Introduction
Ankylosing spondylitis (AS) is a common clinical
rheumatic disease, which can cause spinal joint stiffness deformity
if improperly or untimely treated, with the potential to cause
significant pain for patients. As an autoimmune disease, AS can be
caused through pathological consequences, including complex immune
dysfunction, inflammation, ossification and bone destruction.
Therefore, the key to the treatment of AS lies in immune
regulation, anti-inflammatory effects, and preventing bone
ossification and destruction (1–4). At
present, priority is focused on the identification of effective
treatment and drugs.
According to traditional Chinese medicine, the key
to the treatment of AS is to facilitate blood circulation,
combating chill and inflammation, which are the main functions of
triptolide, to ease swelling and pain, and to exhibit insecticide
and detoxification effects (5).
Therefore, there is an adequate theoretical basis to investigate
the effects of triptolide on AS. Bone morphogenetic proteins (BMPs)
are a family of proteins with multiple biological functions, which
can specifically bind to and activate receptors on the membrane of
target cells, with small mothers against decapentaplegic (Smad)
being the classical pathway of BMP signal transduction (6–8). The
molecular basis and mechanism of control of the BMP/Smad pathway
has been investigated extensively, and it has been suggested that
AS is treated through this pathway. The present study aimed to
investigate the effects of triptolide on AS, and whether the
treatment occurs through the anti-ossification effect of the
BMP/Smad signaling pathway.
Materials and methods
Establishing the rat AS model
A total of 50 Sprague-Dawley rats (25 female, 25
male; age, 8 weeks; weight, 180–200 g) were obtained from the
Animal Center of Sun Yat-Sen University (Guangzhou, China). The
rats were kept in an environment at 26°C with 55% humidity, a12-h
light/dark cycle and free access to food and water. Each rat was
injected subcutaneously, with the exception of rats in the normal
group, with 0.2 ml collagen emulsifier 1–2 cm from the tail base,
following which a small, white skin rash emerged. The emulsifier
contained bovine type II collagen (cat. no. 20032; Chondrex,
Redmond, WA, USA) dissolved in 0.05 mol/l acetic acid, which was
mixed with incomplete Freund's adjuvant (cat. no. 20031; Chondrex)
at a ratio of 1:1 and density of 2 mg/ml. The mixture was placed in
an ice-bath until sufficiently emulsified (4°C overnight). Of the
50 collagen emulsifier-sensitized rats, 40 showed symptoms of
secondary arthritis following immunization. The four limbs of the
animals, particularly the hind limbs, exhibited swelling and
redness. An increased arthritis index (9) indicated successful model
establishment. The experimental rats were divided into five groups
with eight animals in each group: Normal group; model group;
triptolide low dose group (10 mg/kg); triptolide middle dose group
(20 mg/kg); triptolide high dose group (40 mg/kg). The rats were
administrated by gavage daily for 30 days following model
establishment. The present study was approved by the Ethics
Committee of Sun Yat-sen University.
Primary culture of fibroblast
cells
The spinal joint capsules were obtained from the
rats in all groups, which were placed in 0.1% benzalkonium bromide
for 15 mi, and fixed. The capsules were placed into a clean dish
and rinsed with PBS, following which adipose tissues were removed,
cut into a paste with ophthalmic scissors and transferred into 15
ml centrifuge tubes. To each tube, 0.2% Type II collagenase was
added at 2–3 times the volume of the tissues. Following sufficient
agitation, the tubes containing the tissues were placed into a 37°C
incubator with 5% CO2 for 2 h. The tissues were then
centrifuged at 1,000 × g at 4°C for 10 min, and the supernatant was
discarded. The tissues were added to DMEM high glucose medium
containing 10% fetal bovine serum (both from Hyclone; GE Healthcare
Life Sciences, Logan, UT, USA) and 1% penicillin-streptomycin to
resuspend the cells. The cell density was adjusted to
2xl05/cm2. The cells were placed into a 37°C
incubator with 5% CO2 overnight, and the non-adherent
cells were removed. The medium was replaced once every 3–4 days.
The cells were digested and passaged with 0.25% trypsin. When
passaged to the third generation, >99% of the cells were
identified as fibroblast-like synovial cells. Third generation rat
fibroblast-like synovial cells were used in the subsequent
experiments.
Detection of cell proliferation using
a CKK-8 assay
The spine joint capsule fibroblasts were obtained
from well-grown third generation normal rats, and seeded into a
96-well plate at a density of 1×105, with 100 µl in each
well. The plate was placed in a 37°C incubator with 5%
CO2. Proliferation was detected when adherence was
visible, and five wells were assessed once every 24 h. The medium
was suctioned out, and replaced with 100 µl fetal bovine serum-free
DMEM and 10 µl CCK-8. The fibroblasts were incubated for 40 min,
and the absorbance was measured at the wavelength of 450 nm using
an enzyme-linked immunosorbent detector. When the cells were at the
plateau stage, the proliferation of the AS spine joint capsule
fibroblasts were measured.
Determination of rat plasma levels of
TNF-α, IL-1β and IL-6
According to the manufacturer's protocol, Boster
sandwich ELISA kits (TNF-α, cat. no. 050102-RT; IL-1β, cat. no.
070015-D; IL-6, cat. no. 012043-M) from Beijing Saichi
Biotechnology Co., Ltd. (Beijing, China) were used with the
following steps: 0.1 ml plasma was added to the 96-well plate with
the primary antibody (TNF-α, IL-1β and IL-6), with sample dilution
only added to the zero hole. The plate was covered with a
microtiter plate and incubated at 37°C for 1.5 h. The liquid in the
microtiter plate was discarded following incubation, and 0.1 ml
antibody solution (TNF-α, cat. no. sc-52011; IL-1β, cat. no.
sc-52022; IL-6, cat. no. sc-52003) from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA) was added to each well prior to incubation
at 37°C for 1 h. Again, the liquid in the microtiter was removed,
and the plate was rinsed with PBS three times. To each well, 0.1 ml
ABC working solution was added and incubated at 37°C for 30 min.
The liquid in the microtiter was discarded following incubation and
the plate was rinsed with PBS three times. Subsequently, 90 µl TMB
color reagent was added to each well, which was pre-balanced at
37°C for 30 min and then incubated at 37°C in the dark for 30 min.
The optimal coloration time point was determined when the first
3rd-4th standard well exhibited gradient blue, and when no
significant difference was observed in the last 3rd-4th well. To
each well, 0.1 ml TMB stop solution was added, and the liquid in
the microplate turned from blue to yellow. The optical density (OD)
value at a wavelength 450 nm was measured using a microplate
reader.
Detection of the mRNA expression
levels of core-binding factor α1 (Cbfal), BMPRII, Smad1, Smad4,
Smad5, and Smad6 in synovial tissues using reverse
transcription-polymerase chain reaction (RT-PCR) analysis
The rat knee synovial tissues were obtained and
added to TRIzol reagent (BD Biosciences, Franklin Lakes, NJ, USA)
for sufficient lysis. The tissues were added to 0.2 ml chloroform,
agitated, and placed standing in an ice bath for 5 min. The tissues
were then centrifuged (Heraeus AG, Hanau, Germany) at 1,611 × g at
4°C for 20 min. The supernatant was discarded and replaced with an
equal volume of isopropanol. After 5 min standing in an ice bath,
the tissues were centrifuged at 1,119 × g at 4°C for 20 min. The
supernatant was discarded and replaced with 1 ml 75% ethanol. The
tissues were centrifuged at 1,119 × g at 4°C for 5 min. The
supernatant was carefully removed. The EP tube was turned upside
down, and dried under room temperature for 15 min, and 20 µl
RNase-Free ddH2O was added to the dried pellet to
dissolve the precipitate. Subsequently, 1 µl liquid was drawn into
an Eppendorf tube, and the remainder was stored in a −70°C
refrigerator. The 1-µl solution was diluted to 80 µl with
ddH2O. The OD 260 and OD 280 values were measured on a
spectrophotometer (Eppendorf, Hamburg, Germany) and the total RNA
was calculated. cDNA synthesis and the reverse transcription
experiment were performed using the PrimeScript RT reagent kit
(cat. no. DRR031; Sigma-Aldrich; Merck Millipore, Darmstadt,
Germany) according to the manufacturer's protocol. The reaction
sample contained 12.5 µl SYBR Premix Ex Taq, 1 µl PCR forward
primer, 1 µl PCR reverse primer, 2 µl DNA template, 8.5 µl
dH2O to 25 µl total. The RT-PCR steps were as follows:
Amplification profile, initial denaturation at 95°C for 5 min,
denaturation at 95°C for 20 sec, annealing at 60°C for 30 sec, and
extension at 72°C for 20 sec for fluorescence signal acquisition
(40 cycles in total). The dissolution profile was 60–95°C, with
0.5°C per increment, maintained for 20 sec and with 71 cycles in
total, this stage was for fluorescence signal acquisition. In order
to verify the size of the PCR products, 10 µl solution was
collected for electrophoresis on a 1% agarose gel. Quantification
was performed as previously described (10). The primer sequences were as
follows: Cbfal, forward 5′-TCGGATTATGCAGCAGTTGCCT-3′ and reverse
5′-ACTGCCATCCTGATCCACGCTC-3′; BMPRII, forward
5′-GTGCAGACGAGTTGCCAGACTT-3′ and reverse
5′-GGCACGCCTATGACGGTATTTC-3′; Smadl, forward
5′-TAGCAGAGCAGATGCCCAGGTT-3′ and reverse
5′-CGCACGAAGATGTTACTGTCTT-3′; Smad4, forward
5′-AGCAGAGGAGAGGTTCATCTG-3′ and reverse
5′-CGGACCCCGTTGCTGGTGTTTAG-3′; Smad5, forward
5′-CAGCACACCACTTGTTCAGTCT-3′ and reverse
5′-GGTTCGATGATCCTGCTGTAAC-3′; Smad6, forward
5′-CGTAAGATGAGTTGTTCAGATT-3′ and reverse
5′-CCTACGTTGATGGTGGTATCAT-3′; TNF-a, forward
5′-CGTTCGGTCGCAACAACCT-3′ and reverse 5′-CGTTTGGTCGTTTGCTACATA-3′;
IL-1β, forward 5′-CGTGATGTACCAGTTGGGT-3′ and reverse
5′-GTCCATGAGCTTTGTACTACT-3′; IL-6, forward
5′-GCGCTTCACGAACACCCATG-3′ and reverse
5′-GGGAATTGCCATTGCACGGTGG-3′; GAPDH, forward
5′-TTACTGGTCGTGGACGGCCAT-3′ and reverse
5′-AAACGGACACTCACAATGGGCC-3′.
Detection of the protein expression
levels of Cbfal, BMPRII, Smad1, Smad4, Smad5 and Smad6 using
western blot analysis
The fibroblast-like synovial cells were collected
from all rats and washed twice with PBS. To each flask, 400 µl
lysate was added, followed by 40 µl PMSF at a concentration of 10
mmol/l. The flasks were agitated gently, and placed on ice for 10
min to lyse the cells uniformly. The cells were repeatedly drawn
with a sterile syringe and the lysate was added to an EP tube,
which was then placed in an ice bath for 30 min and centrifuged at
1,611 × g at 4°C for 15 min. The supernatant was moved to a new EP
tube, and protein density was measured on a microplate reader and
using BCA assay. To each tube, 20 µl 6X buffer was added for every
100 µl, followed by boiling for 5 min, mixing and storing at −80°C.
These samples (20 µg) were used for electrophoresis (µl) on a 12%
SDS-PAGE gel. The isolated protein bands were then transferred onto
a PVDF membrane using a wet method, and subsequently maintained at
room temperature for 1 h. The membrane was then incubated with
primary antibodies: Cbfal (cat. no. IBSBIOA-1; Sigma-Aldrich; Merck
Millipore), BMPRII (cat. no. PA130036; Sigma-Aldrich; Merck
Millipore), Smad1 (cat. no. sc-1612; Santa-Cruz Biotechnology,
Inc., Dallas, TX, USA), Smad4 (cat. no. sc-0598; Santa-Cruz
Biotechnology, Inc.), Smad5 (cat. no. sc-26412; Santa-Cruz
Biotechnology, Inc.), and Smad6 (cat. no. sc-1304; Santa-Cruz
Biotechnology, Inc.). The dilution for all antibodies was 1:1,000.
The membrane was incubated overnight under 4°C and washed with PBST
three times. Following this, the membrane was incubated with
secondary antibody (cat. no. 563-1; Shanghai Zemai Biotech Co.,
Ltd., Shanghai, China) at a dilution of 1:1,000 for 1 h at 4°C, and
washed with PBST three times. Color development and fixing were
completed using a chemiluminescence assay. The protein expression
levels of Cbfal, BMPRII, Smad1, Smad4, Smad5, and Smad6 were
assessed using ImageJ version 1.48U (National Institutes of Health,
Bethesda, MD, USA).
mRNA expression of the BMP/Smad
signaling pathway in fibroblasts induced by rhBMP-2
The normal and AS fibroblasts in the third passage
logarithmic phase were made into single cell suspensions and seeded
into a 24-well plate at a density of 2×107/well. The
normal and AS fibroblasts were divided into six groups
respectively, and 200 pg/ml rhBMP-2 was added to each well, with
four duplicate wells for each group. The seeded cells were placed
in a CO2 incubator overnight for complete cell
adherence, following which the supernatant was discarded. The cells
in the normal and AS groups were added to 2 ml DMEM containing 10%
FBS and the cells in the remaining group were added to 2 ml DMEM
containing rhBMP-2 and 10% FBS. All groups were placed into a
CO2 incubator for 72 h. The contents were obtained to
measure the mRNA expression levels of Cbfal, BMPRII, Smad1, Smad4,
Smad5 and Smad6.
Statistical analysis
Each experiment was repeated three times. One-way
analysis of variance and the Bonferroni test were performed using
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) for pairwise
comparisons in multiple samples. P<0.05 was considered to
indicate a statistically significant difference.
Results
Proliferation of fibroblast cells
Compared with the normal fibroblasts, the growth
rate of the AS fibroblasts was significantly higher following cell
adherence for 24 h. The significant difference remained for 6 days
(P<0.01). On day 4, the proliferation of the normal and AS
fibroblasts reached a zenith and entered into the plateau phase
(Fig. 1). Intervention with
triptolide at this phase showed that triptolide effectively
inhibited the proliferation of AS fibroblasts, with the highest
concentration resulting in the most marked effect (Fig. 2).
Effects of triptolide on the levels of
TNF-α, IL-1β and IL-6
The ELISA results showed that, compared with the
normal group, the AS spinal arthritis model rats exhibited
significantly higher expression levels of TNF-α, IL-1β and IL-6.
After intervention with triptolide, the TNF-α, IL-1β and IL-6
levels were reduced, especially in the high concentration group
(P<0.01). This suggested that combined medication improved the
inflammatory response (Fig.
3).
Expression levels of BMPRII and
Cbfal
The expression of BMPRII was higher in the AS
fibroblasts and was reduced by triptolide, with the most marked
effect in the highest concentration group. Cbfal was also expressed
at a higher level in the AS fibroblast group, compared with the
significantly lower level in the normal fibroblast group. The
levels of both were decreased following treatment with triptolide
(Fig. 4).
Phosphorylation of pSmad1 and
pSmad5
pSmad1 and pSmad5 were expressed in the normal and
AS fibroblasts, with higher levels in the AS fibroblasts, compared
with those in the normal fibroblasts. The degrees of pSmad1 and
pSmad5 phosphorylation were significantly decreased following
treatment with triptolide (Fig.
5).
Effects of triptolide on the protein
and mRNA expression levels of Smad1, Smad4, Smad5 and Smad6
The mRNA and protein expression levels of Smad1,
Smad4 and Smad5 in the AS fibroblasts were significantly higher,
compared with those in the normal fibroblasts (P<0.01). These
levels were decreased following triptolide treatment, with the most
marked effects in the highest concentration group (P<0.05).
However, the mRNA and protein expression levels of Smad6 were lower
in the AS fibroblasts, compared with those in the normal group
(P<0.01), although the level increased following triptolide
treatment (P<0.05; Figs. 6 and
7).
 | Figure 7.Effects of triptolide on the mRNA
expression of Smad1, Smad4, Smad5 and Smad6. **P<0.01, vs.
normal group; ##P<0.01, vs. drug-10 mg/kg group;
#P<0.05, vs. drug-10 mg/kg group;
&&P<0.01, vs. drug-20 mg/kg group;
&P<0.05, vs. drug-20 mg/kg group;
!!P<0.01, vs. drug-40 mg/kg group;
!P<0.05, vs. drug-40 mg/kg group. Smad, small mothers
against decapentaplegic. |
mRNA expression levels of Smad1,
Smad4, Smad5, Smad6, BMPRII and Cbfal in AS fibroblasts are induced
by rhBMP-2
The phosphorylation of Smad1, Smad4, Smad5, Smad6,
BMPRII and Cbfal were increased in the AS fibroblasts induced by
rhBMP-2, but were decreased following triptolide treatment. This
decrease resulted in lower levels, compared with those in the group
without exposure to rhBMP-2, suggesting that treatment may function
through the BMP/Smad signaling pathway (Fig. 8).
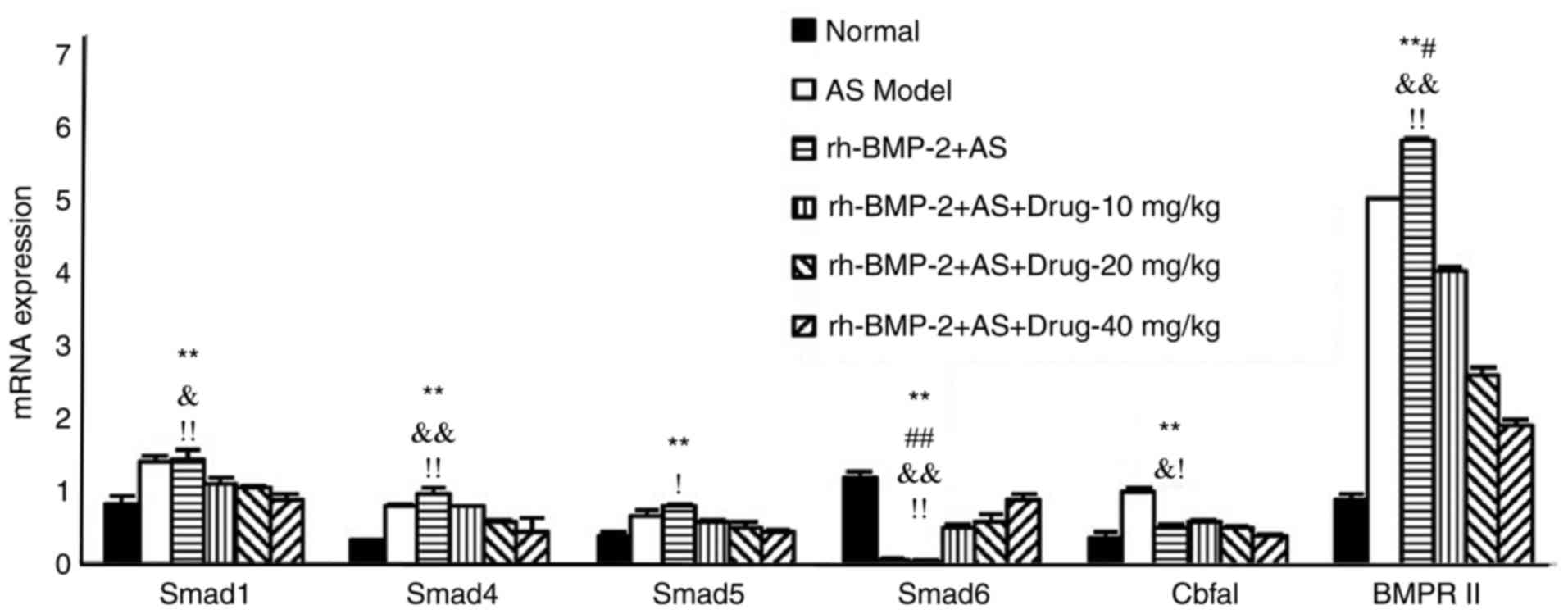 | Figure 8.mRNA expression of Smad1, Smad4,
Smad5, Smad6, BMPRII and Cbfal in AS fibroblasts induced by
rhBMP-2. **P<0.01, vs. normal group; ##P<0.01, vs.
rh-BMP-2-drug-10 mg/kg group; #P<0.05, vs.
rh-BMP-2-drug-10 mg/kg group &&P<0.01, vs.
rh-BMP-2-drug-20 mg/kg group; &P<0.05, vs.
rh-BMP-2-drug-20 mg/kg group; !!P<0.01, vs.
rh-BMP-2-drug-40 mg/kg group; !P<0.05, vs.
rh-BMP-2-drug-40 mg/kg group. AS, ankylosing spondylitis; Smad,
small mothers against decapentaplegic; BMPRII, bone morphogenetic
protein receptor type II; Cbfa1, core-binding factor α1; rh-BMP,
recombinant human BMP. |
Discussion
In AS, heterotopic ossification takes place in the
spine and peripheral joints, which is a pathological atypical bone
formation predominantly occurring in muscle or connective tissues.
Studies have shown that there are three essential conditions for
the formation of heterotopic ossification: Precursor cells, an
osteogenic inducer, and bone tissue environment. Caused by an
initial stimulus, the signal produced in the damaged region
requires non-differentiated mesenchymal cells and an appropriate
tissue environment to facilitate the formation of heterotopic
ossification (11,12). The formation of AS heterotopic
ossification is likely to be derived from a change in the
differentiation direction of mesenchymal cells in soft tissues. The
final formation of ossification is induced by certain osteogenic
factors.
BMPs are a family of >20 types of glycoprotein
isolated and purified from bone matrix, which can regulate the
growth and differentiation of various cell types, including
stimulating chondrocyte matrix synthesis, increasing osteoblast
alkaline phosphatase activity, and facilitating collagen synthesis,
nerve cell differentiation and kidney development. BMPs are
important in inducing bone and cartilage formation, embryonic
development, and organ formation (13). BMP receptors include type I and
type II receptors. The type II receptor is structurally active,
which is activated through binding with a ligand to induce
auto-phosphorylation, through which the activated type II receptor
activates the type I receptor, forming a complex receptor and
completing BMP activation (14,15).
The binding of BMP ligand with heterologous dimers
can activate the Smad-dependent BMP signaling pathway. The Smad
family is widespread and can be divided into three types according
to their functions in signal transduction. Firstly, the
receptor-modulating type includes Smad1, Smad2, Smad3, Smad5 and
Smad8, in which Smad1, Smad5 and Smad8 are involved in the signal
transduction of BMP, whereas Smad2 and Smad3 are involved in
Activin and TGF. Secondly, the common media type comprises Smad4
only and cannot interact with type I receptor or be phosphorylated,
but can hybridize with receptor-modulating type Smads to form
stable polymers. Thirdly, the inhibitory Smads, comprising Smad6
and Smad7, have a negative effect in the Smad signal transduction
pathway through inhibiting receptor-mediated R-Smad carbonation or
interfering with the binding of R-Smad and Co-Smad (16,17).
The Smad-dependent pathway is the classic pathway of
BMP signal transduction. The BMP/Smad pathway is composed of a BMP
signal, BMP receptor, and receptor substrate Smad signaling
molecules (18). The BMP ligand
acts on the membrane type II receptor, the auto-phosphorylation of
which causes activation and subsequent phosphorylation of type I
receptor; this forms heterogenic polymer complexes, which
phosphorylate Smad1, Smad5 and Smad8, thus transducing signals to
cells (19,20). The activated R-Smad and Smad4 then
bind in the cytoplasm, forming an R-Smad/Smad4 complex, which
carries signals to the nucleus and binds with the target gene,
activating target gene transcription and transducing signals
(21).
In the present study, it was found that the protein
and mRNA expression levels of Smad1, Smad4, Smad5, BMPRII and Cbfal
in the AS fibroblasts were higher, compared with those in the
normal group. The levels of both were decreased by triptolide
intervention. BMPRII was inhibited, which was the initiating step
of signal transduction, and the subsequent BMP signal transduction
was inhibited. The receptor-modulating receptor Smad was combined
into a stable hybrid, and the signal was transduced to the nucleus.
The reduction of Smad affected BMP signal transduction, which was
most marked in the combined treatment group.
The protein and mRNA expression levels of Smad6 in
the AS fibroblasts were lower, compared with those in the normal
group, whereas triptolide reversed this effect. Smad6 can regulate
BMP signal transduction through multiple pathways. The decrease of
type II receptors, Smad1, Smad4, Smad5 and BMPRII was inhibited by
combined treatment, as was the phosphorylation of Smad1 and Smad5.
The levels of Smad6 were increased and the osteogenic
differentiation of AS fibroblasts was inhibited, thus exerting an
anti-ossification effect.
Studies have shown that BMP can enhance the
expression of Cbfal. BMP-induced osteogenesis is mediated by Cbfal,
and BMP is necessary for Cbfal transcript initiation (22,23).
The expression of Cbfal in the AS fibroblasts was increased, but
decreased following triptolide treatment, with the most marked
effect in the combined medication group. This suggested that
treatment inhibited the expression of Cbfal or that triptolide
inhibited the osteoblastic differentiation of AS fibroblasts.
In conclusion, triptolide inhibited the expression
levels of BMPRII and Smad1, increased the expression of Smad6,
decreased the phosphorylation of Smad1 and Smad5, regulated
BMP/Smad signal transduction, and reduced downstream BMP signaling,
thus inhibiting the expression of Cbfal. Triptolide may be useful
in the treatment of AS, the mechanism of which may occur via the
BMP/Smad pathway.
References
|
1
|
Meyer K, Niedermann K, Tschopp A and
Klipstein A: Is the work ability index useful to evaluate absence
days in ankylosing spondylitis patients? A cross-sectional study.
BMJ Open. 3:pii: e0022312013. View Article : Google Scholar
|
|
2
|
Gerdan V, Akar S, Solmaz D, Pehlivan Y,
Onat AM, Kisacik B, Sayarlioglu M, Erhan C, Tezcan ME, Ozturk MA,
et al: Initial diagnosis of lumbar disc herniation is associated
with a delay in diagnosis of ankylosing spondylitis. J Rheumatol.
39:1996–1999. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van den Berg R and van der Heijde DM: How
should we diagnose spondyloarthritis according to the ASAS
classification criteria: A guide for practicing physicians. Pol
Arch Med Wewn. 120:452–457. 2010.PubMed/NCBI
|
|
4
|
Arends S, Brouwer E, van der Veer E, Groen
H, Leijsma MK, Houtman PM, Th A, Jansen TL, Kallenberg CG and
Spoorenberg A: Baseline predictors of response and discontinuation
of tumor necrosis factor-alpha blocking therapy in ankylosing
spondylitis: A prospective longitudinal observational cohort study.
Arthritis Res Tlier. 13:R942011. View
Article : Google Scholar
|
|
5
|
Bao J and Dai SM: A Chinese herb
Tripterygium wilfordii Hook F in the treatment of rheumatoid
arthritis: Mechanism, efficacy, and safety. Rheumatol Int.
31:1123–1129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Qin W, Yang F, Deng R, Li D, Song Z, Tian
Y, Wang R, Ling J and Lin Z: Smad 1/5 is involved in bone
morphogenetic protein-2-induced odontoblastic differentiation in
human dental pulp cells. J Endod. 38:66–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ecalco-Cruz AC, Sosa-Garrocho M,
Vázquez-Victorio G, Ortiz-García L, Domínguez-Hüttinger E and
Macías-Silva M: Transforming growth factor-β/SMAD Target gene SKIL
is negatively regulated by the transcriptional cofactor complex
SNON-SMAD4. J Biol Chem. 287:26764–26776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kokabu S, Ohte S, Sasanuma H, Shin M,
Yoneyama K, Murata E, Kanomata K, Nojima J, Ono Y, Yoda T, et al:
Suppression of BMP-Smad signaling axis-induced osteoblastic
differentiation by small C-terminal domain phosphatase 1, a Smad
phosphatase. Mol Endocrinol. 25:474–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Poddubnyy D, Rudwaleit M, Haibel H,
Listing J, Märker-Hermann E, Zeidler H, Braun J and Sieper J:
Effect of non-steroidal anti-inflammatory drags on radiographic
spinal progression in patients with axial spondyloarthritis:
Results from the German Spondyloarthritis Inception Cohort. Ann
Rheum Dis. 71:1616–1622. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yi JF and Tang XL: The effect of
paeoniflorin, collagen, hypothalamus pituitary kidney in rats with
collagen induced arthritis. China Adrenal Axis J Pharmacol Toxicol.
27:668–672. 2013.
|
|
11
|
Wang J: Experimental study of recombinant
expression and anti-tumor effect of ING4 gene (unpublished PhD
thesis). Soochow University; 2005
|
|
12
|
Kroon F, Landewé R, Dougados M and van der
Heijde D: Continuous NSAID use reverts the effects of inflammation
on radiographic progression in patients with ankylosing
spondylitis. Ann Rheum Dis. 71:1623–1629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikushima H and Miyazono K: TGF-β signal
transduction spreading to a wider field: A broad variety of
mechanisms for context-dependent effects of TGF-β. Cell Tissue Res.
347:37–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thatcher JD: The TGF-beta signal
transduction pathway. Sci Signal. 3:tr42010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song B, Estrada KD and Lyons KM: Smad
signaling in skeletal development and regeneration. Cytokine Growth
Factor Rev. 20:379–388. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ekman M, Mu Y, Lee SY, Edlund S, Kozakai
T, Thakur N, Tran H, Qian J, Groeden J, Heldin CH and Landström M:
APC and Smad7 link TGFβ type I receptors to the microtubule system
to promote cell migration. Mol Biol Cell. 23:2109–2121. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vardouli L, Vasilaki E, Papadimitriou E,
Kardassis D and Stournaras C: A novel mechanism of TGFbeta-induced
actin reorganization mediated by Smad proteins and Rho GTPases.
FEBS J. 275:4074–4087. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edlund S, Landström M, Heldin CH and
Aspenström P: Smad7 is required for TGF-beta-induced activation of
the small GTPase Cdc42. J Cell Sci. 117:1835–1847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Konstantinidis G, Moustakas A and
Stournaras C: Regulation of myosin light chain function by BMP
signaling controls actin cytoskeleton remodeling. Cell Physiol
Biochem. 28:1031–1044. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen G, Deng C and Li YP: TGF-β and BMP
signaling in osteoblast differentiation and bone formation. Int J
Biol Sci. 8:272–288. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jun JH, Yoon WJ, Seo SB, Woo KM, Kim GS,
Ryoo HM and Baek JH: BMP2-activated Erk/MAP kinase stabilizes Runx2
by increasing p300 levels and histone acetyltransferase activity. J
Biol Chem. 285:36410–36419. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Q, Zhang H, Pei FX, Chen ZY, Wang
GL, Shen B, Yang J, Zhou ZK and Kong QQ: Use of small interfering
ribonucleic acids to inhibit the adipogenic effect of alcohol on
human bone marrow-derived mesenchymal cells. Int Orthop.
34:1059–1068. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi XM, Blair HC, Yang X, McDonald JM and
Cao X: Tandem repeat of C/EBP binding sites mediates PPAR gamma2
gene transcription in glucocorticoid-induced adipocyte
differentiation. J Cell Biochem. 76:518–527. 2000. View Article : Google Scholar : PubMed/NCBI
|






















