Introduction
Osteoarthritis (OA) is a common degenerative joint
disease that affects the entire joint in patients worldwide
(1). Although the primary
characteristic of OA is the destruction of articular cartilage,
additional characteristics of this disease include synovial
inflammation, osteophyte formation and sclerosis of the subchondral
bone (2). The etiology of OA is
multifactorial and includes joint injury, obesity, aging and
hereditary factors (3), but the
underlying mechanism of pathogenesis of OA remains to elucidated.
There may be a number of initiating events of OA, as several
different factors may lead to a common pathway that leads to the
same disease (4). Previous studies
of OA primarily focused on cartilage alterations; however, this
focus has shifted, and OA is currently considered a pathological
condition of the entire joint, which includes alterations in the
articular cartilage, subchondral bone, ligaments, capsule and
synovial membrane, ultimately leading to joint failure (5). OA remains a worldwide medical
challenge and its definition, risk factors and pathogenesis remain
to be elucidated.
The transforming growth factor-β (TGF-β) family
consists of >35 members, including TGF-βs, activins and bone
morphogenetic proteins (BMPs) (6).
A total of three isoforms of TGF-β have been identified in mammals,
termed TGF-β1, -β2 and -β3, which are secreted as inactive
complexes of a TGF-β dimer, pro-peptide latency associated peptide
(LAP) and latent TGF-β binding proteins (LTBPs) (7). Normal TGF-β signaling has a role in
homeostasis of articular cartilage and excessive activation of
TGF-β in joint tissues, which leads to osteophyte formation,
synovial fibrosis and joint pain in animal models (8). A polymorphism in TGF-β1 at position
29 (T to C, amino acid 10) in the signal peptide sequence is
associated with increased prevalence of spinal osteophytosis and
ossification of the posterior longitudinal ligament (9). The above TGF-β polymorphism has also
been associated with bone mineral density and fracture risk in
postmenopausal Chinese women (10). However, the polymorphism may reduce
morbidity of osteoporosis in Japanese women (11). A previous study indicated that
TGF-β signaling is mediated by both activin-like kinase (ALK)5 and
ALK1 in chondrocytes (12).
Further studies have demonstrated that overexpression of ALK1
increases matrix metalloproteinase (MMP)-13 expression in
vitro and its inhibition reduces MMP-13 expression (12,13).
A total of four LTBP isoforms have been identified
and LTBP-1, −3, and part of LTBP-4 covalently associates with the
LAP of TGF-β via the cysteine-rich domain (14). The large latent complex is secreted
to the extracellular matrix (ECM), where it is targeted, stabilized
and activated (15). Latent TGF-β
cannot be activated unless the mature peptide is released from LAP
and the mechanism underlying this process varies between cell types
and environments (16). A previous
study has indicated that BMP-1 may regulate TGF-β activation by
cleaving LTBP-1 (17). Another
study demonstrated that membrane type-1 (MT1)-MMP-mediated
proteolytic processing of ECM-bound LTBP-1 was a mechanism to
release latent TGF-β from the subendothelial matrix (18). When active TGF-β is released from
the ECM, it binds to TGF-β receptor I and II heterodimers and
induces them to directly activate receptor-regulated Smads
(R-Smads) by phosphorylation. R-Smads in turn form transcriptional
complexes with their common partner Smads to control target gene
expression (19). Therefore, based
on the aforementioned results, the authors of the present study
hypothesized that the TGF-β pathway may have a role in human OA
fibroblast-like synoviocytes (FLS) and thatLTBP-1 may be a
regulator modulating TGF-β activity.
Materials and methods
Cells
Primary OA FLS were extracted from freshly resected
synovial tissues of 31 patients with OA undergoing total knee
arthroplasty and primary normal FLS were obtained from freshly
resected synovial tissues of 5 trauma patients undergoing lower
limb amputation. Tissues were carefully minced, digested with 1%
collagenase I (Worthington Biochemical Corporation, NJ, USA) in
Dulbecco's modified Eagle's medium (DMEM) (Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) for 6 h at 37°C, filtered through a
200-mesh sieve, and subsequently cultured in DMEM with 10% fetal
bovine serum (Hyclone, GE Healthcare Life Sciences). Cells were
cultured to ~90% confluence and split at a 1:3 ratio for the next
passage. All FLS were used during the third to fifth passage. FLS
were analyzed by flow cytometry using Vimentin monoclonal antibody
(cat. no. MA5-11883; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) as a specific marker for FLS and the purity of all FLS was
>95%, which met the requirement of the subsequent
experiments.
Reagents
A total of three selected siRNA duplexes targeting
LTBP-1 with a modification pattern that accounted for off-target
effects caused by both strands and the non-targeting control pool
[the non-siRNA group siRNA(−)] were purchased from Sangon Biotech
Co., Ltd. (Shanghai, China) and used at 100 nM. The following sense
sequences of the siRNA pool targeting LTBP-1 were used in the
present study: 5′-GCCAUCUUCCAUGUAUGAATT−3′,
5′-GCCUAAACUUUAUCAGCAUTT-3′ and 5′-GCCAAUCCCAAGUCUCGUATT-3′.
Transient lipophilic transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). Plasmin from human plasma
(cat. no. P1867) was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Polyclonal LTBP-1 antibody (cat. no. BS60783)
for western blot analysis was purchased from Bioworld Technology,
Inc. (St. Louis Park, MN, USA), and monoclonal LTBP-1 antibody
(cat. no. sc-271140) for immunofluorescence and
immunohistochemistry was purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). TGF-β1 polyclonal antibody (cat. no.
18978-1-AP), TGF-β2 polyclonal antibody (cat. no. 19999-1-AP), and
TGF-β3 polyclonal antibody (cat. no. 18942-1-AP) were purchased
from Wuhan Sanying Biotechnology (Wuhan, China). The antibody
against MMP-13 (cat. no. BS1231) was obtained from Bioworld
Technology, Inc. The phosphorylated (p)-Smad1 (Ser463/465)/Smad5
(Ser463/465)/Smad8 (Ser465/467) (cat. no. 9511) and p-Smad2
(Ser465) (cat. no. 8828) antibodies were purchased from Cell
Signaling Technology, Inc. (Danvers, MA, USA). Antibodies against
β-tubulin (cat. no. ab6046) and GAPDH (cat. no. ab8245) were
obtained from Abcam (Cambridge, UK).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total FLSs RNA was prepared using TRIzol reagent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. cDNA was prepared using the All-In-One RT Master Mix
(Applied Biological Materials, Inc., Richmond, BC, Canada) with a
Bio-Rad MyCycler system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA) as follows: 37°C for 15 min, then 85°C for 5 sec and cooling
to 4°C. Gene expression was measured in a Rotor-Gene Q 2 plex
System (Qiagen GmbH, Hilden, Germany) at 470 nm with Rotor-GenQ
Series software version 2.1.0. The following primer sequences were
used for the qPCR: LTBP-1 long (L)+ short (S) forward:
5′-GCTTCCGTCCAGATACATCAG-3′ (NM001166266, nt499-519), reverse:
5′-CTTGGTACGAGACTTGGGATTG-3′ (NM001166266, nt595-574); LTBP-1 L
forward: 5′-GGTGCACCAAACCTAGCTGTG-3′ (NM206943.1, nt554-574),
reverse: 5′-ACAGGCTTTGCCCTTGG-3′ (NM206943.1, nt636-654); TGF-β1
forward: 5′-GCCCTGGACACCAACTATTG-3′ (NM000660.3, nt1702-1727),
reverse: 5′-CGTGTCCAGGCTCCAAATG-3′ (NM 000660.3, nt1851-1869),
TGF-β2 forward: 5′-AAGCTTACACTGTCCCTGCTGC-3′ (NM003238.1,
nt847-868), reverse: 5′-TGTGGAGGTGCCATCAATACCT-3′ (NM003228.1,
nt934-955), TGF-β3 forward: 5′-GGAAAACACCGAGTCGGAATAC-3′ (NM003239,
nt279-300), reverse: 5′-GCGGAAAACCTTGGAGGTAAT−3′ (NM003239,
nt399-379), GAPDH forward: 5′-GGAAAACACCGAGTCGGAATAC-3′
(NM003238.1, nt847-868), reverse: 5′-GCGGAAAACCTTGGAGGTAAT-3′
(NM003228.1, nt934-955). Thermocycling conditions were:
Denaturation step at 95°C for 10 min, then 40 cycles at 95°C for 15
sec, 65°C for 10 sec and 72°C for 15 sec. Standard curves were
obtained, and relative quantification of gene expression was
assessed compared with threshold values. All results were
normalized to GAPDH, and calculated using the 2−ΔΔCq
method for relative quantification (20).
Western blotting
Proteins from synovial tissues from OA and trauma
patients and the conditioned media of FLSs were combined with
plasmin to solubilize the LTBP-1-containing high-molecular-weight
complexes. For detection of proteins from cell lysates, cells were
lysed using radioimmunoprecipitation assay (RIPA) buffer (Beijing
ComWin Biotech Co., Ltd., Beijing, China) supplemented with a
Protease Inhibitor Cocktail (100X; Beijing ComWin Biotech Co.,
Ltd.) and a phosphatase inhibitor cocktail (cat. no. P1260, Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China).
Centriplus centrifugal filter device YM-3 (3 kDa cut-off; Merck
KGaA) was used to concentrate proteins from conditioned media.
Protein levels were analyzed by western blotting using 20 µg
protein/lane mixed with SDS-PAGE Loading Buffer (Beijing ComWin
Biotech Co., Ltd.). The extracted proteins were loaded onto a 10%
SDS-PAGE gel and electrophoresed for 30 min at 80 V and then
another 40 min at 120 V. Subsequently, the proteins were
transferred to polyvinylidene fluoride (PVDF) membranes. The PVDF
membranes were blocked in 5% skimmed milk in 1X Tris-buffered 0.05%
saline Tween (TBST) for 1 h at room temperature, washed with TBST,
and subsequently incubated for 24 h at 4°C with primary antibodies
against LTBP-1, TGF-βs, MMP-13, p-Smad1/5/8, p-Smad2 and GAPDH at
the following working concentrations: Anti-LTBP-1 antibody, 1:200;
anti-TGF-β1 antibody, 1:500; anti-TGF-β2 antibody, 1:500;
anti-TGF-β3 antibody, 1:500; anti-MMP-13 antibody, 1:300;
anti-p-Smad1/5/8 antibody, 1:800; anti-p-Smad2 antibody, 1:800;
anti-β-Tubulin antibody, 1:5,000; and anti-GAPDH antibody,
1:10,000. The PVDF membranes were subsequently washed in TBST and
incubated at room temperature overnight with horseradish
peroxidase-conjugated secondary antibodies at the following working
concentrations: Goat anti-mouse IgG (ab6789; 1:3,000; Abcam,
Cambridge, UK) and Goat Anti-Rabbit IgG (ab6721; 1:3,000; Abcam).
Protein bands were detected with Immobilon Western Chemiluminescent
HRP Substrate (WBKLS0050; EMD Millipore, Billerica, MA, USA) using
Bio-Rad chemiluminescence imaging system (ChemiDoc XRS; Bio-Rad
Laboratories, Inc., Hercules, CA, USA) according to the
manufacturer's protocol. Densitometry was performed using ImageJ
software (version 1.43; National Institutes of Health, Bethesda,
USA).
Human tissue specimens
A total of 31 OA and 5 normal synovial tissues
obtained from patients including 20 women and 16 men aged between
48 and 69 years old who underwent surgical treatment between May
2016 and January 2017 in the Department of Orthopedics of Tangdu
Hospital (Xi'an, China) were investigated. The diagnoses were
confirmed by a minimum of 2 senior pathologists. Each specimen was
divided into three parts. The first part was fixed in 4% formalin
at room temperature for 24 h (pH 7.4), embedded in paraffin,
sectioned (~4 µm) with a microtome and placed on Super Frost Plus
slides (Microm International GmbH, Walldorf, Germany) and
representative tissue samples were prepared for tissue
immunohistochemistry. The second part was ground for western
blotting using a tissue grinder (Shanghai Jingxiang Industrial
Company Ltd., Shanghai, China). The third part was used for primary
cell culture by the enzyme digestion method (21) as follows: Tissues were minced and
digested with 0.2% collagenase I (Worthington Biochemical
Corporation) in Dulbecco's modified Eagle's medium (DMEM; Hyclone;
GE Healthcare Life Sciences) for 4–6 h at 37°C, filtered through a
200-mesh sieve, and then cultured in DMEM supplemented with 10%
fetal bovine serum (Hyclone; GE Healthcare Life Sciences), 100
units penicillin and 100 µg/ml streptomycin. Finally, the cells
were cultured up to 90% confluence and then split in a 1/3 ratio up
to passage 3–6.
All patients signed written informed consent. All
human material used in the study was handled in accordance with the
policies of the local institutional review board, and all
operations were accepted by the patients and approved by the Ethics
Committee of Tangdu Hospital (Xi'an, China).
Immunohistochemistry
Immunohistochemistry was performed with a monoclonal
LTBP-1 antibody at a pretested dilution. Tissue slides, prepared as
aforementioned, were deparaffinized, rehydrated and incubated in 3%
H2O2 at room temperature for 5 min to
eliminate endogenous peroxidase activity. Subsequently, the slides
were washed with distilled water for 5 min, soaked in bovine serum
albumin (BSA; Beijing Solarbio Science & Technology Co., Ltd.)
at room temperature for 10 min, incubated overnight with LTBP-1
antibody at 1:200 at 4°C, and washed with PBS 3 times for 5 min.
Secondary antibody was added at room temperature for 30 min at the
following working concentrations: Horseradish peroxidase-conjugated
goat anti-mouse IgG (ab6789; 1:1,000; Abcam) and horseradish
peroxidase-conjugated goat anti-rabbit IgG (ab6721; 1:1,000;
Abcam). The samples were washed again with PBS 3 times for 5 min.
Sections were incubated with 3,3,'Diaminobenzidine (Beijing
Solarbio Science & Technology Co., Ltd.) for 4 min at room
temperature, washed and counterstained with hematoxylin for 5 sec
at room temperature. Evaluation of the immunohistochemical staining
and performed using an Olympus BX51 light microscope at
magnifications of 200 and ×400 (Olympus Corporation, Tokyo,
Japan).
Immunofluorescence
Immunofluorescence analysis of FLS was performed
with a monoclonal LTBP-1 antibody at 1:200 dilution. Both OA and
normal FLS were seeded in 6-well culture plates, which were about
104 FLSs per well. Following 24 h of culture, cells
reached ~50% confluence and were washed with PBS 2 times for 3 min
and mixed with 4% paraformaldehyde at room temperature. Following a
30 min incubation at room temperature, the samples were washed
again with PBS 2 times for 3 min and mixed with 0.5% Triton X-100
at room temperature for 30 min. Samples were washed with 0.01 M PBS
2 times for 3 min. For antibody blocking, 5% BSA was added at room
temperature for 30–60 min. LTBP-1 antibody was added at a pretested
dilution at 4°C overnight. Samples were washed with PBST 3 times
for 3 min, and secondary fluorescent antibody was added at room
temperature for 1 h. Samples were washed with 0.01 M PBS Tween-20
(PBST) 3 times for 3 min. Subsequently, cells were incubated with
DAPI for 10 min at room temperature, washed 2 times with 0.01 M PBS
for 5 min and examined using Olympus IX71 fluorescent microscope
(Olympus Corporation).
Cell proliferation assay
To evaluate cell proliferation by Cell Counting
Kit-8 (CCK-8) assays (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), OA and normal FLS were plated in 96-well
flat-bottomed plates for 24 h. The normal FLS were untreated for 24
h and termed normal control (NC). OA FLS were divided into 3
groups, one group of OA FLSs was treated with siRNA for 24 h,
termed siRNA(+), another group of OA FLSs was treated with
non-targeting siRNA for 24 h, termed siRNA(−), and the third group
was untreated for 24 h, termed OA. The number of cells in each well
was ~102, at ~70–80% confluence. FLSs of the four groups
were mixed with 10 µl CCK-8 in each well, and after 1–4 h analyzed
using the Infinite M200 Pro Multifunctional microplate reader at
450 nm (Tecan Group, Ltd., Mannedorf, Switzerland) according to the
manufacturer's protocol.
Statistical analysis
Experiments were repeated 3 times and data are
presented as the mean ± standard deviation. One-way analysis of
variance test followed by Tukey's post hoc test was used to analyze
differences among 3 groups. Two-tailed Student's t-tests was used
for the analysis between two groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Human OA synovial tissues express
increased LTBP-1levels compared with normal synovial tissues in
vivo
LTBP-1 expression was analyzed in human OA and
normal synovial tissues in vivo by immunohistochemistry.
Paraffin-embedded tissue sections were immunostained with LTBP-1
antibody. The present findings demonstrated that LTBP-1 expression
in human OA synovial tissues increased compared with normal
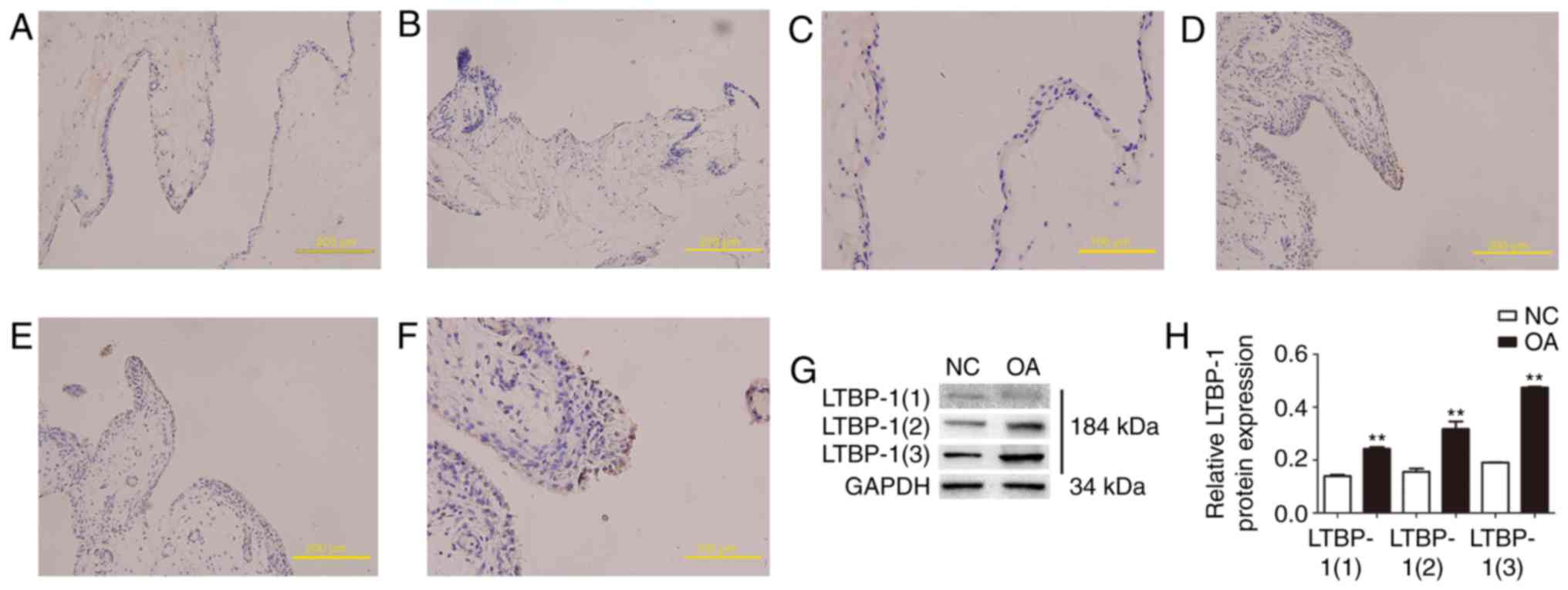
synovial tissues (Fig. 1A-F).
Diagnoses were confirmed by a minimum of 2 senior pathologists.
LTBP-1 expression was also analyzed in human OA and normal synovial
tissues in vivo by western blotting (Fig. 1G and H). To determine whether
LTBP-1 is associated with ECM in synovial tissues, plasmin was used
to solubilize proteins from the ECM. LTBP-1 was identified in
synovial ECM and there was a 2-fold increase in LTBP-1 expression
when human OA synovial tissues were compared with normal synovial
tissues.
Expression levels of LTBP-1, TGF-βs,
p-Smad1/5/8, and MMP-13 are elevated in human OA FLS compared with
normal FLS in vitro
To confirm that OA FLS express LTBP-1, LTBP-1
protein levels were detected by immunofluorescence. It was
determined that LTBP-1expression in OA increased compared with
normal FLS (Fig. 2A and B).
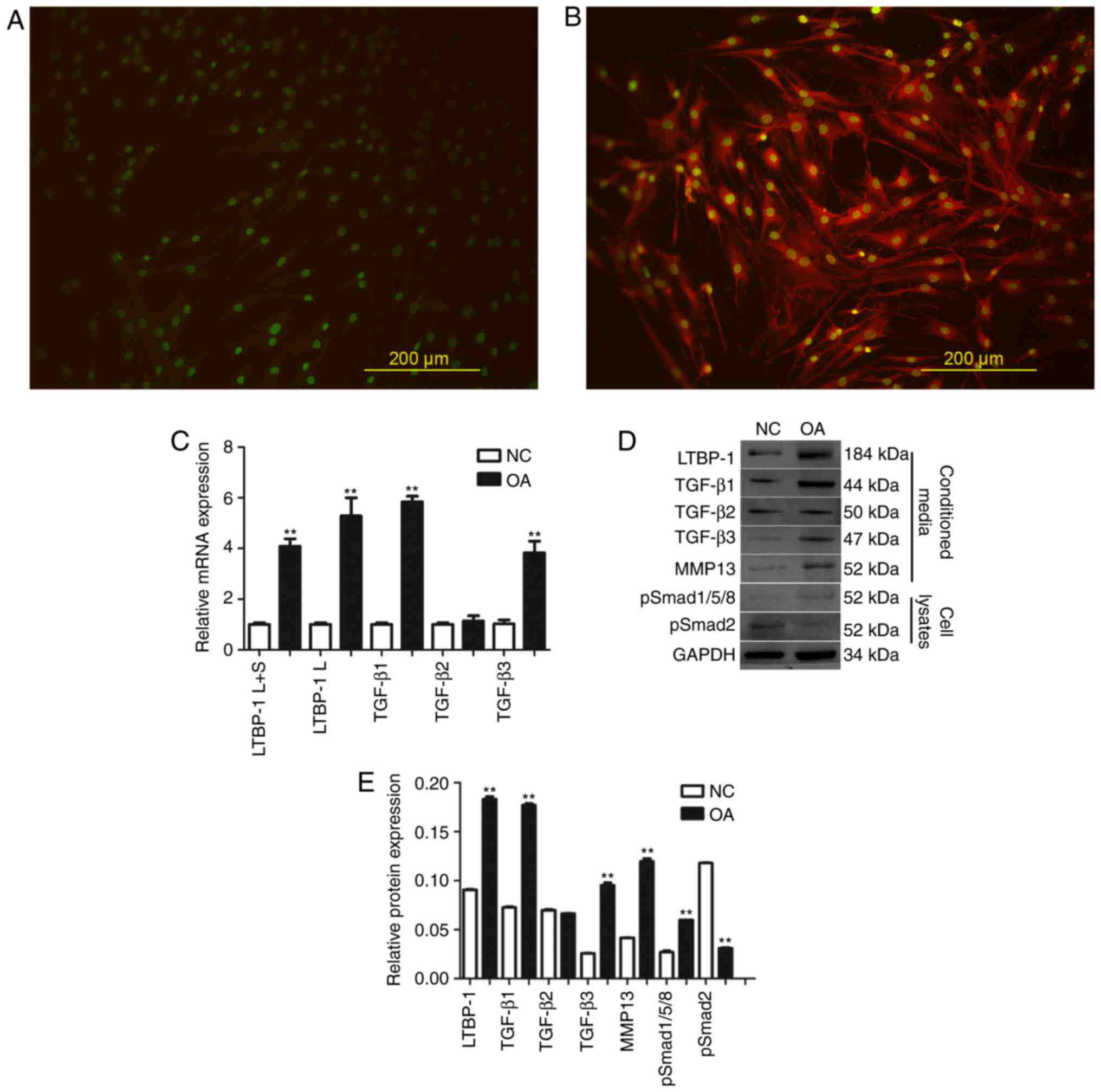 | Figure 2.LTBP-1 expression is higher in OA FLS
in vitro and LTBP-1 influences TGF-β signaling through
p-Smad1/5/8, compared with NC. LTBP-1 expression in FLS in (A) NC
and (B) patients with OA. (C) mRNA expression levels of LTBP-1 and
TGF-β mRNA in FLS of OA and NC groups. (D) LTBP-1, TGF-β1/2/3,
p-Smad1/5/8, p-Smad2 and MMP-13 of OA and NC groups were measured
by western blotting and (E) analyzed to determine relative
expression levels. OA donors, n=31. NC donors, n=5. Scale bar, 200
µm. Data are presented as the mean ± standard deviation.
**P<0.01 vs. the NC group. NC, normal control; TGF-β,
transforming growth factor-β; LTBP-1, latent TGF-β-binding
protein-1; OA, osteoarthritis; MMP, matrix metalloproteinase; p,
phosphorylated; Smad, mothers against decapentaplegic; L, long
isoform; S, short isoform; FLS, fibroblast-like synoviocytes. |
Subsequently, OA and normal FLS were analyzed for
LTBP-1 and TGF-β mRNA expression levels by RT-qPCR (Fig. 2C). Two LTBP-1 forms, Land S, and 3
TGF-β isoforms, TGF-β1, TGF-β2, TGF-β3 were analyzed. It was
determined that LTBP-1 L+S and LTBP-1SmRNAs were expressed in FLS,
and LTBP-1 L+S and LTBP-1S mRNA expression levels in OA FLS
increased ≥4-fold compared with normal FLS. TGF-β1 and TGF-β3
levels also increased in OA FLS compared with normal FLS. However,
there was no significant difference identified in the expression
levels of TGF-β2 between OA and normal FLS.
Expression levels of LTBP-1 and TGF-β signaling
pathway proteins, including TGF-β1, TGF-β2, TGF-β3, p-Smad1/5/8,
p-Smad2 and MMP-13 were analyzed by western blotting (Fig. 2D and E). LTBP-1, TGF-β1, TGF-β3,
p-Smad1/5/8 and MMP-13 levels in OA FLS increased compared with
normal FLS. Expression of TGF-β2 was similar between OA and normal
FLS. Expression of p-Smad2 in OA FLS decreased compared with normal
FLS.
LTBP-1 expression in OA FLS is lowest
24 h after siRNA transfection
LTBP-1 mRNA expression levels were analyzed at 12,
24, and 48 h after siRNA-mediated downregulation of LTBP-1 in OA
FLS. It was determined that LTBP-1 expression was lowest at 24 h
(Fig. 3A). LTBP-1 protein levels
were determined by western blotting, and LTBP-1 protein levels were
lowest 24 h following siRNA transfection (Fig. 3B and C).
OA FLS demonstrate increased
proliferation compared with normal FLS in vitro and siRNA-mediated
downregulation of LTBP-1 reduces proliferation of OA FLS
Proliferation of OA and normal FLS was determined 3
h following addition of CCK-8. Proliferation of OA FLS
significantly increased compared with normal FLS in vitro
(Fig. 4). However, siRNA-mediated
downregulation of LTBP-1 significantly reduced the proliferation of
OA FLS (Fig. 4).
siRNA-mediated downregulation of
LTBP-1 reduces expression levels of TGF-βs and MMP-13 in OA
FLS
To determine whether downregulation of LTBP-1 may
modulate TGF-β mRNA levels in OA FLS, mRNA levels of TGF-β1, TGF-β2
and TGF-β3 were quantified using RT-qPCR 24 h following
siRNA-mediated downregulation of LTBP-1. No significant difference
in the expression of TGF-βs was identified (Fig. 5A).
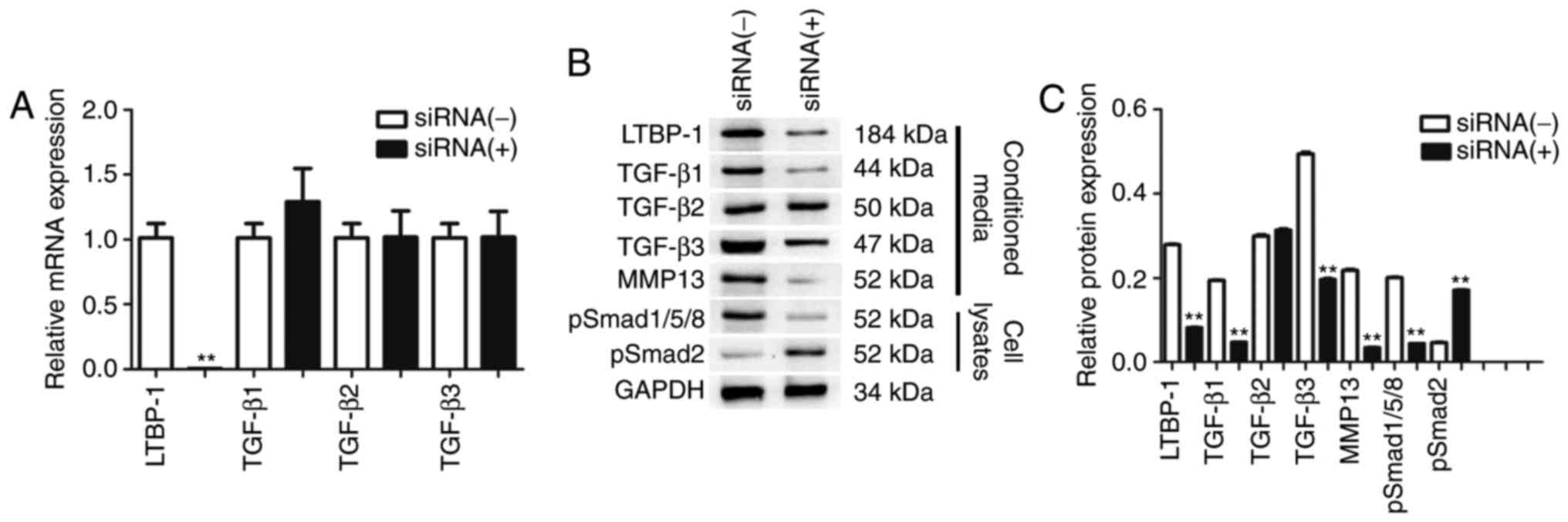 | Figure 5.siRNA-mediated downregulation of
LTBP-1 led to expression of TGF-β1-p-Smad1/5/8-MMP-13 in FLS of OA.
(A) LTBP-1 and TGF-β mRNA levels. Expression of LTBP-1, TGF-βs,
p-Smad1/5/8, p-Smad2 and MMP-13 in OA and NC were determined using
(B) western blotting and were (C) quantified. Data are presented as
the mean ± standard deviation of three independent experiments. OA
donors, n=31. NC donors, n=5. **P<0.01 vs. the siRNA(−)group.
siRNA, short interfering RNA; LTBP-1, latent transforming growth
factor-β-binding protein-1; OA, osteoarthritis; Smad, mothers
against decapentaplegic; MMP, matrix metalloproteinase; p,
phosphorylated; NC, normal controls. |
Subsequently, it was determined whether
downregulation of LTBP-1 may alter protein levels of TGF-βs and
MMP-13 in OA FLS. The results demonstrated that following
transfection, LTBP-1, TGF-β1, TGF-β3 and MMP-13 protein levels were
significantly reduced, but TGF-β2 expression levels were not
affected. siRNA-mediated downregulation of LTBP-1 reduced
p-Smad1/5/8 and increased p-Smad2 in OA FLS (Fig. 5B and C).
p-Smad1/5/8 and p-Smad2 expression levels were
determined in cell lysates by western blotting 24 h following
siRNA-mediated downregulation of LTBP-1 in OA FLS. Following
transfection, p-Smad1/5/8 protein levels significantly decreased in
the transfected group compared with the siRNA(−)OA FLS group.
p-Smad2 protein levels increased 4-fold compared with siRNA(−)OA
FLS (Fig. 5B and C).
Discussion
The present study demonstrated that in human OA FLS,
TGF-β signals via p-Smad2 and p-Smad1/5/8. Furthermore, it was
demonstrated that LTBP-1 may modulate the activity of TGF-β in
human OA FLS. The results of the present study contribute to the
efforts to elucidate the mechanism underlying the development and
pathology of OA.
TGF-β signaling is a pathway which contributes to
the development of OA (22). The
association of TGF-β and OA varies with the stage of OA (3). TGF-β has been identified in cartilage
and synovial fluid of patients with OA at elevated levels compared
with healthy control patients (4,23).
Therefore, the authors of the present study hypothesized that FLS,
components of an articulation, may participate in the TGF-β
signaling pathway. The results of the present study demonstrated
that the TGF-β signaling pathway has an important role in OA FLS.
Similar to other types of cells (12), TGF-β signals not only via p-Smad2
but also via p-Smad1/5/8 in OA FLS. Previous studies demonstrated
that the TGF-β signaling pathway has a role in stimulation of
chondrocytes to renew the ECM in the cartilage of young animals
(24–30). This process is considered a
function of the TGF-β signaling pathway in cartilage. However,
previous studies have suggested that the TGF-β signaling pathway
may signal not only via ALK5 but also via ALK1, to phosphorylate
Smad1/5/8, which may additionally stimulate the expression of
MMP-13 and aggravate OA (31,32).
Other studies have demonstrated that among elderly patients and
patients with OA, TGF-β signaling via ALK5 is reduced in cartilage,
as demonstrated by reduced Smad2 phosphorylation. The switch from
ALK5 to ALK1 precedes cartilage degradation and may be necessary
for OA development (33,34). In other cell types, including
endothelial cells, the ratio of ALK5 to ALK1 may affect the
response to TGF-β (29). Since
TGF-β expression levels in synovial fluid and chondrocytes are
elevated in patients with OA (23), the authors of the present study
hypothesized that TGF-β secreted from FLS may degrade articular
cartilage primarily via ALK1 using autocrine and paracrine
mechanisms. Therefore, the alteration in the balance between ALK1
and ALK5 in chondrocytes, and between p-Smad2 and p-Smad1/5/8 in
FLS may have a role in the TGF-β signaling pathway in the
development of OA.
The present study demonstrated an increase in TGF-β
and LTBP-1 in the conditioned media solubilized by plasmin of human
OA FLS. Tumor studies have revealed that LTBP-1 associates with
TGF-β in complex with its pro-peptide and targets TGF-β to specific
extracellular structures (16,35).
In epithelial cells, LTBP-1 needs to be matrix-bound for
αvβ6-integrin-mediated activation (36). Protease-mediated activation leads
to secretion of active TGF-β from the ECM. Subsequently, a latent
complex is released from the ECM, with cleavage of LTBP-1 at
protease-sensitive sites, which results in truncated LTBP-1
associating with SLC (37,38). The latent TGF-β releases active
TGF-β from its noncovalent complex, which may be regulated by an
interaction with proteases, thrombospondin or integrins in a cell
type-specific manner (16,37). The present study investigated the
role of LTBP-1 derived from OA FLS in the process of autocrine and
paracrine TGF-β activation. Previous studies demonstrated that in
glioma cells, FLPs were not only involved in the processing of
TGF-β but also in the proteolytic truncation of LTBP-1, and human
glioblastoma cells secreted different forms of TGF-β associated
with LTBP-1 (39,40). Therefore, the authors of the
present study hypothesized that in OA FLS, LTBP-1 may participate
in TGF-β activation. The present study reported the expression of
LTBP-1 in OA FLS and normal FLS. The expression of LTBP-1 markedly
increased in human OA FLS compared with normal FLS. In vitro
LTBP-1 protein levels were elevated in synovial tissues. The
present study demonstrated that levels of free TGF-β in the
conditioned media were associated with the LTBP-1 level and this
association may involve a positive feedback loop. When LTBP-1 was
knocked down, TGF-β signaling was altered via downregulation of
TGF-βs, p-Smad1/5/8 and MMP-13 and upregulation of p-Smad2. LTBP-1
depletion in OA FLS led to alterations in TGF-β protein levels.
Despite similar TGF-β1, -β2 and -β3 mRNA levels compared with the
control group, OA FLS with LTBP-1 knockdown secreted fewer TGF-βs
in the conditioned medium solubilized by plasmin. Furthermore,
LTBP-1 depletion significantly reduced Smad1/5/8 phosphorylation
levels. Therefore, LTBP-1 knockdown in OA FLS altered the paracrine
and autocrine TGF-β signaling. The aforementioned data are
consistent with a hypothesis that a there is positive feedback loop
between LTBP-1 expression and TGF-β bioavailability in OA FLS.
The present study also demonstrated that
proliferation of OA FLS was enhanced compared with normal FLS.
Previous studies demonstrated that TGF-β signaling via ALK5
represses chondrocyte hypertrophic differentiation and promotes
cell survival (12,26). However, the role TGF-β signaling
via ALK1 was demonstrated to aggravate the formation of osteophytes
and synovial fibrosis, which may enhance the proliferation of OA
FLS (24,29–41).
In addition, OA FLS with LTBP-1 knockdown demonstrated decreased
proliferation compared with the control group, indicating that
reduced TGF-β signaling via p-Smad1/5/8 may inhibit proliferation
of OA FLS.
Although LTBPs are secreted proteins and cell
confluency affects LTBP secretion and ECM deposition, prior to
seeding cells in 6-well plates, the cells were counted and it was
ensured that cell numbers in both groups were similar. Therefore,
cell confluency should not have affected LTBP secretion or ECM
deposition in the present study. Although, there may be a potential
influence of Lipofectamine® 2000, both groups were
treated with it; therefore, both groups would have been affected in
the same way. Further studies on the mechanism underlying TGF-β
activation should aid in identification of therapeutic treatments
for OA.
Acknowledgements
The authors of the present study would like to thank
Professor Hua Long of Tangdu Hospital (Shaanxi, China) for
collection of human tissue samples.
References
|
1
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar :
|
|
2
|
Loeser RF: Age-related changes in the
musculoskeletal system and the development of osteoarthritis. Clin
Geriat Med. 26:371–386. 2010. View Article : Google Scholar
|
|
3
|
Wang TY and Chen D: Differential roles of
TGF-β signalling in joint tissues during osteoarthritis
development. Ann Rheum Dis. 75:e722016. View Article : Google Scholar :
|
|
4
|
Davidson Blaney EN, van der Kraan PM and
van den Berg WB: TGF-beta and osteoarthritis. Osteoarthritis
Cartilage. 15:597–604. 2007. View Article : Google Scholar
|
|
5
|
Martel-Pelletier J, Wildi LM and Pelletier
JP: Future therapeutics for osteoarthritis. Bone. 51:297–311. 2012.
View Article : Google Scholar
|
|
6
|
de Caestecker M: The transforming growth
factor-β superfamily of receptors. Cytokine Growth Factor Rev.
15:1–11. 2004. View Article : Google Scholar
|
|
7
|
Lawrence DA: Latent-TGF-beta: An overview.
Mol Cell Biochem. 219:163–170. 2001. View Article : Google Scholar
|
|
8
|
Davidson Blaney EN, van Caam AP, Vitters
EL, Bennink MB, Thijssen E, van den Berg WB, Koenders MI, van Lent
PL, van de Loo FA and van der Kraan PM: TGF-β is a potent inducer
of nerve growth factor in articular cartilage via the ALK5-Smad2/3
pathway. Potential role in OA related pain? Osteoarthritis
Cartilage. 23:478–486. 2015. View Article : Google Scholar
|
|
9
|
Kamiya M, Harada A, Mizuno M, Iwata H and
Yamada Y: Association between a polymorphism of the transforming
growth factor-beta1 gene and genetic susceptibility to ossification
of the posterior longitudinal ligament in Japanese patients. Spine.
26:1264–1266. 2001. View Article : Google Scholar
|
|
10
|
Lau HH, Ho AY, Luk KD and Kung AW:
Transforming growth factor-beta1 gene polymorphisms and bone
turnover, bone mineral density and fracture risk in southern
Chinese women. Calcif Tissue Int. 74:516–521. 2004. View Article : Google Scholar
|
|
11
|
Yamada Y: Association of a Leu(10)->Pro
polymorphism of the transforming growth factor-beta1 with genetic
susceptibility to osteoporosis and spinal osteoarthritis. Mech
Geing Dev. 116:113–123. 2000. View Article : Google Scholar
|
|
12
|
Davidson Blaney EN, Remst DF, Vitters EL,
van Beuningen HM, Blom AB, Goumans MJ, van den Berg WB and van der
Kraan PM: Increase in ALK1/ALK5 ratio as a cause for elevated
MMP-13 expression in osteoarthritis in humans and mice. J Immunol.
182:7937–7945. 2009. View Article : Google Scholar
|
|
13
|
Das R, Timur UT, Edip S, Haak E, Wruck C,
Weinans H and Jahr H: TGF-β2 is involved in the preservation of the
chondrocyte phenotype under hypoxic conditions. Ann Anat. 198:1–10.
2015. View Article : Google Scholar
|
|
14
|
Saharinen J and Keski-Oja J: Specific
sequence motif of 8-Cys repeats of TGF-beta binding proteins,
LTBPs, creates a hydrophobic interaction surface for binding of
small latent TGF-beta. Mol Biol Cell. 11:2691–2704. 2000.
View Article : Google Scholar :
|
|
15
|
Rifkin DB: Latent transforming growth
factor-beta (TGF-beta) binding proteins: Orchestrators of TGF-beta
availability. J Biol Chem. 280:7409–7412. 2005. View Article : Google Scholar
|
|
16
|
Annes JP, Munger JS and Rifkin DB: Making
sense of latent TGFbeta activation. J cell Sci. 116:217–224. 2003.
View Article : Google Scholar
|
|
17
|
Ge G and Greenspan DS: BMP1 controls
TGFbeta1 activation via cleavage of latent TGFbeta-binding protein.
J Cell Biol. 175:111–120. 2006. View Article : Google Scholar :
|
|
18
|
Tatti O, Vehvilainen P, Lehti K and
Keski-Oja J: MT1-MMP releases latent TGF-beta1 from endothelial
cell extracellular matrix via proteolytic processing of LTBP-1. Exp
Cell Res. 314:2501–2514. 2008. View Article : Google Scholar
|
|
19
|
Schmierer B and Hill CS: TGFbeta-SMAD
signal transduction: Molecular specificity and functional
flexibility. Nat Rev Mol Cell Biol. 8:970–982. 2007. View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Peng HZ, Yun Z, Wang W and Ma Ba: Dual
specificity phosphatase 1 has a protective role in osteoarthritis
fibroblast-like synoviocytes via inhibition of the MAPK signaling
pathway. Mol Med Rep. 2017. View Article : Google Scholar
|
|
22
|
Vinatier C, Merceron C and Guicheux J:
Osteoarthritis: from pathogenic mechanisms and recent clinical
developments to novel prospective therapeutic options. Drug Discov
Today. 21:1932–1937. 2016. View Article : Google Scholar
|
|
23
|
Punzi L, Oliviero F and Ramonda R:
Transforming growth factor-beta levels in synovial fluid of
osteoarthritis with or without calcium pyrophosphate dihydrate
crystals. J Rheumatol. 30:4202003.
|
|
24
|
van Beuningen HM, van der Kraan PM, Arntz
OJ and van den Berg WB: Transforming growth factor-beta 1
stimulates articular chondrocyte proteoglycan synthesis and induces
osteophyte formation in the murine knee joint. Lab Invest.
71:279–290. 1994.
|
|
25
|
Frenkel SR, Saadeh PB, Mehrara BJ, Chin
GS, Steinbrech DS, Brent B, Gittes GK and Longaker MT: Transforming
growth factor beta superfamily members: Role in cartilage modeling.
Plastic Reconstr Surg. 105:980–990. 2000. View Article : Google Scholar
|
|
26
|
Yang X, Chen L, Xu X, Li C, Huang C and
Deng CX: TGF-beta/Smad3 signals repress chondrocyte hypertrophic
differentiation and are required for maintaining articular
cartilage. J Cell Biol. 153:35–46. 2001. View Article : Google Scholar :
|
|
27
|
Davidson Blaney EN, Vitters EL, van den
Berg WB and van der Kraan PM: TGF beta-induced cartilage repair is
maintained but fibrosis is blocked in the presence of Smad7.
Arthritis Res Ther. 8:R652006. View
Article : Google Scholar :
|
|
28
|
Grimaud E, Heymann D and Rédini F: Recent
advances in TGF-beta effects on chondrocyte metabolism. Potential
therapeutic roles of TGF-beta in cartilage disorders. Cytokine
Growth Factor Rev. 13:241–257. 2002. View Article : Google Scholar
|
|
29
|
Scharstuhl A, Glansbeek HL, van Beuningen
HM, Vitters EL, van der Kraan PM and van den Berg WB: Inhibition of
endogenous TGF-beta during experimental osteoarthritis prevents
osteophyte formation and impairs cartilage repair. J Immunol.
169:507–514. 2002. View Article : Google Scholar
|
|
30
|
van Beuningen HM, Glansbeek HL, van der
Kraan PM and van den Berg WB: Differential effects of local
application of BMP-2 or TGF-beta 1 on both articular cartilage
composition and osteophyte formation. Osteoarthritis Cartilage.
6:306–317. 1998. View Article : Google Scholar
|
|
31
|
Oh SP, Seki T, Goss KA, Imamura T, Yi Y,
Donahoe PK, Li L, Miyazono K, ten Dijke P, Kim S and Li E: Activin
receptor-like kinase 1 modulates transforming growth factor-beta 1
signaling in the regulation of angiogenesis. Proc Natl Acad Sci
USA. 97:2626–2631. 2000. View Article : Google Scholar :
|
|
32
|
Goumans MJ, Valdimarsdottir G, Itoh S,
Rosendahl A, Sideras P and ten Dijke P: Balancing the activation
state of the endothelium via two distinct TGF-beta type I
receptors. EMBO J. 21:1743–1753. 2002. View Article : Google Scholar :
|
|
33
|
Davidson Blaney EN, Scharstuhl A, Vitters
EL, van der Kraan PM and van den Berg WB: Reduced transforming
growth factor-beta signaling in cartilage of old mice: Role in
impaired repair capacity. Arthritis Res Ther. 7:R1338–R1347. 2005.
View Article : Google Scholar :
|
|
34
|
Davidson Blaney EN, Vitters EL, van der
Kraan PM and van den Berg WB: Expression of transforming growth
factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P
in spontaneous and instability-induced osteoarthritis: Role in
cartilage degradation, chondrogenesis and osteophyte formation. Ann
Rheum Dis. 65:1414–1421. 2006. View Article : Google Scholar :
|
|
35
|
Taipale J, Saharinen J and Keski-Oja J:
Extracellular matrix-associated transforming growth factor-beta:
Role in cancer cell growth and invasion. Adv Cancer Res. 75:87–134.
1998. View Article : Google Scholar
|
|
36
|
Annes JP, Chen Y, Munger JS and Rifkin DB:
Integrin alphaVbeta6-mediated activation of latent TGF-beta
requires the latent TGF-beta binding protein-1. J Cell Biol.
165:723–734. 2004. View Article : Google Scholar :
|
|
37
|
Koli K, Saharinen J, Hyytiäinen M,
Penttinen C and Keski-Oja J: Latency, activation and binding
proteins of TGF-beta. Microsc Res Tech. 52:354–362. 2001.
View Article : Google Scholar
|
|
38
|
Solovyan VT and Keski-Oja J: Apoptosis of
human endothelial cells is accompanied by proteolytic processing of
latent TGF-beta binding proteins and activation of TGF-beta. Cell
Death Differ. 12:815–826. 2005. View Article : Google Scholar
|
|
39
|
Leitlein J, Aulwurm S, Waltereit R,
Naumann U, Wagenknecht B, Garten W, Weller M and Platten M:
Processing of immunosuppressive pro-TGF-beta 1, 2 by human
glioblastoma cells involves cytoplasmic and secreted furin-like
proteases. J Immunol. 166:7238–7243. 2001. View Article : Google Scholar
|
|
40
|
Olofsson A, Miyazono K, Kanzaki T,
Colosetti P, Engström U and Heldin CH: Transforming growth
factor-beta 1, -beta 2 and -beta 3 secreted by a human glioblastoma
cell line. Identification of small and different forms of large
latent complexes. J Biol Chem. 267:19482–19488. 1992.
|
|
41
|
Scharstuhl A, Vitters EL, van der Kraan PM
and van den Berg WB: Reduction of osteophyte formation and synovial
thickening by adenoviral overexpression of transforming growth
factor beta/bone morphogenetic protein inhibitors during
experimental osteoarthritis. Arthritis Rheum. 48:3442–3451. 2003.
View Article : Google Scholar
|