Introduction
Hydronephrosis is the distension of the renal pelvis
and calyces due to the obstruction of the free flow urine out of
the kidney. If left untreated, it leads to the progressive atrophy
of the kidney and decreased kidney function, ultimately leading to
kidney failure (1,2). One of the methods of choice for
diagnosing hydronephrosis is computed tomography (CT) scan.
However, 1–2% of individuals with normal renal function will suffer
from contrast-induced acute kidney injury (CI-AKI) after the use of
contrast medium (3–5), and this proportion is even higher for
individuals with renal dysfunction (6,7).
CI-AKI is the acute deterioration of renal function within 3 days
after the use of a contrast agent, without any other identifiable
cause (5).
The pathogenesis of CI-AKI is unclear, but three
mechanisms have been suggested (5). Contrasts agents induce renal
vasoconstriction, leading to renal medulla ischemia (8). Hypoxia can lead to increased amounts
of secreted reactive oxygen species (ROS), aggravating organ injury
when the oxidative stress overwhelms the antioxidative capacities
of the organ (9,10). Contrast agents may also have direct
kidney toxicity that leads to mitochondrial dysfunction and
apoptosis (10,11).
Clinically, there is no effective way to treat AKI;
therefore, prevention should be the best choice. N-acetylcysteine
(NAC) has strong antioxidant effects and it is currently recognized
as a protective drug against contrast medium induced renal damage
(12). Previous studies have shown
that NAC could be useful to prevent renal damage in a rat model of
kidney obstruction (13), and to
prevent CI-AKI in patients with normal or impaired kidney function
undergoing CT scan (14,15). However, the effect of NAC on CI-AKI
in complete unilateral ureteral obstruction (UUO) has not been
reported.
Therefore, the present study aimed to investigate
the protective effects of NAC on CI-AKI in rat models of unilateral
hydronephrosis. The results of this study could provide new ways of
preventing CI-AKI in patients with impaired kidney function.
Materials and methods
Experimental animals
Male Sprague-Dawley (SD) rats (n=82, body weight of
250–290 g) of specific pathogen-free (SPF) grade were provided by
the Animal Center of the Guangdong Medical Laboratory (Foshan,
China; quality certificate no. 0113875). SD rats were kept in the
SPF animal house of the Guangdong Provincial Medical Experimental
Animal Center (license number for experimental animals: SYXK
(Guangdong) 2008–0002). All animals were quarantined for 3 days.
During the period, animals were inspected once in a day, and
unhealthy rats were removed immediately if found. Only healthy rats
were used in the experiment. All animal experiments were performed
according to the animal experimental guide of the Ethics Committee
of the Southern Medical University. All experimental procedures
were approved by this committee.
Animal model
The model of UUO was induced as previously described
(16). Briefly, the rats were
adapted to their new environment for 1 week. Rats were fasted for
12 h before modeling, but they had free access to water. After
anesthesia with injection of 3% pentobarbital sodium, the middle
abdomen was shaved, disinfected, and incised to expose the
conjunction of the left renal pelvis and ureter (UPJ). Then, the
abdomen of the 14 rats in the sham-operated group was closed. For
the remaining 68 rats in the model group, a 1.8-cm plastic epidural
catheter for anesthesia was folded into a ‘V-shape’ with a plastic
catheter, gently sheathed within the ureter to obtain a
dissociative ureter with 5 mm in length at 1 cm below UPJ. The ends
of the V tube were ligated with no. 1 silk thread leading to
occlusion of the ureteral lumen. Care was taken to keep the
distance between the end of the line and the knot to only 1–2 mm in
all animals. Animals were placed in the lateral position after
operation and kept one animal/cage. Penicillin (8×104
U/rat) was injected for 3 days to prevent infection.
Grouping and treatments
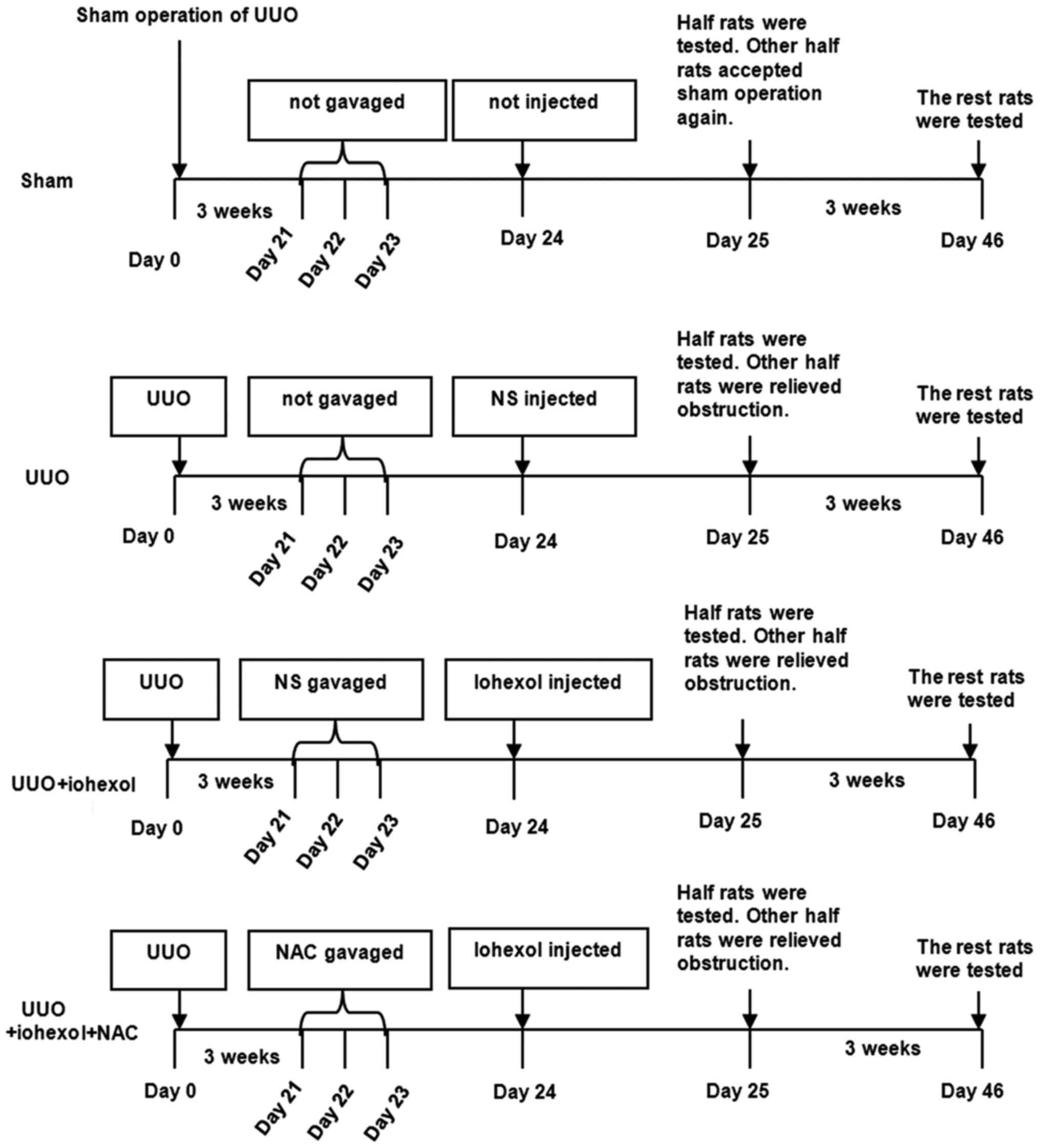
Fig. 1 presents the
study design and grouping. The model was considered ready after 3
weeks, and 68 rats were randomly divided into three groups: The NAC
gastric perfusion group (UUO+iohexol+NAC, n=24), which was
administered with NAC by gavage for 3 days [H20090620, 600
mg/kg/day (Zambon Co., SpA, Bresso, Italy), according to
Pattharanitima and Tasanarong (5),
a 6.3 coefficient was used to convert the NAC human dose to rats];
the normal saline (NS) gastric perfusion group (UUO+iohexol, n=24),
which was administered with the same amount of NS by gavage for 3
days; and the control group (UUO, n=20), which was not
administrated by gavage and was caged under the same conditions as
the other groups.
After 3 days, the UUO+iohexol+NAC and UUO+iohexol
rats were injected with contrast medium via the tail vein [iohexol
(Yangtze River Pharmaceutical Group Co., Ltd., Taizhou, China),
H10970326, 300 mg/ml/kg, according to Efrati et al (17)]. Rats in the UUO group were not
injected with contrast medium but the same amount of NS. Fourteen
sham-operated rats were not treated by gavage administration as
well as any drug. One day after injection, half of the rats in each
group were randomly selected to collect serum and kidney
samples.
Release of obstruction
The remaining animals underwent laparotomy under
anesthesia on the same day (1 day after contrast administration).
After exposing the obstruction part, the V tube was found, and the
ligation line was cut, resulting in obstruction relief. Finally,
the suture was removed. For sham-operated rats, all of them
underwent sham operation. Postoperatively, penicillin
(8×104 U/rat) was injected for 3 days to prevent
infection.
Sample collection
Three weeks after obstruction relief, 5 ml of blood
was taken from the abdominal aorta, and centrifuged at 4°C and
3,000 rpm for 10 min to obtain serum. The rats were sacrificed by
cervical dislocation and both kidneys were taken.
Renal morphology and pathology
Length, width, and height of the kidney were
measured to calculate the volume of the kidney [size = (length ×
width × height) × 0.523]. The kidney was cut along the dorsal
longitudinal section to release the hydrops completely. Then, the
thickness of the renal parenchyma (the average of the thickest and
thinnest values) was measured after the kidney was dried with
tissue paper. Kidney sections were analyzed by H&E
staining.
Serum creatinine
An automatic biochemical analyzer (AEROSET; Abbott
Laboratories, Abbott Park, IL, USA) was used to detect serum
creatinine (Creatinine Assay kit; Sigma-Aldrich, St. Louis, MO,
USA).
Serum neutrophil gelatinase-associated
lipocalin (NGAL)
The Rat NGAL kit (Alpco, Salem, NH, USA) was used to
test for NGAL levels. Microplates were read on a Bio-Rad plate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Apoptosis detection in kidney
tissues
A TUNEL apoptosis detection kit (TdT-mediated dUTP
nick end labeling, #12156792910; Roche Applied Science, Penzberg,
Germany) was used to detect the apoptosis of renal tubular cells.
The number of apoptotic cells and the total number of cells were
counted to calculate apoptosis index (AI; AI = number of apoptotic
cells/total number of cells × 100%). Each sample was measured with
three fields of view, and the average value was calculated.
Real-time Qpcr
A PCR kit (PikoReal; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) was used to detect the expression of Bcl-2 mRNA
and Bax mRNA in left kidney tissues. TRIzol was used to extract
total RNA [(Invitrogen, Inc., Carlsbad, CA, USA); fluorescence
quantitative PCR kit (Takara Bio, Inc., Otsu, Japan); cellulose
nitrate membrane (Millipore Corp., Billerica, MA, USA)] according
to the manufacturer's instructions. After RNA extraction, UV
spectrophotometry was used to measure the OD values at 260 and 280
nm. The RNA sample was stored at −80°C for later use.
Reverse transcription of RNA to cDNA was performed
according to the instructions of the reverse transcription kit.
RNAase-free water was used to dilute the product 10 times. For
fluorescence quantitative PCR amplification, the Livak method
(2−ΔΔCt) was used for relative quantitation (β-actin as
control). Bcl-2 gene was amplified with: Forward,
5′-GTGGTGGAGGAACTCTTCAGGGATG-3′ and reverse,
5′-GGTCTTCAGAGACAGCCAGGAGAAATC-3′ (226 bp); Bax gene was amplified
with: Forward, 5′-GGGTTTCATCCAGGATCGAGCAG-3′ and reverse,
5′-GAGTCCGTGTCCACGTCAGCAAT-3′ (288 bp); and β-actin gene was
amplified with: Forward, 5′-ATGTGGCCGAGGACTTTGATT-3′ and reverse,
5′-AGTGGGGTGGCTTTTAGGATG-3′ (107 bp).
Western blotting
Western blotting was used to detect the expression
of Bcl-2 and Bax proteins in the left kidney tissues (Invitrogen
Inc.). FlourChem V2.0 gel imaging analysis software (Alpha
Innotech, San Leandro, CA, USA) was used for analysis. The
grayscale values of targeted bands and β-actin band were used to
quantify the relative expression of targeted proteins.
Statistical analysis
Data are represented as mean ± standard deviation.
Effect of contrast medium on the rats among the different groups
and different time points was determined by the factorial variance
analysis method. Single effect analysis was based on the results of
mean LSD correction after factorial analysis. The Welch test was
used when the variance was not homogeneous. SPSS 21.0 (IBM Corp.,
Armonk, NY, USA) was used for all statistical analyses. Two-sided
P-values <0.05 were considered statistically significant.
Results
Morphological changes of kidney in
rats
No significant abnormalities were found in renal
section from sham-operated rats (Fig.
2A). In the model rats, the left kidney showed thinner
parenchyma, glomeruli atrophy, and lower number of glomeruli
(Fig. 2B). Furthermore, expansion
of some part of the left renal tubular, flattened tubular
epithelia, tubular protein casts, and cellular casts in some renal
tubular; collapse of some renal tubular lumen, renal tubular
vacuolation; infiltrated renal interstitial inflammatory cell, and
severe renal interstitial fibrosis were found in rats from the
UUO+iohexol group (Fig. 2C). The
UUO+iohexol+NAC showed less damage (Fig. 2D).
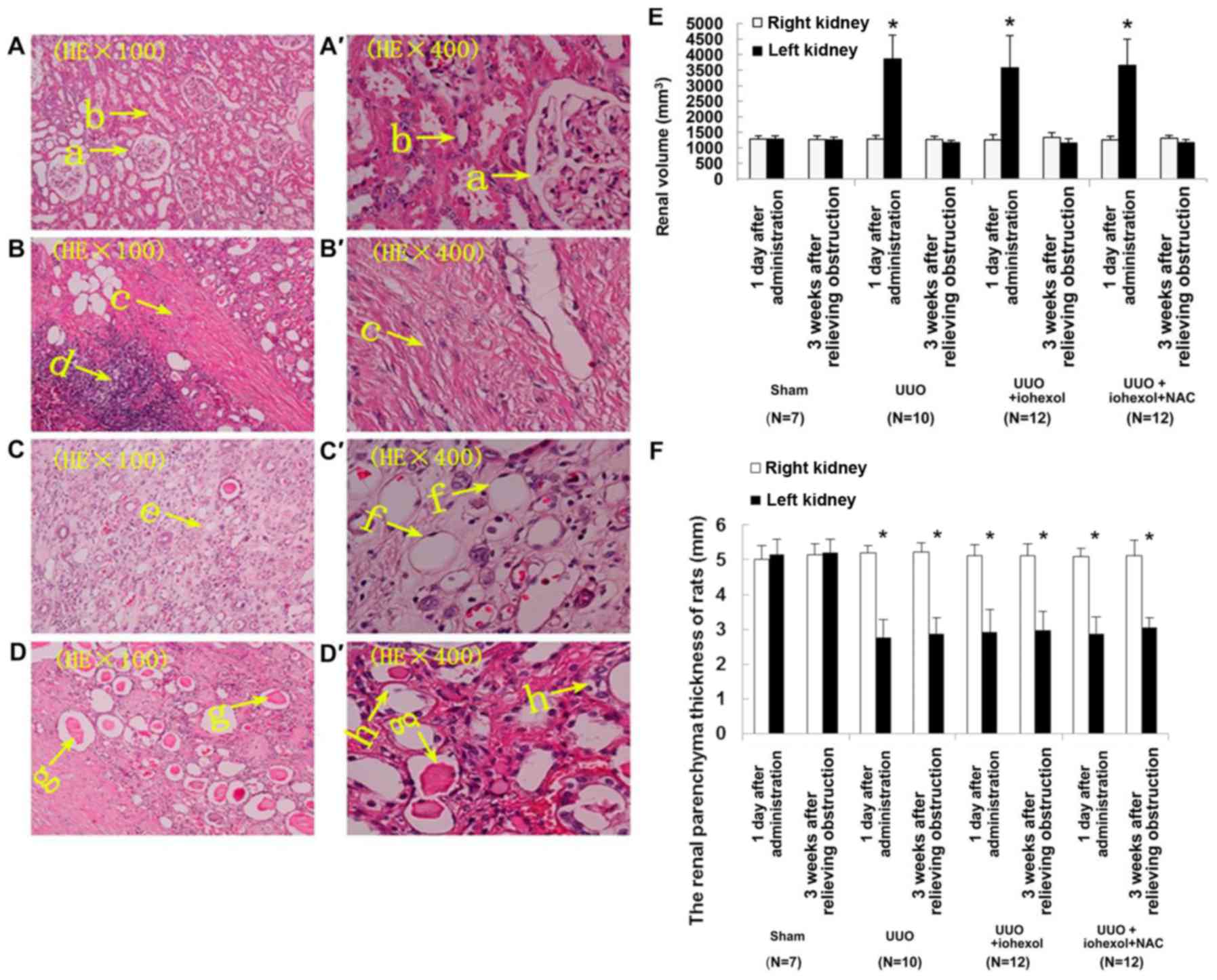 | Figure 2.Histological sections of the left
kidneys (1 day after contrast administration) of the sham-operated
group (n=7) (A) the UUO group (n=10), (B) the UUO+iohexol group
(n=12), (C) and the UUO+iohexol+NAC group (n=12), (D) (H&E,
×100). (A'-D') show (A-D) at higher magnification (H&E, ×400).
‘a’ indicates normal glomeruli, ‘b’ indicates normal tubules, ‘c’
indicates renal interstitial fibrosis, ‘d’ indicates infiltrated
renal interstitial inflammatory cells, ‘e’ indicates dilated renal
tubules, ‘f’ indicates flattened tubular epithelia, ‘g’ indicates
cellular casts in some renal tubules, and ‘h’ indicates renal
tubular vacuolation. (E) Renal volume of the rat models of UUO. (F)
Cortical thickness of the rat models of UUO. Data are presented as
mean ± standard deviation.*P<0.05 vs. right kidney. UUO,
unilateral ureteral obstruction; NAC, N-acetylcysteine. |
Except for the sham-operated group, the left kidney
volume of the rats in each group was significantly increased
compared with the right kidney 1 day after obstruction (Fig. 2E). Similarly, the parenchyma
thickness of the left kidney was significantly thinner than that of
the right kidney (Fig. 2F),
indicating that the model was successful and reliable. Except for
the sham-operated rats, the left kidney volume of the rats in each
group was significantly decreased 3 weeks after obstruction relief,
indicating that relief of obstruction was successful.
Changes of kidney function after
modeling
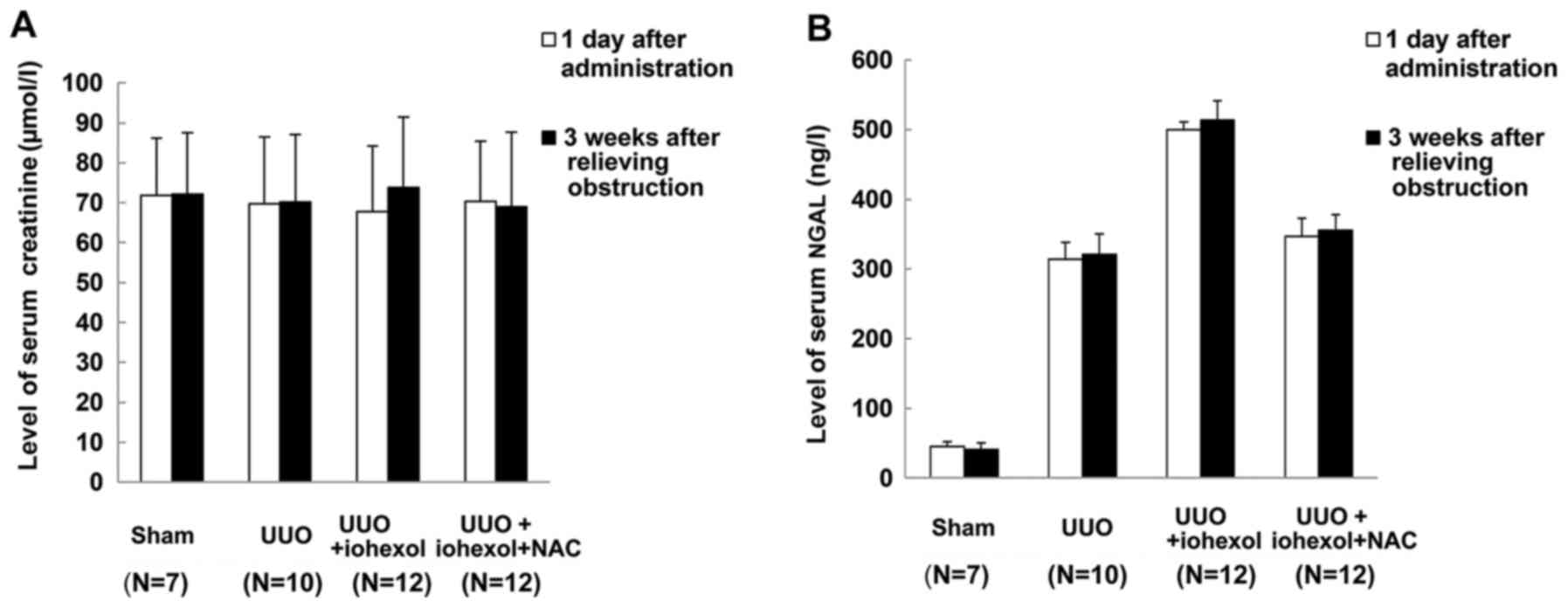
No significant differences were found in the serum
creatinine and NGAL levels of the four groups on the day after drug
administration and 3 weeks after relieving obstruction (Fig. 3), suggesting that unilateral
obstruction and contrast medium-mediated injury did not
significantly affect the overall renal function due to the
compensation of the healthy kidney.
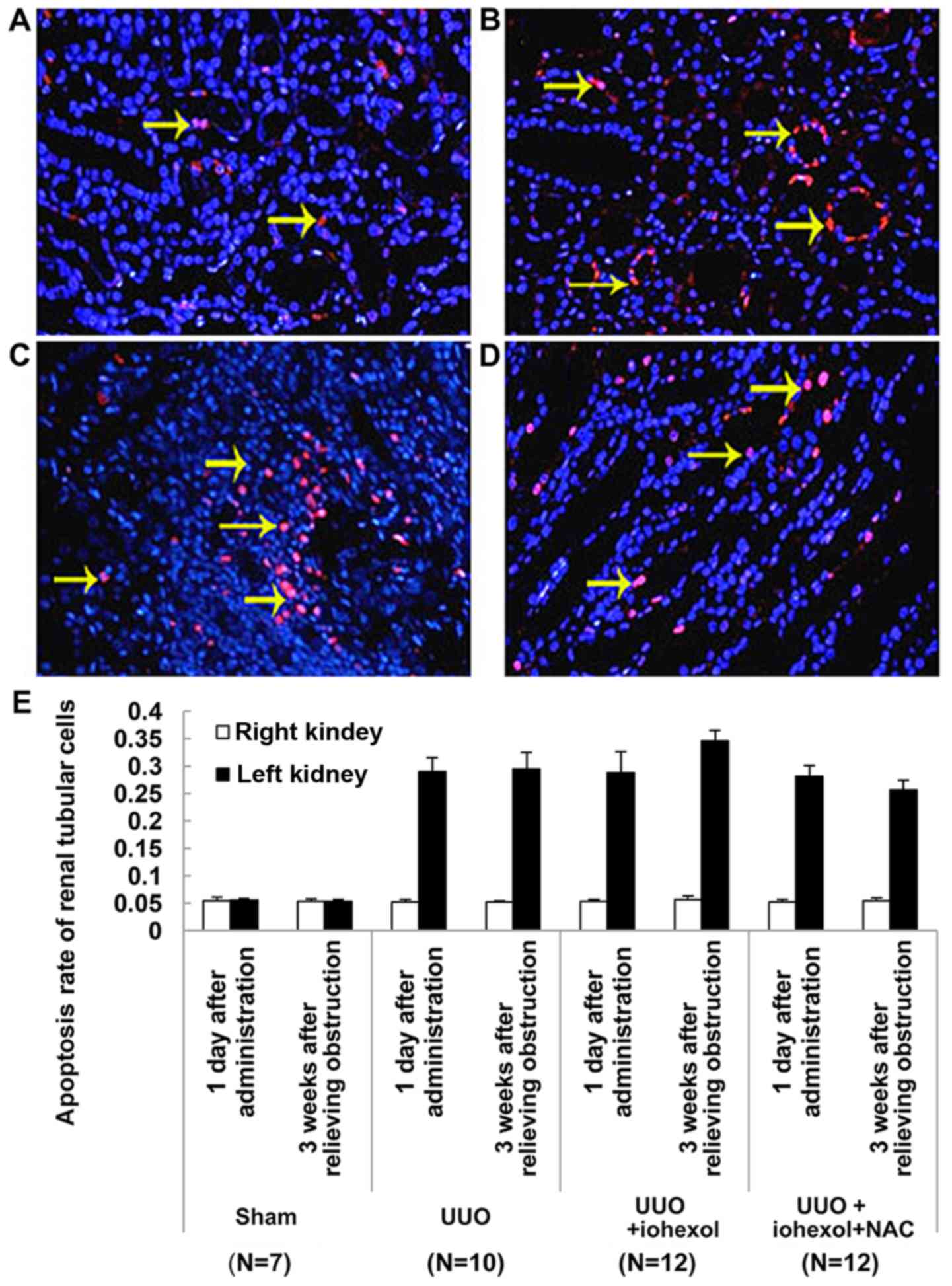
Renal tubular cell apoptosis
Fig. 4A-D shows the
renal tubular cell apoptosis detected by TUNEL assay 3 weeks after
relieving obstruction in the left kidneys of the sham-operated
group (n=7) (Fig. 4A), the UUO
group (n=10) (Fig. 4B), the
UUO+iohexol group (n=12) (Fig.
4C), and the UUO+iohexol+NAC group (n=12) (Fig. 4D). As shown in Fig. 4E, except for the sham-operated
group, there were differences in apoptosis rates of the left and
right kidney renal tubular cells in rats of the three UUO groups on
the two time points (1 day after drug administration and 3 weeks
after relief of obstruction, P<0.001). The apoptosis rate of the
left renal tubular cells of the sham-operated rats was
significantly lower than that of the other three groups 1 day after
drug administration (P<0.001), but there was no significant
difference in the other three groups. The apoptosis rate of left
renal tubular cells in UUO+iohexol rats increased 3 weeks after
relief of obstruction compared with 1 day after drug administration
and it was also higher than that of CG rats (both P<0.05). The
apoptosis rate of left renal tubular cells in UUO+iohexol+NAC rats
3 weeks were decreased after obstruction relief compared with 1 day
after drug administration; it was also lower than that of
UUO+iohexol rats (P<0.05) (Fig.
4E).
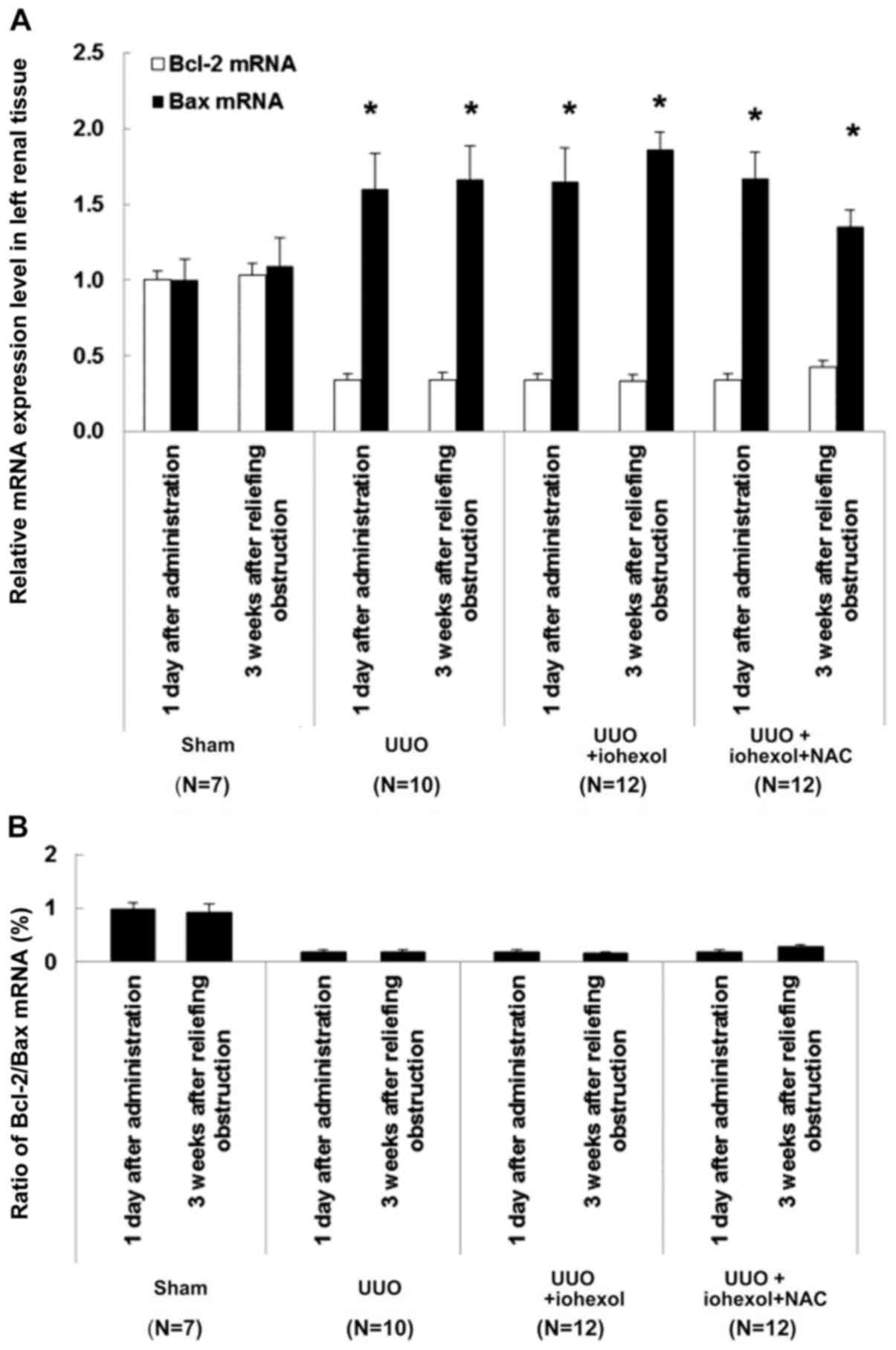
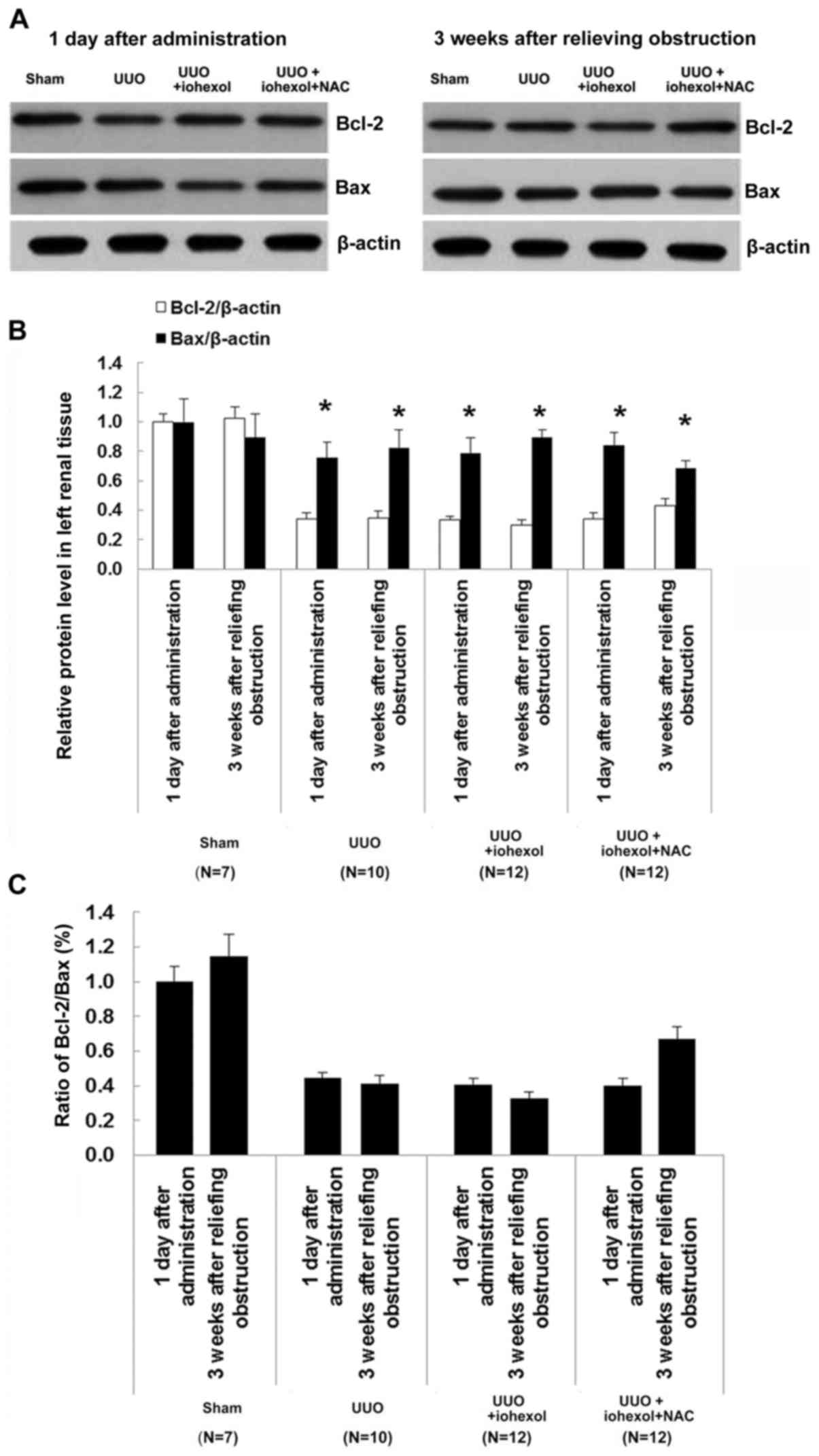
Changes of Bcl-2/Bax expression
Bax mRNA expression in the left kidney tissues of
SOG rats at two time points (1 day after drug administration and 3
weeks after relief of obstruction) was significantly lower than in
the three UUO groups (Fig. 5A).
The ratio of Bcl-2 mRNA expression and Bcl-2/Bax expression was
significantly higher than in the three UUO groups (Fig. 5A). For the three UUO groups 1 day
after drug administration, there were no significant differences
between the groups. Three weeks after relief of obstruction, Bcl-2
mRNA expression of the left kidney tissues in UUO+iohexol rats was
slightly increased, but without significant difference, while the
expression of Bax mRNA was decreased remarkably, represented by
decreased ratio of Bcl-2/Bax (Fig.
5B). The Bax mRNA expression of the left kidney tissues in
UUO+iohexol+NAC rats was decreased (Fig. 5A), but the expression of Bcl-2 mRNA
and ratio of Bcl-2/Bax were increased (Fig. 5).
Western blotting results of Bcl-2/Bax in the left
kidney tissues in each group of rats are shown in Fig. 6A-C. Results showed that Bcl-2
protein expression was decreased in all three UUO groups 1 day
after drug administration. Compared with 1 day after drug
administration, the Bcl-2/Bax ratio decreased in the UUO+iohexol
group at 3 weeks after obstruction relief, while the ratio was
increased in the UUO+iohexol+NAC group.
Discussion
The objective of this study was to investigate the
protective effects of NAC on CI-AKI in rats with unilateral
hyronephrosis. Results showed that compared with controls, serum
NGAL levels were high in UUO+iohexol rats 1 day after injection and
3 weeks after obstruction relief, but UUO+iohexol+NAC rats had
lower serum NGAL levels compared with UUO+iohexol rats. After
modeling, UUO+iohexol rats had significantly higher apoptosis rate
of renal tubular cells, higher expression of Bax mRNA (P<0.05),
and lower ratio of Bcl-2/Bax. Three weeks after obstruction relief,
UUO+iohexol+NAC rats had lower apoptosis rate, lower Bax mRNA
expression, higher expression of Bcl-2 mRNA, and higher ratio of
Bcl-2/Bax when compared with day 1 after drug administration. Only
one previous study showed a role of NAC in preventing renal
impairment in a model of kidney obstruction (18), but the animal model was different
from the present study, which used rats instead of mice. In
addition, the study by Shen et al (18) focused on the protective effect of
NAC on renal fibrosis, while the present study focused on the
protective role of NAC on the apoptosis of renal cells. Therefore,
we explored different mechanisms of the protective effects of
NAC.
When the ureters are obstructed, urine renal
excretion are blocked, resulting in increase of intrarenal
pressure, expansion of renal pelvis and calyx, renal interstitial
edema, infiltration of focal inflammatory cell, fibrosis of renal
tubular, renal vasoconstriction, renal parenchymal hypoxia
ischemia, impaired renal function, and progressive atrophy of renal
parenchyma (3). Even if the upper
urinary tract obstruction is relieved, some cases are still
suffering from renal atrophy, which was also observed in the
present study. A number of studies have indicated that ROS-mediated
apoptosis is involved in the renal pathological changes after
urinary tract obstruction (5,19).
In the present study, the apoptosis rate of the left renal tubular
cells in the UUO rats was increased, and the Bcl-2 expression
decreased while the expression of Bax increased. Renal tubular cell
apoptosis may be the main cause of obstructive renal atrophy, which
is consistent with the conclusions of other studies (5,19).
NGAL, also known as Lipocalin-2, is encoded by a
gene on chromosome 9q34. NGAL is one of the main inducer genes in
the early stage of ischemic renal injury. The increase of NGAL mRNA
expression and NGAL protein secretion in the rat kidney with early
ischemic injury can be detected in urine and blood, and is directly
proportional to the level and duration of ischemia (20). In the present study, serum NGAL
levels of UUO rats were higher than in sham-operated rats,
suggesting that ureteral obstruction caused kidney injury. NGAL
levels did not decrease after obstruction relief. Taken together,
these data suggest that the ureteral obstruction-caused kidney
injury is not significant in the early stage only due to the
compensation of the healthy side kidney, and the injury does not
significantly affect the overall renal function, confirmed by the
absence of difference in serum creatinine levels.
In clinical practice, enhanced CT is one of the main
imaging modalities for urinary tract obstruction examination.
Currently, iodine contrast agents are widely used for enhanced CT.
Adverse reactions are often observed with the use of contrast
agents, including allergic reaction and neurotoxicity, vascular
toxicity and renal toxicity; among them, allergic reaction is the
most common and renal toxicity is the most serious (21). The mechanism of acute renal injury
induced by iodine contrast agent has not been fully clarified. Most
authors believe that the process includes at least three cascades
of pathophysiological processes. First, the contrast agent induce
renal vascular contraction, resulting in renal medullary ischemia
(22). Second, ischemia and
hypoxia can cause increase of ROS, which further aggravates the
injury of ischemic kidney. When organ injury occurs, ROS produced
by inadequate tissue perfusion overwhelm the antioxidant reserves
(10). Third, contrast
agent-mediated renal tubular toxicity leads to mitochondrial
dysfunction, producing reactive oxygen and cell apoptosis (10). The incidence and severity of renal
injury are correlated with the level of renal dysfunction before
contrast agent injection (23).
For individuals with unilateral urinary tract obstruction, overall
renal function is normal. However, there are some injuries to the
renal function of the obstructed side that can lead to decreased
glomerular filtration rate (24),
and infusion of contrast agent at this time increases the risk for
injuries of renal ischemia and direct toxicity. In the present
study, NGAL levels of UUO+iohexol rats were increased significantly
1 day after injection of the contrast agent, which was an acute
reaction resulting in rental injury. Cell apoptosis is a
time-dependent process, and cell apoptosis rate was not changed at
this time. Three weeks later, although the obstruction was
relieved, the apoptosis rate of renal tubular cells was
significantly increased, suggesting CI-AKI.
There is no known treatment for CI-AKI, but
interventions that could decrease ischemia and/or oxidative damage
have been suggested to prevent CI-AKI. Animal models and clinical
trials have studied a variety of prevention methods (6,11,14,15,17).
Researches mainly focus on anti-vasoconstriction, enhancing renal
blood flow, or preventing ROS damage. Because ROS play an important
role in CI-AKI (25), removing ROS
has become one of the most promising ways to prevent CI-AKI. NAC,
as an antioxidant, has become a commonly recognized protective drug
for kidney injury induced by contrast agents (6,11,14,15,17).
Presently, NAC has been used in several studies to prevent CI-AKI
(26–28). NAC contains a thiol group (-SH)
that can deactivate ROS, and plays the role in antioxidant directly
(29). Second, NAC can promote
glutathione synthesis, and through glutathione, plays an indirect
role in antioxidation (29).
Third, through NO and S-nitrosothiols, NAC can play roles in
vascular dilation, inhibiting the generation of
angiotensin-converting enzyme, and stabilizing NO to reduce the
effect of contrast agents on renal functions (29). A study used NAC to prevent kidney
damage induced by obstruction (13). The present study showed that the
NGAL serum levels of UUO+iohexol+NAC rats were significantly lower
than in UUO+iohexol rats, which supports that NAC could reduce
CI-AKI.
Low levels of ROS can promote cell proliferation to
some extent, but relatively high levels of ROS can induce cell
apoptosis, and even higher levels of ROS might directly cause cell
necrosis (30). An important
regulatory mechanism of apoptosis activation by oxidative stress is
the imbalance of Bcl-2 and Bax expression (31,32),
but the exact mechanisms remain unclear. NAC-induced decreased
oxidative stress is accompanied by reduced renal apoptosis
(5,24), which is represented by changes in
the expression of Bcl-2 and Bax. Bcl-2 and Bax are anti-apoptosis
and pro-apoptosis proteins, respectively (19). Bcl-2 is an anti-apoptotic protein
and inhibits membrane permeability and blocks the destruction of
cellular components by oxidation by stabilizing the mitochondrial
membrane (33). Bax is a regulator
of Bcl-2 activity, and the ratio of Bcl-2 to Bax determines the
occurrence of apoptosis (33). In
the present study, in the obstructed kidney of the UUO+iohexol+NAC
rats, the Bcl-2/Bax ratio was significantly higher after
obstruction relief than before, resulting that the apoptosis rate
of renal tubular cells was decreased. Taken together, it suggests
that the antioxidant effect of NAC upregulates the expression of
Bcl-2 mRNA and down-regulates the expression of Bax mRNA, showing
the protective effect of NAC against CI-AKI.
Of course, the present study is not without
limitations. It was performed in animal models and clinical trials
are necessary to confirm these results. In addition, the mechanisms
of ROS leading to apoptosis through changes in the Bcl-2/Bax ratio
remain unclear. The present study was not designed to
comprehensively assess the Bcl-2/Bax pathway and additional studies
are necessary to shed light on these mechanisms.
In conclusion, the prophylactic use of NAC reduced
the apoptotic rate of renal tubular cells after contrast
exposition, which was accompanied by change in the expression of
Bcl-2/Bax mRNA.
Acknowledgements
The authors acknowledge the help of Dr Zhiyong Zhong
from Comparative Medical Laboratory of Guangdong Medical Laboratory
Animal Center and Dr Hua Yuan from Wuxi Maternal and Child
Health-Care Hospital.
References
|
1
|
Kumar V, Fausto N and Abbas AK: Robbins
and Cotran Pathologic Basis of Disease. 7th. Elsevier Saunders;
Philadelphia: 2005
|
|
2
|
Chevalier RL: Pathophysiology of
obstructive nephropathy in the newborn. Semin Nephrol. 18:585–593.
1998.PubMed/NCBI
|
|
3
|
Rudnick MR, Goldfarb S and Tumlin J:
Contrast-induced nephropathy: Is the picture any clearer? Clin J Am
Soc Nephrol. 3:261–262. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berg KJ: Nephrotoxicity related to
contrast media. Scand J Urol Nephrol. 34:317–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pattharanitima P and Tasanarong A:
Pharmacological strategies to prevent contrast-induced acute kidney
injury. Biomed Res Int. 2014:2369302014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gurm HS, Smith DE, Berwanger O, Share D,
Schreiber T, Moscucci M and Nallamothu BK; BMC2 (Blue Cross Blue
Shield of Michigan Cardiovascular Consortium), : Contemporary use
and effectiveness of N-acetylcysteine in preventing
contrast-induced nephropathy among patients undergoing percutaneous
coronary intervention. JACC Cardiovasc Interv. 5:98–104. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tepel M, Aspelin P and Lameire N:
Contrast-induced nephropathy: A clinical and evidence-based
approach. Circulation. 113:1799–1806. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Persson PB, Hansell P and Liss P:
Pathophysiology of contrast medium-induced nephropathy. Kidney Int.
68:14–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brezis M and Rosen S: Hypoxia of the renal
medulla-its implications for disease. N Engl J Med. 332:647–655.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tumlin J, Stacul F, Adam A, Becker CR,
Davidson C, Lameire N and McCullough PA: CIN Consensus Working
Panel: Pathophysiology of contrast-induced nephropathy. Am J
Cardiol. 98:14K–20K. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Romano G, Briguori C, Quintavalle C, Zanca
C, Rivera NV, Colombo A and Condorelli G: Contrast agents and renal
cell apoptosis. Eur Heart J. 29:2569–2576. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kelly AM, Dwamena B, Cronin P, Bernstein
SJ and Carlos RC: Meta-analysis: Effectiveness of drugs for
preventing contrast-induced nephropathy. Ann Intern Med.
148:284–294. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sunay M, Karakan T, Aydin A, Koca G,
Borcek P and Öğüş E: Do montelukast sodium and N-acetylcysteine
have a nephroprotective effect on unilateral ureteral obstruction?
A placebo controlled trial in a rat model. J Urol. 194:1132–1137.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inda-Filho AJ, Caixeta A, Manggini M and
Schor N: Do intravenous N-acetylcysteine and sodium bicarbonate
prevent high osmolal contrast-induced acute kidney injury? A
randomized controlled trial. PLoS One. 9:e1076022014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rehman T, Fought J and Solomon R:
N-acetylcysteine effect on serum creatinine and cystatin C levels
in CKD patients. Clin J Am Soc Nephrol. 3:1610–1614. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chaabane W, Praddaude F, Buleon M, Jaafar
A, Vallet M, Rischmann P, Galarreta CI, Chevalier RL and Tack I:
Renal functional decline and glomerulotubular injury are arrested
but not restored by release of unilateral ureteral obstruction
(UUO). Am J Physiol Renal Physiol. 304:F432–F439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Efrati S, Berman S, Ilgiyeav I, Siman-Tov
Y, Averbukh Z and Weissgarten J: Differential effects of
N-acetylcysteine, theophylline or bicarbonate on contrast-induced
rat renal vasoconstriction. Am J Nephrol. 29:181–191. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen Y, Miao NJ, Xu JL, Gan XX, Xu D, Zhou
L, Xue H, Zhang W and Lu LM: N-acetylcysteine alleviates
angiotensin II-mediated renal fibrosis in mouse obstructed kidneys.
Acta Pharmacol Sin. 37:637–644. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liapis H, Yu H and Steinhardt GF: Cell
proliferation, apoptosis, Bcl-2 and Bax expression in obstructed
opossum early metanephroi. J Urol. 164:511–517. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skott M, Nørregaard R, Sorensen HB, Kwon
TH, Frøkiaer J and Nielsen S: Pre-existing renal failure worsens
the outcome after intestinal ischaemia and reperfusion in rats.
Nephrol Dial Transplant. 25:3509–3517. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Goldenberg I and Matetzky S: Nephropathy
induced by contrast media: Pathogenesis, risk factors and
preventive strategies. CMAJ. 172:1461–1471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Devrim E, Cetin M, Namuslu M, Ergüder IB,
Cetin R and Durak I: Oxidant stress due to non ionic low osmolar
contrast medium in rat kidney. Indian J Med Res. 130:433–436.
2009.PubMed/NCBI
|
|
23
|
Nicholson T and Downes M: Contrast
nephrotoxicity and iso-osmolar contrast agents: Implications of
NEPHRIC. Clin Radiol. 58:659–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh CH, Chiang HS, Lai TY and Chien CT:
Unilateral ureteral obstruction evokes renal tubular apoptosis via
the enhanced oxidative stress and endoplasmic reticulum stress in
the rat. Neurourol Urodyn. 30:472–479. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weisbord SD and Palevsky PM: Prevention of
contrast-induced nephropathy with volume expansion. Clin J Am Soc
Nephrol. 3:273–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baliga R, Ueda N, Walker PD and Shah SV:
Oxidant mechanisms in toxic acute renal failure. Am J Kidney Dis.
29:465–477. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tepel M, van der Giet M, Schwarzfeld C,
Laufer U, Liermann D and Zidek W: Prevention of
radiographic-contrast-agent-induced reductions in renal function by
acetylcysteine. N Engl J Med. 343:180–184. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shyu KG, Cheng JJ and Kuan P:
Acetylcysteine protects against acute renal damage in patients with
abnormal renal function undergoing a coronary procedure. J Am Coll
Cardiol. 40:1383–1388. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kay J, Chow WH, Chan TM, Lo SK, Kwok OH,
Yip A, Fan K, Lee CH and Lam WF: Acetylcysteine for prevention of
acute deterioration of renal function following elective coronary
angiography and intervention: A randomized controlled trial. JAMA.
289:553–558. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Chen J, Xiong C, Wei H, Yin C and
Ruan J: Apoptosis induction by the total flavonoids from
arachniodes exilis in HepG2 cells through reactive oxygen
species-mediated mitochondrial dysfunction involving MAPK
activation. Evid Based Complement Alternat Med. 2014:9069412014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yuan Y, Wang Y, Hu FF, Jiang CY, Zhang YJ,
Yang JL, Zhao SW, Gu JH, Liu XZ, Bian JC and Liu ZP: Cadmium
activates reactive oxygen species-dependent AKT/mTOR and
mitochondrial apoptotic pathways in neuronal cells. Biomed Environ
Sci. 29:117–126. 2016.PubMed/NCBI
|
|
32
|
Gao H, Li LY, Zhang M and Zhang Q:
Inactivated Sendai virus induces apoptosis mediated by reactive
oxygen species in murine melanoma cells. Biomed Environ Sci.
29:877–884. 2016.PubMed/NCBI
|
|
33
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep. 9:2265–2272.
2014.PubMed/NCBI
|