Introduction
Allergic diseases, including bronchial asthma,
atopic dermatitis and rhinitis, affect 30–40% of the global
population (1,2). Allergens from house dust mites
(HDMs); in particular those from the most common HDMs,
Dermatophagoides farinae (Der f) and Dermatophagoides
pteronyssinus (Der p), are major environmental factors for
allergic diseases (3–5). At least 34 groups of HDM allergens
have been identified and listed in the Allergen Nomenclature
database (http://www.allergen.org). Der f 20,
identified and denominated from D. farinae, belongs to the
group 20 allergens. Der f 20 is a 40-kDa arginine kinase, however,
its physiological function remains to be fully elucidated.
Allergen extracts of various mite species, including
mite bodies, eggs and culture media, have been used to diagnose and
treat IgE-mediated allergic diseases. Certain patients may be
sensitized to one or two mite allergens, whereas others respond to
a spectrum of allergens (6–10).
However, these extracts have limitations in terms of safety and
validity in allergen-specific immunotherapy (SIT) (6–10).
SIT is the only etiological therapy, which suppresses allergic
responses in rhinitis and asthma (8,11).
By contrast, pure and standardized recombinant allergens,
containing the majority of the IgE-binding epitopes of an allergen
source, can be used to replace natural extracts, offering a safer
and more valid approach to SIT (9,10).
Several SIT-based studies have focused on using
recombinant allergens to develop epitope-based vaccines (12,13).
These vaccines contain multiple B-cell and/or T-cell linear antigen
epitopes and can thus overcome virulence return or spread, and
induce more efficient presentation when detected and combined by
host major histocompatibility complex (MHC) molecules (14,15).
These findings suggest that B-cell and T-cell epitopes from one
major component of an allergen may be necessary for immunotherapy
of allergic diseases. Therefore, the identification of exact
epitopes of HDM allergens can benefit the preparation of
epitope-based vaccines and treatment of allergic diseases.
Previous studies have identified several HDM
allergen epitopes (16), although
no Der f 20 epitopes have been reported. Therefore, the present
study used bioinformatics approaches to identify B-cell and T-cell
epitopes of Der f 20.
Materials and methods
Sequence retrieval and analyses
The amino acid sequence of Der f 20 (accession no.
AIO08850.1) was obtained from the International Union of
Immunological Societies nomenclature database and the protein
database of the National Center for Biotechnology Information
(www.allergen.org). The family classification of
Der f 20 was analyzed using Pfam v29.0 (pfam.xfam.org) (17),
Superfamily v1.75 (supfam.cs.bris.ac.uk/SUPERFAMILY/hmm.html)
(18) and InterPro v56.0
(www.ebi.ac.uk/interpro/) (19). The TMHMM server 2.0 (www.cbs.dtu.dk/services/TMHMM/) was used
to predict transmembrane protein helices (20).
Physiochemical and patterns
analyses
Physiochemical analyses, including molecular weight,
negatively charged residues, positively charged residues,
theoretical pI, aliphatic index, grand average of hydropathicity
(GRAVY) and instability index of Der f 20, were predicted using
ProtParam (web.expasy.org/protparam/) (21). The characteristic patterns,
functional motifs and active sites of Der f 20 were assessed using
Prosite (prosite.expasy.org/) (22).
Structure prediction and homology
modeling
The TMHMM server 2.0 (www.cbs.dtu.dk/services/TMHMM/) was used to predict
transmembrane protein helices (20). The PredictProtein server
(www.predictprotein.org/) was used to
predict the secondary structure of Der f 20 (23). Homology modeling was used to
construct a tertiary structure of Der f 20. A BLASTP (blast.ncbi.nlm.nih.gov/Blast.cgi) search
with default parameters was performed against the Protein Data Bank
(PDB) (www.rcsb.org/pdb/) to identify suitable
templates of Der f 20. The appropriate templates were selected
based on the high score, low e-value, and maximum sequence
identity. MODELLER v9.16 (salilab.org/modeller/) (24) was used to predict the tertiary
structure of Der f 20. The predicted structure was imported into
Chiron (redshift.med.unc.edu/chiron/login.php) (25) to rectify unfavorable clashes and
improve stereochemistry quality.
Estimating the quality of the structural models is a
vital step in protein structure construction. PROCHECK (services.mbi.ucla.edu/SAVES) (26) was used to verify the stereochemical
quality of the structure of Der f 20. ERRAT (services.mbi.ucla.edu/SAVES) (27) was used to analyze the statistics of
non-bonded interactions between different atom types. VERIFY_3D
(services.mbi.ucla.edu/SAVES)
(28) was used to determine the
compatibility of an atomic model (3D) with its the amino acid
sequence (1D) and to compare the results with favorable structures.
ProSA (prosa.services.came.sbg.ac.at/prosa.php) (29) was used to analyze the Z-score to
determine the degree of match between the template protein and Der
f 20. QMEAN (swissmodel.expasy.org/qmean) (30) is a composite scoring function,
which provides the global (for the entire structure) and local (per
residue) error estimates on the basis of a single model.
Superimposition of the query and template structure, and
visualization of the generated models was performed using UCSF
Chimera 1.10.2 (www.cgl.ucsf.edu/chimera/) (31).
Prediction of B-cell epitopes
BcePred (http://crdd.osdd.net/raghava/bcepred) (32), ABCpred (crdd.osdd.net/raghava/abcpred) (33), BCPreds (ailab.ist.psu.edu/bcpred) (34) and the Bioinformatics Predicted
Antigenic Peptides (BPAP) system (imed.med.ucm.es/Tools/antigenic.pl) (35) were used to predict the B-cell
antigenic epitopes of Der f 20. BcePred predicts B-cell epitopes
using physicochemical properties, including hydrophilicity,
flexibility/mobility, accessibility, polarity, exposed surfaceand
turns, or a combination of properties. ABCpred predicts B-cell
epitopes in antigen sequences using artificial neural networks.
BCPREDS selects three prediction methods of the AAP method
(35), BCPred (36) and FBCPred (37) predict B-cell antigenic epitopes.
The BPAP system combines the physicochemical properties of amino
acids to predict epitopes.
Prediction of T-cell epitopes
The T-cell epitopes were predicted by identifying
peptide binding to MHC molecules. The binding significance of each
peptide to the given MHC molecule was based on the estimated
strength of binding exhibited by a predicted nested core peptide at
a set threshold level. NetMHCII 2.2 (www.cbs.dtu.dk/services/NetMHCII) (38) predicted the binding of epitope
peptides to HLA-DQ alleles using artificial neuron networks.
NetMHCIIpan-3.1(www.cbs.dtu.dk/services/NetMHCIIpan) (39) was used for HLA-DR-based epitope
prediction. In these two software programs, peptides with a high
binding ability had a half maximal inhibitory concentration (IC50)
value <50 nM and weak binding peptides had an IC50 value <500
nM. The ultimate T-cell epitopes were obtained by combining the
results of the HLA-DR and HLA-DQ allele epitopes.
Results
Amino acid sequence analysis
Family classification showed that Der f 20 belongs
to the ATP: guanido phosphotransferase family (InterPro no.
IPR000749) and arginine kinase superfamily (InterPro no.
IPR023660). Prosite was used to analyze characteristic motifs or
patterns and revealed that Der f 20 contained a PHOSPHAGEN_KINASE
pattern (PS00112; 271–277; CPTNLGT) and active site at
residue 271 (Fig. 1). The
phosphorylation sites of Der f 20 included Ser residues 20, 260 and
282; Thr residues 44, 49, 177, 269, 278, 311 and 334; and Tyr
residues 75 and 145. DNA-PK kinase (T 177; LLGMDKATQQQLIDD)
was predicted as phosphorylated for Der f 20.
Der f 20 includes 356 amino acids and has a
molecular weight of 40177.8 Da. The protein contains 49 negatively
charged residues (Asp and Glu) and 46 positively charged residues
(Arg and Lys). Der f 20 had a theoretical pI of 6.24 and an
aliphatic index of 95.06. GRAVY was −0.103, indicating that Der f
20 exhibits a hydrophilic characteristic. The instability index was
30.57, indicating that the amino acid sequence of Der f 20 was
stable.
Structural analysis and homology
modeling
The Der f 20 protein sequences were entered into the
TMHMM Server 2.0 to predict transmembrane helices. The computed
results showed that Der f 20 had no transmembrane helices, and all
protein sequences were located outside of the membrane (Fig. 1). The percentages of overall amino
acids located in α-helices, β-sheets and random coils were 41.57%
(12 domains), 15.73% (10 domains) and 42.70%, respectively
(Table I).
 | Table I.Secondary and tertiary structural
elements of Der f 20. |
Table I.
Secondary and tertiary structural
elements of Der f 20.
| Structure | α-helices (%) | β-sheets (%) | Random coils
(%) |
|---|
| Secondary | 41.57 (12
domains) | 15.73 (10
domains) | 42.70 |
| Tertiary | 41.01 (14
domains) | 13.48 (10
domains) | 45.51 |
The tertiary structure of Litopenaeus
vannamei arginine kinase (PDB accession no. 4BG4) had a high
sequence identity (78%) with Der f 20 and was therefore used as the
template for homology modeling. Following homology modeling, a
Ramachandran plot showed that 89.4% of the amino acid residues
within the tertiary structure of Der f 20 were within the most
favored regions; 9.9% of residues were in additional allowed
regions; 0.6% of residues were in generously allowed regions; and
0% of residues were in disallowed regions. The ERRAT results showed
that the overall quality factor was 96.264, indicating that the
tertiary structure of Der f 20 had high resolution. The VERIFY 3D
results showed that 96.63% of residues had an average 3D-1D score
≥0.2, indicating that the structures were favorable. As indicated
by ProSa, the Z-scores of template and Der f 20 showed high
matching between the tertiary structures of the protein. The QMEAN
server results showed that the QMEAN Z-score was −0.82 and the
standard deviation value was <1, indicating that the protein
model variation rate was low, overall folding and local structures
had high accuracy rates and stereochemistry was reasonable. In
addition, the Q value was 0.753, indicating that the predicted
model of Der f 20 was reliable. Therefore, based on these results,
the tertiary structure model of Der f 20 was reliable and suitable
for use in the present study (Table
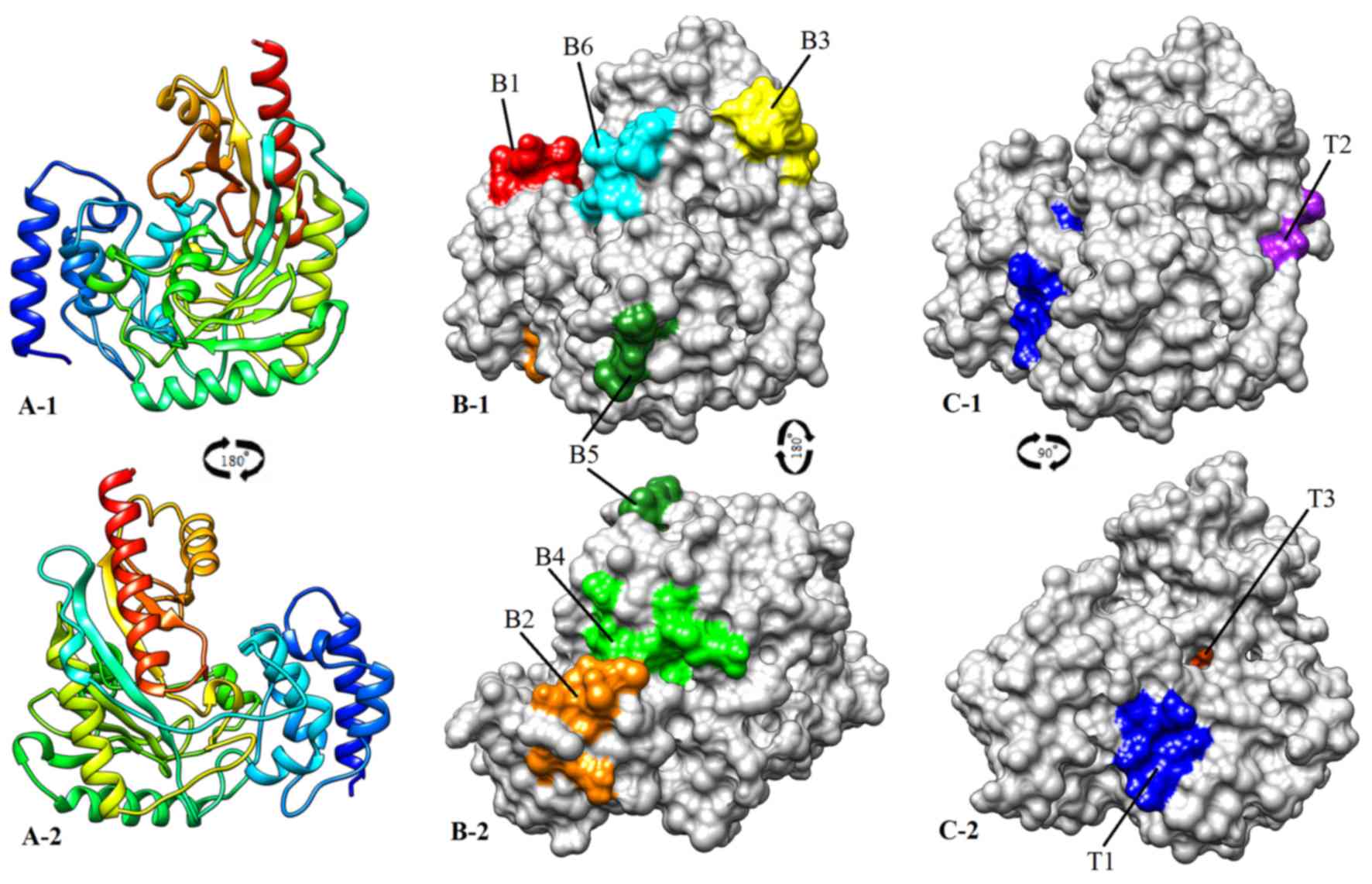
II; Fig. 2A).
 | Table II.Evaluation parameters for the
tertiary structure of Der f 20. |
Table II.
Evaluation parameters for the
tertiary structure of Der f 20.
| Protein | Structural
assessment method | Ramachandran plot
% | ERRAT value | VERIFY 3D (%) | Z-score | Q-value |
|---|
| Der f 20 | PROCHECK | 89.4 core | 96.264 | 96.63 |
|
|
|
|
| 9.9 allowed |
|
|
|
|
|
|
| 0.6 generously
allowed |
|
|
|
|
|
|
| 0.0 disallowed |
|
|
|
|
|
| ProSa |
|
|
| −10.17 |
|
|
| QMEAN |
|
|
| −0.12 | 0.753 |
| 4BG4 | PROCHECK | 92.0 core | 98.127 | 95.21 |
|
|
|
|
| 7.6 allowed |
|
|
|
|
|
|
| 0.5 generously
allowed |
|
|
|
|
|
|
| 0.0 disallowed |
|
|
|
|
|
| ProSa |
|
|
| −10.53 |
|
|
| QMEAN |
|
|
| −0.14 | 0.748 |
The tertiary structure of Der f 20 was also found to
contain α-helices, β-sheets and random coils, although the amino
acid numbers of these three elements were marginally different,
compared with the secondary structure. The percentages of overall
amino acids located in α-helices, β-sheets and random coils were
41.01% (14 domains), 13.48% (10 domains) and 45.51%, respectively
(Table I).
B-cell epitope prediction
Hydrophobicity, fragment flexibility/mobility,
surface accessibility, polarity, exposed surface and turns are
important features for B-cell antigenic epitope identification.
These antigenic indices were used to determine the epitope forming
capacity of the Der f 20 amino acid sequence. Based on antigenic
indices, BcePred, ABCpred, BCPred and BPAP were used in the present
study to predict B-cell epitopes. Ultimately, seven antigenic
epitope peptides were predicted, including 20–25, 43–49, 110–118,
131–142, 170–174, 203–210 and 311–321 (Tables III and IV; Fig.
2B).
 | Table III.B-cell and T-cell epitope
prediction. |
Table III.
B-cell and T-cell epitope
prediction.
| Epitope
prediction | Prediction
server | Prediction
result |
|---|
| B-cell
epitopes | BcePred | 12–18, 36–45,
143–152, 229–235, 256–263, 290–297, 321–330 |
|
| ABCpred | 3–19, 24–40, 37–53,
61–77, 69–85, 83–99, 94–110, 127–143, 158–174, 164–180, 179–195,
185–201, 203–219, 228–244, 252–268, 309–325, 320–336, 329–345 |
|
| BCPred | 99–119, 131–151,
311–331, 207–227 |
|
| BPAP system | 11–29, 31–38,
47–56, 73–87, 106–113, 117–142, 164–172, 177–187, 193–210, 238–249,
264–273, 279–290, 296–308, 317–324, 345–351 |
| T-cell epitopes
(HLA-DR) | NetMHCIIpan-3.0
(DRB1*01:01) | 14–22, 167–175,
194–202, 239–247, 243–251, 267–275 |
|
| NetMHCIIpan-3.0
(DRB3*01:01) |
|
|
| NetMHCIIpan-3.0
(DRB4*01:01) |
|
|
| NetMHCIIpan-3.0
(DRB5*01:01) | 194–202,
279–287 |
| T-cell epitopes
(HLA-DQ) | NetMHCII-2.2
(HLA-DQA10101-DQB10501) |
|
|
| NetMHCII-2.2
(HLA-DQA10102-DQB10602) | 228–236,
274–282 |
|
| NetMHCII-2.2
(HLA-DQA10301-DQB10302) |
|
|
| NetMHCII-2.2
(HLA-DQA10401-DQB10402) | 217–225 |
|
| NetMHCII-2.2
(HLA-DQA10501-DQB10201) |
|
|
| NetMHCII-2.2
(HLA-DQA10501-DQB10301) | 45–53, 66–74 |
 | Table IV.Predicted B-cell and T-cell epitopes
of Dermatophagoides farina 20. |
Table IV.
Predicted B-cell and T-cell epitopes
of Dermatophagoides farina 20.
| Peptide | Type of
epitope | Position |
Sequencea |
|---|
| P1 | B | 20–25 | SAECHS |
| P2 | B | 41–49 | GRKTGMGAT |
| P3 | B | 111–118 | CNVDPNNE |
| P4 | B | 131–141 | LQGYPFNPCLT |
| P5 | B | 170–174 | LLGMD |
| P6 | B | 312–321 | AGEHTESVGG |
| P7 | T | 194–202 | FLQAANACR |
| P8 | T | 239–247 | LKQVFSRLI |
| P9 | T | 274–282 | NLGTTIRAS |
T-cell epitope prediction
NetMHCIIpan 3.1 was used to predict T-cell epitopes
in the regions of HLA-DR DRB101, HLA-DRB301, HLA-DRB40 and
HLA-DRB501. NetMHCII 2.2 was used to predict T-cell epitopes in the
regions of HLA-DQA10101-DQB10501, HLA-DQA10102-DQB10602,
HLA-DQA10301-DQB10302, HLA-DQA10401-DQB10402, HLA-DQA10501-DQB10201
and HLA-DQA10501-DQB10301. Combined with the software results, two
T-cell epitope peptides were ultimately predicted, including
194–202 and 274–282 (Tables III
and IV; Fig. 2C).
Discussion
Type I allergic diseases, including rhinitis, asthma
and atopic dermatitis, are increasing worldwide. HDM antigens are
responsible for the sensitization of >50% of patients with
airway allergic disease (7,40).
Therefore, the prediction and characterization of specific B-cell
and T-cell epitopes of HDM allergens, including Der f 20, can
assist in mechanistic investigations of immune responses and the
design of epitope-based vaccines.
Der f 20 is a member of the ATP:guanido
phosphotransferase family and arginine kinase superfamily, and the
protein contains a phosphagen kinase motif pattern and an active
site at residue 271. Der f 20 is a hydrophilic and stable protein
with no transmembrane helices and all protein sequences located
outside of the membrane. Homology modeling, or comparative protein
modeling, construct a Der f 20 structure based on comparisons with
data extracted from homologous sequences with known structures
(parents or templates) (41).
The quality of a homology model is dependent on high
quality sequence alignment and template structure. Therefore, the
present study used the crystal structure of 4BG4 as a template, as
it has 78% sequence identity with Der f 20. Following homology
modeling with MODELLER, various additional parameters/programs were
incorporated to establish a reliable model of Der f 20. Although
Der f 20 contains α-helices, β-sheets and random coils, the numbers
of amino acids in these three elements in the tertiary structure
were found to vary marginally from the secondary structure. This
discrepancy may be due to different structural prediction
methods.
Epitopes or antigenic determinants, which represent
the immune-active regions of antigen molecules, are the regions of
an antigen, which are recognized by the immune system, specifically
by antibodies and lymphocyte (B-cell or T-cell) surface antigen
receptors. The properties of the antigen epitope, their number and
their spatial configuration determine antigen specificity (42,43).
Epitopes usually contain 6–8 amino acids residues and in general
contain <20 amino acid residues.
In the present study, BcePred, ABCpred, BCPreds and
BPAP were used to predict the B-cell epitopes of Der f 20.
Secondary and tertiary protein structures also contain important
information for B-cell epitope prediction. For example, α-helices
and β-sheets have higher chemical bond energy and have difficulty
forming epitope sequences. By contrast, β-turns and random coils
are located in surface-exposed regions of a protein, which often
contain epitope sequences (44).
Integrating the shared results of the four servers, and combining
information from secondary and tertiary structures, the present
study ultimately predicted six B-cell epitope peptides: 20–25,
41–49, 111–118, 131–141, 170–174 and 312–321. A total of three
T-cell epitope peptides were predicted: 194–202, 239–247 and
274–282. In addition, allergen epitopes usually contain high
proportions of hydrophobic amino acid residues, including Ala, Ser,
Asn, Gly and Lys (45). The
predictions showed that the majority of the B-cell and T-cell
epitopes identified in the present study contained multiple
hydrophobic amino acids. However, these predicted epitopes require
further experimental verification.
In conclusion, the present study constructed a
reasonable tertiary structure of Der f 20. Using bioinformatics,
the B-cell epitopes (20–25, 41–49, 111–118, 131–141, 170–174 and
312–321) and T-cell epitopes (194–202, 239–247 and 274–282) were
predicted based on the secondary and tertiary structures of Der f
20. These results represent a significant step towards the design
of Der f 20 epitope-based vaccines for allergic diseases.
Glossary
Abbreviations
Abbreviations:
|
HDM
|
house dust mite
|
|
PDB
|
Protein Data Bank
|
|
3D structure
|
tertiary structure
|
References
|
1
|
Arlian LG: House-dust-mite allergens: A
review. Exp Appl Acarol. 10:167–186. 1991. View Article : Google Scholar
|
|
2
|
Platts-Mills TAE, Thomas WR, Aalberse R,
Vervloet D and Champman MD: Dust mite allergens and asthma: Report
of a second international workshop. J Allergy Clin Immunol.
89:1046–1060. 1992. View Article : Google Scholar
|
|
3
|
Thomas WR, Hales BJ and Smith WA: House
dust mite allergens in asthma and allergy. Trends Mol Med.
16:321–328. 2010. View Article : Google Scholar
|
|
4
|
Nadchatram M: House dust mites, our
intimate associates. Trop Biomed. 22:23–37. 2005.
|
|
5
|
Tovey ER, Chapman MD and Platts-Mills TA:
Mite faeces are a major source of house dust mite allergens.
Nature. 289:592–593. 1981. View
Article : Google Scholar
|
|
6
|
Marth K, Focke-Tejkl M, Lupinek C, Valenta
R and Niederberger V: Allergen peptides, recombinant allergens and
hypoallergens for allergen-specific immunotherapy. Curr Treat
Options Allergy. 1:91–106. 2014. View Article : Google Scholar :
|
|
7
|
Vrtala S, Huber H and Thomas WR:
Recombinant house dust mite allergens. Methods. 66:67–74. 2014.
View Article : Google Scholar
|
|
8
|
Jutel M, Solarewicz-Madejek K and
Smolinska S: Recombinant allergens: The present and the future. Hum
Vaccin Immunother. 8:1534–1543. 2012. View
Article : Google Scholar :
|
|
9
|
Valenta R, Niespodziana K, Focke-Tejkl M,
Marth K, Huber H, Neubauer A and Niederberger V: Recombinant
allergens: What does the future hold? J Allergy Clin Immunol.
127:860–864. 2011. View Article : Google Scholar
|
|
10
|
Focke-Tejkl M and Valenta R: Safety of
engineered allergen-specific immunotherapy vaccines. Curr Opin
Allergy Clin Immunol. 12:555–563. 2012. View Article : Google Scholar :
|
|
11
|
Lee J, Park CO and Lee KH: Specific
immunotherapy in atopic dermatitis. Allergy Asthma Immunol Res.
3:221–229. 2015. View Article : Google Scholar
|
|
12
|
Zhao J, Li C, Zhao B, Xu P, Xu H and He L:
Construction of the recombinant vaccine based on T-cell epitope
encoding Der p1 and evaluation on its specific immunotherapy
efficacy. Int J Clin Exp Med. 4:6436–6443. 2015.
|
|
13
|
Koffeman EC, Genovese M, Amox D, Keogh E,
Santana E, Matteson EL, Kavanaugh A, Molitor JA, Schiff MH, Posever
JO, et al: Epitope-specific immunotherapy of rheumatoid arthritis:
Clinical responsiveness occurs with immune deviation and relies on
the expression of a cluster of molecules associated with T-cell
tolerance in a double-blind, placebo-controlled, pilot phase II
trial. Arthritis Rheum. 11:3207–3216. 2009. View Article : Google Scholar
|
|
14
|
Sharmin R and Islam AB: A highly conserved
WDYPKCDRA epitope in the RNA directed RNA polymerase of human
coronaviruses can be used as epitope-based universal vaccine
design. BMC Bioinformatics. 1:1612014. View Article : Google Scholar
|
|
15
|
Alexander C, Kay AB and Larche M:
Peptide-based vaccines in the treatment of specific allergy. Curr
Drug Targets Inflamm Allergy. 4:353–361. 2002. View Article : Google Scholar
|
|
16
|
Cui Y: Immunoglobulin e-binding epitopes
of mite allergens: From characterization to immunotherapy. Clin Rev
Allergy Immunol. 3:344–353. 2014. View Article : Google Scholar
|
|
17
|
Finn RD, Coggill P, Eberhardt RY, Eddy SR,
Mistry J, Mitchell AL, Potter SC, Punta M, Qureshi M,
Sangrador-Vegas A, et al: The Pfam protein families database:
Towards a more sustainable future. Nucleic Acids Res. 44:D279–D285.
2016. View Article : Google Scholar
|
|
18
|
Gough J, Karplus K, Hughey R and Chothia
C: Assignment of homology to genome sequences using a library of
hidden Markov models that represent all proteins of known
structure. J Mol Biol. 313:903–919. 2001. View Article : Google Scholar
|
|
19
|
Mitchell A, Chang HY, Daugherty L, Fraser
M, Hunter S, Lopez R, McAnulla C, McMenamin C, Nuka G, Pesseat S,
et al: The InterPro protein families database: The classification
resource after 15 years. Nucleic Acids Res. 43:(Database Issue).
D213–D221. 2015. View Article : Google Scholar
|
|
20
|
Krogh A, Larsson B, von Heijne G and
Sonnhammer EL: Predicting transmembrane protein topology with a
hidden Markov model: Application to complete genomes. J Mol Biol.
305:567–580. 2001. View Article : Google Scholar
|
|
21
|
Wilkins MR, Gasteiger E, Bairoch A,
Sanchez JC, Williams KL, Appel RD and Hochstrasser DF: Protein
identification and analysis tools in the ExPASy server. Methods Mol
Biol. 112:531–552. 1999.
|
|
22
|
De Castro E, Sigrist CJ, Gattiker A,
Bulliard V, Langendijk-Genevaux PS, Gasteiger E, Bairoch A and Hulo
N: ScanProsite: Detection of PROSITE signature matches and
ProRule-associated functional and structural residues in proteins.
Nucleic Acids Res. 34:(Web Server issue). W362–W365. 2006.
View Article : Google Scholar :
|
|
23
|
Yachdav G, Kloppmann E, Kajan L, Hecht M,
Goldberg T, Hamp T, Hönigschmid P, Schafferhans A, Roos M, et al:
PredictProtein-an open resource for online prediction of protein
structural and functional features. Nucleic Acids Res. 42:(Web
Server issue). W337–W343. 2014. View Article : Google Scholar :
|
|
24
|
Webb B and Sali A: Protein structure
modeling with MODELLER. Methods Mol Biol. 1137:1–15. 2014.
View Article : Google Scholar
|
|
25
|
Ramachandran S, Kota P, Ding F and
Dokholyan NV: Automated minimization of steric clashes in protein
structures. Proteins. 1:261–270. 2011. View Article : Google Scholar
|
|
26
|
Laskowski RA, Rullmannn JA, MacArthur MW,
Kaptein R and Thornton JM: AQUA and PROCHECK-NMR: Programs for
checking the quality of protein structures solved by NMR. J Biomol
NMR. 4:477–486. 1996.
|
|
27
|
Colovos C and Yeates TO: Verification of
protein structures: Patterns of nonbonded atomic interactions.
Protein Sci. 9:1511–1519. 1993. View Article : Google Scholar
|
|
28
|
Bowie JU, Lüthy R and Eisenberg D: A
method to identify protein sequences that fold into a known
three-dimensional structure. Science. 253:164–170. 1991. View Article : Google Scholar
|
|
29
|
Wiederstein M and Sippl MJ: ProSA-web:
Interactive web service for the recognition of errors in
three-dimensional structures of proteins. Nucleic Acids Res.
35:(Web Server issue). W407–W410. 2007. View Article : Google Scholar :
|
|
30
|
Benkert P, Tosatto SC and Schomburg D:
QMEAN: A comprehensive scoring function for model quality
assessment. Proteins. 1:261–277. 2008. View Article : Google Scholar
|
|
31
|
Pettersen EF, Goddard TD, Huang CC, Couch
GS, Greenblatt DM, Meng EC and Ferrin TE: UCSF Chimera-a
visualization system for exploratory research and analysis. J
Comput Chem. 13:1605–1612. 2004. View Article : Google Scholar
|
|
32
|
Saha S and Raghava GP: BcePred: Prediction
of continuous B-cell epitopes in antigenic sequences using
physico-chemical properties. Artificial Immune Systems.
3239:197–204. 2004. View Article : Google Scholar
|
|
33
|
Saha S and Raghava GP: Prediction of
continuous B-cell epitopes in an antigen using recurrent neural
network. Proteins. 65:40–48. 2006. View Article : Google Scholar
|
|
34
|
Chen J, Liu H, Yang J and Chou KC:
Prediction of linear B-cell epitopes using amino acid pair
antigenicity scale. Amino Acids. 3:423–428. 2007. View Article : Google Scholar
|
|
35
|
Zheng LN, Lin H, Pawar R, Li ZX and Li MH:
Mapping IgE binding epitopes of major shrimp (Penaeus monodon)
allergen with immunoinformatics tools. Food Chem Toxicol.
49:2954–2960. 2011. View Article : Google Scholar
|
|
36
|
EI-Manzalawy Y, Dobbs D and Honavar V:
Predicting linear B-cell epitopes using string kernels. J Mol
Recognit. 21:243–255. 2008. View
Article : Google Scholar :
|
|
37
|
EI-Manzalawy Y, Dobbs D and Honavar V:
Predicting flexible length linear B-cell epitopes. Comput Syst
Bioinformatics Conf. 7:121–132. 2008. View Article : Google Scholar :
|
|
38
|
Nielsen M and Lund O: NN-align. An
artificial neural network-based alignment algorithm for MHC class
II peptide binding prediction. BMC Bioinformatics. 10:2962009.
View Article : Google Scholar :
|
|
39
|
Andreatta M, Karosiene E, Rasmussen M,
Stryhn A, Buus S and Nielsen M: Accurate pan-specific prediction of
peptide-MHC class II binding affinity with improved binding core
identification. Immunogenetics. 67:641–650. 2015. View Article : Google Scholar :
|
|
40
|
An S, Shen C, Liu X, Chen L, Xu X, Rong M,
Liu Z and Lai R: Alpha-actinin is a new type of house dust mite
allergen. Plos One. 8:e813772013. View Article : Google Scholar :
|
|
41
|
Wong A, Gehring C and Irving HR: Conserved
functional motifs and homology modeling to predict hidden
moonlighting functional sites. Front Bioeng Biotechnol. 3:822015.
View Article : Google Scholar :
|
|
42
|
Brusic V, Bajic VB and Petrovsky N:
Computational methods for prediction of T-cell epitopes-a framework
for modelling, testing, and applications. Methods. 34:436–443.
2004. View Article : Google Scholar
|
|
43
|
Zhao H, Verma D, Li W, Choi Y, Ndong C,
Fiering SN, Bailey-Kellogg C and Griswold KE: Depletion of T-cell
epitopes in lysostaphin mitigates anti-drug antibody response and
enhances antibacterial efficacy in vivo. Chem Biol. 5:629–639.
2015. View Article : Google Scholar
|
|
44
|
Sikic K, Tomic S and Carugo O: Systematic
comparison of crystal and NMR protein Structures deposited in the
protein data bank. Open Biochem J. 4:83–95. 2010. View Article : Google Scholar :
|
|
45
|
Oezguen N, Zhou B, Negi SS, Ivanciuc O,
Schein CH, Labesse G and Braun W: Comprehensive 3D-modeling of
allergenic proteins and amino acid composition of potential
conformational IgE epitopes. Mol Immunol. 45:3740–3747. 2008.
View Article : Google Scholar :
|