Introduction
As a functional gastrointestinal disorder, irritable
bowel syndrome (IBS) may be divided into three primary types:
Constipation predominant (C-IBS), diarrhea predominant (D-IBS) and
mixed/alternating IBS, which have significant influences on life
quality for nearly 10–20% of the population (1). The diagnosis of IBS is on the basis
of the exclusion of the Rome I, II and III criteria in addition to
other organic or functional disorders (2). Current data indicates that IBS is
increasing in the Asia-Pacific region, particularly in developing
countries, and a study conducted in China has reported that the
prevalence of IBS (according to the Rome III criteria) is 15.9% in
outpatient clinics (3). IBS is
characterized by discomfort, recurrent abdominal pain, and altered
bowel habits in the absence of any organic disorder, and D-IBS
patients demonstrate visceral hypersensitivity and damaged colonic
motility with elevated frequency and enhanced amplitude of giant
migrating contractions (GMCs) which leads to mass movements, stool
propulsion and the initiation of defecation (1,4). The
concrete pathogenesis of IBS is multifaceted and not completely
understood, however several risk factors for IBS have been
identified including genetic, epigenetic, environmental and
behavioral factors (2). The
development of D-IBS is correlated with psychosocial stress,
altered gut flora, intestinal barrier dysfunction, disturbed
gastrointestinal motility, mucosal immune activation, visceral
hypersensitivity, euro-endocrine abnormality and genetic
susceptibility (5). There is still
no agreement over optimal pharmacological treatment for D-IBS and
as ion channels are important in gastrointestinal function,
disrupted ion channels may result in disease, therefore the present
study aimed to investigate whether a gene associated with ion
channels may act as a novel target to treat the disease (4,6).
Sodium voltage-gated channel alpha subunit 9 (SCN9A)
encodes the subunit of the voltage-gated sodium channel (VGSC
NaV1.7), is expressed at a rather high density in
sensory, sympathetic and nociceptive neurons and may be important
in nociception and vasomotor regulation (7,8).
SCN5A-encoded Nav1.5 has previously been demonstrated to
exist in human intestinal interstitial cells of Cajal, and ion
channels may be associated with a subset of patients with IBS, a
heterogeneous and poorly understood disorder. Due to the fact that
SCN5A and SCN9A encode sodium channels which share common
evolutionary origins, it is plausible to conjecture that SCN9A may
be important in IBS (9–11). Nerve growth factor (NGF) is
critical to the functional regulation and development of sensory
neuron (nociceptor) signaling events that result in pain and has a
primary role in the pathophysiology of inflammatory pain (12). Chronic abdominal pain and low-grade
mucosal inflammation are the predominant manifestations of D-IBS
and NGF is associated with chronic inflammatory pain, therefore
there is a possibility that NGF may be a potential therapeutic
target in treating D-IBS, and a study conducted by Willot et
al (13) verifies this
conjecture. The present study, aimed to investigate the effects of
SCN9A gene modification on sodium ion channels (Na+
channel) and the expression of NGF in a rat model of D-IBS.
Materials and methods
Ethical statement
All experiments in the present study were conducted
in accordance with public institution conventions and strictly
complied with relevant standards for the care and use of laboratory
animals according to the National Research Council or National
laws. The study was approved by the Ethics Committee of Cangzhou
Central Hospital (Cangzhou, China).
Study subjects
A total of 56 healthy, mature, specific
pathogen-free Sprague-Dawley rats, male (n=28) and female (n=28),
aged 3 months, weighted 172±15 g, were provided by the Laboratory
Animal Center of Xiangya Medical College in Central South
University [Changsha, China; animal certificate no. SCXK (XIANG)
2016-0007]. All rats were randomly kept in cages with 8 rats in
each and they had free access to food and water. Rats were fed
under controlled temperature (25±3°C) and humidity (55–68%). All
cages were cleaned once a day; windows were opened twice a day for
ventilation for 0.5 h each time. A complete disinfection of all
cages, equipment and the room was conducted once a week.
Model establishment and grouping
A total of 56 rats were divided into two groups: 32
were assigned for the model group and 24 for the normal control
group. From the 32 rats assigned to the model group, 8 were
randomly selected and were used to conduct the model evaluation.
The remaining 24 rats were divided into 3 sub-groups, each
containing 8 rats as follows: SCN9A gene modification group (model
rats with insertion of cells with SCN9A gene modification),
negative control (NC) group (model rats with insertion of cells
with non-transfected SCN9A gene modification), the blank group
(model rats without any treatment) and the normal group (normal
rats without any treatment).
Rats in the model group had free access to water
however a 12 h fasting period was required prior to experiments. On
the first day of experimentation, all rats were put in the
specifically prepared plastic containers with tail position higher
than head. The rat tail was raised for 30 sec to expose the anus
for inserting the infusion catheter (with 8 cm outside anus) with
the other end connected to a syringe. Following this, 1 ml glacial
acetic acid (40 ml/l, analytically pure, provided by Tianjin Bodi
Chemical Co. Ltd. (Tianjin, China) was infused into the colon, then
the infusion catheter was removed by pressing the anus with a
cotton swab soaked in normal saline, and 1 ml PBS (0.01 mol/l,
provided by Beijing Zhongshan Jiqiao Biotechnology Co. Ltd.,
Beijing, China; batch no. ZLI.9062) was used to clean the feces or
other waste in the colon. From the fourth day of experiment,
restraint stress treatment was applied for 5 days consecutively for
2 h each day (front shoulders, front limbs and chest were
restrained by a wide paper tape to keep rat from scratching head
and face, other body parts were free to move). Model rats had
increased rectal sensitivity, defecation and visceral sensitivity
which is consistent with the characteristics of IBS patients.
Model evaluation
For the model evaluation, rats were sacrificed under
anesthesia with 10% pentobarbital sodium (300 µl/100 g) following
blooding. Rat abdomens were cut open for the observation of organs
with the naked eye. Colon tissue (2 cm; 1 cm distance from cecum)
was cut and opened longitudinally, and then was conventionally
fixed with 10% neutral formalin solution for 4–6 h, dehydrated by
85% ethanol, cleared by xylene twice (each for 15 min),
paraffin-embedded at 54–56°C, sliced into 4 µm thickness and stored
at −80°C.
The following indicators were observed: i) The color
and texture of rat fur and rat mental status and activity; ii)
calculation of body mass growth rate: Following finish of feeding,
rat body mass was weighed and recorded as the initial body mass;
rat body mass was then observed and weighed every day during the
experimental period. Growth rate of body mass = (body mass of the
day – initial body mass)/initial body mass ×100%; iii) Comparison
of the loose stool rates: Starting time of diarrhea on each day was
recorded according to the filter paper marks; total frequencies of
stools and the frequencies of mucks were recorded in 24 h a day.
Loose stools rate = frequencies of mucks/total frequencies of
stools ×100%; iv) calculation of viscera indexes of thymus and
spleen: Following finish of the experiment, rat body mass was
weighed following a 24 h fasting period and recorded as final body
mass.
Rats were sacrificed via exsanguination; thymus and
spleen were removed from body and then rats were weighed following
clearing damp with filter paper. Viscera indexes = viscera
weight/final body mass ×100%.
Cell separation and culture
Colon tissues of 8 normal rats were used for cell
separation and culture. Following removal of connective tissues,
colons were put in the culture dish with prepared D-hanks (Hyclone;
GE Healthcare, Logan, UT, USA; batch no. NSG0054). Colons were cut
open longitudinally and D-hanks solution with antibiotics was used
to clean feces and other waste. Colons were then cut into tissue
blocks of 1 mm3 and transferred to the 50-ml centrifuge
tube following washing with D-hanks solution with antibiotics 2–3
times. D-hanks solution containing 10 mM dithiothreitol (cat no.
10197777001; Beijing Solarbio Science & Technology Co., Ltd.;
solarbio.bioon.com.cn) and 1 mM EDTA
(batch no. B1227012; Beijing Meike Mei Biotechnology Development
Co. Ltd.) was added to the centrifuge tube with colon tissues to 20
ml in total. Following a still-standing period at room temperature
for 15 min, colon tissue blocks were centrifuged for 5 min (4°C,
300 × g) using a centrifugal machine (L420; Hunan Xiangyi
Instrument Development Co., Ltd., Hunan, China). Sediments were
transferred to a 100-ml conical beaker, shaken and digested
following addition of 50 ml hyaluronidase (cat. no. A0701203;
Shanghai Gaochuang Chemical Technology Co. Ltd., Shanghai, China;
www.gaochem.cn) resulting in a turbid solution.
The turbid solution was then centrifuged for 5 min (4°C, 100 ×
g) and then sediments were transferred to the conical beaker
with 20 ml type I collagenases (batch no. 080203; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany). It was then shaken for 30 min at
37°C (80 r/min), and centrifuged for 5 min (4°C, 200 × g).
Sediments were taken, triturated for 5 min with 10 ml icy
precooling Hanks solution (batch no. 971272; Sino American Shanghai
Squibb Pharmaceuticals Ltd., Shanghai, China), filtered using a
200-mesh sieve, and then washed with icy precooling Hanks. The
washing fluid was collected and centrifuged for 10 min (150 × g).
Cells sediments were suspended and put into a T25 cell culture
bottle (batch no. TCF-25 Shanghai Baiyan Biotechnology Co., Ltd.,
Shanghai, China) for 90 min. And cell supernatants were then
transferred to a 5 ml Dulbecco's modified Eagle's medium containing
10% fetal bovine serum for another culture. After trituration with
a pipette, cells were counted by an inverted microscope (MI12,
Mshot Technology Ltd., Guangzhou, China). Then cells were
inoculated into a 25 cm2 culture bottle containing
1×106 cells at 37°C in 5% CO2. Three days
later, cells were observed under an inverted microscope and medium
was changed.
Construction of SCN9A gene adenovirus
vector
DNA of SCN9A gene was extracted using Easy Pure
Genomic DNA Kit (EE101-01; Trans Gen Biotech Co., Ltd., Beijing,
China) in accordance with the manufacturer's protocol and gene
modifications were done according to the manufacturer's protocol,
using hydrosulfite (American Epigentek Company, Farmingdale, NY,
USA). Polymerase chain reaction (PCR) (14) amplifications were applied to the
modified DNAs with methylation specific primers and non-methylation
specific primers. Double enzyme digestions of adenovirus vector
pDC316-EGFP (Microbix Biosystems Inc., Mississauga, ON, USA) and
SCN9A gene segment/SCN9A gene modified segment were performed with
BglII and HindIII endonucleases (New England Biolabs,
Hitchin, Herts, UK), followed by collection with agarose gel
electrophoresis. The collected vector segments and target gene cDNA
were connected with T4Ligase (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) at 16°C overnight. TG1 competent bacteria
(Guangzhou Medical University, Guangzhou, China) was directly
transformed from 5 µl connected products and 3 clones were selected
following shaking overnight, through a lysogeny broth culture dish
(Gibco; Thermo Fisher Scientific, Inc.) with ampicillin. The
plasmids were extracted and recombinant adenovirus vectors were
correctly verified by double-enzyme digestion and termed
pDC316-EGFP-SCN9A/hSCN9A. The obtained recombinant adenoviruses
were amplified and purified, and the infectious titers (TCID50)
were tested. Virus titer was calculated according to the Karber
method: Titer (T)=101+d(s-0.5).
Adenovirus transfection of target
cells and grouping
Cells deriving from the gene modification SCN9A
(transfected with plasmid modified by SCN9A), NC (transfected with
plasmid not modified by SCN9A gene) and the blank group were used
for this experiment. Briefly, cells were put under a fluorescence
microscope for the observation of green fluorescent protein
expression. When cell density increased to 30–50%, cell
transfection was conducted using Lipofectamin 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.). Cells of each group (100 pmol)
were incubated with 250 µl serum-free Opti-MEM (Gibco; Thermo
Fisher Scientific, Inc.) at a final concentration of 50 nM at room
temperature for 5 min. Lipofectamin 2000 (5 µl) was incubated with
250 µl serum-free Opti-MEM (Gibco; Thermo Fisher Scientific, Inc.)
at a final concentration of 50 nM at room temperature for 5 min.
Then the aforementioned two compositions were mixed and incubated
at room temperature for two min and then transplanted into a cell
culture. After incubation at 37°C in 5% CO2 for 6~8 h,
medium was replaced by complete medium, followed by a 24 h
transfection incubation. Rates of green fluorescence cells in 400
cells were calculated under different magnetic optic imaging (MOI)
values (MOI=0.0%; MOI=5, 15.9±1.6%; MOI=20, 42.5±2.1%; MOI=40,
95.2±1.9%; MOI=80, 99.1±1.7%; MOI=100, 99.4±2.5%), and results
demonstrated that the rates of cells expressing green fluorescence
were increased with the increasing of MOI values. When MOI was 40,
green fluorescence appeared in various cells (Fig. 1); when MOI was 80 and 100, almost
100% of cells revealed green fluorescence; however, following a
culture period of 48 h, cells with MOI of 80 and 100 decreased
significantly and floating dead cells in the culture dish were
observed. It was hypothesized that the virulence may have been too
strong to inhibit cell proliferation and finally led to cell
apoptosis. Therefore, considering green fluorescence expression and
cell growth, MOI=40 was selected for the optimum transfection
time.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNAs were extracted with TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.). Reverse transcription
reactions were conducted according to the manufacturer's protocol
of the reverse transcription kit (18091200; Thermo Fisher
Scientific, Inc., Shanghai, China). PCR reactions (20 µl) were
performed in a mixture containing 10 µl SYBR-Green Mix (Thermo
Fisher Scientific, Shanghai, China), 1 µl of forward primer, 1 µl
of reverse primer, 1 µl of cDNA and 7 µl of ddH2O (batch
no. 4385618; Shanghai solarbio Bioscience & Technology Co.,
Ltd., Shanghai, China). PCR reactions were performed in ABI7 100
real time PCR equipment (Applied Biosystems; Thermo Fisher
Scientific, Inc.) as follows: pre-denaturation at 95°C for 5 min,
95°C for 40 sec, 57°C for 40 sec, 72°C for 40 sec; extension at
72°C for 10 min and at 4°C for 5 min. GAPDH was used as internal
reference. PCR primer are listed in Table I.
 | Table I.RT-qPCR primer sequences. |
Table I.
RT-qPCR primer sequences.
| Name | Sequence (5′-3′) |
|---|
| NGF |
|
|
Forward |
CCGAGCCCCGAATCCTGTA |
|
Reverse |
GGGAAGGGGGCTGCAGGCAAG |
| SCN9A |
|
Forward |
TCTCCCTTCAGTCCTCTAA |
|
Reverse |
AACAAAGTCCAGCCAGTT |
| Nav1.8 |
|
|
Forward |
GGACTCCCTGAAGACCAATATGGAG |
|
Reverse |
GCATTGAGCTAGATGGGTTAATGTTG |
| Nav1.9 |
|
|
Forward |
CCCTGCTGCGCTCGGTGAAGAA |
|
Reverse |
GACAAAGTAGATCCCAGAGG |
| GAPDH |
|
|
Forward |
CAAGGTCATCCATGACAATTTG |
|
Reverse |
GTCCACCACCCTGTTGCTGTAG |
Western blotting
Colon tissues of all groups were cut into small
fragments (measuring about 1 mm3). Following washing
with PBS (0.01 mol/l) and precooling with ice, colon tissue
fragments were incubated at 4°C for 2 h, prior to addition of an
adequate amount of protein extracting solution (including 50 mmol/l
Tris-HCl, 1% SDS, 50 mmol/l NaCl and 0.5% proteinase inhibitor).
Centrifugation of the prepared samples was performed at 94,553 × g
for 10 min. Then, buffer solution (20% glycerinum, 1 mmol/l
Tris-HCl, 10% SDS, 10% β-mercaptoethanol) was added for
degeneration at 100°C for 4 min. The supernatant was collected with
sediments removed and kept for use, or preserved at −80°C.
Polypropylene gel (10%) was prepared, and electrophoresis was done
with addition of sample (10 µl/well) and 3 µl Marker (Tiangen
Biotech Co., Ltd.). Quantitative analysis of proteins was performed
by Kjeldahl method. Protein was transferred to a polyvinylidene
fluoride membrane (PVDF) (Jiangsu Jie LV Mo Technology Co., Ltd.,
Jiangsu, China). Subsequently, PVDF were soaked in 5% non-fat milk
and sealed at 37°C for 2 h. Subsequently, the PVDF nitrocellulose
membrane was incubated with rabbit polyclonal antibody Anti-SCN9A
(1:1,000, cat. no. ab65167; Abcam, Cambridge, UK) diluted with
Tris-buffered saline with Tween-20 (TBST), on a shaking table at
4°C overnight. Following the overnight incubation, the membrane was
washed three times with TBS with Tween-20 (10 min each). Following
this, the membrane was soaked in the prepared secondary antibody
horseradish peroxidase labeled sheep anti-rabbit IgG (HRP-IgG)
diluent (1:1,000; cat. no. DF109489; Shanghai Yaoyun Biological
Technology Co., Ltd., Shanghai, China), stirred at 4°C (WD-9405B;
Beijing Liuyi Instrument Factory, Beijing, China) and incubated for
1 h. The nitrocellulose membrane was fully cleaned, anti-incubated,
and cleaned with TBST 5 times (5 min each time). Adequate amounts
of developing solution A and B (Tiangen Biotech Co., Ltd.) were
mixed evenly away from light. Following addition of the mixed
solution, the PVDF was put into UV Transilluminator (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) for imaging, and then was
preserved for analysis. Detection procedure of Nav1.7,
Nav1.8 and Nav1.9 protein levels were the
same as the aforementioned one.
Statistical analysis
SPSS software, version 20.0 (IBM SPSS, Armonk, NY,
USA) was used for statistical analysis. All measurement data were
presented as the mean ± standard deviation. A paired Student's
t-test was used for comparison between two groups. As for the
comparisons among multiple groups, one-way analysis of variance was
applied. P<0.05 was considered to indicate a statistically
significant difference.
Results
Comparisons of model evaluation based
on rat fur, mental status, activity, growth rate of body mass,
loose stool rate and viscera indexes between the normal control and
model groups
Fur color and texture, mental status and activity of
rats in all groups were observed. Results demonstrated that rats in
the normal control group had good mental status, well-shaped body
and shining dorsal fur; whereas rats in the model group were
slightly dispirited and exhausted, the glossiness of dorsal fur was
decreased markedly, and bodies were comparatively thinner. No
mortalities occurred during experiments.
Comparison of growth rate of body mass revealed a
persistent increasing of body mass for rats in the normal control
group from day 1 to day 5; whereas body mass of rats in the model
group was significantly decreased compared with the normal control
group (P<0.05) and presented a continuously decreasing trend
(Table II).
 | Table II.Comparisons of mean growth rate of
body mass of rats between the normal control and model groups. |
Table II.
Comparisons of mean growth rate of
body mass of rats between the normal control and model groups.
| Groups | n | 1 day | 2 days | 3 days | 4 days | 5 days |
|---|
| Normal control | 8 | 0.935±0.098 | 2.573±0.265 | 3.507±0.382 | 5.796±0.623 | 6.878±0.731 |
| Model | 8 |
−5.856±0.623a |
−7.395±0.754a |
−8.171±0.925a |
−8.580±0.792a |
−8.835±0.956a |
Comparison of loose stool rate revealed that stools
of rats in the normal control group were dry and in particle forms,
and there was no stain on filter papers; whereas loose stool rate
of rats in the model group increased significantly compared with
that in the normal control group (P<0.05; Table III).
 | Table III.Comparisons of mean loose stool rates
of rats between the normal control and model groups. |
Table III.
Comparisons of mean loose stool rates
of rats between the normal control and model groups.
| Groups | n | 1 day | 2 days | 3 days | 4 days | 5 days |
|---|
| Normal control | 8 | 0 | 0 | 0 | 0 | 0 |
| Model | 8 |
22.597±3.630a |
28.432±3.344a |
28.784±3.455a |
29.457±3.068a |
31.771±2.797a |
Results demonstrated that the spleen index of rats
in the normal control group was 0.251±0.023 and the thymus index
was 0.354±0.041; whereas spleen index of rats in the model group
was 0.153±0.017 and the thymus index was 0.285±0.036. Compared with
the normal control group, viscera indexes of the model group
decreased significantly (P<0.05; Table IV), which demonstrated that (D-IBS
models were successfully established.
 | Table IV.Comparisons of viscera indexes of
rats between the normal control and model groups. |
Table IV.
Comparisons of viscera indexes of
rats between the normal control and model groups.
| Groups | n | Spleen index | Thymus index |
|---|
| Normal control | 8 | 0.251±0.023 | 0.354±0.041 |
| Model | 8 |
0.153±0.017a |
0.285±0.036a |
Titer determination of recombinant
adenovirus
Cells with recombinant adenovirus were collected,
frozen and dissolved repeatedly 3 times at 37°C and −80°C.
Following centrifugation at 560 × g and precipitation, part of the
virus supernatant was used to infect cells for the amplification of
recombinant virus, as previously described (15). The aforementioned procedures were
repeated various times to acquire recombinant adenoviruses with
high titers. Virus titer tested by TCID50 was 2.8×108 PFU/ml.
SCN9A expression levels increase in
SCN9A transfected group of rats
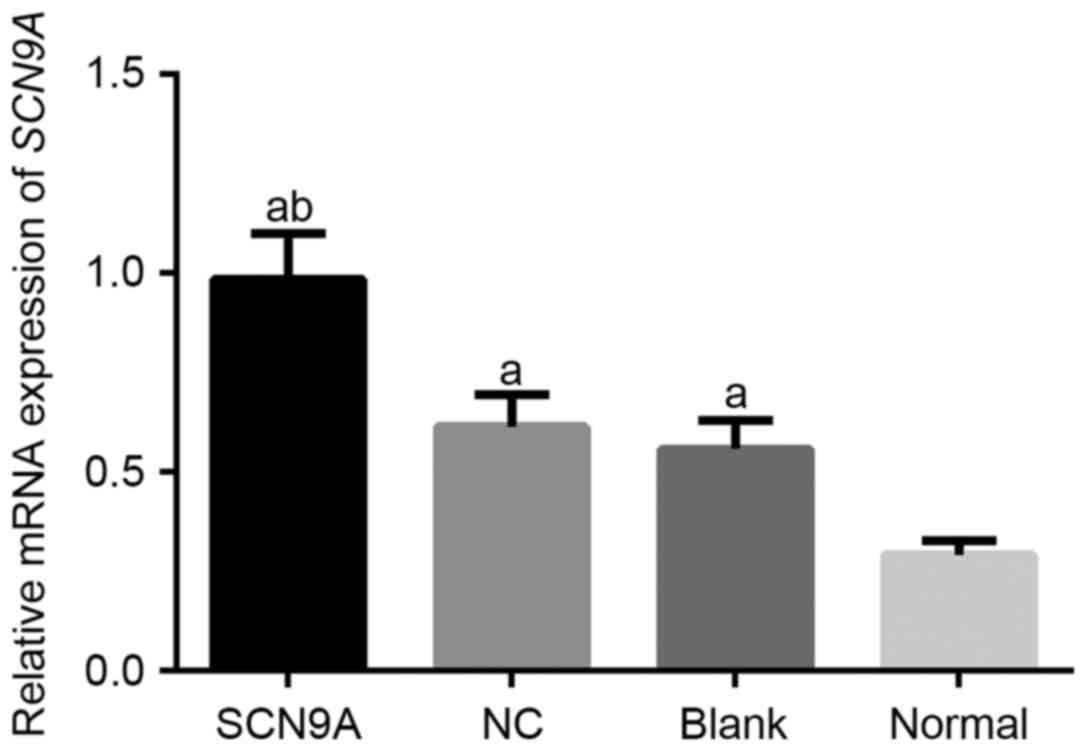
RT-qPCR demonstrated that the SCN9A mRNA expression
in the SCN9A group, the NC group, the blank group and the normal
group were 0.985±0.116, 0.614±0.081, 0.558±0.073 and 0.292±0.035.
Compared with the NC group and the blank group, SCN9A mRNA
expression in the SCN9A group was comparatively higher (P<0.05;
Fig. 2). Western blotting revealed
that the expression of SCN9A in the SCN9A, the NC, the blank and
the normal groups were 1.115±0.146, 0.637±0.083, 0.591±0.075 and
0.344±0.029. Compared with the normal group, SCN9A protein
expression levels in the SCN9A, the NC and the blank groups were
comparatively increased (P<0.05), and the protein expression in
the SCN9A group was further increased compared with the NC and the
blank groups (P<0.05; Fig. 3A and
B). These results demonstrated that transfection had been
successful.
NGF expression levels increase in
SCN9A transfected group of rats
RT-qPCR demonstrated that the NGF expression levels
in the SCN9A, the NC, the blank and the normal groups were
2.215±0.324, 1.162±0.152, 1.089±0.127 and 0.576±0.078. Compared
with the normal group, the NGF mRNA expression in the SCN9A group,
the NC and the blank group were increased (P<0.05), and NGF mRNA
expression in SCN9A group was further enhanced compared with in the
NC group and the blank group (P<0.05; Fig. 4). Western blotting revealed that
the expression levels of NGF in the SCN9A, the NC, the blank and
the normal group were 0.876±0.092, 0.671±0.074, 0.625±0.058 and
0.413±0.053. Compared with the normal group, the NGF protein
expression in the blank, the SCN9A and the NC groups were increased
(P<0.05), and protein expression in the SCN9A group was
significantly increased compared with the NC group and the blank
group (P<0.05; Fig. 5A and B).
Results demonstrated that SCN9A gene modification of colon tissues
of rats with D-IBS stimulated the expression of NGF.
SCN9A gene modification promotes
expression of Nav1.8 and Nav1.9 in rats
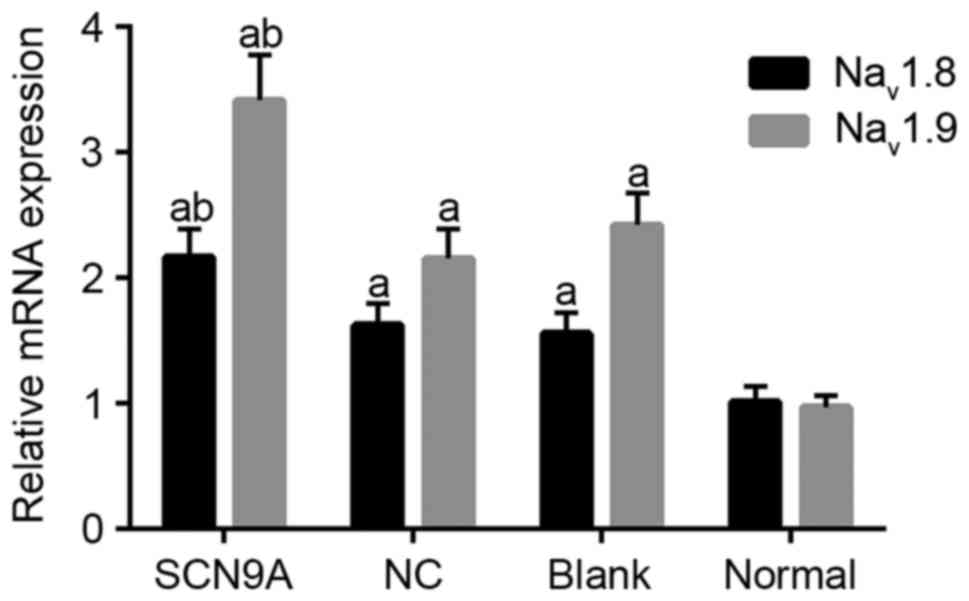
RT-qPCR demonstrated that the Nav1.8
expression levels in the SCN9A, the NC, the blank and the normal
groups were 2.167±0.224, 1.624±0.173, 1.559±0.167 and 1.016±0.123.
Compared with the normal group, Nav1.8 and
Nav1.9 mRNA expression levels in the SCN9A, the NC and
the blank group were relatively increased (P<0.05), and
Nav1.8 and Nav1.9 mRNA expressions in the
SCN9A group was significantly increased compared with the NC group
and the blank group (P<0.05; Fig.
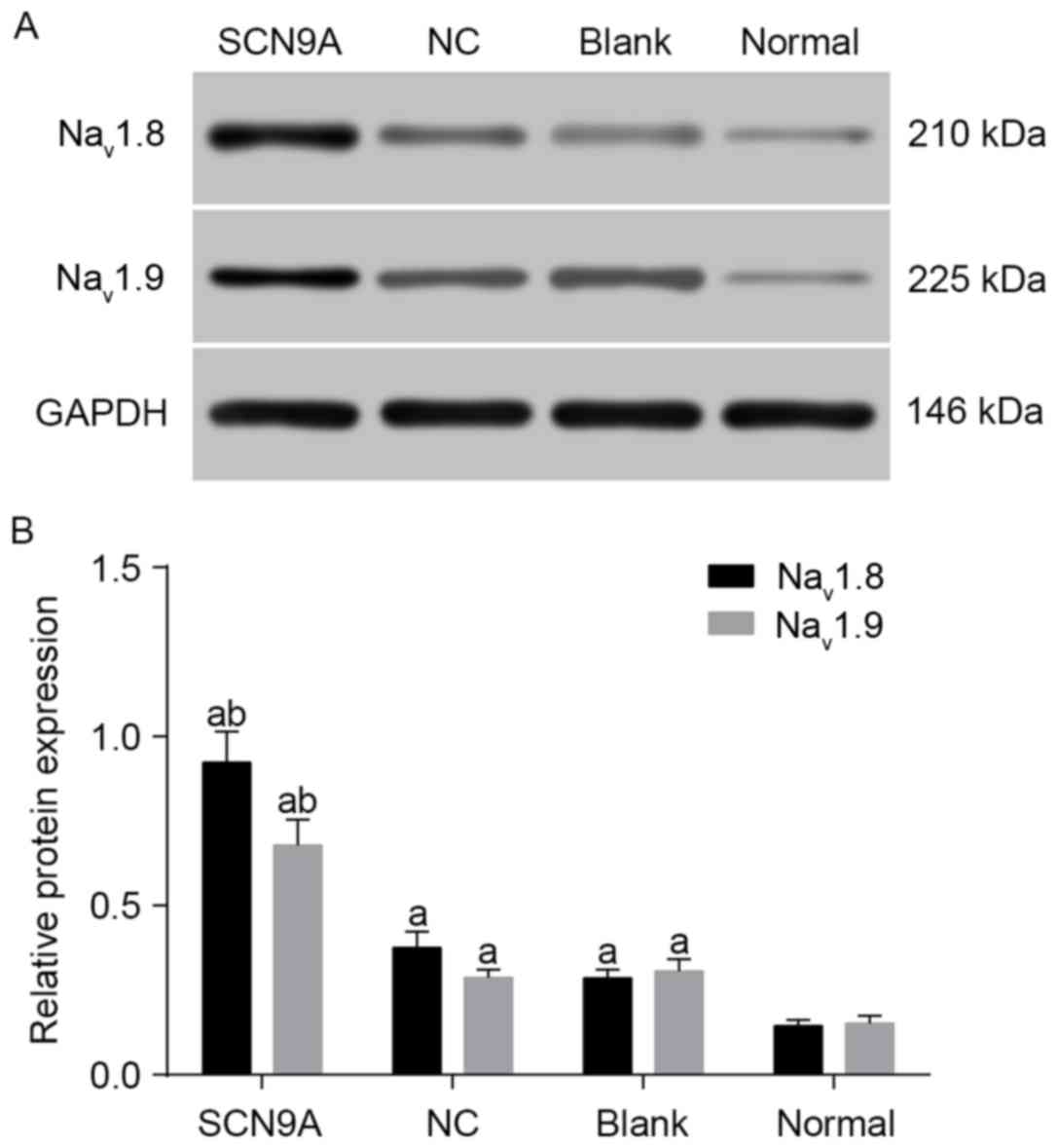
6). Western blotting demonstrated that Nav1.8
protein levels in the SCN9A, the NC, the blank and the normal
groups were 0.923±0.091, 0.375±0.048, 0.286±0.025 and 0.145±0.017.
Nav1.8 and Nav1.9 protein expression levels
in the SCN9A, the NC and the blank group were significantly
increased compared with the normal group (P<0.05), and compared
with the NC and the blank group, protein expression in the SCN9A
group was increased (P<0.05; Fig.
7A and B). Results demonstrated that SCN9A gene modification
promoted the expression of Nav1.8 and Nav1.9
in rats with D-IBS.
Discussion
The present study aimed to investigate the role of
the SCN9A gene in Na+ channels and the expression of NGF
in D-IBS rats, and the data suggested that SCN9A gene modification
promoted the expression of sodium channels-Nav1.8,
Nav1.9, and increased the expression level of NGF in
rats with D-IBS.
VGSCs, which are additionally termed Nav,
are responsible for the conversion of chemical and/or mechanical
stimuli into electrical signals in excited cells, and currently 9
different sodium channels which are encoded by 9 different genes
named SCN (1–9A) have been identified (16). SCN9A (sodium channel
NaV1.7) with physiological traits including slow
closed-state inactivation has previously been demonstrated to be
expressed in trigeminal ganglia, dorsal root ganglia (DRG) and
sympathetic ganglion neurons, and its mutations have been suggested
to be associated with pain due to its ability to amplify stimuli
(17,18). Sodium channel Nav1.8 is
abundantly expressed in DRG neurons and peripheral nerve axons
(19). NaV1.9 has an
important role in regulating afferent sensitivity in visceral pain
(a rather common symptom for patients with gastrointestinal
diseases including IBS-D) to inflammatory and mechanical stimuli
(20). It has been acknowledged
that highly conserved NaV channels sharing common
evolutionary origins and a large variety of voltage-sensitive ion
channels are expressed in contractile organs including
gastrointestinal organs, which may lead to the consideration of
mechanical modulation of ion channel function (11,21).
Ion channels existing in gastrointestinal smooth muscle (including
rat and human colon) are excitatory for slow waves, therefore ion
channels may act as a therapeutic and pathophysiological target, as
they directly participate in gastrointestinal motility and visceral
pain, thus ion channelopathies including Nav1.8 and
NaV1.9 have the potential to result in IBS (22). In addition, disturbed
gastrointestinal motility is a risk factor for D-IBS, and ion
channels including Nav1.8 and NaV1.9 may
influence the contractile ability of these organs, suggesting that
the development of D-IBS may require the active participation of
ion channels. Since expression of Nav1.8 and
Nav1.9 channels, as well as NGF were enhanced by SCN9A
gene modification, this may lead to a novel direction for D-IBS
treatment (5). Designer genetic
recombination tools and mammalian genetic modification technology
are currently novel, reliable and efficient methods to target
genomic sequences, which may result in further advances in
understanding of gene expression and its associated influence
(23).
Furthermore, it was demonstrated that NGF mRNA was
expressed most frequently in the SCN9A group compared with the
other groups, following the establishment of D-IBS in rats,
implicating that the expression of NGF may be stimulated in SCN9A
gene-modified colon tissue cells. As a protein with a high degree
of conservation and homology, NGF is produced by a single gene
located at chromosome 1, which codes for 2 transcripts that
generate 27 kDa and 35 kDa precursors (24). NGF may exert influence on
paracellular permeability via altering the expression of claudins
in tight junctions or on the transcellular uptake route via
increasing macro-pinocytosis during stress and inflammation mast
cell mediators (25). Therefore,
NGF may interrupt the barrier to antigens and bacteria, and as
D-IBS has risk factors including psychosocial stress, intestinal
barrier dysfunction and altered gut flora, it may be hypothesized
that NGF is closely associated with D-IBS (5). A previous study reveals that NGF as a
pro-inflammatory mediator is critical in the sensitization of
peripheral visceral pain hypersensitivity, and as colonic
hypersensitivity is generally regarded as a biological marker for
IBS, NGF thus may be considered as a potential novel therapeutic
target for IBS treatment (26).
Furthermore, NGF is expressed in colonic mucosa and its influences
on the induction and maintenance of visceral sensitivity in animals
have been acknowledged, and it has been identified to be
upregulated in the colonic wall and rectal mucosa in conditions of
chronic stress present in IBS patients (13,27).
In conclusion, the present study investigated the
influence of SCN9A gene modification on Na+ channels and
NGF in rats with D-IBS. Results demonstrated that
Nav1.8, Nav1.9 and NGF were expressed more
frequently in the SCN9A group, providing a promising novel
therapeutic target for the clinical treatment of D-IBS. However,
the present study did not investigate how the SCN9A gene regulates
and influences associated signaling pathways and factors, therefore
further studies are required in the future.
Acknowledgements
The authors would like to acknowledge the helpful
comments on the paper received from reviewers.
References
|
1
|
Moore NA, Sargent BJ, Manning DD and Guzzo
PR: Partial agonism of 5-HT3 receptors: A novel approach to the
symptomatic treatment of IBS-D. ACS Chem Neurosci. 4:43–47. 2013.
View Article : Google Scholar
|
|
2
|
Pitzurra R, Fried M, Rogler G, Rammert C,
Tschopp A, Hatz C, Steffen R and Mutsch M: Irritable bowel syndrome
among a cohort of European travelers to resource-limited
destinations. J Travel Med. 18:250–256. 2011. View Article : Google Scholar
|
|
3
|
Basandra S and Bajaj D: Epidemiology of
dyspepsia and irritable bowel syndrome (IBS) in medical students of
northern India. J Clin Diagn Res. 8:JC13–JC16. 2014.
|
|
4
|
Clavé P: Treatment of IBS-D with 5-HT3
receptor antagonists vs spasmolytic agents: Similar therapeutical
effects from heterogeneous pharmacological targets.
Neurogastroenterol Motil. 23:1051–1055. 2011. View Article : Google Scholar
|
|
5
|
Xu XJ, Liu L and Yao SK: Nerve growth
factor and diarrhea-predominant irritable bowel syndrome (IBS-D): A
potential therapeutic target? J Zhejiang Univ Sci B. 17:1–9. 2016.
View Article : Google Scholar :
|
|
6
|
Beyder A and Farrugia G: Ion
channelopathies in functional GI disorders. Am J Physiol
Gastrointest Liver Physiol. 311:G581–G586. 2016. View Article : Google Scholar :
|
|
7
|
Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L,
Fan J, Bu D, Liu B, Fan Z, et al: Mutations in SCN9A, encoding a
sodium channel alpha subunit, in patients with primary
erythermalgia. J Med Genet. 41:171–174. 2004. View Article : Google Scholar :
|
|
8
|
Reimann F, Cox JJ, Belfer I, Diatchenko L,
Zaykin DV, McHale DP, Drenth JP, Dai F, Wheeler J, Sanders F, et
al: Pain perception is altered by a nucleotide polymorphism in
SCN9A. Proc Natl Acad Sci USA. 107:5148–5153. 2010. View Article : Google Scholar :
|
|
9
|
Saito YA, Strege PR, Tester DJ, Locke GR
III, Talley NJ, Bernard CE, Rae JL, Makielski JC, Ackerman MJ and
Farrugia G: Sodium channel mutation in irritable bowel syndrome:
Evidence for an ion channelopathy. Am J Physiol Gastrointest Liver
Physiol. 296:G211–G218. 2009. View Article : Google Scholar
|
|
10
|
Catterall WA: Voltage-gated sodium
channels at 60: Structure, function and pathophysiology. J Physiol.
590:2577–2589. 2012. View Article : Google Scholar :
|
|
11
|
Brunklaus A, Ellis R, Reavey E, Semsarian
C and Zuberi SM: Genotype phenotype associations across the
voltage-gated sodium channel family. J Med Genet. 51:650–658. 2014.
View Article : Google Scholar
|
|
12
|
Lewin GR, Lechner SG and Smith ES: Nerve
growth factor and nociception: From experimental embryology to new
analgesic therapy. Handb Exp Pharmacol. 220:251–282. 2014.
View Article : Google Scholar
|
|
13
|
Willot S, Gauthier C, Patey N and Faure C:
Nerve growth factor content is increased in the rectal mucosa of
children with diarrhea-predominant irritable bowel syndrome.
Neurogastroenterol Motil. 24:734–739. 2012. View Article : Google Scholar
|
|
14
|
Zhang J, Ho JC, Chan YC, Lian Q, Siu CW
and Tse HF: Overexpression of myocardin induces partial
transdifferentiation of human-induced pluripotent stem cell-derived
mesenchymal stem cells into cardiomyocytes. Physiol Rep.
2:e002372014. View
Article : Google Scholar :
|
|
15
|
Vernon SK, Murthy S, Wilhelm J, Chanda PK,
Kalyan N, Lee SG and Hung PP: Ultrastructural characterization of
human immunodeficiency virus type 1 gag-containing particles
assembled in a recombinant adenovirus vector system. J Gen Virol.
72:1243–1251. 1991. View Article : Google Scholar
|
|
16
|
Kurban M, Wajid M, Shimomura Y and
Christiano AM: A nonsense mutation in the SCN9A gene in congenital
insensitivity to pain. Dermatology. 221:179–183. 2010. View Article : Google Scholar :
|
|
17
|
Estacion M, Han C, Choi JS, Hoeijmakers
JG, Lauria G, Drenth JP, Gerrits MM, Dib-Hajj SD, Faber CG, Merkies
IS and Waxman SG: Intra- and interfamily phenotypic diversity in
pain syndromes associated with a gain-of-function variant of
NaV1.7. Mol Pain. 7:922011. View Article : Google Scholar :
|
|
18
|
Snyder LM, Ross SE and Belfer I: An SCN9A
variant, known to cause pain, is now found to cause itch. Pain.
155:1677–1678. 2014. View Article : Google Scholar
|
|
19
|
Faber CG, Lauria G, Merkies IS, Cheng X,
Han C, Ahn HS, Persson AK, Hoeijmakers JG, Gerrits MM, Pierro T, et
al: Gain-of-function Nav1.8 mutations in painful neuropathy. Proc
Natl Acad Sci USA. 109:19444–19449. 2012. View Article : Google Scholar :
|
|
20
|
Hockley JR, Winchester WJ and Bulmer DC:
The voltage-gated sodium channel NaV 1.9 in visceral pain.
Neurogastroenterol Motil. 28:316–326. 2016. View Article : Google Scholar
|
|
21
|
Beyder A, Rae JL, Bernard C, Strege PR,
Sachs F and Farrugia G: Mechanosensitivity of Nav1.5, a
voltage-sensitive sodium channel. J Physiol. 588:4969–4985. 2010.
View Article : Google Scholar :
|
|
22
|
Beyder A, Mazzone A, Strege PR, Tester DJ,
Saito YA, Bernard CE, Enders FT, Ek WE, Schmidt PT, Dlugosz A, et
al: Loss-of-function of the voltage-gated sodium channel NaV1.5
(channelopathies) in patients with irritable bowel syndrome.
Gastroenterology. 146:1659–1668. 2014. View Article : Google Scholar :
|
|
23
|
Smith KR, Chan S and Harris J: Human
germline genetic modification: Scientific and bioethical
perspectives. Arch Med Res. 43:491–513. 2012. View Article : Google Scholar
|
|
24
|
Iulita MF and Cuello AC: Nerve growth
factor metabolic dysfunction in Alzheimer's disease and down
syndrome. Trends Pharmacol Sci. 35:338–348. 2014. View Article : Google Scholar
|
|
25
|
Camilleri M, Madsen K, Spiller R,
Greenwood-Van Meerveld B and Verne GN: Intestinal barrier function
in health and gastrointestinal disease. Neurogastroenterol Motil.
24:503–512. 2012. View Article : Google Scholar :
|
|
26
|
Matricon J, Muller E, Accarie A, Meleine
M, Etienne M, Voilley N, Busserolles J, Eschalier A, Lazdunski M,
Bourdu S, et al: Peripheral contribution of NGF and ASIC1a to
colonic hypersensitivity in a rat model of irritable bowel
syndrome. Neurogastroenterol Motil. 25:e740–e754. 2013. View Article : Google Scholar
|
|
27
|
Winston JH, Xu GY and Sarna SK: Adrenergic
stimulation mediates visceral hypersensitivity to colorectal
distension following heterotypic chronic stress. Gastroenterology.
138:294–304.e3. 2010. View Article : Google Scholar
|