpression in cancer tissues. Of course, the
results need to be further confirmed with other experiments.
The present study mined the publicly available data,
including GEO, TCGA, published study and the authors' own PCR data
together to study the possible clinical role of miR-133a-3p. The
results suggested that miR-133a-3p may play as a tumor suppressor
in HCC. Furthermore, the prospective novel pathways of miR-133a-3p
could offer potential biomarkers for HCC. To the best of the
authors' knowledge, this is the first study to combine multiple
resources to validate miR-133a-3p suppressive role in HCC and to
explore its molecular mechanism by functional pathway analysis.
However, the findings need further experimental confirmation.
Acknowledgements
This study was supported partly by the Fund of
Guangxi Medical University Students Innovative Project (grant no.
201610598092).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar
|
|
2
|
Yu G, Chen X, Chen S, Ye W, Hou K and
Liang M: Arsenic trioxide reduces chemo-resistance to
5-fluorouracil and cisplatin in HBx-HepG2 cells via complex
mechanisms. Cancer Cell Int. 15:1162015. View Article : Google Scholar :
|
|
3
|
Bao J, Lu Y, Deng Y, Rong C, Liu Y, Huang
X, Song L, Li S and Qin X: Association between IL-18 polymorphisms,
serum levels, and HBV-related hepatocellular carcinoma in a Chinese
population: A retrospective case-control study. Cancer Cell Int.
15:722015. View Article : Google Scholar :
|
|
4
|
Luo Y, Zhang X, Tan Z, Wu P, Xiang X, Dang
Y and Chen G: Astrocyte elevated gene-1 as a novel
clinicopathological and prognostic biomarker for gastrointestinal
cancers: A meta-analysis with 2999 patients. PLoS One.
10:e01456592015. View Article : Google Scholar :
|
|
5
|
Yu MA, Liang P, Yu XL, Han ZY, Dong XJ,
Wang YU, Cheng C and Li X: Multiple courses of immunotherapy with
different immune cell types for patients with hepatocellular
carcinoma after microwave ablation. Exp Ther Med. 10:1460–1466.
2015. View Article : Google Scholar :
|
|
6
|
Luo X, Yang S, Zhou C, Pan F, Li Q and Ma
S: MicroRNA-328 enhances cellular motility through
posttranscriptional regulation of PTPRJ in human hepatocellular
carcinoma. Onco Targets Ther. 8:3159–3167. 2015.
|
|
7
|
Li T, Zhao S, Song B, Wei Z, Lu G, Zhou J
and Huo T: Effects of transforming growth factor β-1 infected human
bone marrow mesenchymal stem cells on high- and low-metastatic
potential hepatocellular carcinoma. Eur J Med Res. 20:562015.
View Article : Google Scholar :
|
|
8
|
Zhang JW, Li Y, Zeng XC, Zhang T, Fu BS,
Yi HM, Zhang Q and Jiang N: miR-630 overexpression in
hepatocellular carcinoma tissues is positively correlated with
alpha-fetoprotein. Med Sci Monit. 21:667–673. 2015. View Article : Google Scholar :
|
|
9
|
Hou YF, Wei YG, Li B, Yang JY, Wen TF, Xu
MQ, Yan LN and Wang WT: Upper abdominal shape as a risk factor of
extended operation time and severe postoperative complications in
HCC hepatectomy through subcostal incision. World J Surg Oncol.
13:2982015. View Article : Google Scholar :
|
|
10
|
Zhao Y, Fang Z, Luo J, Liu Q, Xu G, Pan H,
Wei W and Yan Z: Evaluation of extrahepatic collateral arteries in
hepatocellular carcinoma in three independent groups in a single
center. Exp Ther Med. 10:2366–2374. 2015. View Article : Google Scholar :
|
|
11
|
Wang L, Wang J, Zhang X, Li J, Wei X,
Cheng J, Ling Q, Xie H, Zhou L, Xu X and Zheng S: Diagnostic value
of preoperative needle biopsy for tumor grading assessment in
hepatocellular carcinoma. PLoS One. 10:e01442162015. View Article : Google Scholar :
|
|
12
|
Selitsky SR, Baran-Gale J, Honda M, Yamane
D, Masaki T, Fannin EE, Guerra B, Shirasaki T, Shimakami T, Kaneko
S, et al: Small tRNA-derived RNAs are increased and more abundant
than microRNAs in chronic hepatitis B and C. Sci Rep. 5:76752015.
View Article : Google Scholar :
|
|
13
|
Zuo D, Chen L, Liu X, Wang X, Xi Q, Luo Y,
Zhang N and Guo H: Combination of miR-125b and miR-27a enhances
sensitivity and specificity of AFP-based diagnosis of
hepatocellular carcinoma. Tumour Biol. 37:6539–6549. 2016.
View Article : Google Scholar
|
|
14
|
Jiang L, Cheng Q, Zhang BH and Zhang MZ:
Circulating microRNAs as biomarkers in hepatocellular carcinoma
screening: A validation set from China. Medicine (Baltimore).
94:e6032015. View Article : Google Scholar :
|
|
15
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar
|
|
16
|
Fornari F, Ferracin M, Trerè D, Milazzo M,
Marinelli S, Galassi M, Venerandi L, Pollutri D, Patrizi C, Borghi
A, et al: Circulating microRNAs, miR-939, miR-595, miR-519d and
miR-494, Identify Cirrhotic Patients with HCC. PLoS One.
10:e01414482015. View Article : Google Scholar :
|
|
17
|
He R, Yang L, Lin X, Chen X, Lin X, Wei F,
Liang X, Luo Y, Wu Y, Gan T, et al: miR-30a-5p suppresses cell
growth and enhances apoptosis of hepatocellular carcinoma cells via
targeting AEG-1. Int J Clin Exp Pathol. 8:15632–15641. 2015.
|
|
18
|
de Carvalho AC, Scapulatempo-Neto C, Maia
DC, Evangelista AF, Morini MA, Carvalho AL and Vettore AL: Erratum:
Accuracy of microRNAs as markers for the detection of neck lymph
node metastases in patients with head and neck squamous cell
carcinoma. BMC Med. 13:1552015. View Article : Google Scholar :
|
|
19
|
de Carvalho AC, Scapulatempo-Neto C, Maia
DC, Evangelista AF, Morini MA, Carvalho AL and Vettore AL: Accuracy
of microRNAs as markers for the detection of neck lymph node
metastases in patients with head and neck squamous cell carcinoma.
BMC Med. 13:1082015. View Article : Google Scholar :
|
|
20
|
Liu H, Li W, Chen C, Pei Y and Long X:
MiR-335 acts as a potential tumor suppressor miRNA via
downregulating ROCK1 expression in hepatocellular carcinoma. Tumour
Biol. 36:6313–6319. 2015. View Article : Google Scholar
|
|
21
|
Yin J, Hou P, Wu Z, Wang T and Nie Y:
Circulating miR-375 and miR-199a-3p as potential biomarkers for the
diagnosis of hepatocellular carcinoma. Tumour Biol. 36:4501–4507.
2015. View Article : Google Scholar
|
|
22
|
Liu Y, Ren F, Luo Y, Rong M, Chen G and
Dang Y: Down-Regulation of MiR-193a-3p Dictates Deterioration of
HCC: A Clinical Real-Time qRT-PCR Study. Med Sci Monit.
21:2352–2360. 2015. View Article : Google Scholar :
|
|
23
|
Zhang X, Tang W, Li R, He R, Gan T, Luo Y,
Chen G and Rong M: Downregulation of microRNA-132 indicates
progression in hepatocellular carcinoma. Exp Ther Med.
12:2095–2101. 2016. View Article : Google Scholar :
|
|
24
|
Motawi TK, Shaker OG, El-Maraghy SA and
Senousy MA: Serum MicroRNAs as potential biomarkers for early
diagnosis of hepatitis C Virus-related hepatocellular carcinoma in
egyptian patients. PLoS One. 10:e01377062015. View Article : Google Scholar :
|
|
25
|
Tu H, Wei G, Cai Q, Chen X, Sun Z, Cheng
C, Zhang L, Feng Y, Zhou H, Zhou B and Zeng T: MicroRNA-212
inhibits hepatocellular carcinoma cell proliferation and induces
apoptosis by targeting FOXA1. Onco Targets Ther. 8:2227–2235.
2015.
|
|
26
|
Zhang JG, Shi Y, Hong DF, Song M, Huang D,
Wang CY and Zhao G: miR-148b suppresses cell proliferation and
invasion in hepatocellular carcinoma by targeting WNT1/β-catenin
pathway. Sci Rep. 5:80872015. View Article : Google Scholar :
|
|
27
|
Guo GX, Li QY, Ma WL, Shi ZH and Ren XQ:
MicroRNA-485-5p suppresses cell proliferation and invasion in
hepatocellular carcinoma by targeting stanniocalcin 2. Int J Clin
Exp Pathol. 8:12292–12299. 2015.
|
|
28
|
Liu Y, Ren F, Rong M, Luo Y, Dang Y and
Chen G: Association between underexpression of microrna-203 and
clinicopathological significance in hepatocellular carcinoma
tissues. Cancer Cell Int. 15:622015. View Article : Google Scholar :
|
|
29
|
Yao H, Liu X, Chen S, Xia W and Chen X:
Decreased expression of serum miR-424 correlates with poor
prognosis of patients with hepatocellular carcinoma. Int J Clin Exp
Pathol. 8:14830–14835. 2015.
|
|
30
|
Huang CS, Yu W, Cui H, Wang YJ, Zhang L,
Han F and Huang T: Increased expression of miR-21 predicts poor
prognosis in patients with hepatocellular carcinoma. Int J Clin Exp
Pathol. 8:7234–7238. 2015.
|
|
31
|
Chen Z, Huang Z, Ye Q, Ming Y, Zhang S,
Zhao Y, Liu L, Wang Q and Cheng K: Prognostic significance and
anti-proliferation effect of microRNA-365 in hepatocellular
carcinoma. Int J Clin Exp Pathol. 8:1705–1711. 2015.
|
|
32
|
Lagos-Quintana M, Rauhut R, Yalcin A,
Meyer J, Lendeckel W and Tuschl T: Identification of
tissue-specific microRNAs from mouse. Curr Biol. 12:735–739. 2002.
View Article : Google Scholar
|
|
33
|
Koutsoulidou A, Mastroyiannopoulos NP,
Furling D, Uney JB and Phylactou LA: Expression of miR-1, miR-133a,
miR-133b and miR-206 increases during development of human skeletal
muscle. BMC Dev Biol. 11:342011. View Article : Google Scholar :
|
|
34
|
Babiarz JE, Ravon M, Sridhar S, Ravindran
P, Swanson B, Bitter H, Weiser T, Chiao E, Certa U and Kolaja KL:
Determination of the human cardiomyocyte mRNA and miRNA
differentiation network by fine-scale profiling. Stem Cells Dev.
21:1956–1965. 2012. View Article : Google Scholar
|
|
35
|
Li Y, Cai X, Guan Y, Wang L, Wang S, Li Y,
Fu Y, Gao X and Su G: Adiponectin upregulates miR-133a in cardiac
hypertrophy through AMPK activation and reduced ERK1/2
phosphorylation. PLoS One. 11:e01484822016. View Article : Google Scholar :
|
|
36
|
Wang LK, Hsiao TH, Hong TM, Chen HY, Kao
SH, Wang WL, Yu SL, Lin CW and Yang PC: MicroRNA-133a suppresses
multiple oncogenic membrane receptors and cell invasion in
non-small cell lung carcinoma. PLoS One. 9:e967652014. View Article : Google Scholar :
|
|
37
|
Guo J, Xia B, Meng F and Lou G: miR-133a
suppresses ovarian cancer cell proliferation by directly targeting
insulin-like growth factor 1 receptor. Tumour Biol. 35:1557–1564.
2014. View Article : Google Scholar
|
|
38
|
Zhang W, Liu K, Liu S, Ji B, Wang Y and
Liu Y: MicroRNA-133a functions as a tumor suppressor by targeting
IGF-1R in hepatocellular carcinoma. Tumour Biol. 36:9779–9788.
2015. View Article : Google Scholar
|
|
39
|
Yoshino H, Chiyomaru T, Enokida H,
Kawakami K, Tatarano S, Nishiyama K, Nohata N, Seki N and Nakagawa
M: The tumour-suppressive function of miR-1 and miR-133a targeting
TAGLN2 in bladder cancer. Br J Cancer. 104:808–818. 2011.
View Article : Google Scholar :
|
|
40
|
Cui W, Zhang S, Shan C, Zhou L and Zhou Z:
microRNA-133a regulates the cell cycle and proliferation of breast
cancer cells by targeting epidermal growth factor receptor through
the EGFR/Akt signaling pathway. FEBS J. 280:3962–3974. 2013.
View Article : Google Scholar
|
|
41
|
Kojima S, Chiyomaru T, Kawakami K, Yoshino
H, Enokida H, Nohata N, Fuse M, Ichikawa T, Naya Y, Nakagawa M and
Seki N: Tumour suppressors miR-1 and miR-133a target the oncogenic
function of purine nucleoside phosphorylase (PNP) in prostate
cancer. Br J Cancer. 106:405–413. 2012. View Article : Google Scholar
|
|
42
|
Cheng H, Fertig EJ, Ozawa H, Hatakeyama H,
Howard JD, Perez J, Considine M, Thakar M, Ranaweera R, Krigsfeld G
and Chung CH: Decreased SMAD4 expression is associated with
induction of epithelial-to-mesenchymal transition and cetuximab
resistance in head and neck squamous cell carcinoma. Cancer Biol
Ther. 16:1252–1258. 2015. View Article : Google Scholar :
|
|
43
|
Guo Y, Sheng Q, Li J, Ye F, Samuels DC and
Shyr Y: Large scale comparison of gene expression levels by
microarrays and RNAseq using TCGA data. PLoS One. 8:e714622013.
View Article : Google Scholar :
|
|
44
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:(Database issue). D447–D452. 2015. View Article : Google Scholar
|
|
45
|
Zhao L and Wang W: miR-125b suppresses the
proliferation of hepatocellular carcinoma cells by targeting
Sirtuin7. Int J Clin Exp Med. 8:18469–18475. 2015.
|
|
46
|
Chen Y, Dong X, Yu D and Wang X: Serum
miR-96 is a promising biomarker for hepatocellular carcinoma in
patients with chronic hepatitis B virus infection. Int J Clin Exp
Med. 8:18462–18468. 2015.
|
|
47
|
Gan TQ, Tang RX, He RQ, Dang YW, Xie Y and
Chen G: Upregulated MiR-1269 in hepatocellular carcinoma and its
clinical significance. Int J Clin Exp Med. 8:714–721. 2015.
|
|
48
|
Mao B and Wang G: MicroRNAs involved with
hepatocellular carcinoma (Review). Oncol Rep. 34:2811–2820. 2015.
View Article : Google Scholar
|
|
49
|
Yang W, Dou C, Wang Y, Jia Y, Li C, Zheng
X and Tu K: MicroRNA-92a contributes to tumor growth of human
hepatocellular carcinoma by targeting FBXW7. Oncol Rep.
34:2576–2584. 2015. View Article : Google Scholar
|
|
50
|
Yang J, Liu X, Yuan X and Wang Z: miR-99b
promotes metastasis of hepatocellular carcinoma through inhibition
of claudin 11 expression and may serve as a prognostic marker.
Oncol Rep. 34:1415–1423. 2015. View Article : Google Scholar
|
|
51
|
Jin Q, Li XJ and Cao PG: MicroRNA-26b
enhances the radiosensitivity of hepatocellular carcinoma cells by
targeting EphA2. Tohoku J Exp Med. 238:143–151. 2016. View Article : Google Scholar
|
|
52
|
Yang F, Li QJ, Gong ZB, Zhou L, You N,
Wang S, Li XL, Li JJ, An JZ, Wang DS, et al: MicroRNA-34a targets
Bcl-2 and sensitizes human hepatocellular carcinoma cells to
sorafenib treatment. Technol Cancer Res Treat. 13:77–86. 2014.
View Article : Google Scholar
|
|
53
|
Wu G, Wang Y, Lu X, He H, Liu H, Meng X,
Xia S, Zheng K and Liu B: Low mir-372 expression correlates with
poor prognosis and tumor metastasis in hepatocellular carcinoma.
BMC Cancer. 15:1822015. View Article : Google Scholar :
|
|
54
|
Drakaki A, Hatziapostolou M, Polytarchou
C, Vorvis C, Poultsides GA, Souglakos J, Georgoulias V and
Iliopoulos D: Functional microRNA high throughput screening reveals
miR-9 as a central regulator of liver oncogenesis by affecting the
PPARA-CDH1 pathway. BMC Cancer. 15:5422015. View Article : Google Scholar :
|
|
55
|
Wang W, Wang X, Zhang Y, Wang D, Gao H,
Wang L and Gao S: Prognostic role of microRNA-150 in various
carcinomas: a meta-analysis. Onco Targets Ther. 9:1371–1379. 2016.
View Article : Google Scholar :
|
|
56
|
Mirghasemi A, Taheriazam A, Karbasy SH,
Torkaman A, Shakeri M, Yahaghi E and Mokarizadeh A: Down-regulation
of miR-133a and miR-539 are associated with unfavorable prognosis
in patients suffering from osteosarcoma. Cancer Cell Int.
15:862015. View Article : Google Scholar :
|
|
57
|
Fujiwara T, Katsuda T, Hagiwara K, Kosaka
N, Yoshioka Y, Takahashi RU, Takeshita F, Kubota D, Kondo T,
Ichikawa H, et al: Clinical relevance and therapeutic significance
of microRNA-133a expression profiles and functions in malignant
osteosarcoma-initiating cells. Stem Cells. 32:959–973. 2014.
View Article : Google Scholar
|
|
58
|
Ji F, Zhang H, Wang Y, Li M, Xu W, Kang Y,
Wang Z, Wang Z, Cheng P, Tong D, et al: MicroRNA-133a,
downregulated in osteosarcoma, suppresses proliferation and
promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone. 56:220–226.
2013. View Article : Google Scholar
|
|
59
|
Lan D, Zhang X, He R, Tang R, Li P, He Q
and Chen G: miR-133a is downregulated in non-small cell lung
cancer: A study of clinical significance. Eur J Med Res. 20:502015.
View Article : Google Scholar :
|
|
60
|
Akanuma N, Hoshino I, Akutsu Y, Murakami
K, Isozaki Y, Maruyama T, Yusup G, Qin W, Toyozumi T, Takahashi M,
et al: MicroRNA-133a regulates the mRNAs of two invadopodia-related
proteins, FSCN1 and MMP14, in esophageal cancer. Br J Cancer.
110:189–198. 2014. View Article : Google Scholar
|
|
61
|
Wang G, Zhu S, Gu Y, Chen Q, Liu X and Fu
H: MicroRNA-145 and microRNA-133a inhibited proliferation,
migration, and invasion, while promoted apoptosis in hepatocellular
carcinoma cells via targeting FSCN1. Dig Dis Sci. 60:3044–3052.
2015. View Article : Google Scholar
|
|
62
|
Lin XJ, Chong Y, Guo ZW, Xie C, Yang XJ,
Zhang Q, Li SP, Xiong Y, Yuan Y, Min J, et al: A serum microRNA
classifier for early detection of hepatocellular carcinoma: A
multicentre, retrospective, longitudinal biomarker identification
study with a nested case-control study. Lancet Oncol. 16:804–815.
2015. View Article : Google Scholar
|
|
63
|
Kaufmann R, Mussbach F, Henklein P and
Settmacher U: Proteinase-activated receptor 2-mediated calcium
signaling in hepatocellular carcinoma cells. J Cancer Res Clin
Oncol. 137:965–973. 2011. View Article : Google Scholar
|
|
64
|
Wu R, Duan L, Cui F, Cao J, Xiang Y, Tang
Y and Zhou L: S100A9 promotes human hepatocellular carcinoma cell
growth and invasion through RAGE-mediated ERK1/2 and p38 MAPK
pathways. Exp Cell Res. 334:228–238. 2015. View Article : Google Scholar
|
|
65
|
Huang WT, Wang HL, Yang H, Ren FH, Luo YH,
Huang CQ, Liang YY, Liang HW, Chen G and Dang YW: Lower expressed
miR-198 and its potential targets in hepatocellular carcinoma: A
clinicopathological and in silico study. Onco Targets Ther.
9:5163–5180. 2016. View Article : Google Scholar :
|