Introduction
Glioma, the most common form of brain malignancy, is
one a major contributor to cancer-associated deaths worldwide
(1). The strategies employed for
the diagnosis and treatment of glioma have improved over the past
several decades (2). However, the
prognosis of patients with glioma remains poor, with a survival
time of 12–18 months post-diagnosis (3). Therefore, the identification of the
exact mechanisms underlying the malignant phenotype of glioma cells
is required.
Neuroglobin (Ngb), a novel tumor-associated protein,
functions as an oncogene or tumor suppressor in human cancer.
Previous reports have demonstrated that overexpression of Ngb
enhances reactive oxygen species scavenging and reverses oxidative
stress-induced cell death in neuroblastoma cells (4–6). The
expression of Ngb is upregulated under hypoxic conditions in
glioblastoma cells and tumor xenografts, indicating a potential
role of Ngb in cancer cell survival in hypoxic microenvironments
(7,8). In addition to brain tumors, aberrant
expression of Ngb has also been reported in other types of
malignancies. For example, Ngb is reported to be overexpressed in
certain non-small cell lung cancer cases, particularly in squamous
cell carcinomas (9). Notably,
17β-estradiol induces Ngb upregulation, which renders cancer cells,
including MCF-7, HepG2, SK-N-BE, HeLa and DLD-1, resistant to
oxidative stress (10–13). However, Ngb expression is
downregulated in hepatocellular carcinoma tissues and its silencing
promotes the proliferation and cell cycle progression of cancer
cells (14). Our previous study
demonstrated that Ngb functions as an independent prognostic
biomarker for patients with glioma and promotes the growth of
cancer cells by suppressing apoptosis (15). However, the mechanisms underlying
the survival-enhancing effect of Ngb in glioma remains a challenge,
therefore, the present study aimed to investigate the effect and
mechanisms of Ngb in glioma.
The results of the present study demonstrated that
Ngb promoted the proliferation and inhibited the apoptosis of
glioma cells, which may occur through effects on the
phosphatidylinositol 3-kinase (PI3K)/AKT pathway. To the best of
our knowledge, the presents study is the first to indicate that Ngb
may be a potential therapeutic target for glioma.
Materials and methods
Cell culture and transfection
U87MG ATCC and U251MG human glioma cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA) and were cultivated in Dulbecco's modified Eagle's medium
(DMEM; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal calf serum (Gibco; Thermo Fisher
Scientific, Inc.) and antibiotics (100 units/ml penicillin and 100
µg/ml streptomycin; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
at 37°C in a humidified incubator containing 5% CO2.
Short hairpin RNA (shRNA) targeting Ngb
(5′-GUGAGUCCCUGCUCUACAU-3′) and non-targeting (NT) shRNA
(5′-GCCACACGAUUGCUGUCUU-3′) were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Vectors (1 µg) were
transfected into cells at 50–70% confluency using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. Retroviral vector
pMMP-Ngb was generated, packaged and transduced as previously
described (16). pMMP is a
MFG-based vector with modifications from the myeloproliferative
sarcoma virus (17) and primer
binding sequence (18) vector
systems. pMMP vector alone was used as the control for Ngb
overexpression experiments. AKT inhibitor, MK2206 (1 µM; 37°C for
48 h; Selleck Chemicals, Houston, TX, USA), and insulin-like growth
factor-1 (IGF-1; 10 ng/ml; 37°C for 48 h; Sigma-Aldrich; Merck
KGaA) were used to treat glioma cells 24 h post-transfection,
according to the manufacturer's protocol. Control cells were
treated with dimethylsulphoxide (DMSO; EMD Millipore, Billerica,
MA, USA).
Bioinformatics analysis
KEGG PathwayFinder by gene correlation analysis in
the R2: Genomics Analysis and Visualization Platform (http://r2.amc.nl) was performed to investigate the
association between Ngb and various signaling pathways according to
glioma data (GSE4290) (19).
MTT assay
Glioma cells (2×103 cells/well) were
seeded in 96-well plates containing 100 µl DMEM per well. U87 cells
were treated with either DMSO or MK2206 (1 µM; 37°C for 48 h) 24 h
following Ngb retrovirus infection. U251 cells were treated with
either DMSO or IGF-1 (10 ng/ml; 37°C for 48 h) 24 h
post-transfection with Ngb shRNA. Following transfection for 24,
48, 72 and 96 h time intervals, 10 µl of MTT was added into each
well and incubated at 37°C for 4 h. Subsequently, 150 µl DMSO was
added per well and the absorbance was determined using microplate
reader at 490 nm.
Colony formation assay
Glioma cells (1×103 cells/well) that were
transfected with the corresponding vectors were cultured in 6-well
plates and maintained at 37°C in humidified cell incubators
containing 5% CO2 for 14–21 days. U87 cells were treated
with either DMSO or MK2206 (1 µM; 37°C for 48 h) 24 h following
infection with Ngb retroviruses. U251 cells were treated with
either DMSO or IGF-1 (10 ng/ml; 37°C for 48 h) 24 h following
transfection with Ngb shRNA. The formed cell colonies were stained
with crystal violet (0.05%; 20 min at room temperature) and counted
to represent the cell proliferation of glioma cells.
Apoptosis analysis
Apoptosis in glioma cells following transfection was
detected by using an Annexin V/propidium iodide (PI) kit (BD
Pharmingen; BD Biosciences, San Jose, CA, USA). U87 cell were
treated with either DMSO or MK2206 (1 µM; 37°C for 48 h) 24 h
post-infection with Ngb retroviruses. U251 cells were treated with
DMSO or IGF-1 (10 ng/ml; 37°C for 48 h) 24 h post-transfection with
Ngb shRNA. Briefly, glioma cells were resuspended in 1X binding
buffer at a concentration of 1×106 cells/ml. A total of
100 µl of solution (1×105 cells) was transferred to a 5
ml culture tube and supplemented with 5 µl of Annexin V-fluorescein
isothiocyanate and 5 µl of PI. Following incubation for 15 min at
25°C in the dark and with supplementation of 1X binding buffer (400
µl), the percentage ratio of apoptotic glioma cells was detected
using FACSCalibur flow cytometer (BD Biosciences) and CellQuest Pro
software (version 5.1.1; BD Biosciences).
Western blotting
U87 cells were treated with either DMSO or MK2206 (1
µM; 37°C for 48 h) 24 h following infection with Ngb retroviruses.
U251 cells were treated with either DMSO or IGF-1 (10 ng/ml; 37°C
for 48 h) 24 h post-transfection with Ngb shRNA. The transfected
cells were lysed by radioimmunoprecipitation assay lysis buffer
(Beyotime Institute of Biotechnology, Haimen, China) and
phenylmethylsulfonyl fluoride (Beyotime Institute of Biotechnology)
for total protein extracts, followed by quantification with a
Bradford Protein assay kit (Beyotime Institute of Biotechnology).
Cell lysates (40 µg/lane) were separated by 10% SDS-PAGE. After
being transferred to polyvinylidene fluoride membranes
(Sigma-Aldrich; Merck KGaA) and blocked with 5% non-fat milk for 1
h at room temperature, the membranes were incubated with primary
antibodies against GAPDH (1:5,000; cat. no. G8140-01; US
Biological, Salem, MA, USA), Ngb (1:1,000; cat. no. ab37258; Abcam,
Cambridge, MA, USA), AKT (1:1,000; cat. no. 9272; Cell Signaling
Technology, Inc., Danvers, MA, USA), phosphorylated (p)-AKT
(Ser473; 1:2,000; cat. no. 4060; Cell Signaling Technology, Inc.),
mammalian target of rapamycin (mTOR; 1:1,000; cat. no. 2983; Cell
Signaling Technology, Inc.), p-mTOR (Ser2448; 1:1,000; cat. no.
5536; Cell Signaling Technology, Inc.), Bcl-2 (1:1,000; cat. no.
15071; Cell Signaling Technology, Inc.), Bcl-2-associated X (Bax;
1:1,000; cat. no. 5023; Cell Signaling Technology, Inc.), cleaved
poly(ADP-ribose) polymerase 1 (PARP; 1:1,000; cat. no. 5625; Cell
Signaling Technology, Inc.), cleaved caspase-3 (1:1,000, cat. no.
9664; Cell Signaling Technology, Inc.), cleaved caspase-7 (1:1,000;
cat. no. 8438; Cell Signaling Technology, Inc.) and cleaved
caspase-8 (1:1,000; cat. no. 9496; Cell Signaling Technology, Inc.)
at 4°C overnight. Subsequently, membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit and horse
anti-mouse secondary antibodies for 1 h at room temperature
(1:1,000; cat. nos. 7074 and 7076, respectively; Cell Signaling
Technology, Inc.). GAPDH was employed as a loading control.
Luminata Fe Western HRP Substrate (EMD Millipore, Billerica, MA,
USA) was used to visualize proteins. A Bio-Rad Gel imaging system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to
quantify western blotting data. Image J software (version 1.41;
National Institutes of Health, Bethesda, MD, USA) was used to
quantify protein levels.
Statistical analysis
Data are presented as the mean ± standard deviation
and were analyzed using GraphPad Prism 5 software (GraphPad
Software, Inc., La Jolla, CA, USA). Student's t-test or one-way
ANOVA followed by the post hoc Tukey's test were employed to
analyze continuous variables. P<0.05 was considered to indicate
a statistically significant difference.
Results
PI3K/AKT pathway is a candidate target
of Ngb in glioma
To investigate the potential mechanisms underlying
the survival-enhancing effect of Ngb in glioma, KEGG PathwayFinder
by gene correlation analysis using the R2: Genomics Analysis and
Visualization Platform (http://r2.amc.nl)
was performed to investigate the association between Ngb and
various signaling pathways in glioma. Base on the Gene Expression
Omnibus (GEO) data (GSE4290) from the R2: Genomics Analysis and
Visualization Platform, the results demonstrated that Ngb was
strongly associated with the PI3K/AKT pathway in glioma
(P<0.001; Table I). Therefore,
the PI3K/AKT pathway is a candidate target of Ngb in glioma.
 | Table I.KEGG PathwayFinder by Gene correlation
of the GSE4290 dataset. |
Table I.
KEGG PathwayFinder by Gene correlation
of the GSE4290 dataset.
| Group | In_Set | Total | Percentage, % | P-value |
|---|
|
Retrograde_endocannabinoid_signaling | 79 | 92 | 85.90 |
1.30×10−6 |
|
Nicotine_addiction | 33 | 35 | 94.30 |
6.10×10−5 |
|
PI3K_Akt_signaling_pathway | 167 | 225 | 74.20 |
6.70×10−5 |
|
GABAergic_synapse | 65 | 79 | 82.30 |
1.30×10−4 |
|
Synaptic_vesicle_cycle | 51 | 60 | 85.00 |
1.60×10−4 |
|
Glutamatergic_synapse | 83 | 106 | 78.30 |
3.20×10−4 |
|
Dopaminergic_synapse | 88 | 114 | 77.20 |
4.90×10−4 |
| Axon_guidance | 94 | 123 | 76.40 |
5.70×10−4 |
| DNA_replication | 32 | 36 | 88.90 |
6.70×10−4 |
|
Circadian_entrainment | 65 | 82 | 79.30 |
8.30×10−4 |
|
Morphine_addiction | 66 | 84 | 78.60 |
1.10×10−3 |
|
Long_term_potentiation | 49 | 60 | 81.70 |
1.20×10−3 |
| Spliceosome | 89 | 118 | 75.40 |
1.60×10−3 |
|
Cholinergic_synapse | 74 | 97 | 76.30 |
2.40×10−3 |
| Endocytosis | 167 | 236 | 70.80 |
2.80×10−3 |
|
Pancreatic_cancer | 52 | 66 | 78.80 |
3.50×10−3 |
|
Protein_processing_in_endoplasmic_reticulum | 110 | 151 | 72.80 |
3.50×10−3 |
|
Serotonergic_synapse | 67 | 88 | 76.10 |
4.20×10−3 |
|
Amphetamine_addiction | 46 | 58 | 79.30 |
4.80×10−3 |
|
Oxytocin_signaling_pathway | 102 | 140 | 72.90 |
4.90×10−3 |
|
Long_term_depression | 45 | 57 | 78.90 |
6.20×10−3 |
|
Amyotrophic_lateral_sclerosis__ALS_ | 40 | 50 | 80.00 |
6.60×10−3 |
|
Aldosterone_synthesis_and_secretion | 50 | 65 | 76.90 |
9.60×10−3 |
|
Fc_gamma_R_mediated_phagocytosis | 63 | 84 | 75.00 |
9.80×10−3 |
| Hepatitis_B | 98 | 136 | 72.10 |
9.90×10−3 |
Ngb regulates the PI3K/AKT pathway in
glioma cells
Subsequently, the present study investigated the
activation of the PI3K/AKT pathway following modulation of Ngb
levels by transfection. Ngb was silenced in U251 cells following
transfection with shRNA targeting Ngb, compared with the NT shRNA
group, as protein levels were reduced (P<0.05; Fig. 1). In addition, Ngb knockdown
notably reduced the level of p-AKT (Ser473) compared with the NT
shRNA group, without affecting total AKT levels, in U251 cells
(P<0.05; Fig. 1). Furthermore,
p-mTOR (Ser2448), Bcl-2 and Bax, which are downstream targets of
the PI3K/ATK pathway, were also modulated by Ngb knockdown. Ngb
knockdown led to reduced levels of p-mTOR (Ser2448) and Bcl-2, and
increased BAX expression, in U251 cells, compared with the NT shRNA
group (P<0.05; Fig. 1). By
contrast, Ngb overexpression promoted the activation of the
PI3K/AKT pathway, with increased levels of p-AKT (Ser473), p-mTOR
(Ser2448) and Bcl-2, and decreased BAX expression, in U87 cells,
compared with the empty vector control cells (P<0.05; Fig. 1). These data indicate that Ngb may
promote the activation of the PI3K/AKT pathway in glioma cells.
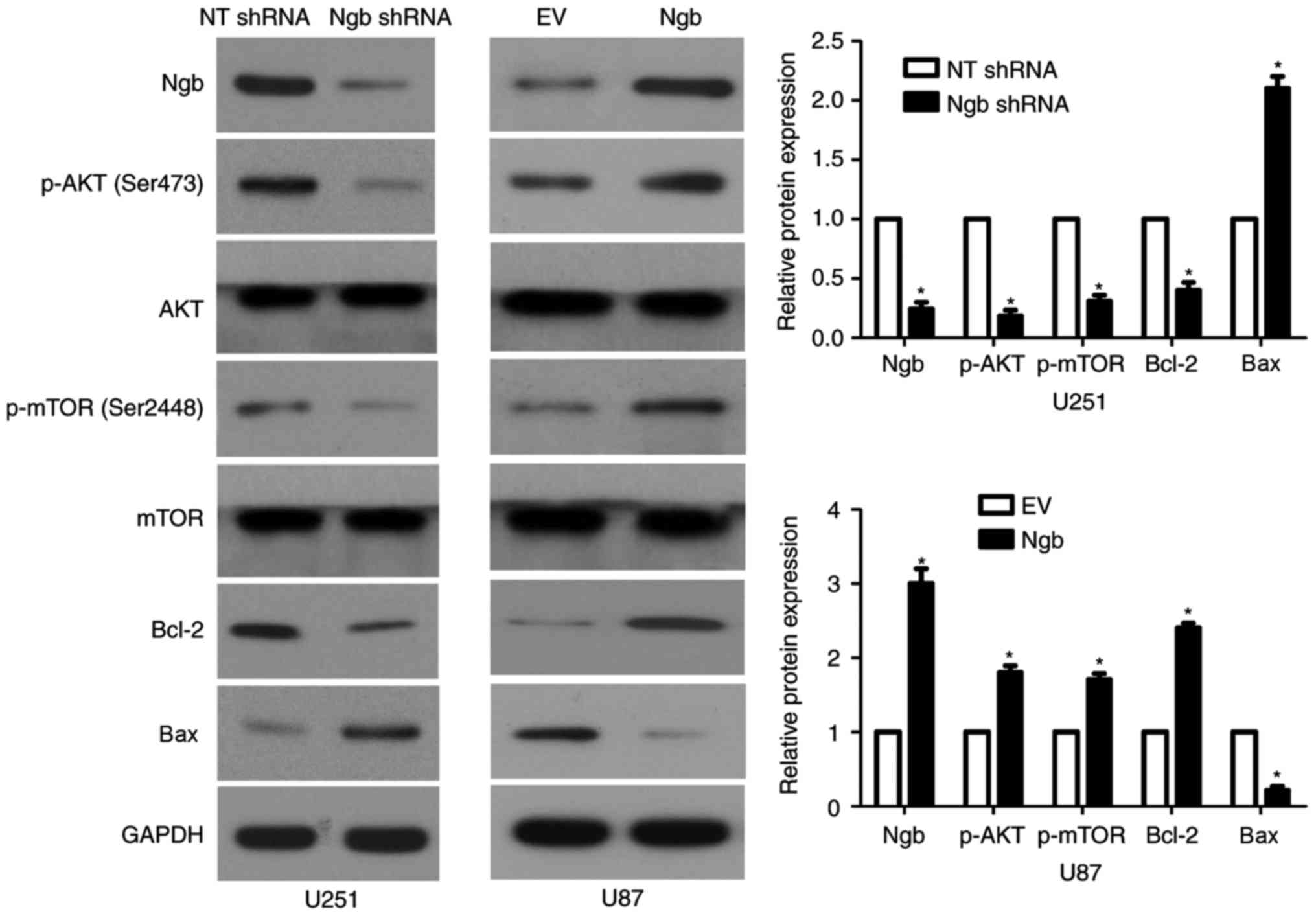 | Figure 1.Ngb regulates the activation of the
PI3K/AKT pathway in glioma cells. U251 cells that were transfected
with NT shRNA and Ngb shRNA were subjected to western blot
analysis. Ngb knockdown led to decreased levels of p-AKT, p-mTOR
and Bcl-2, and increased Bax expression, in U251 cells. U87 cells
that were transfected with EV and Ngb overexpression vector were
subjected to western blot analysis. Ngb overexpression enhanced the
activation of the PI3K/AKT pathway in U87 cells, as p-AKT levels
were increased compared with the EV group. *P<0.05 vs. NT shRNA
group for U251 cells; *P<0.05 vs. EV group for U87 cells. PI3K,
phosphatidylinositol 3-kinase; NT, non-targeting; shRNA, short
hairpin RNA; Ngb, neuroglobin; p-, phosphorylated-; mTOR, mammalian
target of rapamycin; Bax, Bcl-2-associated X; EV, empty vector. |
Ngb promotes cellular malignant
phenotypes of glioma by targeting the PI3K/AKT pathway
The present study further investigated whether Ngb
regulated the proliferation and apoptosis of glioma cells through
targeting the PI3K/AKT pathway. Overexpression of Ngb led to
increased p-AKT expression, and reduced levels of cleaved PARP and
cleaved caspase-3/7/8, in U87 cells, compared with cells
transfected with empty vector (P<0.05; Fig. 2A). Functionally, Ngb overexpression
promoted the proliferation and reduced the apoptosis of U87 cells,
compared with cells transfected with empty vector (P<0.05;
Fig. 2B-D). An AKT inhibitor,
MK2206, was employed to block the activation of PI3K/AKT in
Ngb-overexpressing U87 cells. MK2206 treatment led to reduced p-AKT
expression and increased levels of cleaved PARP, cleaved caspase-3,
cleaved caspase-7 and cleaved caspase-8 in Ngb-overexpressing U87
cells (P<0.05; Fig. 2A).
Furthermore, MK2206 treatment reduced the proliferation and induced
the apoptosis of Ngb-overexpressing U87 cells (P<0.05; Fig. 2B-D). Ngb knockdown using shRNA led
to reduced expression of p-AKT, and increased levels of cleaved
PARP and cleaved caspase-3/7/8, in U251 cells, compared with cells
transfected with NT shRNA (P<0.05; Fig. 3A). In addition, Ngb knockdown
reduced the proliferation and induced the apoptosis of U251 cells,
compared with cells transfected with NT shRNA (P<0.05; Fig. 3B-D). IGF-1, an activator of the
PI3K/AKT pathway, increased p-AKT levels, and decreased cleaved
PARP and cleaved caspase-3/7/8 expression, in Ngb-knockdown U251
cells (P<0.05; Fig. 3A).
Furthermore, IGF-1 treatment resulted in enhanced proliferation and
reduced apoptosis in Ngb-knockdown U251 cells (P<0.05; Fig. 3A-D). Therefore, these results
further confirm that Ngb may promote a cellular malignant phenotype
in glioma, which may occur via the PI3K/AKT pathway.
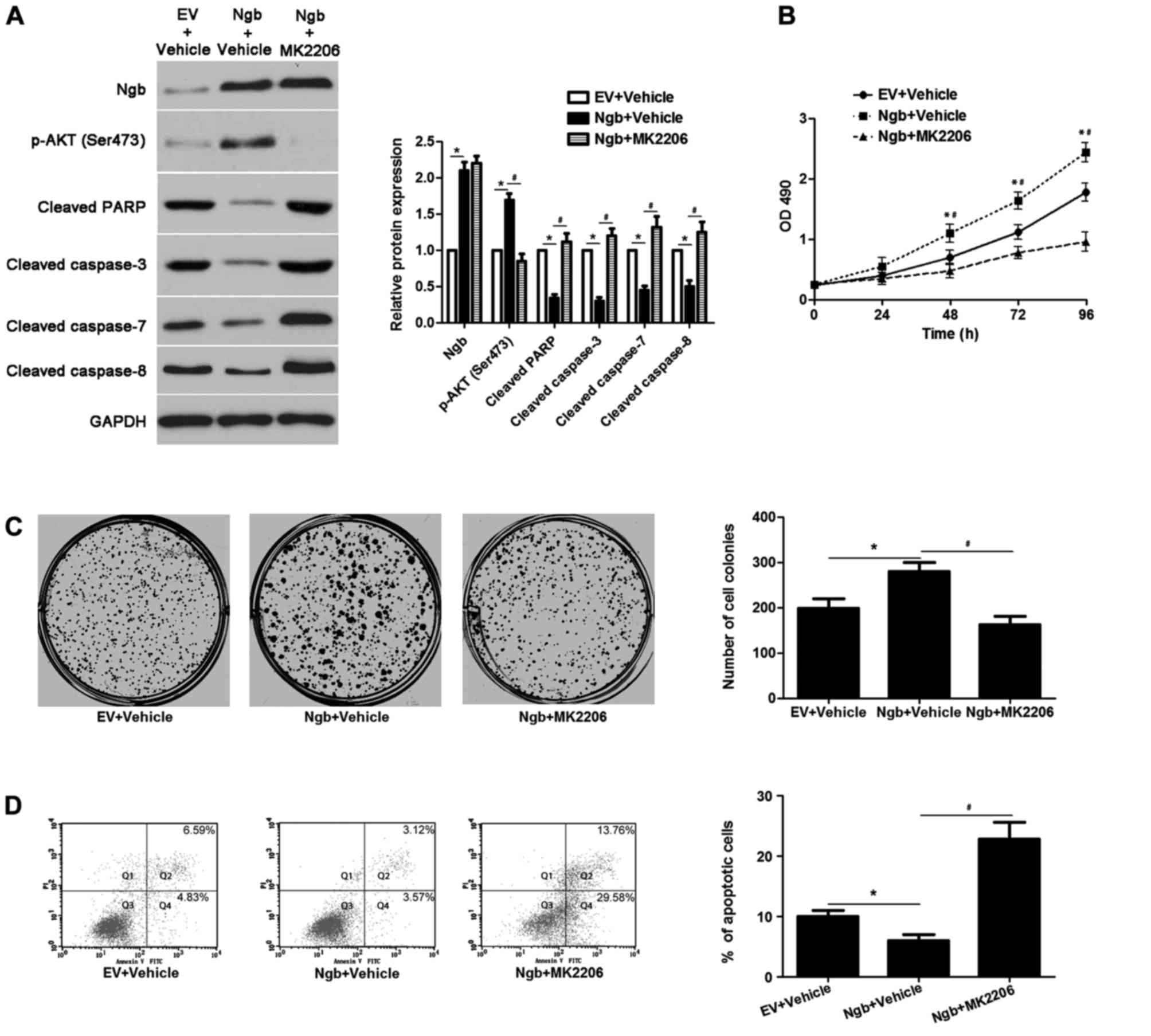 | Figure 2.MK2206 treatment abolishes the effects
of Ngb in U87 cells. (A) Ngb-overexpressing U87 cells that were
treated with vehicle or the AKT inhibitor MK2206, respectively,
were detected by western blotting. MK2206 treatment reduced p-AKT
(Ser473) expression, and increased the levels of cleaved PARP and
cleaved caspase-3/7/8, without affecting Ngb expression. *P<0.05
vs. EV + vehicle group; #P<0.05 vs. Ngb + vehicle
group, as indicated; n=3. (B) MK2206 treatment restrained the
proliferation of Ngb-overexpressing U87 cells. *P<0.05 vs. EV +
vehicle group; #P<0.05 vs. Ngb + vehicle group; n=3.
(C) The colony formation ability of Ngb-overexpressing U87 cells
was weakened by MK2206 treatment. *P<0.05 vs. EV + vehicle
group; #P<0.05 vs. Ngb + vehicle group; n=3. (D) The
proportion of apoptotic cells was increased following MK2206
treatment in Ngb-overexpressing U87 cells. Q2 and Q4 quadrants were
considered to indicate apoptotic cells. *P<0.05 vs. EV + vehicle
group; #P<0.05 vs. Ngb + vehicle group; n=3. Ngb,
neuroglobin; p-, phosphorylated-; PARP, poly(ADP-ribose) polymerase
1; EV, empty vector; OD, optical density; PI, propidium iodide;
FITC, fluorescein isothiocyanate. |
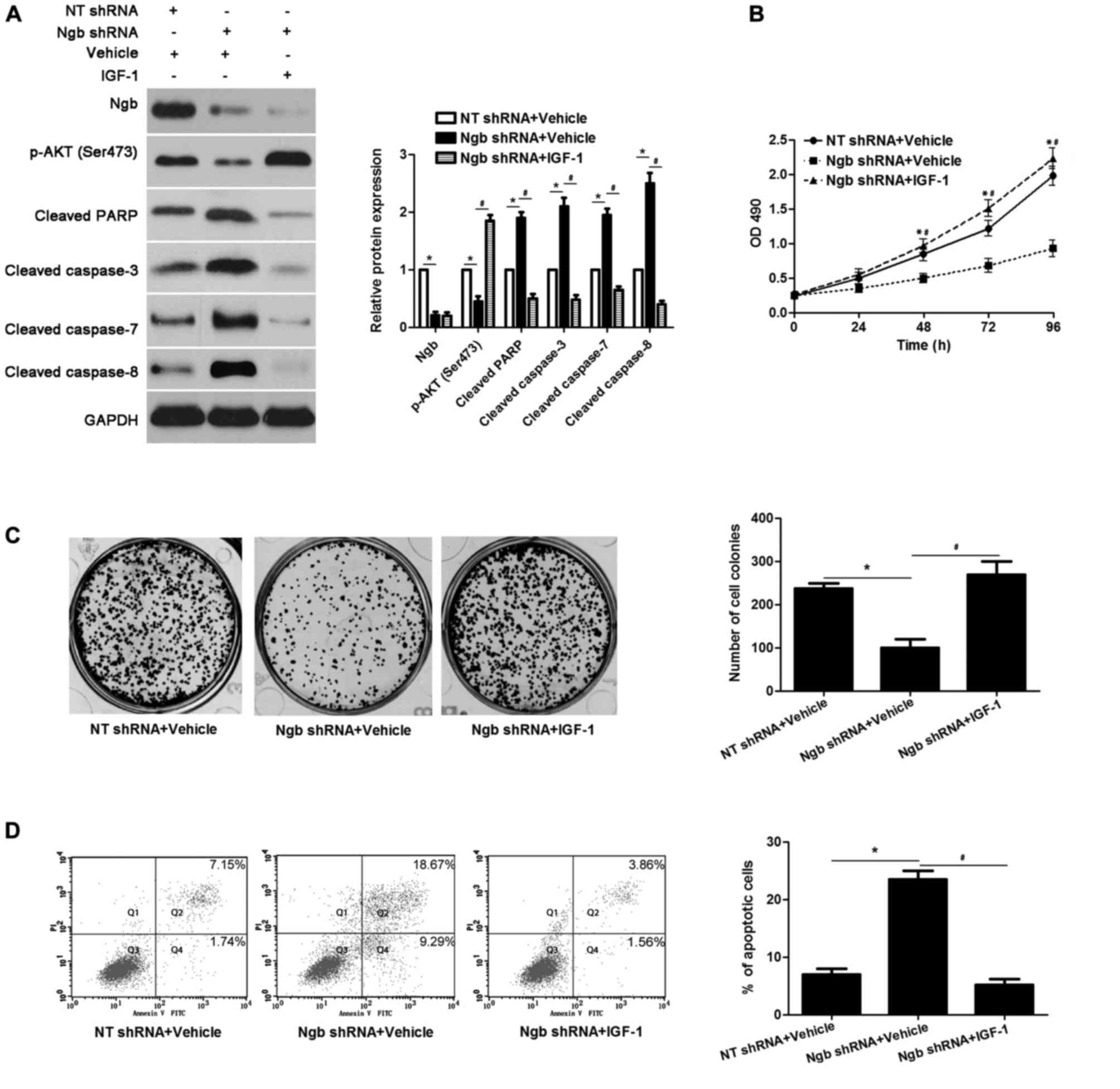 | Figure 3.IGF-1 treatment reverses the effects
of Ngb knockdown in U251 cells. (A) U251 cells with knockdown of
Ngb using shRNA were treated with IGF-1, which is an activator of
the PI3K/AKT pathway, or vehicle. Western blotting revealed that
IGF-1 treatment enhanced the activation of the PI3K/AKT pathway,
and reduced levels of cleaved PARP and cleaved caspase-3/7/8, in
Ngb-knockdown U251 cells. *P<0.05 vs. NT shRNA + vehicle group;
#P<0.05 vs. Ngb shRNA + vehicle group, as indicated;
n=3. (B) IGF-1 treatment facilitated the proliferation of
Ngb-knockdown U251 cells. *P<0.05 vs. NT shRNA + vehicle group;
#P<0.05 vs. Ngb shRNA + vehicle group; n=3. (C) The
colony formation ability in Ngb-knockdown U251 cells was increased
following IGF-1 treatment. *P<0.05 vs. NT shRNA + vehicle group;
#P<0.05 vs. Ngb shRNA + vehicle group, as indicated;
n=3. (D) IGF-1 treatment significantly decreased the apoptosis of
Ngb-knockdown U251 cells. Q2 and Q4 quadrants were considered to
indicate apoptotic cells. *P<0.05 vs. NT shRNA + vehicle group;
#P<0.05 vs. Ngb shRNA + vehicle group; n=3. IGF-1,
insulin-like growth factor-1; Ngb, neuroglobin; shRNA, short
hairpin RNA; PI3K, phosphatidylinositol 3-kinase; PARP,
poly(ADP-ribose) polymerase 1; NT shRNA; non-targeting shRNA; p-,
phosphorylated-; OD, optical density; PI, propidium iodide; FITC,
fluorescein isothiocyanate. |
Discussion
The expression and role of Ngb in human cancer is a
novel and controversial topic. Several studies have reported that
Ngb may protect against oxidative stress-induced cell injury in
brain cancer (4,5,7). In
addition, our previous study demonstrated that Ngb was
overexpressed in glioma tissues compared with normal brain tissues,
and its overexpression was associated with poor prognostic features
and shorter overall survival (15). Ngb has also been reported to
promote the proliferation and inhibit the apoptosis of glioma cells
in vitro and in vivo (15). However, the potential mechanisms
underlying the effects of Ngb in glioma are yet to be established.
The present study investigated the molecular mechanisms involved in
the antiapoptotic effect of Ngb in glioma cells. KEGG PathwayFinder
by gene expression analysis using the R2: Genomics Analysis and
Visualization Platform revealed that Ngb was associated with the
PI3K/AKT pathway in glioma tissues from the GSE4290 dataset of the
GEO database. Further experiments in the current study demonstrated
that Ngb enhanced the activation of the PI3K/AKT pathway in glioma
cells.
Aberrant activation of the PI3K/AKT pathway has been
widely reported in various cancers types during progression,
including glioma (20–22). The PI3K/AKT pathway has roles in
the cell proliferation, cell cycle progression and apoptosis
resistance of glioma cells via its downstream targets, which
include mTOR, Bcl-2, Bax, cyclin D1 and Bcl-2-like 1 (20,23–26).
mTOR was reported to have an essential role in the cell survival,
proliferation and apoptosis of glioma cells (27,28).
Bcl-2 is an antiapoptotic protein, while Bax is a proapoptotic
factor. Altered Bcl-2/Bax expression was associated with altered
apoptosis levels glioma cells (29). In the present study, treatment with
the AKT inhibitor MK2206 blocked the activation of the PI3K/AKT
pathway, and subsequently resulted in decreased proliferation and
increased apoptosis in Ngb-overexpressing U87 cells. Furthermore,
IGF-1 treatment in U251 cells with Ngb knockdown enhanced the
PI3K/AKT pathway activation, cell proliferation and apoptosis
resistance. Therefore, Ngb may promote malignant phenotypes of
glioma cells by targeting the PI3K/AKT pathway.
In conclusion, the results of the present study
demonstrated that Ngb enhanced the activation of the PI3K/AKT
pathway in glioma cells. Furthermore, Ngb regulated the
proliferation and apoptosis of glioma cells, and these effects may
occur via the PI3K/AKT pathway. Therefore, Ngb may serve as a
potential target for the treatment of glioma.
Acknowledgements
The present study was supported by the Scientific
Research Plan Projects of Shaanxi Education Department (grant nos.
14JK1629, 2010JK811 and 11JK0715) and the Edge Discipline
Construction Project in Shaanxi Province.
References
|
1
|
Awad AJ, Burns TC, Zhang Y and Abounader
R: Targeting MET for glioma therapy. Neurosurg Focus. 37:E102014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huse JT and Aldape KD: The evolving role
of molecular markers in the diagnosis and management of diffuse
glioma. Clin Cancer Res. 20:5601–5611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chistiakov DA and Chekhonin VP:
Extracellular vesicles shed by glioma cells: Pathogenic role and
clinical value. Tumour Biol. 35:8425–8438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fordel E, Thijs L, Martinet W, Lenjou M,
Laufs T, Van Bockstaele D, Moens L and Dewilde S: Neuroglobin and
cytoglobin overexpression protects human SH-SY5Y neuroblastoma
cells against oxidative stress-induced cell death. Neurosci Lett.
410:146–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fordel E, Thijs L, Martinet W, Schrijvers
D, Moens L and Dewilde S: Anoxia or oxygen and glucose deprivation
in SH-SY5Y cells: A step closer to the unraveling of neuroglobin
and cytoglobin functions. Gene. 398:114–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peroni D, Negro A, Bähr M and Dietz GP:
Intracellular delivery of Neuroglobin using HIV-1 TAT protein
transduction domain fails to protect against oxygen and glucose
deprivation. Neurosci Lett. 421:110–114. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Emara M, Salloum N and Allalunis-Turner J:
Expression and hypoxic up-regulation of neuroglobin in human
glioblastoma cells. Mol Oncol. 3:45–53. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Emara M, Turner AR and Allalunis-Turner J:
Hypoxic regulation of cytoglobin and neuroglobin expression in
human normal and tumor tissues. Cancer Cell Int. 10:332010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oleksiewicz U, Daskoulidou N, Liloglou T,
Tasopoulou K, Bryan J, Gosney JR, Field JK and Xinarianos G:
Neuroglobin and myoglobin in non-small cell lung cancer:
Expression, regulation and prognosis. Lung Cancer. 74:411–418.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiocchetti M, Nuzzo MT, Totta P, Acconcia
F, Ascenzi P and Marino M: Neuroglobin, a pro-survival player in
estrogen receptor α-positive cancer cells. Cell Death Dis.
5:e14492014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fiocchetti M, Cipolletti M, Leone S,
Naldini A, Carraro F, Giordano D, Verde C, Ascenzi P and Marino M:
Neuroglobin in breast cancer cells: Effect of hypoxia and oxidative
stress on protein level, localization, and anti-apoptotic function.
PLoS One. 11:e01549592016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fiocchetti M, Cipolletti M, Leone S,
Ascenzi P and Marino M: Neuroglobin overexpression induced by the
17β-Estradiol-Estrogen receptor-α pathway reduces the sensitivity
of MCF-7 breast cancer cell to paclitaxel. IUBMB Life. 68:645–651.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fiocchetti M, Camilli G, Acconcia F, Leone
S, Ascenzi P and Marino M: ERβ-dependent neuroglobin up-regulation
impairs 17β-estradiol-induced apoptosis in DLD-1 colon cancer cells
upon oxidative stress injury. J Steroid Biochem Mol Biol.
149:128–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Lan SJ, Liu QR, Liu JM and Chen
XQ: Neuroglobin, a novel intracellular hexa-coordinated globin,
functions as a tumor suppressor in hepatocellular carcinoma via
Raf/MAPK/Erk. Mol Pharmacol. 83:1109–1119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang B, Chang M, Wang J and Liu Y:
Neuroglobin functions as a prognostic marker and promotes the tumor
growth of glioma via suppressing apoptosis. Biomed Pharmacother.
88:173–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu K, Li J, Verma VK, Liu C, Billadeau DD,
Lamprecht G, Xiang X, Guo L, Dhanasekaran R, Roberts LR, et al:
Vasodilator-stimulated phosphoprotein promotes activation of
hepatic stellate cells by regulating Rab11-dependent plasma
membrane targeting of transforming growth factor beta receptors.
Hepatology. 61:361–374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Riviere I, Brose K and Mulligan RC:
Effects of retroviral vector design on expression of human
adenosine deaminase in murine bone marrow transplant recipients
engrafted with genetically modified cells. Proc Natl Acad Sci USA.
92:pp. 6733–6737. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Colicelli J and Goff SP: Isolation of a
recombinant murine leukemia virus utilizing a new primer tRNA. J
Virol. 57:37–45. 1986.PubMed/NCBI
|
|
19
|
Sun L, Hui AM, Su Q, Vortmeyer A,
Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey
R, et al: Neuronal and glioma-derived stem cell factor induces
angiogenesis within the brain. Cancer Cell. 9:287–300. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu M and Wang J, Qi Q, Huang B, Chen A,
Li X and Wang J: Nitidine chloride inhibits the malignant behavior
of human glioblastoma cells by targeting the PI3K/AKT/mTOR
signaling pathway. Oncol Rep. 36:2160–2168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang J, Yang Q, Yu J, Li X, Yu S and Zhang
X: SPOCK1 promotes the proliferation, migration and invasion of
glioma cells through PI3K/AKT and Wnt/β-catenin signaling pathways.
Oncol Rep. 35:3566–3576. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu JS and Cui W: Proliferation, survival
and metabolism: The role of PI3K/AKT/mTOR signalling in
pluripotency and cell fate determination. Development.
143:3050–3060. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luo M, Liu Q, He M, Yu Z, Pi R, Li M, Yang
X, Wang S and Liu A: Gartanin induces cell cycle arrest and
autophagy and suppresses migration involving PI3K/Akt/mTOR and MAPK
signalling pathway in human glioma cells. J Cell Mol Med. 21:46–57.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zanotto-Filho A, Braganhol E, Battastini
AM and Moreira JC: Proteasome inhibitor MG132 induces selective
apoptosis in glioblastoma cells through inhibition of PI3K/Akt and
NFkappaB pathways, mitochondrial dysfunction, and activation of
p38-JNK1/2 signaling. Invest New Drugs. 30:2252–2262. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yu Z, Xie G, Zhou G, Cheng Y, Zhang G, Yao
G, Chen Y, Li Y and Zhao G: NVP-BEZ235, a novel dual PI3K-mTOR
inhibitor displays anti-glioma activity and reduces chemoresistance
to temozolomide in human glioma cells. Cancer Lett. 367:58–68.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding L, Ding L, Wang S, Wang S, Wang W,
Wang W, Lv P, Lv P, Zhao D, Zhao D, et al: Tanshinone IIA affects
autophagy and apoptosis of glioma cells by inhibiting
phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin
signaling pathway. Pharmacology. 99:188–195. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao N, Guo Y, Zhang M, Lin L and Zheng Z:
Akt-mTOR signaling is involved in Notch-1-mediated glioma cell
survival and proliferation. Oncol Rep. 23:1443–1447.
2010.PubMed/NCBI
|
|
28
|
Koul N, Sharma V, Dixit D, Ghosh S and Sen
E: Bicyclic triterpenoid Iripallidal induces apoptosis and inhibits
Akt/mTOR pathway in glioma cells. BMC Cancer. 10:3282010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang P, Zhen H, Jiang X, Zhang W, Cheng X,
Guo G, Mao X and Zhang X: Boron neutron capture therapy induces
apoptosis of glioma cells through Bcl-2/Bax. BMC Cancer.
10:6612010. View Article : Google Scholar : PubMed/NCBI
|

















