Introduction
Myocardial infarction is a common cardiovascular
event, which occurs following prolonged ischemia of the coronary
arteries. It is responsible for heart failure and sudden death, and
is associated with longer hospital stays (1). Timely and effective blood flow
restoration to the ischemic myocardium helps cardiomyocyte
survival, and reduces cardiac morbidity and mortality. However,
reperfusion leads to ischemia and reperfusion (I/R) injury, which
can cause irreversible damage and subsequent tissue remodeling
(2). It has been well demonstrated
that myocardial apoptosis is observed in human acute myocardial
infarcts and is primarily triggered during reperfusion through
various mechanisms (3–5). Increasing evidence suggests that
suppression of myocardial apoptosis decreases infarct size and
improves regional contractile dysfunction during reperfusion
(3). Therefore, it is important to
study how to inhibit myocardial apoptosis associated with
reperfusion.
MicroRNAs (miRNAs/miRs) are a class of short (19–25
nucleotides), single-stranded, non-coding RNA molecules, which
modulate gene expression at the post-transcriptional level by base
pairing with the 3′untranslated region (6). It has been acknowledged that miRNAs
are involved in diverse physiological and pathological processes,
including cell proliferation and apoptosis (7,8).
Emerging evidence has suggested that various miRNAs serve a
critical role in I/R (9–12). Among miRNAs, miR-146 has been
reported to protect against liver (13,14)
small intestine (15) and
myocardial I/R injury (16). In
addition, miR-146 has been reported to negatively regulate the
production of proinflammatory cytokines, including interleukin
(IL)-1, IL-6 and tumor necrosis factor (TNF)-α, by the nuclear
factor (NF)-κB signaling pathway (17). Activation of NF-κB has been
reported to be involved in myocardial I/R (18). However, little information is
available regarding the protective effects of miR-146 against I/R
injury in the myocardium via inhibiting NF-κB pathway and
inflammatory cytokine production.
The current study aimed to investigate whether
miR-146 overexpression offered protection against myocardial I/R
injury by inhibiting the NF-κB pathway and production of the
inflammatory cytokine, TNF-α. A cellular oxygen-glucose
deprivation/recovery (OGD/R) model of I/R was induced in H9c2 rat
myocardial cells. Following overexpression of miR-146 in the cells,
cell viability and cell apoptosis, as well as the underlying
mechanism, were analyzed.
Materials and methods
Cell culture and treatment
H9c2 rat myocardial cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). The cells
were cultured in Dulbecco's modified Eagle's medium (DMEM;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
10% fetal bovine serum (FBS; Sigma-Aldrich; Merck KgaA), 100 U/ml
penicillin G (Gibco; Thermo Fisher Scientific Inc., Waltham MA,
USA), 100 µg/ml streptomycin (Gibco; Thermo Fisher Scientific Inc.)
and 2 mM glutamine (Gibco; Thermo Fisher Scientific Inc.). The
cells were allowed to grow to 80% confluence and were then used for
further experiments. The cells in the control group were untreated,
and the cells in the miR-Ctrl and miR-146 mimics groups were first
stimulated by OGD/R and then transfected with miR-146 mimic or
mimic control. The cells were stimulated by OGD/R as previously
described (19). Briefly, cells
were washed twice with PBS and incubated in glucose-free DMEM.
Thereafter, the cells were placed in an anaerobic chamber
containing a mixture of 95% N2 and 5% CO2 at
37°C for 6 h, after which 4.5 mg/ml glucose was added.
Subsequently, the cells were incubated in an atmosphere containing
95% air and 5% CO2 for another 18 h.
Transfection
Mature miR-146 mimics and mimic control (miR-Ctrl)
were designed and synthesized by Shanghai GenePharma Co., Ltd.,
(Shanghai, China). For stable transfection, the cells
(5×105 cells/well) were seeded on 6-well plates and were
transiently transfected with 100 nM miR-146 mimic or miR-Ctrl for
48 h using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific Inc.) according to the manufacturer's protocol.
The sequences were as follows: miR-146 mimic, sense
5′-UGAGAACUGAAUUCCAUGGGUU-3′ and antisense
5′-CCCAUGGAAUUCAGUUCUCAUU-3′; and miR-Ctrl, sense
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense
5′-ACGUGACACGUUCGGAGAATT-3′. The cell suspension was collected for
further analyses. Untreated cells were considered the control
group.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Transfection efficiency was confirmed by RT-qPCR.
Total RNA, including miRNA, was extracted from the cells with
TRIzol (Invitrogen; Thermo Fisher Scientific Inc.) according to the
manufacturer's protocol. RT-PCR was performed using the Multiscribe
RT kit (Applied Biosystems; Thermo Fisher Scientific Inc.)
according to the manufacturer's protocol. The contents of the kit
included 1×100 µl MultiScribe TM Reverse Transcriptase (50 U/µl),
1×100 µl RNase Inhibitor (20 U/µl), 1×1 ml Dntp Mixture (2.5 mM
each dNTP), 1×100 µl Oligo d(T)16 (50 µM), 1×100 µl Random Hexamers
(50 µM), 1×1.5 ml 10X RT Buffer, and 1×1.5 ml MgCl2
Solution. The RNA was denatured at 70°C for 5 min and then put on
ice for 2 min. Thereafter, the solution was added followed by
incubation for 5 min at 25°C then at 42°C for 60 min and finally at
70°C for 5 min. The levels of miRNA were determined using SYBR
Advantage qPCR Premix (Takara Bio Inc., Otsu, Japan), and the
reactions were carried out with an ABI PRISM 7900HT Sequence
Detection system (Applied Biosystems; Thermo Fisher Scientific
Inc.). The thermocycling conditions were as follows: 1 min at 95°C,
34 cycles of 94°C for 30 sec, 62°C for 30 sec and 72°C for 2 min,
and a final extension at 72°C for 5 min. MiR expression was
quantified by the comparative 2−∆∆Cq method (20) and U6 small nuclear RNA was used as
a loading control. The primers used were as follows: miR-146,
forward, 5′-CCGATGTGTATCCTCAGCTTTG-3′ and reverse,
5′-GCTGAAGAACTGAATTTCAGAGGTC-3′; and U6, forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′.
Cell viability
Following stimulation with OGD/R and transfection
with miR-146 mimics or miR-Ctrl, cell viability was analyzed by an
MTT colorimetric assay based on a standardized method (21). Briefly, the cells were seeded in
96-well plates and treated with OGD/R and transfected with miR-146
mimic or miR-Ctrl. After treatment for 0 and 72 h, and 7 days, the
cells were treated with 5 mg/ml MTT (20 µl; Invitrogen; Thermo
Fisher Scientific Inc.) and were incubated at 37°C for 4 h.
Subsequently, dimethyl sulfoxide (100 µl; Sigma-Aldrich; Merck
KGaA) was added to dissolve the formazan crystals. Absorbance at
590 nm was measured using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Terminal
deoxynucleotidyl-transferase-mediated dUTP nick-end labeling
(TUNEL) assay
A TUNEL assay was performed to analyze apoptosis
using the In Situ Cell Death Detection kit (Roche
Diagnostics, Basel, Switzerland) according to a standard protocol
(22). Briefly, H9c2 cells were
treated with OGD/R and transfected with miR-146 mim or miR-Ctrl.
Thereafter, the cells were incubated with terminal deoxynucleotidyl
transferase and biotinylated dUTP. Flow cytometric analysis
(FACSCalibur; BD Biosciences, Franklin Lakes, NJ, USA) was
performed to analyze the apoptotic cells.
Western blotting
Following treatment with OGD/R and transfection with
miR-146 mimics or miR-Ctrl, the cell suspension was harvested,
centrifuged at 6,400 × g for 10 min at 4°C, and lysed in
radioimmunoprecipitation assay lysis buffer with protease inhibitor
(Merck & Co., Whitehorse Station, NJ, USA). The proteins were
quantified using a Bicinchoninic Acid Protein Assay kit (Pierce;
Thermo Fisher Scientific Inc.). Equal amounts of protein (20
µg/lane) were separated by 10–12% SDS-PAGE followed by transfer to
a polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.).
Thereafter, the membranes were blocked with 5% skim milk in
Tris-buffered saline with 1% Tween (TBST) for 1 h and probed with
the following primary antibodies overnight at 4°C: Anti-B-cell
lymphoma 2 (Bcl-2)-associated X protein (Bax; cat. no. ab32503;
1:1,000; Abcam, Cambridge, UK), anti-Bcl-2 (cat. no. ab32124;
1:1,000; Abcam) and anti-phosphorylated NF-κB (cat. no. ab16502;
1:1,000; Abcam). GAPDH (cat. no. ab9485; 1:2,000; Abcam) was used
as an internal control. After washing with TBST, the membranes were
exposed to goat anti-rabbit immunoglobulin G horseradish
peroxidase-conjugated secondary antibody (cat. no. ab6721; dilution
1:5,000; Abcam) for 2 h at room temperature. Protein bands were
visualized with WEST-ZOL-plus Western Blot Detection system (Intron
Biotechnology, Inc., Seongnam, South Korea) using enhanced
chemiluminescent reagents (ECL-Plus; Beyotime Institute of
Biotechnology, Shanghai, China) according to the manufacturer's
protocol. Optical band densities were quantified using ImageJ
software v1.41 (National Institutes of Health, Bethesda, MD,
USA).
ELISA
Following treatment with OGD/R and transfection with
miR-146 mimic or miR-Ctrl, the cell suspension was collected and
centrifuged at 600 × g for 5 min. The supernatant was removed and
stored at −80°C until further use. The levels of TNF-α were
analyzed by sandwich ELISA (cat. no. RTA00; R&D Systems, Inc.,
Minneapolis, MN, USA) according to the manufacturer's protocol.
Statistical analysis
Each experiment was run in triplicate. The data are
presented as the mean ± standard deviation. Statistic Package for
Social Science (SPSS, version 16.0; SPSS, Inc., Chicago, IL, USA)
statistical software was used to analyze statistical differences.
One-way analysis of variance with a Bonferroni's post hoc test were
performed for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression levels of miR-146
To explore the potential functional role of miR-146
in myocardial I/R, a cellular OGD/R model of I/R was generated
using H9c2 cells. The cells were first stimulated by OGD/R and were
then transfected with miR-146 mimics or miR-Ctrl. The levels of
miR-146 were analyzed by RT-qPCR. Transfection with miR-146 mimics
significantly increased the levels of miR-146 compared with in the
miR-Ctrl group (P<0.05; Fig.
1), indicating a high transfection efficiency.
Effects of miR-146 overexpression on
cell viability
Following miR-146 overexpression, cell viability was
analyzed by MTT. As shown in Fig.
2, the results revealed that compared to the untreated cells,
cell viability was significantly reduced by OGD/R stimulation and
transfection with miR-Ctrl (P<0.05). However, cell viability
significantly increased following transfection with miR-146 mimics
compared with in the miR-Ctrl group (P<0.05). These results
demonstrated that overexpression of miR-146 promoted cell viability
following I/R.
Effects of miR-146 overexpression on
cell apoptosis, and on TNF-α and p-NF-κB levels
A TUNEL assay was performed to determine the effects
of miR-146 overexpression on cell apoptosis. As demonstrated in
Fig. 3A, it was observed that the
percentage of apoptotic cells was significantly greater following
OGD/R stimulation and transfection with miR-Ctrl. However,
apoptosis was significantly decreased by overexpression of miR-146
compared with the miR-Ctrl group (P<0.05). The underlying
mechanisms of cell apoptosis were further explored through the
expression of Bax and Bcl-2. The results revealed that miR-146
overexpression significantly decreased the protein expression
levels of Bax but significantly increased the levels of Bcl-2
(Fig. 3B and C). This indicated
that miR-146 overexpression inhibited apoptosis of ischemic cells
by decreasing Bax and increasing Bcl-2. The underlying signaling
pathway associated with the effects of miR-146 overexpression on
ischemia cells was then investigated. TNF-α and p-NF-κB levels were
analyzed following miR-146 overexpression by ELISA and western
blotting, respectively. Stimulation with OGD/R markedly upregulated
the expression of TNF-α and p-NF-κB (P<0.05) in comparison to
the untreated cells. p-NF-κB levels were normalized to total NF-κB
levels (data not shown). Total NF-κB protein was measured,
including phosphorylated and non-phosphorylated forms. The ratio of
phosphorylated form and non-phosphorylated form indicates the
activity of NF-κB. There effects were reversed by overexpressing
miR-146 when compared with the miR-Ctrl group (P<0.05; Fig. 3D and E). Representative images of
the western blot are shown in Fig.
3F. Analysis of the data indicated that the overexpression of
miR-146 inactivated the NF-κB/TNF-α signaling pathway.
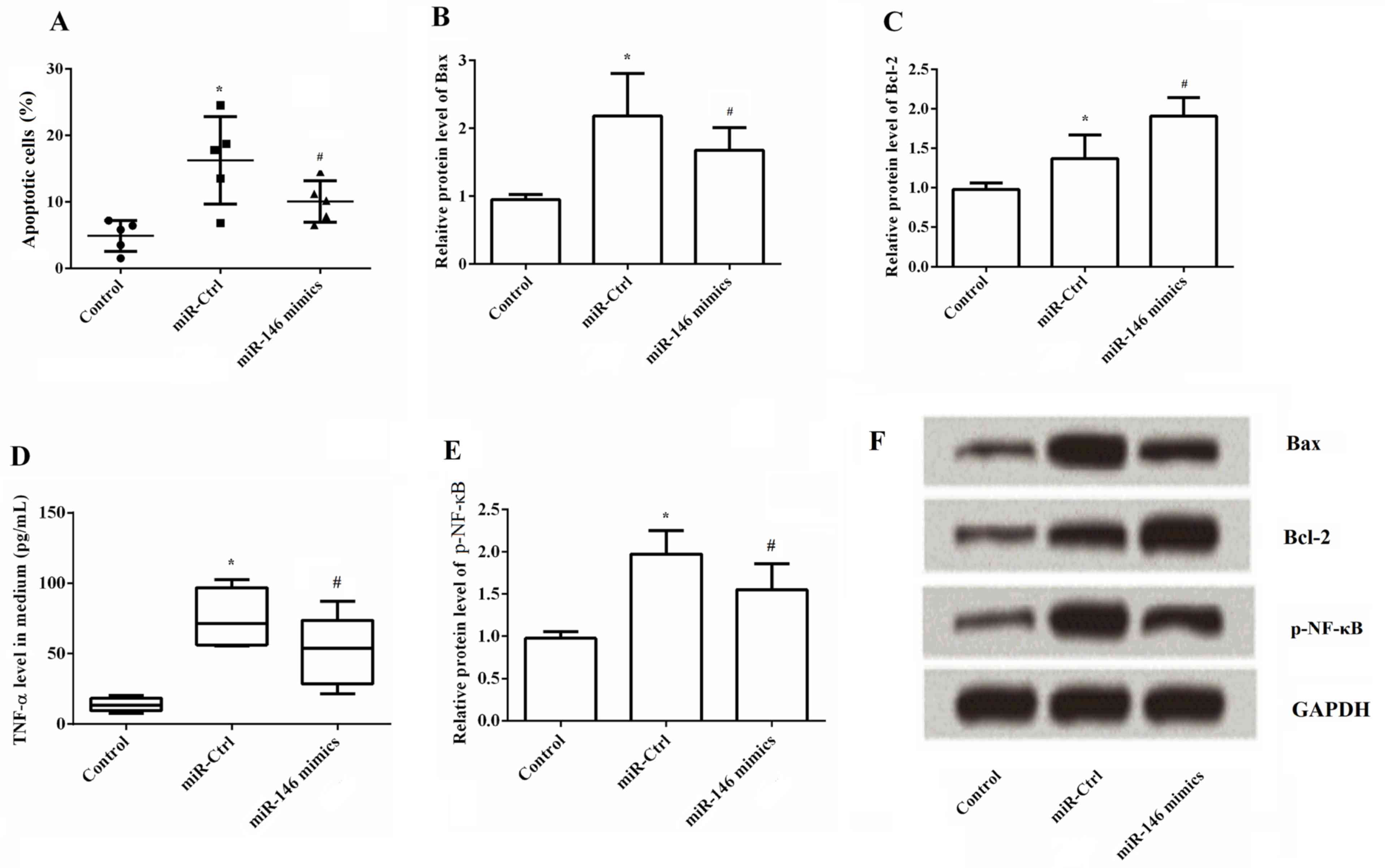 | Figure 3.Effects of miR-146 overexpression on
cell apoptosis, TNF-α and p-NF-κB levels. A terminal
deoxynucleotidyl-transferase-mediated dUTP nick-end labeling assay,
ELISA and western blotting were used to determine the effects of
miR-146 overexpression on cell apoptosis, TNF-α and p-NF-κB levels,
respectively. (A) Percentage of apoptotic cells significantly
decreased by overexpression of miR-146 compared with the miR-Ctrl
group. (B) Semi-quantitative analysis of Bax protein expression,
which was significantly reduced by overexpression of miR-146
compared with the miR-Ctrl group, (C) Semi-quantitative analysis of
Bcl-2 protein expression, which was significantly increased by
overexpression of miR-146 compared with the miR-Ctrl group, (D)
Overexpression of miR-146 markedly decreased the levels of TNF-α,
(E) Overexpression of miR-146 significantly reduced the levels of
p-NF-κB. (F) Representative western blot images. *P<0.05 vs.
control group; #P<0.05 vs. miR-Ctrl group. Bax,
B-cell lymphoma 2-associated X protein; Bcl-2, B-cell lymphoma 2;
p-NF-κB, phosphorylated nuclear factor-κB; TNF-α, tumor necrosis
factor-α. |
Discussion
In the present study, the potential cytoprotective
effects of miR-146 against OGD/R-induced myocardial cell injury, as
well as the underlying mechanisms, were investigated. The results
demonstrated that miR-146 overexpression significantly increased
cell viability and decreased apoptosis of H9c2 rat myocardial
cells. In addition to this, it was observed that overexpression of
miR-146 could significantly reduce the protein levels of Bax, TNF-α
and p-NF-κB, and increase the levels of Bcl-2. The results of the
current study suggested that miR-146 overexpression may protect
against OGD/R-induced cardiomyocyte apoptosis, which may be
achieved by inhibiting the NF-κB/TNF-α signaling pathway.
miRNAs are critically involved in the pathological
alterations of the heart, including cardiac hypertrophy (23), arrhythmogenesis (24), heart failure (25) and angiogenesis (26), as well as myocardial I/R injury
(27). It has been reported that
specific protectomiRs may serve as potential therapeutic tools for
treating I/R injury (28). For
example, locked nucleic acid-modified antisense miR-92a exerts
cell-protective, proangiogenic and anti-inflammatory effects on a
porcine model of myocardial I/R. Inhibition of miR-92a may be a
novel therapeutic tool to preserve cardiac function following
ischemia (11). miR-146 was first
identified as an immune system modulator that affects the mammalian
response to microbial infection (29). It has been reported that miR-146
serves a critical role in regulating numerous cell functions,
including cell proliferation and apoptosis, and is involved in
various human diseases, such as cancers, rheumatoid arthritis and
Alzheimer's disease (30–32). In addition, increasing evidence has
indicated that miR-146 is also involved in I/R. Jiang et al
(13) proposed that miR-146a
ameliorated liver I/R injury in vivo and
hypoxia/reoxygenation injury in vitro by directly
suppressing IL-1 receptor-associated kinase 1 (IRAK1) and TNF
receptor-associated factor 6 (TRAF6). In addition, Wang et
al (16) observed that the
increased expression of miR-146a protected against myocardial I/R
injury by significantly reducing myocardial infarct size and
preventing I/R-induced cardiac dysfunction. The underlying
mechanisms may be associated with the weakening of NF-κB activation
and inflammatory cytokine production through the inhibition of
IRAK1 and TRAF6. The protective role of miR-146 in myocardial I/R
injury in the current study was partly similar. miR-146 exerted its
cytoprotective effects against OGD/R-induced cardiomyocyte
apoptosis by inhibiting the NF-κB/TNF-α signaling pathway.
In addition to necrosis, several lines of evidence
suggest that myocardial apoptosis is also initiated and enhanced
during reperfusion through numerous mechanisms (3,33,34).
Therefore, an improved understanding of the cellular mechanisms
underlying apoptosis may aid in the prevention of
reperfusion-associated irreversible injury. Apoptosis is a highly
regulated process and the balance between apoptosis-promotion and
apoptosis-inhibition decides the cell fate. Bcl-2 and Bax are two
such regulatory proteins that serve pivotal roles in apoptosis, and
have been investigated in myocardial I/R injury (35,36).
Bcl-2 is an apoptosis inhibitor, whereas Bax is a proapoptotic
protein. Overexpression of Bcl-2 and downregulation of Bax
attenuate apoptosis. The results the present study demonstrated
that overexpression of miR-146 increases cell viability and
decreases cell apoptosis. The mechanism underlying cell apoptosis
may be associated with increasing and decreasing levels of Bcl-2
and Bax, respectively.
It has been demonstrated that reperfusion injury
initiated by inflammatory cascades results in perpetual damage to
cardiac tissue following ischemia (37). Therefore, regulation of the
inflammatory response may be a potential pharmacological target to
protect the heart from I/R injury (38). Among the inflammatory cascades, the
transcription factor NF-κB is one of the central players in this
cascade and is known for modulating the transduction of
inflammatory signals and cytokine production. NF-κB is activated
early on in reperfusion. Activation of NF-κB results in
translocation to the nucleus, where numerous effector genes are
stimulated, many of which are important regulators of cell
apoptosis and inflammation (39).
For example, some cytokines, including TNF-α and IL-6, are
motivated by NF-κB (40), which
then enhance the production of proinflammatory cytokines. Previous
studies have confirmed higher levels of TNF-α following I/R
(41,42) and upregulation of TNF-α
subsequently results in amplified cytokine effects. TNF-α is
responsible for myocardial dysfunction by direct inhibition of
contractility and stimulation of myocyte apoptosis (43). Furthermore, it has been reported
that a neutralizing antibody against TNF-α exerts protective
anti-apoptotic effects in cardiomyocytes (44,45).
Because of the important role of NF-κB/TNF-α in I/R, the effects of
overexpression of miR-146 on the expression of TNF-α and p-NF-κB
were analyzed. The expression levels of TNF-α and p-NF-κB were
significantly decreased through the overexpression of miR-146,
which suggested an inactivating effect of miR-146 on
NF-κB/TNF-α.
In conclusion, the results from the current study
suggested that miR-146 protects against OGD/R-induced cardiomyocyte
apoptosis, and the effects may be achieved by inhibiting the
NF-κB/TNF-α signaling pathway. Overexpression of miR-146 may
provide a novel therapeutic and preventive target for the treatment
of myocardial I/R injury.
Acknowledgements
The present study was supported by The Project of
Wenzhou Science and Technology Bureau (grant no. Y20130270).
References
|
1
|
Gaughan J, Mason A and Ward P: English
Hospitals Can Improve Their Use of Resources: An Analysis of Costs
and Length of Stay for Ten Treatments. Working Papers. 2012.
|
|
2
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar
|
|
3
|
Zhao ZQ, Morris CD, Budde JM, Wang NP,
Muraki S, Sun HY and Guyton RA: Inhibition of myocardial apoptosis
reduces infarct size and improves regional contractile dysfunction
during reperfusion. Cardiovasc Res. 59:132–142. 2003. View Article : Google Scholar
|
|
4
|
Fliss H and Gattinger D: Apoptosis in
ischemic and reperfused rat myocardium. Circ Res. 79:949–956. 1996.
View Article : Google Scholar
|
|
5
|
Zhao ZQ, Nakamura M, Wang NP, Wilcox JN,
Shearer S, Ronson RS, Guyton RA and Vinten-Johansen J: Reperfusion
induces myocardial apoptotic cell death. Cardiovasc Res.
45:651–660. 2000. View Article : Google Scholar
|
|
6
|
Pasquinelli AE: MicroRNAs and their
targets: Recognition, regulation and an emerging reciprocal
relationship. Nat Rev Genet. 13:271–282. 2012.
|
|
7
|
Hwang HW and Mendell JT: MicroRNAs in cell
proliferation, cell death, and tumorigenesis. Br J Cancer.
94:776–780. 2006. View Article : Google Scholar :
|
|
8
|
Bueno MJ, de Castro Pérez I and Malumbres
M: Control of cell proliferation pathways by microRNAs. Cell Cycle.
7:3143–3148. 2008. View Article : Google Scholar
|
|
9
|
Ye Y, Perez-Polo JR, Qian J and Birnbaum
Y: The role of microRNA in modulating myocardial
ischemia-reperfusion injury. Physiol Genomics. 43:534–542. 2011.
View Article : Google Scholar
|
|
10
|
Ren XP, Wu J, Wang X, Sartor MA, Jones K,
Qian J, Nicolaou P, Pritchard TJ and Fan GC: MicroRNA-320 is
involved in the regulation of cardiac ischemia/reperfusion injury
by targeting heat-shock protein 20. Circulation. 119:2357–2366.
2009. View Article : Google Scholar :
|
|
11
|
Hinkel R, Penzkofer D, Zühlke S, Fischer
A, Husada W, Xu QF, Baloch E, van Rooij E, Zeiher AM, Kupatt C and
Dimmeler S: Inhibition of MicroRNA-92a protects against
ischemia/reperfusion injury in a large-animal model. Circulation.
128:1066–1075. 2013. View Article : Google Scholar
|
|
12
|
Weiss JB, Eisenhardt SU, Stark GB, Bode C,
Moser M and Grundmann S: MicroRNAs in ischemia-reperfusion injury.
Am J Cardiovasc Dis. 2:237–247. 2012.
|
|
13
|
Jiang W and Kong L, Ni Q, Lu Y, Ding W,
Liu G, Pu L, Tang W and Kong L: miR-146a ameliorates liver
ischemia/reperfusion injury by suppressing IRAK1 and TRAF6. PLoS
One. 9:e1015302014. View Article : Google Scholar :
|
|
14
|
Chen Q, Kong L, Xu X, Geng Q, Tang W and
Jiang W: Down-regulation of MicroRNA-146a in the early stage of
liver ischemia-reperfusion injury. Transplant Proc. 45:492–496.
2013. View Article : Google Scholar
|
|
15
|
Chassin C, Hempel C, Stockinger S, Dupont
A, Kübler JF, Wedemeyer J, Vandewalle A and Hornef MW:
MicroRNA-146a-mediated downregulation of IRAK1 protects mouse and
human small intestine against ischemia/reperfusion injury. EMBO Mol
Med. 4:1308–1319. 2012. View Article : Google Scholar :
|
|
16
|
Wang X, Ha T, Liu L, Zou J, Zhang X,
Kalbfleisch J, Gao X, Williams D and Li C: Increased expression of
microRNA-146a decreases myocardial ischaemia/reperfusion injury.
Cardiovasc Res. 97:432–442. 2013. View Article : Google Scholar
|
|
17
|
Xie YF, Shu R, Jiang SY, Song ZC, Guo QM,
Dong JC and Lin ZK: miRNA-146 negatively regulates the production
of pro-inflammatory cytokines via NF-κB signalling in human
gingival fibroblasts. J Inflamm (Lond). 11:382014. View Article : Google Scholar :
|
|
18
|
Chandrasekar B, Smith JB and Freeman GL:
Ischemia-reperfusion of rat myocardium activates nuclear
factor-kappaB and induces neutrophil infiltration via
lipopolysaccharide-induced CXC chemokine. Circulation.
103:2296–2302. 2001. View Article : Google Scholar
|
|
19
|
Wu WY, Wang WY, Ma YL, Yan H, Wang XB, Qin
YL, Su M, Chen T and Wang YP: Sodium tanshinone IIA silate inhibits
oxygen-glucose deprivation/recovery-induced cardiomyocyte apoptosis
via suppression of the NF-κB/TNF-α pathway. Br J Pharmacol.
169:1058–1071. 2013. View Article : Google Scholar :
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Inada T, Kubo K and Shingu K: Possible
link between cyclooxygenase-inhibiting and antitumor properties of
propofol. J Anesth. 25:569–575. 2011. View Article : Google Scholar
|
|
22
|
Li X, Jiang L, Lin S, He Y, Shen G, Cai Z,
Ling M, Ni J, Zhang H and Zhang M: Inhibition of mTORC1 renders
cardiac protection against lipopolysaccharide. Int J Clin Exp
Pathol. 7:8432–8442. 2014.
|
|
23
|
Cheng Y, Ji R, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNAs are aberrantly expressed in
hypertrophic heart: Do they play a role in cardiac hypertrophy? Am
J Pathol. 170:1831–1840. 2007. View Article : Google Scholar :
|
|
24
|
Yang B, Lin H, Xiao J, Lu Y, Luo X, Li B,
Zhang Y, Xu C, Bai Y, Wang H, et al: The muscle-specific microRNA
miR-1 regulates cardiac arrhythmogenic potential by targeting GJA1
and KCNJ2. Nat Med. 13:486–491. 2007. View
Article : Google Scholar
|
|
25
|
Thum T, Galuppo P, Wolf C, Fiedler J,
Kneitz S, van Laake LW, Doevendans PA, Mummery CL, Borlak J,
Haverich A, et al: MicroRNAs in the human heart: A clue to fetal
gene reprogramming in heart failure. Circulation. 116:258–267.
2007. View Article : Google Scholar
|
|
26
|
Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen
H, Dean DB and Zhang C: MicroRNA expression signature and
antisense-mediated depletion reveal an essential role of MicroRNA
in vascular neointimal lesion formation. Circ Res. 100:1579–1588.
2007. View Article : Google Scholar
|
|
27
|
Yin C, Wang X and Kukreja RC: Endogenous
microRNAs induced by heat-shock reduce myocardial infarction
following ischemia-reperfusion in mice. FEBS Lett. 582:4137–4142.
2008. View Article : Google Scholar :
|
|
28
|
Varga ZV, Zvara A, Faragó N, Kocsis GF,
Pipicz M, Gáspár R, Bencsik P, Görbe A, Csonka C, Puskás LG, et al:
MicroRNAs associated with ischemia-reperfusion injury and
cardioprotection by ischemic pre- and postconditioning:
ProtectomiRs. Am J Physiol Heart Circ Physiol. 307:H216–H227. 2014.
View Article : Google Scholar
|
|
29
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:12481–12486. 2006.
View Article : Google Scholar :
|
|
30
|
Wang LL, Huang Y, Wang G and Chen SD: The
potential role of microRNA-146 in Alzheimer's disease: Biomarker or
therapeutic target? Med Hypotheses. 78:398–401. 2012. View Article : Google Scholar
|
|
31
|
Hurst DR, Edmonds MD, Scott GK, Benz CC,
Vaidya KS and Welch DR: Breast cancer metastasis suppressor 1
up-regulates miR-146, which suppresses breast cancer metastasis.
Cancer Res. 69:1279–1283. 2009. View Article : Google Scholar :
|
|
32
|
Nakasa T, Miyaki S, Okubo A, Hashimoto M,
Nishida K, Ochi M and Asahara H: Expression of microRNA-146 in
rheumatoid arthritis synovial tissue. Arthritis Rheum.
58:1284–1292. 2008. View Article : Google Scholar :
|
|
33
|
Krijnen PA, Nijmeijer R, Meijer CJ, Visser
CA, Hack CE and Niessen HW: Apoptosis in myocardial ischaemia and
infarction. J Clin Pathol. 55:801–811. 2002. View Article : Google Scholar :
|
|
34
|
Eefting F, Rensing B, Wigman J, Pannekoek
WJ, Liu WM, Cramer MJ, Lips DJ and Doevendans PA: Role of apoptosis
in reperfusion injury. Cardiovasc Res. 61:414–426. 2004. View Article : Google Scholar
|
|
35
|
Chen Z, Chu CC, Ho YS, Hamdy RC and Chua
BH: Overexpression of Bcl-2 attenuates apoptosis and protects
against myocardial I/R injury in transgenic mice. Am J Physiol
Heart Circ Physiol. 280:H2313–H2320. 2001.
|
|
36
|
Wang N, Minatoguchi S, Chen X, Uno Y, Arai
M, Lu C, Takemura G, Fujiwara T and Fujiwara H: Antidiabetic drug
miglitol inhibits myocardial apoptosis involving decreased hydroxyl
radical production and Bax expression in an ischaemia/reperfusion
rabbit heart. Br J Pharmacol. 142:983–990. 2004. View Article : Google Scholar :
|
|
37
|
Carden DL and Granger DN: Pathophysiology
of ischaemia-reperfusion injury. J Pathol. 190:255–266. 2000.
View Article : Google Scholar
|
|
38
|
Park JL and Lucchesi BR: Mechanisms of
myocardial reperfusion injury. Ann Thorac Surg. 68:1905–1912. 1999.
View Article : Google Scholar
|
|
39
|
Zeng M, Wei X, Wu Z, Li W, Li B, Zhen Y,
Chen J, Wang P and Fei Y: NF-κB-mediated induction of autophagy in
cardiac ischemia/reperfusion injury. Biochem Biophys Res Commun.
436:180–185. 2013. View Article : Google Scholar
|
|
40
|
Li C, Gao Y, Tian J, Shen J, Xing Y and
Liu Z: Sophocarpine administration preserves myocardial function
from ischemia-reperfusion in rats via NF-κB inactivation. J
Ethnopharmacol. 135:620–625. 2011. View Article : Google Scholar
|
|
41
|
Kawamura T, Kadosaki M, Nara N, Wei J,
Endo S and Inada K: Nicorandil attenuates NF-kappaB activation,
adhesion molecule expression, and cytokine production in patients
with coronary artery bypass surgery. Shock. 24:103–108. 2005.
View Article : Google Scholar
|
|
42
|
Liu X, Shen J, Jin Y, Duan M and Xu J:
Recombinant human erythropoietin (rhEPO) preconditioning on nuclear
factor-kappa B (NF-kB) activation & proinflammatory cytokines
induced by myocardial ischaemia-reperfusion. Indian J Med Res.
124:343–354. 2006.
|
|
43
|
Meldrum DR: Tumor necrosis factor in the
heart. Am J Physiol. 274:R577–R595. 1998.
|
|
44
|
Minamino T, Yujiri T, Papst PJ, Chan ED,
Johnson GL and Terada N: MEKK1 suppresses oxidative stress-induced
apoptosis of embryonic stem cell-derived cardiac myocytes. Proc
Natl Acad Sci USA. 96:15127–15132. 1999. View Article : Google Scholar :
|
|
45
|
Li S, Jiao X, Tao L, Liu H, Cao Y, Lopez
BL, Christopher TA and Ma XL: Tumor necrosis factor-alpha in
mechanic trauma plasma mediates cardiomyocyte apoptosis. Am J
Physiol Heart Circ Physiol. 293:H1847–H1852. 2007. View Article : Google Scholar
|

















