Introduction
Pelvic organ prolapse (POP) refers to a collection
of conditions whereby the uterine, anterior vaginal wall and/or
posterior vaginal wall prolapses. POP incidence is more common in
middle-aged and elderly females, with a worldwide frequency of ~37%
in women who have experienced vaginal birth delivery (1), and may result in physical and
psychological harm to patients, seriously decreasing their quality
of life. Epidemiological studies have revealed well-established
risk factors of the disease including birth delivery injury,
obesity, chronic increased abdominal pressure and age (2). Currently, the treatment of POP is
dominated by surgical repair of the native tissue with vaginal
meshes, which typically has positive outcomes. The use of meshes,
however, is associated with considerable risk of erosion, pain,
infection and vaginal stenosis. In addition, the curative effect of
drug therapy, a therapeutic method for patients with mild POP,
remains unclear. Therefore, the establishment of effective
treatments and prevention methods are required for patients with
POP.
Kudzu root, a Chinese herb containing puerarin
(7,4-dihydroxy-8-β-D-glucosylisoflavone,
C21H20O9) as its main isoflavone
glycoside, has been used for thousands of years in traditional
Chinese medicine for the treatment of fever, diarrhea,
cardiovascular diseases and diabetes in (3), and has been previously been used for
its antioxidant (4),
anti-hyperglycemic (5) and
anti-inflammatory (6) properties.
Additionally, its influence on proliferation and differentiation of
osteoblasts has been demonstrated (7).
It has been reported that estrogen may significantly
increase the ratio of collagen (COL)I/III in urethral and anal
vasculature of young adult rats (8), and its function of increasing the
rate of fibroblast proliferation has also been reported (9). The association between intrinsic
connective tissue abnormality and the development of POP is widely
understood (10). HoxAl1 is
essential for the formation of uterosacral ligaments, which
supports the vaginal wall in mice (11). Furthermore, as a phytoestrogen,
puerarin has been demonstrated to affect loss of bone density in
ovariectomized mice (12).
Puerarin may also induce inflammation in mouse mesangial cells
associated with the production of matrix metalloproteinase (MMP)-2
and −9 during advanced glycation, and N-carboxymethyllysine
(13). The increased invasiveness
of endometriotic stromal cells by vimentin E2 may be significantly
reversed by puerarin by increasing MMP-9 accumulation and
decreasing tissue inhibitor of metalloproteinase (TIMP)-1
accumulation (14).
In addition, under certain conditions including
inflammation and endometriosis, the expression of MMP-2 and −9 are
inhibited by puerarin, and the expression of TIMP-1 increased
(15,16). Furthermore, puerarin has been
studied for its wide-ranging effects on angiogenesis (17). It has been demonstrated that
administration of puerarin reduces spinal ischemia-reperfusion
injury by exerting neuroprotective properties (18). Incubation of isolated human
mononuclear cells with 0.1–3 mmol/l puerarin may increase the
number of endothelial progenitor cells, and enhance proliferation,
migration, adhesion and in vitro vasculogenesis (7,19).
Therefore, it was hypothesized that puerarin may
inhibit POP by regulating the metabolism of fibroblasts. To verify
this, an in vitro cell culture was established to evaluate
the potential effects of puerarin against POP and the underlying
mechanisms involved, which may provide a novel pharmacological
therapeutic approach for the treatment of POP.
Materials and methods
Ethics statement
Human protocols were approved by the Ethics
Committee of Renmin Hospital of Wuhan University (Wuhan, China).
All samples were obtained from patients undergoing routine
hysterectomy following obtaining signed informed consent forms.
Patients
A total of 8 patients who underwent hysterectomy
surgery for reasons excluding the presence of malignant tumors and
POP, served as controls, and 10 patients who underwent hysterectomy
surgery only for POP (POP-Q standard: POP-IV) in the Obstetric and
Gynecological Department of Renmin Hospital of Wuhan University,
were enrolled in the present study. None of these recruited women
had any connective tissue diseases, pathologically confirmed
endometriosis or estrogen-associated ovarian tumors. Furthermore,
these patients were free from any complications that may lead to
collagen depleted-associated diseases, including diabetes and
hyperthyroidism. Patients who had received surgery in the
uterosacral ligamental site ever or had a history of estrogen
application within the past three months were excluded from the
present study.
Primary cell culture
A modified enzyme digestion method was used in the
present study for the establishment of primary cell culture. Tissue
specimens (0.5×0.5×0.2 cm3) were dissected from part of
the parametrial ligament (including sacral and associated
ligaments) during surgery. The tissues were washed with
phosphate-buffered saline (HyClone; GE Healthcare Life Sciences,
Logan, UT, USA) containing 1% antibiotics (100 KU/ml penicillin G
and 100 mg/ml streptomycin; Hangzhou Ginom Biomedical Technology
Co., Ltd., Hangzhou, China) 3–5 times to clear the underlying
blood, axungia and necrotic tissue, following which tissues were
sectioned into ~1 mm3 thick pieces. Sections were
digested with 1% collagenase-I (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 3 h at 37°C in 5%
CO2, followed by further digestion with 0.25% trypsin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 5 min.
Subsequently, 2 ml fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) was added to halt digestion. Dulbecco's Modified
Eagle's medium (DMEM; Hangzhou Ginom Biomedical Technology Co.,
Ltd.) supplemented with 15% FBS was slowly added to the culture
bottle. The culture medium was replaced every two days and human
parametrial ligament fibroblasts (HPLFs) were cultured to 70%
confluence for passage. The stable primary cells were obtained
after ~15 days. The HPLFs were used at passage 4–8 for subsequent
experiments.
Cell viability assay
HPLF viability following treatment with puerarin was
detected using Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc., Shanghai, China). Cells at a density of
~3×103 were seeded into a 96-well plate containing 100
µl DMEM. Cells were treated with 0.01, 0.1 or 1.0 mmol/l puerarin
for 24, 48 or 72 h, following which 100 µl fresh medium containing
10 µl CCK-8 solution was added into each well and cells were
incubated for 1 h at 37°C. The optical density was measured at
wavelength of 450 nm using a spectrophotometric plate reader. Each
group was repeated in 5 wells.
Puerarin treatment of HPLFs
Puerarin was purchased from Chengdu Must
Bio-technology Co., Ltd. (Changdu, China; purity, HPLC ≥98%). Based
on previous studies (7,19–21)
and the results of the CCK-8 assay, the concentration of puerarin
in the drug group was set at 0.01, 0.1 and 1 mmol/l. Healthy
passage 4 HPLFs were used in the drug experiments. Cells
(2×105/ml) were seeded into six-well plates and
incubated for 24 h at 37°C in 5% CO2. Following this,
1.5 ml medium containing 0.01, 0.1 or 1 mmol/l was added to the
each well, and HPLFs were incubated for 48 h at 37°C in 5%
CO2.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Gene expression of TIMP-1, MMP-2, MMP-9, COL I and
III, and GAPDH in tissues was evaluated by RT-qPCR. The primers
used for amplification were purchased from Beijing SBS Genetech
Co., Ltd. (Beijing, China). Following drug treatment, total RNA of
HPLFs was isolated using TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the
manufacturer's protocol. RNA was reverse transcribed to cDNA (n=3)
using a RevertAid First Strand cDNA Synthesis kit (cat. no. k1622;
Thermo Fisher Scientific, Inc.). Primer sequences for TIMP-1,
MMP-2, MMP-9, COL I and III, and GAPDH were purchased from SBS
Genetech Co., Ltd. (Table I). qPCR
was performed using SYBR® Premix Ex Taq™
reagent (cat. no. DRR041; Takara Bio, Inc., Otsu, Japan) and an
Applied Biosystems 7500 Real-Time system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Normalized threshold (Cq) values
were used for comparison (22).
Each sample was analyzed in triplicate to ensure accuracy.
 | Table I.Primer sequences for RT-qPCR. |
Table I.
Primer sequences for RT-qPCR.
| Gene | Species | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| GAPDH | Human |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
| TIMP-1 | Human |
CCAAAGCAGTGAGCGAGA |
ACGCTGGTATAAGGTGGTCTG |
| MMP-2 | Human |
AGTTTCCATTCCGCTTCCAG |
CGGTCGTAGTCCTCAGTGGT |
| MMP-9 | Human |
GTCCACCCTTGTGCTCTTCC |
GACTCTCCACGCATCTCTGC |
| COL I | Human |
CAAGACGAAGACATCCCACCAATC |
ACAGATCACGTCATCGCACAACA |
| COL III | Human |
TCGCTCTGCTTCATCCCACTAT |
CTTCCAGACATCTCTATCCGCAT |
MMP zymography assay
Serum-free medium was added to cells following drug
treatment, following which they were incubated for 24 h at 37°C.
The cell supernatant was extracted by centrifugation for 10 min at
37°C and 12,000 × g to detect the activity of MMP-2 and −9
by zymography and proteins were loaded onto acrylamide gels.
Quantification of protein was performed using the BCA method.
Proteins (20 µg per lane) were separated by 8% SDS-PAGE containing
collagen enzyme substrates to electrophoresis. Subsequently, gels
were washed with zymogram renaturing buffer (10X, diluted 1:9 with
deionized water) for 30 min and incubated with enzyme reaction
buffer for 6 h at 37°C, stained by Coomassie Blue R-250 for 30 min,
incubated with destaining solution (100% methanol:acetic
acid:water, 50:10:40) for 30 min at room temperature and visualized
using a YLN-5000 Electrophoretic Optical Density Scanner (cat. no.
YLN-26; Beijing Yu Langnuo Technology Co., Ltd., Beijing,
China).
Western blot analysis
HPLFs were harvested with RIPA lysis buffer (Wuhan
Boster Biological Technology, Ltd., Wuhan, China) containing a
protein inhibitor cocktail (Sigma-Aldrich; Merck KGaA.). Protein
was extracted by centrifugation for 10 min at 4°C and 12,000 ×
g. The protein concentration was quantified using a
bicinchoninic acid assay kit (cat. no. P0010; Beyotime Institute of
Biotechnology, Haimen, China). Total cellular proteins (20 µg) were
mixed with 5X gel loading buffer, separated by 10% SDS-PAGE and
transferred onto polyvinylidene fluoride membranes (Merck KGaA).
Membranes were blocked in 5 g/l skimmed milk for 1 h at room
temperature and washed in TBS. Membranes were incubated with the
following rabbit primary antibodies: Anti-TIMP-1 (1:2,000; cat. no.
ab12684), anti-COL I (1:2,000; cat. no. ab34710) and anti-COL III
(1:5,000; cat. no. ab7778), all purchased from Abcam (Cambridge,
MA, USA) overnight at 4°C, followed by incubation with
fluorescence-labeled secondary antibodies (1:20,000;
IRDye® 800CW goat anti-rabbit IgG; cat. no. 926-32211;
LI-COR Biosciences, Lincoln, NE, USA) for 1 h at 37°C after
washing. A rabbit anti-GAPDH primary antibody (1:2,500; cat. no.
ab9485; Abcam) served as an internal reference control. The
reactive bands were detected with an Odyssey® infrared
imaging system (LI-COR Biosciences).
Statistical analysis
Data are presented as the mean ± standard deviation
for each group and were analyzed by one-way analysis of variance
using SPSS software version 21.0 (IBM SPSS, Armonk, NY, USA).
Differences between two groups were determined using Dunnett's
test, and multiple means were compared by Tukey's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Identification of HPLFs
A previous study of the authors identified stable
and accurate HPLFs by positive staining of vimentin and negative
staining of cytokeratin (23).
They primarily exhibit long spindles connecting to each other to
form a network structure with a similar behavior to fibroblasts, as
observed by light microscopy.
Puerarin promotes HPLF
proliferation
To confirm the effect of puerarin on proliferation
of HPLFs, a CCK-8 assay was performed. Increased proliferation was
detected in the HPLF culture 24 h after treatment with puerarin. At
48 and 72 h, proliferation rates further significantly increased.
Proliferation rates of HPLFs increased markedly following 0.1
mmol/l puerarin treatment compared with 1 mmol/l and 0.01 mmol/l
puerarin at 24, 48 and 72 h (P<0.01; Fig. 1A). These results indicated that
HPLF proliferation was enhanced by puerarin in a time-dependent
manner, and all the three concentration of puerarin may promote
HPLF proliferation.
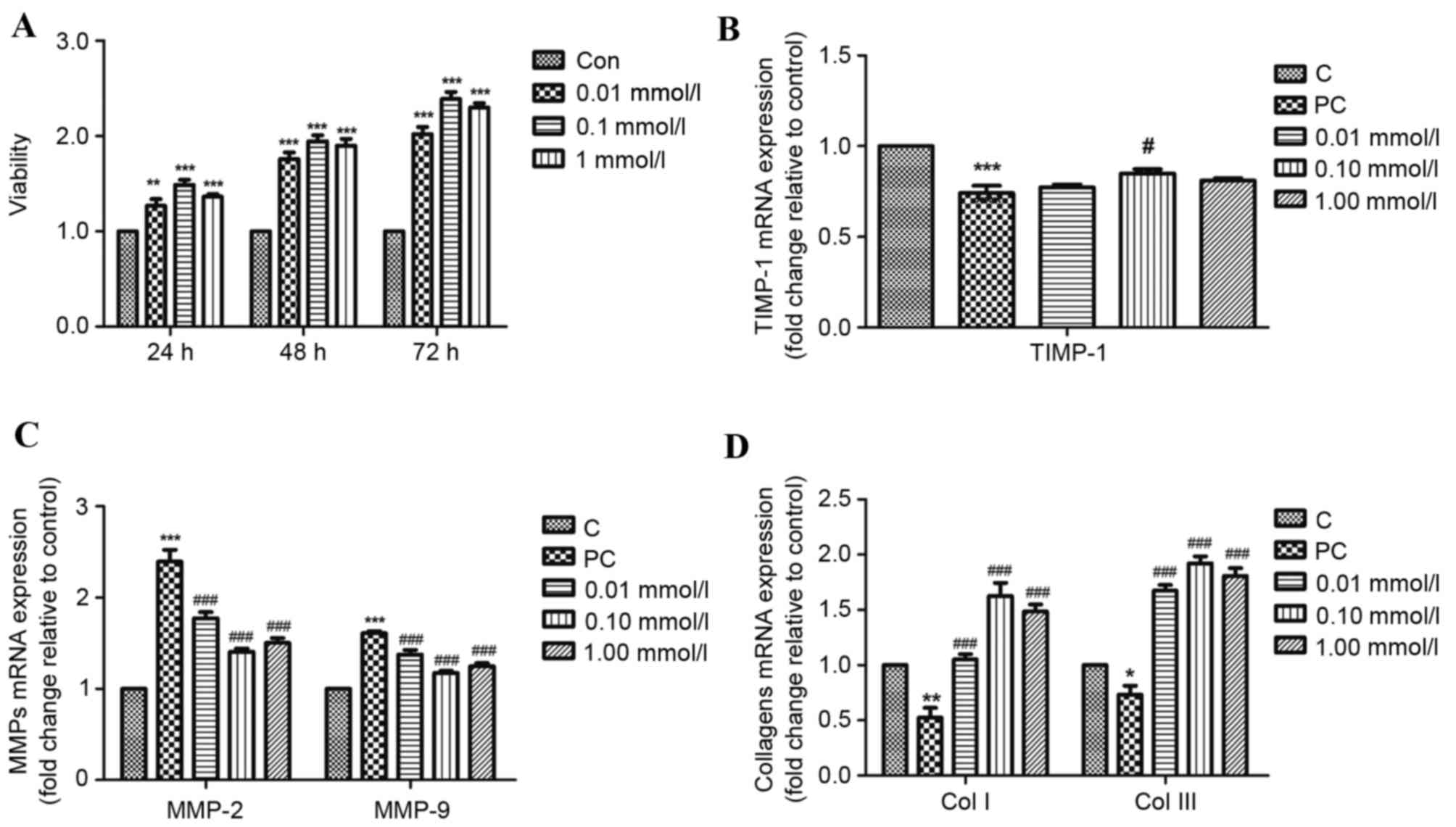 | Figure 1.Effects of puerarin treatment on
proliferation of HPLFs and mRNA expression of pelvic organ
prolapse-associated genes in HPLFs. (A) Following incubation for
24, 48 or 72 h with 0.01, 0.10 or 1.00 mmol/l puerarin, HPLF
viability was assessed by Cell Counting Kit-8 assay. Following
incubation for 48 h with 0.01, 0.10 or 1.00 mmol/l puerarin, mRNA
expression levels of (B) TIMP-1 (C) MMP-2 and −9, and (D) COL I and
III were detected by reverse transcription-quantitative polymerase
chain reaction. The C and PC groups were treated with common medium
without puerarin. Data are presented as the mean ± standard
deviation (n=5). *P<0.05, **P<0.01 and ***P<0.001 vs. C;
#P<0.05 and ###P<0.001 vs. PC. C,
control; PC, positive control; HPLF, human parametrial ligament
fibroblast; TIMP-1, tissue inhibitor of metalloproteinase-1; MMP,
matrix metalloproteinase; COL, collagen. |
Effects of puerarin on HPLFs in pelvic
floor tissue of POP patients
The effects of puerarin on TIMP-1, MMP-2, MMP-9 and
COL I and III mRNA expression levels in HPLFs derived from pelvic
floor tissue of POP patients were tested to investigate its effects
on resistance to degradation of the extracellular matrix (ECM). As
presented in Fig. 1B, 0.10 mmol/l
puerarin treatment significantly increased mRNA expression levels
of TIMP-1 (P<0.05), and MMP-2 and −9 mRNA expression levels were
significantly decreased following 0.01, 0.10 and 1.00 mmol/l
puerarin treatment (P<0.01; Fig.
1C), compared with the positive control. COL I and III mRNA
expression levels were significantly increased following all three
concentrations of puerarin (P<0.01; Fig. 1D) compared with the positive
control. Therefore, puerarin may regulate the degradation and
synthesis of ECM components at the level of transcription. These
results suggested that to resist ECM degradation, the most suitable
concentration of puerarin is ~0.10 mmol/l.
Varying expressions of POP-associated
genes in HPLFs between patients with and without POP
To detect whether the regulatory factors and
metabolism of collagens alter in HPLFs between patients with or
without POP, RT-qPCR, zymography assay and western blotting were
performed to detect levels of TIMP-1, MMP-2, MMP-9, and COL I and
III. As collagens are vital elements in pelvic connective tissue to
maintain the fastness of ligament, significant decreases in COL I
(P<0.01) and III (P<0.05) mRNA expression levels were
observed in HPLFs from patients with POP (Fig. 1D). Similarly, as observed on a
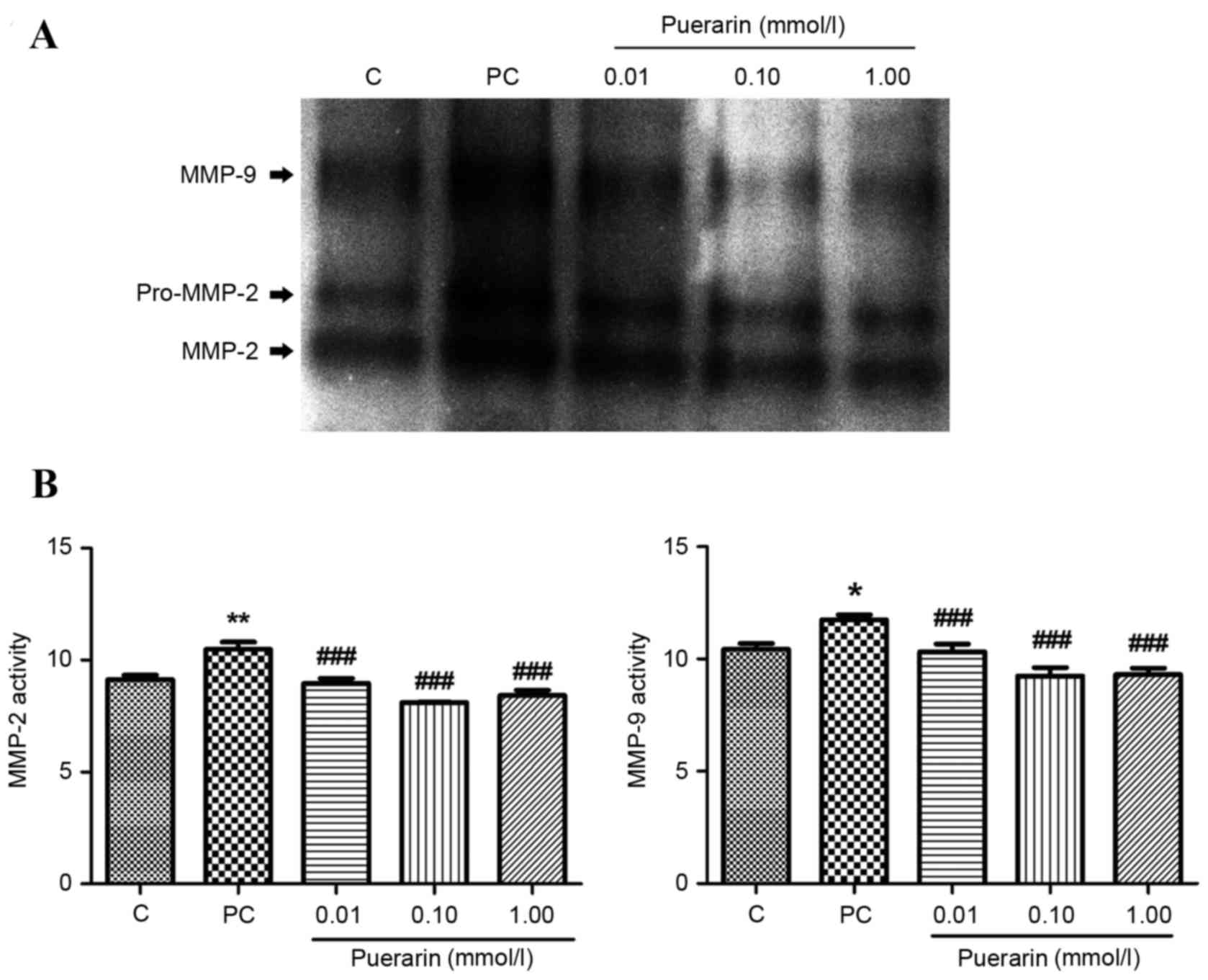
gelatin gel (Fig. 2A), MMP-2 and
−9 (Fig. 2B) activity was
inhibited by puerarin treatment at all concentrations compared with
the positive control (P<0.01). Additionally, MMP-2 and MMP-9
protein and mRNA expression levels were increased in the HPLFs of
POP patients compared with controls (P<0.001).
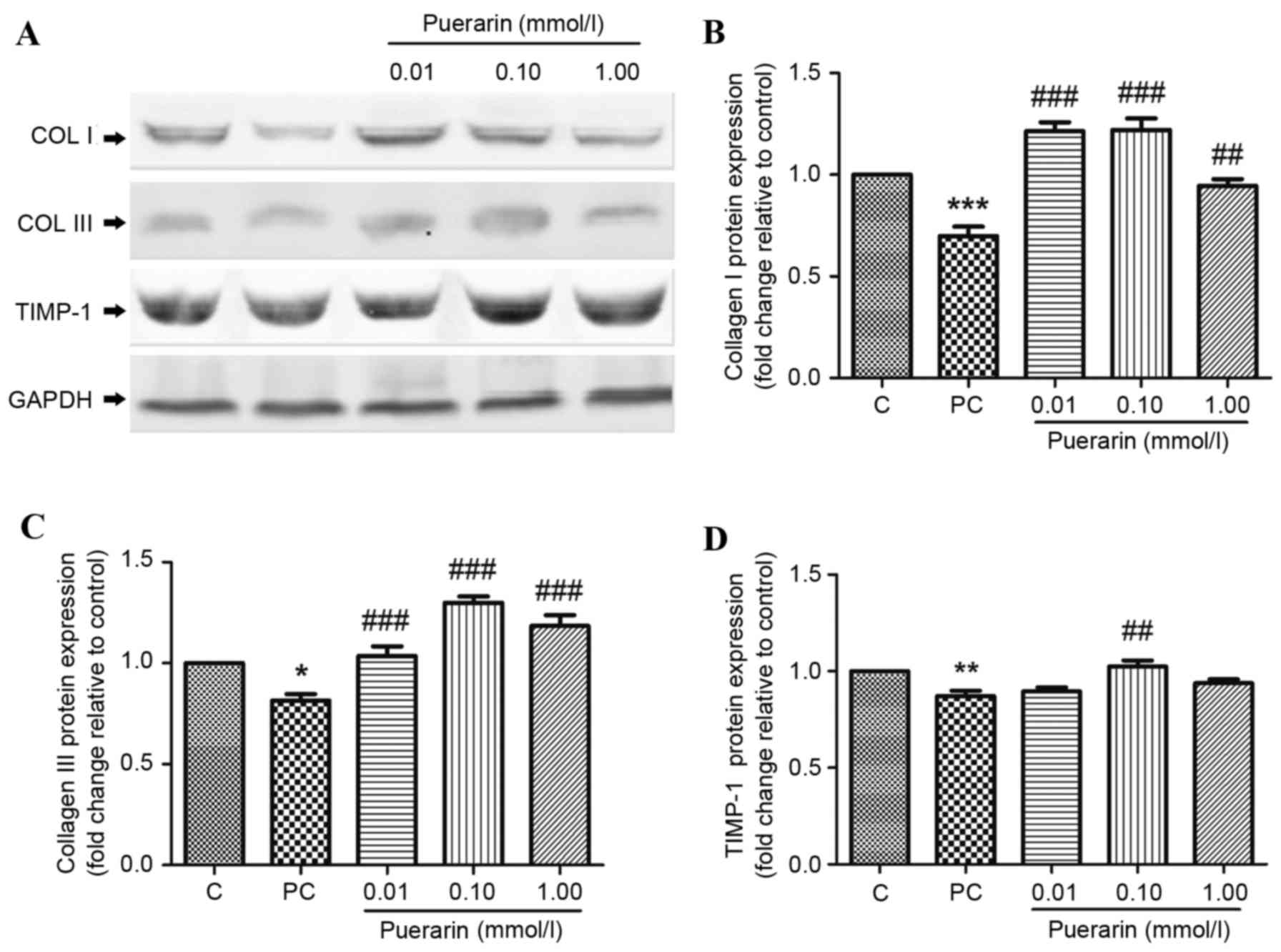
Protein expression levels of TIMP-1, and COL I and
II, were additionally detected by western blot analysis (Fig. 3A). COL I (Fig. 3B) and III (Fig. 3C) protein expression levels were
significantly decreased in POP patients compared with controls
(P<0.001 and P<0.05, respectively); however, 0.01, 0.10 and
1.00 mmol/l puerarin treatment significantly reversed this effect.
As presented in Fig. 3D, protein
expression levels of TIMP-1 were markedly decreased in HPLFs of POP
patients compared with controls (P<0.001).
ECM remodeling is a vital factor in the development
of POP. These results demonstrated that MMP-2 and −9 transcription
was upregulated, whereas TIMP-1, and COL I and III expression
levels were downregulated in POP patients. Furthermore, the
activity of MMPs was increased in POP patients, indicating more
collagen degradation than collagen synthesis.
Discussion
Fibroblasts deposit ECM components, including
elastic fibers and collagen, to maintain tissue elasticity and
toughness of connective tissue (24,25).
It has previously been demonstrated that abnormalities with
intrinsic connective tissue composed of fibroblasts are associated
with the development of POP (10).
Fibroblasts serve a pivotal role, particularly in pelvic connective
tissue, in ECM remodeling and mechanical force resisting by
regulating the balance between collagen synthesis and degradation
following tissue injury (26,27),
and fibroblasts exhibit different functional characteristics in the
vaginal tissue of women with POP compared with women without POP
(28). Even within a single
tissue, fibroblasts exhibit remarkable functional diversity.
Therefore, once the integrity and function of fibroblasts are
destroyed by factors including pregnancy, childbirth and dystocia,
the risk of POP markedly increases (1,29,30).
Histologically, POP is characterized by degradation
of fibroblasts and an imbalance between deposition and degradation
of the ECM (31). Tissue
remodeling is closely associated to modifications in cell
proliferation, differentiation, ECM expression and the release of
signaling molecules (32). The
overexpression of MMPs or underexpression of TIMPs may disrupt the
dynamic balance of the ECM. MMPs are able to cleave almost all ECM
components, mediating pelvic tissue remodeling in health and
disease (33,34), and can degrade collagen and other
ECM proteins. Elevated levels of TIMPs have a potent inhibitory
effect on MMP activation (35).
During ECM remodeling, the balance between MMPs and their
inhibitors, TIMPs, is as important as appropriate production of the
ECM, which is regulated by numerous cytokines and growth factors
(36). The activity of MMPs is
balanced by TIMPs (37), and the
binding of TIMPs to inactive MMPs inhibits the proteolytic activity
and accordingly limits ECM degradation. Thus, TIMP-1 controls the
activity of MMPs and appears to be an important regulator of ECM
production (38,39). Furthermore, the primary collagens
in pelvic tissue, COL I and II, provide powerful tension and
strength for pelvic tissue. However, MMPs impact the tension and
resilience of pelvic tissue by regulating the metabolism of COL I
and II. Therefore, the present study selected to examine these
interrelated factors.
Secretion and remodeling of the ECM are important
functions of fibroblasts. The present study demonstrated that ECM
metabolism and associated metabolism molecules including TIMP-1,
MMPs and collagens, were altered in HPLFs of POP patients compared
with controls. Other research groups assessing collagen content in
pelvic floor connective tissue additionally concluded that total
collagen content is decreased in the pelvic floor connective tissue
of women with POP in comparison with asymptomatic controls
(40,41). Loss of the ECM leads to dysfunction
of attachment and support of pelvic connective tissue (31). Thus, it was hypothesized the
excessive degradation of collagen may result from decrease of the
TIMP:MMP ratio in pelvic floor tissues, as previously reported by
Lin et al (42). It has
been demonstrated that abnormality of the COL III and MMP-2
signaling pathway contributes to weakness of connective tissues of
patients with POP (43). The
metabolism of COL I and III depends on the activity of the
interstitial MMPs including gelatinases, MMP-2 and MMP-9. In POP,
it has been consistently demonstrated that the expression of MMP-2
is increased in uterosacral ligaments, and may be involved in
altering collagen metabolism (44,45).
Furthermore, TIMP-1 may regulate cellular processes including cell
growth, apoptosis and differentiation, and may serve a role in
urethral scar formation independent of its metalloproteinase
inhibitory activity (38,46). At the genetic level, the TIMP-1
protein is encoded from six exons that may be functionally defined
as an N-terminal domain (amino acids 1 to 126) that is sufficient
to retain its inhibitory function of MMPs (47). Furthermore, MMP-2 and −9, known as
gelatinases, are key enzymes resulting in weak ductility and lack
of integrity in the collagen tissues (48). In addition, increased MMP-2 or −9:
TIMP-1 ratios downregulate COL I and III levels, mediating the
development of POP (49).
Therefore, TIMP-1, and MMP-2 and −9 were selected for examination
in the present study. As a primary active ingredient extracted from
a Kudzu root, puerarin was demonstrated to protect against
degradation of collagen. Furthermore, the ratio of TIMP-1: MMP-2
and −9 was increased by puerarin treatment. The present study
demonstrated that the expression of MMPs, which are closely
associated with ECM degradation, is regulated at the
transcriptional level by growth factors and transcriptional
factors, and its activity may additionally be inhibited by TIMPs
following its secretion. Therefore, upstream regulators of MMPs may
be involved in this process.
TIMP-1 has been the most extensively studied of the
four reported TIMPs, which has been demonstrated to inhibit MMP
activity by forming 1:1 stoichiometric non-covalent complexes with
endopeptidase. Consequently, TIMP-1 serves a vital role in
maintaining the balance between ECM accumulation and degradation in
various physiological processes (50). Thus, the activity of MMPs may be
regulated at transcriptional and protein levels to maintain ECM
stability. Although the underlying mechanisms of POP remain
unclear, degradation of the ECM may be involved (51,52).
The present study demonstrated that puerarin treatment,
particularly 0.10 mmol/l, enhances TIMP-1, and COL-I and III
expression levels in HPLFs, and inhibits the activity of MMP-2 and
−9, strongly supporting the effects of puerarin in preventing ECM
degradation.
In conclusion, the present study provided evidence
for increased ECM degradation in the pelvic tissue of patients with
POP compared with healthy controls. Furthermore, puerarin, an
isoflavonoid, exhibited a protective effect against POP via its
anti-degradation effects on collagens, implicating puerarin as a
potential effective therapeutic agent for the treatment of POP.
Further research, including animal experiments, is required to
validate these findings.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81471442) and the
Foundation of Collaborative and Innovation Projects of Wuhan
University School of Medicine (grant no. 523266078).
References
|
1
|
Swift S, Woodman P, O'Boyle A, Kahn M,
Valley M, Bland D, Wang W and Schaffer J: Pelvic organ support
study (POSST): The distribution, clinical definition and
epidemiologic condition of pelvic organ support defects. Am J
Obstet Gynecol. 192:795–806. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mallett VT: Female urinary incontinence:
What the epidemiologic data tell us. Int J Fertil Womens Med.
50:12–17. 2005.PubMed/NCBI
|
|
3
|
Wong KH, Li GQ, Li KM, Razmovski-Naumovski
V and Chan K: Kudzu root: Traditional uses and potential medicinal
benefits in diabetes and cardiovascular diseases. J Ethnopharmacol.
134:584–607. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang J, Guo W, Tian B, Sun M, Li H, Zhou
L and Liu X: Puerarin attenuates cognitive dysfunction and
oxidative stress in vascular dementia rats induced by chronic
ischemia. Int J Clin Exp Pathol. 8:4695–4704. 2015.PubMed/NCBI
|
|
5
|
Hsu FL, Liu IM, Kuo DH, Chen WC, Su HC and
Cheng JT: Antihyperglycemic effect of puerarin in
streptozotocin-induced diabetic rats. J Nat Prod. 66:788–792. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao LN, Zhou X, Zhang Y, Cui YL, Yu CQ and
Gao S: The anti-inflammatory activities of ethanol extract from
dan-lou prescription in vivo and in vitro. BMC Complement Altern
Med. 15:3172015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang C, Meng MX, Tang XL, Chen KM, Zhang
L, Liu WN and Zhao YY: The proliferation, differentiation and
mineralization effects of puerarin on osteoblasts in vitro. Chin J
Nat Med. 12:436–442. 2014.PubMed/NCBI
|
|
8
|
Rizk DE, Hassan HA, Al-Marzouqi AH,
Ramadan GA, Al-Kedrah SS, Daoud SA and Fahim MA: Combined estrogen
and ghrelin administration restores number of blood vessels and
collagen type I/III ratio in the urethral and anal canal submucosa
of old ovariectomized rats. Int Urogynecol J Pelvic Floor Dysfunct.
19:547–552. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ewies AA, Elshafie M, Li J, Stanley A,
Thompson J, Styles J, White I and Al-Azzawi F: Changes in
transcription profile and cytoskeleton morphology in pelvic
ligament fibroblasts in response to stretch: The effects of
estradiol and levormeloxifene. Mol Hum Reprod. 14:127–135. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bai SW, Choe BH, Kim JY and Park KH:
Pelvic organ prolapse and connective tissue abnormalities in Korean
women. J Reprod Med. 47:231–234. 2002.PubMed/NCBI
|
|
11
|
Connell KA, Guess MK, Chen H, Andikyan V,
Bercik R and Taylor HS: HOXA11 is critical for development and
maintenance of uterosacral ligaments and deficient in pelvic
prolapse. J Clin Invest. 118:1050–1055. 2008.PubMed/NCBI
|
|
12
|
Wang X, Wu J, Chiba H, Umegaki K, Yamada K
and Ishimi Y: Puerariae radix prevents bone loss in ovariectomized
mice. J Bone Miner Metab. 21:268–275. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim KM, Jung DH, Jang DS, Kim YS, Kim JM,
Kim HN, Surh YJ and Kim JS: Puerarin suppresses AGEs-induced
inflammation in mouse mesangial cells: A possible pathway through
the induction of heme oxygenase-1 expression. Toxicol Appl
Pharmacol. 244:106–113. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Strinic T, Vulic M, Tomic S, Capkun V,
Stipic I and Alujevic I: Increased expression of matrix
metalloproteinase-1 in uterosacral ligament tissue from women with
pelvic organ prolapse. Acta Obstet Gynecol Scand. 89:832–834. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang X, Zhang H, Wang J, Zhang Z and Li C:
Puerarin decreases bone loss and collagen destruction in rats with
ligature-induced periodontitis. J Periodontal Res. 50:748–757.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang D, Liu Y, Han J, Zai D, Ji M, Cheng
W, Xu L, Yang L, He M, Ni J, et al: Puerarin suppresses invasion
and vascularization of endometriosis tissue stimulated by
17β-estradiol. PLoS One. 6:e250112011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang S, Chen S, Shen Y, Yang D, Liu X,
Sun-Chi AC and Xu H: Puerarin induces angiogenesis in myocardium of
rat with myocardial infarction. Biol Pharm Bull. 29:945–950. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tian F, Xu LH, Zhao W, Tian LJ and Ji XL:
The neuroprotective mechanism of puerarin treatment of acute spinal
cord injury in rats. Neurosci Lett. 543:64–68. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang MY, Qiang H, Yang HQ, Dang XQ and
Wang KZ: In vitro and in vivo effects of puerarin on promotion of
osteoblast bone formation. Chin J Integr Med. 18:276–282. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu JH, Wang XX, Chen JZ, Shang YP, Zhu
JH, Guo XG and Sun J: Effects of puerarin on number and activity of
endothelial progenitor cells from peripheral blood. Acta Pharmacol
Sin. 25:1045–1051. 2004.PubMed/NCBI
|
|
21
|
Zhang Y, Zeng X, Zhang L and Zheng X:
Stimulatory effect of puerarin on bone formation through activation
of PI3K/Akt pathway in rat calvaria osteoblasts. Planta Med.
73:341–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong S, Li H, Wu D, Li B, Liu C, Guo W,
Min J, Hu M, Zhao Y and Yang Q: Oxidative damage to human
parametrial ligament fibroblasts induced by mechanical stress. Mol
Med Rep. 12:5342–5348. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Watt FM and Fujiwara H: Cell-extracellular
matrix interactions in normal and diseased skin. Cold Spring Harb
Perspect Biol. 3:a0051242011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Parsonage G, Filer AD, Haworth O, Nash GB,
Rainger GE, Salmon M and Buckley CD: A stromal address code defined
by fibroblasts. Trends Immunol. 26:150–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gao E, Lei YH, Shang X, Huang ZM, Zuo L,
Boucher M, Fan Q, Chuprun JK, Ma XL and Koch WJ: A novel and
efficient model of coronary artery ligation and myocardial
infarction in the mouse. Circ Res. 107:1445–1453. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sassoli C, Chellini F, Pini A, Tani A,
Nistri S, Nosi D, Zecchi-Orlandini S, Bani D and Formigli L:
Relaxin prevents cardiac fibroblast-myofibroblast transition via
notch-1-mediated inhibition of TGF-β/Smad3 signaling. PLoS One.
8:e638962013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kufaishi H, Alarab M, Drutz H, Lye S and
Shynlova O: Comparative characterization of vaginal cells derived
from premenopausal women with and without severe pelvic organ
prolapse. Reprod Sci. 23:931–943. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mant J, Painter R and Vessey M:
Epidemiology of genital prolapse: Observations from the oxford
family planning association study. Br J Obstet Gynaecol.
104:579–585. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Swift SE, Tate SB and Nicholas J:
Correlation of symptoms with degree of pelvic organ support in a
general population of women: What is pelvic organ prolapse? Am J
Obstet Gynecol. 189:372–379. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Budatha M, Roshanravan S, Zheng Q,
Weislander C, Chapman SL, Davis EC, Starcher B, Word RA and
Yanagisawa H: Extracellular matrix proteases contribute to
progression of pelvic organ prolapse in mice and humans. J Clin
Invest. 121:2048–2059. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Proff P and Romer P: The molecular
mechanism behind bone remodelling: A review. Clin Oral Investig.
13:355–362. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ruiz-Zapata AM, Kerkhof MH,
Zandieh-Doulabi B, Brolmann HA, Smit TH and Helder MN: Functional
characteristics of vaginal fibroblastic cells from premenopausal
women with pelvic organ prolapse. Mol Hum Reprod. 20:1135–1143.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Li Y, Chen J, Guo X, Guan H and Li
C: Differential expression profiling of matrix metalloproteinases
and tissue inhibitors of metalloproteinases in females with or
without pelvic organ prolapse. Mol Med Rep. 10:2004–2008. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Page-McCaw A, Ewald AJ and Werb Z: Matrix
metalloproteinases and the regulation of tissue remodelling. Nat
Rev Mol Cell Biol. 8:221–233. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lu KV, Jong KA, Rajasekaran AK, Cloughesy
TF and Mischel PS: Upregulation of tissue inhibitor of
metalloproteinases (TIMP)-2 promotes matrix metalloproteinase
(MMP)-2 activation and cell invasion in a human glioblastoma cell
line. Lab Invest. 84:8–20. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gaultier F, Foucault-Bertaud A, Lamy E,
Ejeil AL, Dridi SM, Piccardi N, Piccirilli A, Msika P, Godeau G and
Gogly B: Effects of a vegetable extract from Lupinus albus (LU105)
on the production of matrix metalloproteinases (MMP1, MMP2, MMP9)
and tissue inhibitor of metalloproteinases (TIMP1, TIMP2) by human
gingival fibroblasts in culture. Clin Oral Investig. 7:198–205.
2003.PubMed/NCBI
|
|
38
|
Ries C: Cytokine functions of TIMP-1. Cell
Mol Life Sci. 71:659–672. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Arthur MJ and Iredale JP: Hepatic
lipocytes, TIMP-1 and liver fibrosis. J R Coll Physicians Lond.
28:200–208. 1994.PubMed/NCBI
|
|
40
|
Chen BH, Wen Y, Li H and Polan ML:
Collagen metabolism and turnover in women with stress urinary
incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor
Dysfunct. 13:80–87. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kannan K, McConnell A, McLeod M and Rane
A: Microscopic alterations of vaginal tissue in women with pelvic
organ prolapse. J Obstet Gynaecol. 31:250–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lin SY, Tee YT, Ng SC, Chang H, Lin P and
Chen GD: Changes in the extracellular matrix in the anterior vagina
of women with or without prolapse. Int Urogynecol J Pelvic Floor
Dysfunct. 18:43–48. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ma Y, Guess M, Datar A, Hennessey A,
Cardenas I, Johnson J and Connell KA: Knockdown of Hoxa11 in vivo
in the uterosacral ligament and uterus of mice results in altered
collagen and matrix metalloproteinase activity. Biol Reprod.
86:1002012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Phillips CH, Anthony F, Benyon C and Monga
AK: Collagen metabolism in the uterosacral ligaments and vaginal
skin of women with uterine prolapse. BJOG. 113:39–46. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gabriel B, Watermann D, Hancke K, Gitsch
G, Werner M, Tempfer C and Zur Hausen A: Increased expression of
matrix metalloproteinase 2 in uterosacral ligaments is associated
with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct.
17:478–482. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Li C, Xu YM and Li HB: Preliminary
experimental study of urethral reconstruction with tissue
engineering and RNA interference techniques. Asian J Androl.
15:430–433. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Murphy G, Houbrechts A, Cockett MI,
Williamson RA, O'Shea M and Docherty AJ: The N-terminal domain of
tissue inhibitor of metalloproteinases retains metalloproteinase
inhibitory activity. Biochemistry. 30:8097–8102. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Amalinei C, Caruntu ID, Giuşcă SE and
Balan RA: Matrix metalloproteinases involvement in pathologic
conditions. Rom J Morphol Embryol. 51:215–228. 2010.PubMed/NCBI
|
|
49
|
Jackson SR, Avery NC, Tarlton JF, Eckford
SD, Abrams P and Bailey AJ: Changes in metabolism of collagen in
genitourinary prolapse. Lancet. 347:1658–1661. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Batra J, Robinson J, Soares AS, Fields AP,
Radisky DC and Radisky ES: Matrix metalloproteinase-10 (MMP-10)
interaction with tissue inhibitors of metalloproteinases TIMP-1 and
TIMP-2: Binding studies and crystal structure. J Biol Chem.
287:15935–15946. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Alarab M, Kufaishi H, Lye S, Drutz H and
Shynlova O: Expression of extracellular matrix-remodeling proteins
is altered in vaginal tissue of premenopausal women with severe
pelvic organ prolapse. Reprod Sci. 21:704–715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Goepel C, Johanna Kantelhardt E, Karbe I,
Stoerer S and Dittmer J: Changes of glycoprotein and collagen
immunolocalization in the uterine artery wall of postmenopausal
women with and without pelvic organ prolapse. Acta Histochem.
113:375–381. 2011. View Article : Google Scholar : PubMed/NCBI
|