Introduction
Osteosarcoma (OS), also termed bone sarcoma,
originates from bone and particularly from the mesenchymal stem
cell lineage (1). OS, the most
common bone tumor, is highly aggressive and usually has poor
prognosis (2). Additionally, OS
primarily affects adolescents and children and ~60% of neoplasms
occur in patients under the age of 20 (3,4).
Current treatment frequently involves a combination of surgery and
chemotherapy; however, OS still leads to a high mortality and
morbidity, particularly in children and adolescents (1).
Currently, considerable progress has been made in
identifying the critical factors in the development and progression
of OS, including genes, pathways and copy number variants (CNVs)
(5). Alterations of tumor
suppressor gene expression including protein kinase,
cAMP-dependent, regulatory, and type I a and deregulation of major
signaling pathways such as the wingless-type MMTV integration site
family, transforming growth factor-β, Notch and sonic hedgehog have
been previously associated with OS (6,7). It
has also been previously demonstrated that OS development is
dependent on loss of P53 and enhanced by loss of retinoblastoma 1
(RB1) (8). CNVs are DNA segments 1
kb in length which are present in a variable population frequency
in the genome (9). During the
1990s, CNVs with duplications and deletions were expressed as an
inducement of a quantity of single gene disorders (10). Various differentially expressed
genes (DEGs) and several candidate CNVs in OS have been identified
to be involved in the development of OS by analyzing the microarray
data and high-resolution single nucleotide polymorphism (SNP)/CNV
arrays (11,12). However, frequently only one of
these approaches has been used in previous studies to identify the
candidate molecule, and the molecular mechanism of OS remains to be
elucidated (9–11).
The proto-oncogene MET protein, a hepatocyte growth
factor receptor, encodes tyrosine-kinase activity (13), which has been revealed to be
aberrantly expressed in OS and closely associated with cancer
(14–16). Therefore, overexpression of the MET
oncogene may convert human primary osteoblasts (HOB) into OS
cells.
The present study extracted the transcriptional and
CNV profiles from Gene Expression Omnibus (GEO) database. The
differentially expressed genes (DEGs) and CNVs were screened in the
transcriptional profile of MET-HOB cells, which were previously
turned into OS cells by lentiviral vector (LV)-driven
overexpression of the MET oncogene. Subsequently, the shared genes
in the expression and CNV profiles were analyzed. The present study
obtained a series of candidate markers in OS and may provide the
foundation for treatment of OS.
Materials and methods
Microarray and CNV data
Transcriptional profile (ID: GSE28256) was
downloaded from the GEO database (www.ncbi.nlm.nih.gov/geo/) which was based on the
platform of GPL6098 (Illumina humanRef-8 v1.0 expression beadchip)
(17). The dataset contained 15
samples, including 6 HOB cell lines and 9 MET-HOBs clones, which
were previously turned into osteosarcoma cells by over-expression
of MET oncogene driven by a LV. The CNV data were extracted from
the GSE32964 dataset in the GEO database, which included 36 samples
for detecting SNP and 32 samples for CNV. A total of 32 CNV samples
of OS tumor tissues based on the platform of GPL6985 (Illumina
HumanCNV370-QuadV3 DNA Analysis BeadChip) were analyzed in the
present study.
Data preprocessing
The probe-level data of the transcriptional profile
were initially converted into expression values. Probes that mapped
with the gene names labeled in the annotation platform were
transformed using log2 and normalized using
preprocess-Core package in R version 2.9.0. According to the
annotation platform, the values of probes corresponding to the same
transcript were averaged and then defined as the final expression
value of a transcript. The PennCNV tool (version 2014 May 07;
http://penncnv.openbioinformatics.org) was used in the
subsequent processing of data, the profile of CNV samples was
converted into specific format for PennCNV, which contained log R
Ratio: LRR and B Allele frequency: BAF. In addition, to investigate
the differences among samples, a heatmap was generated to compare
their expression values using the Gplots package in R.
Identification of DEGs and CNV
A Student's t-test was conducted on the gene
expression values between testing and control samples. The Linear
Models for Microarray Data (LIMMA) package was used to normalize
the data and identify the DEGs in MET-HOB samples compared with
control samples using cut-offs of P<0.05 and |log2
fold-change (FC)|>2. Additionally, detect_cnv.pl in PennCNV was
applied to select CNV areas and CNVs were identified with the
cut-off criteria of >30% overlap within the cases. Genes shared
in CNVs and DEGs were identified as critical genes associated with
the development of OS.
Functional enrichment of DEGs
The Database for Annotation, Visualization and
Integrated Discovery (DAVID) provides numerous comprehensive
functional annotation which contributes to the understanding of the
biological meanings behind abundant genes (18). Gene Ontology (GO) and the Kyoto
Encyclopedia of Genes and Genomes (KEGG) analyses have become
commonly used approaches for functional and pathway studies of
large-scale genomic or transcription data, respectively (19). Therefore, they were used in the
present study. Next, DAVID was used to screen the enriched GO terms
and KEGG pathways in the DEGs. P<0.05 was used as a cut-off
criterion.
Results
Data preprocessing and DEGs
screening
In the present study, 24,350 probes were detected in
the original data and 21,454 non-redundant genes were obtained
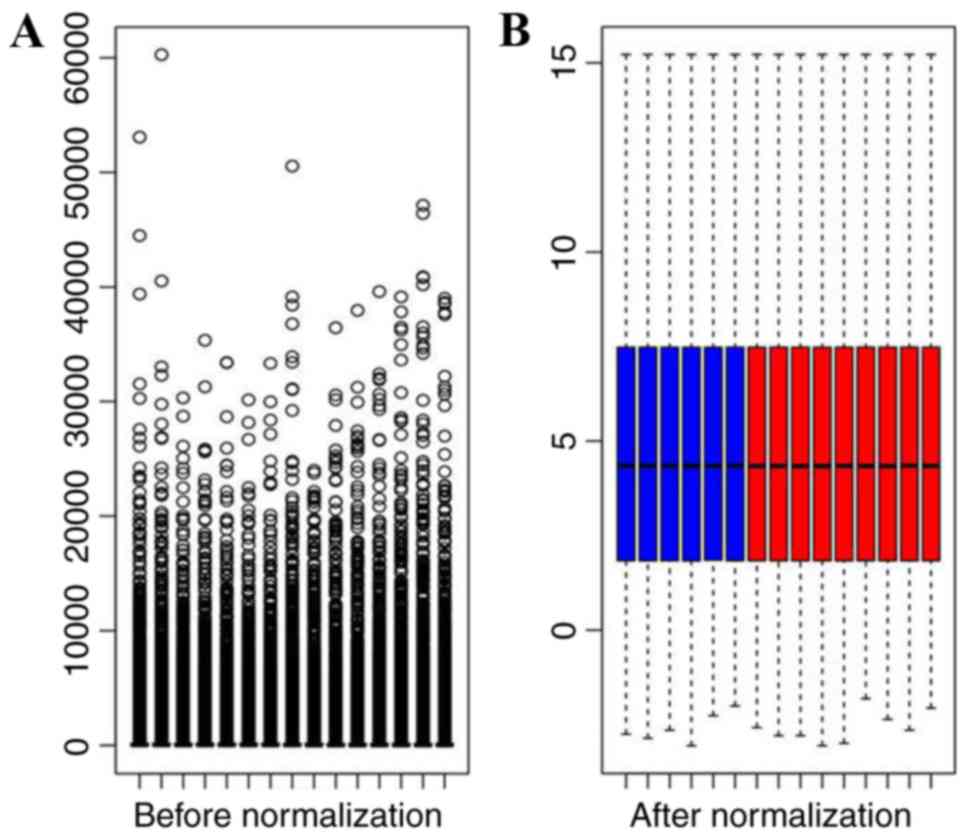
following data preprocessing. The raw data in all samples have been
normalized (Fig. 1). A total of
1601 DEGs were screened out in MET-HOBs compared with controls.
Among these genes, 784 were upregulated in MET-HOBs and 817 were
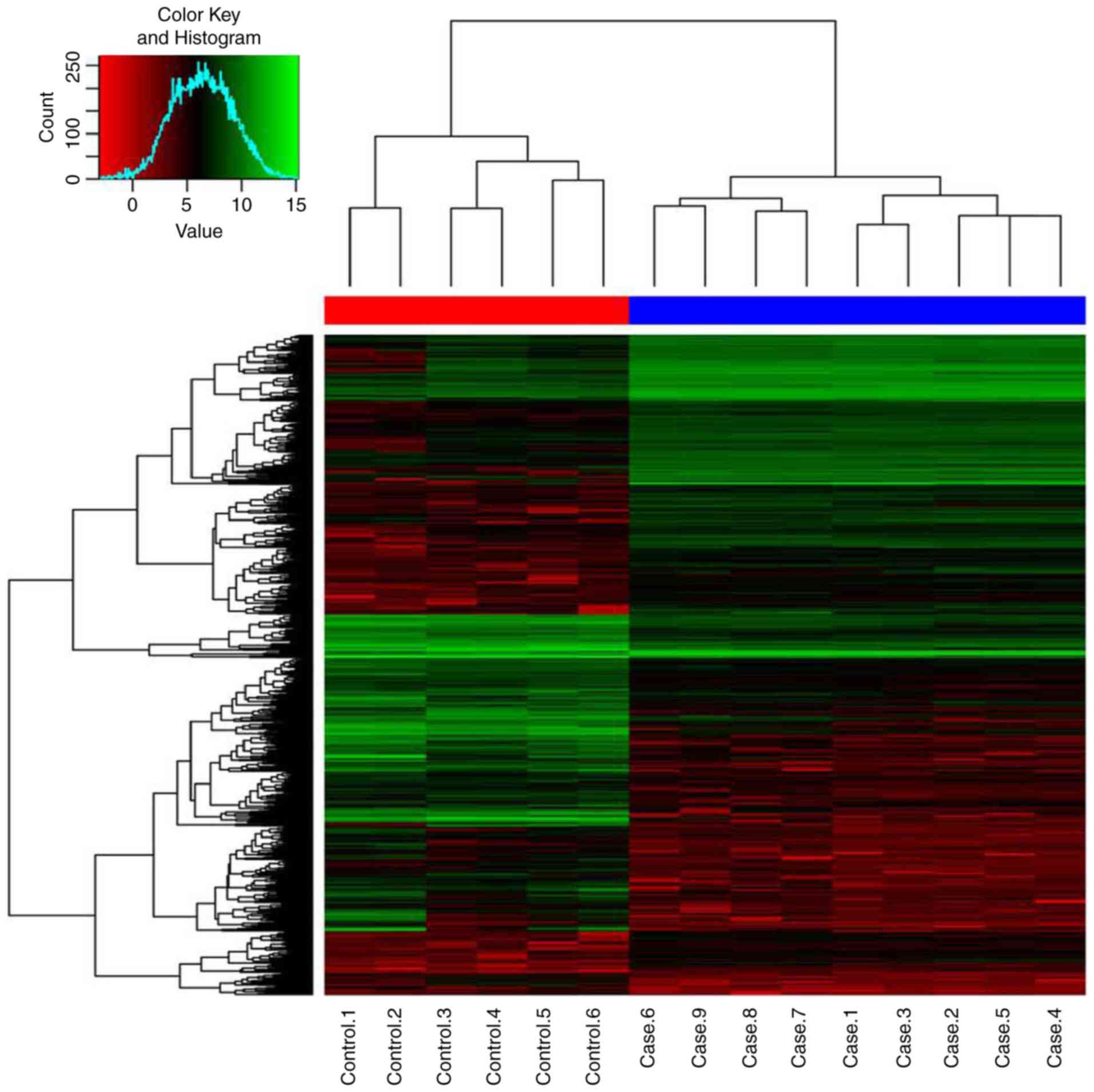
downregulated. The hierarchical clustering analysis revealed a
clearly distinct expression of all DEGs between MET-HOBs and HOBs
(Fig. 2).
Function enrichment of DEGs
In order to identify the functions of the DEGs, they
were performed GO (P<0.01) and KEGG (P<0.01) enrichment
analyses. The results indicated that 344 GO terms were obtained and
the top 10% terms were listed in Table
I, such as extracellular region (P=2.68E-24), extracellular
matrix (ECM; P=4.08E-24) and proteinaceous extracellular matrix
(P=4.79E-24). Besides, 14 KEGG pathways were obtained and most of
them were related to cancers, such as hsa05200: pathways in cancer
(P=4.12E-09), hsa04512: ECM-receptor interaction (P=2.41E-08) and
hsa05222: small cell lung cancer (P=1.06E-05; Table II).
 | Table I.The top 10% enriched GO terms for
DEGs. |
Table I.
The top 10% enriched GO terms for
DEGs.
| Category | GO ID | GO name | Gene number | P-value |
|---|
| CC | GO:0044421 | Extracellular
region part | 134 |
2.68×10−24 |
| CC | GO:0031012 | Extracellular
matrix | 74 |
4.08×10−24 |
| CC | GO:0005578 | Proteinaceous
extracellular matrix | 71 |
4.79×10−24 |
| BP | GO:0001501 | Skeletal system
development | 60 |
4.57×10−15 |
| MF | GO:0019838 | Growth factor
binding | 32 |
2.32×10−14 |
| BP | GO:0001944 | Vasculature
development | 50 |
1.22×10−13 |
| BP | GO:0001568 | Blood vessel
development | 49 |
1.88×10−13 |
| MF | GO:0005201 | Extracellular
matrix structural constituent | 28 |
2.11×10−13 |
| BP | GO:0042127 | Regulation of cell
proliferation | 98 |
4.21×10−12 |
| BP | GO:0006260 | DNA
replication | 40 |
8.03×10−12 |
| BP | GO:0007049 | Cell cycle | 96 |
1.09×10−11 |
| BP | GO:0051726 | Regulation of cell
cycle | 55 |
1.23×10−11 |
| BP | GO:0022403 | Cell cycle
phase | 63 |
1.57×10−11 |
| CC | GO:0005576 | Extracellular
region | 179 |
1.87×10−11 |
| CC | GO:0044427 | Chromosomal
part | 57 |
2.60×10−11 |
| BP | GO:0051270 | Regulation of cell
motion | 39 |
5.52×10−11 |
| BP | GO:0006259 | DNA metabolic
process | 70 |
8.61×10−11 |
| CC | GO:0044420 | Extracellular
matrix part | 28 |
1.55×10−10 |
| BP | GO:0040012 | Regulation of
locomotion | 38 |
1.89×10−10 |
| BP | GO:0051301 | Cell division | 49 |
1.93×10−10 |
| BP | GO:0030334 | Regulation of cell
migration | 35 |
3.13×10−10 |
| BP | GO:0007155 | Rell adhesion | 85 |
5.11×10−10 |
| BP | GO:0022610 | Biological
adhesion | 85 |
5.66×10−10 |
| CC | GO:0005615 | Extracellular
space | 79 |
6.44×10−10 |
| BP | GO:0006928 | Cell motion | 65 |
6.97×10−10 |
| BP | GO:0022402 | Cell cycle
process | 73 |
7.17×10−10 |
| CC | GO:0005694 | Chromosome | 60 |
1.09×10−9 |
| BP | GO:0000278 | Mitotic cell
cycle | 54 |
2.53×10−9 |
| BP | GO:0000279 | M phase | 49 |
7.98×10−9 |
| BP | GO:0048514 | Blood vessel
morphogenesis | 37 |
1.03×10−8 |
| BP | GO:0065004 | Protein-DNA complex
assembly | 23 |
1.29×10−8 |
| CC | GO:0005581 | Collagen | 14 |
1.97×10−8 |
| BP | GO:0030198 | Extracellular
matrix organization | 24 |
3.69×10−8 |
| BP | GO:0008283 | Cell
proliferation | 57 |
4.64×10−8 |
 | Table II.Enriched Kyoto Encyclopedia of Genes
and Genomes pathways for DEGs. |
Table II.
Enriched Kyoto Encyclopedia of Genes
and Genomes pathways for DEGs.
| KEGG ID | Pathway name | Gene number | P-value |
|---|
| hsa05200 | Pathways in
cancer | 53 |
4.12×10−9 |
| hsa04512 | ECM-receptor
interaction | 23 |
2.41×10−8 |
| hsa05222 | Small cell lung
cancer | 19 |
1.06×10−5 |
| hsa03030 | DNA
replication | 12 |
1.72×10−5 |
| hsa04510 | Focal adhesion | 30 |
8.78×10−5 |
| hsa04115 | p53 signaling
pathway | 15 |
1.62×10−4 |
| hsa05210 | Colorectal
cancer | 16 |
4.93×10−4 |
| hsa05218 | Melanoma | 14 |
9.09×10−4 |
| hsa05219 | Bladder cancer | 10 |
1.79×10−3 |
| hsa00980 | Metabolism of
xenobiotics by cytochrome P450 | 12 |
2.20×10−3 |
| hsa05215 | Prostate
cancer | 15 |
2.68×10−3 |
| hsa05217 | Basal cell
carcinoma | 11 |
3.69×10−3 |
| hsa05214 | Glioma | 11 |
9.89×10−3 |
Identification of CNVs
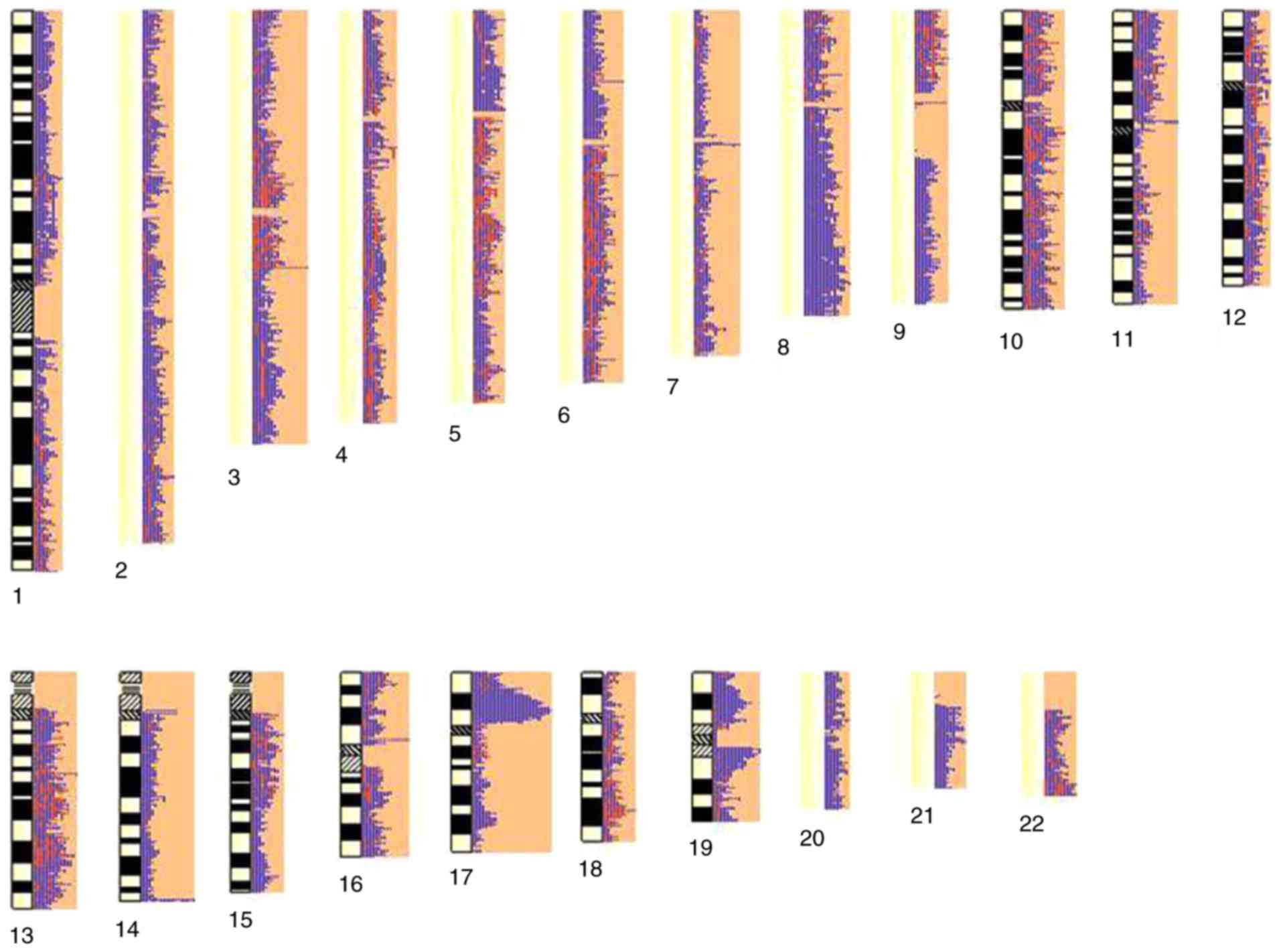
A total of 1,313 chromosome regions were identified
and 678 genes (239 duplications and 439 deletions) were obtained,
which were spread among 22 pairs of autosomes (Fig. 3). Then these CNVs were checked for
overlap with the DEGs. Finally, 12 genes were identified in both in
CNVs and DEGs, including the six upregulated genes cadherin 18
(CDH18), spectrin β, erythrocytic (SPTB), ciliary rootlet
coiled-coil, rootletin pseudogene 2 (CROCCP2),
β-1,4-N-acetyl-galactosaminyltransferase 1 (B4GALNT), G protein
regulated inducer of neurite outgrowth 1 (GPRIN1) and growth factor
independent 1 (GFI1). A total of six downregulated genes were
identified, including laminin subunit α 1 (LAMA1), EH domain
binding protein 1-like 1 (EHBP1L1), cathepsin Z (CTSZ), WNK lysine
deficient protein kinase 1 (PRKWNK1), glutathione S-transferase µ 2
(GSTM2) and microsomal glutathione S-transferase 1 (MGST1)
(Table III).
 | Table III.CNV-driven genes. |
Table III.
CNV-driven genes.
| Gene | logFC |
|---|
| LAMA1 | −2.583 |
| EHBP1L1 | −5.75702 |
| SPTB | 5.224308 |
| CTSZ | −7.39063 |
| CROCCP2 | 2.491733 |
| B4GALNT | 3.6299 |
| CDH18 | 2.227242 |
| GPRIN1 | 2.361558 |
| PRKWNK1 | −2.75581 |
| GFI1 | 3.577933 |
| GSTM2 | −2.62289 |
| MGST1 | −2.30508 |
Discussion
OS is a universally fatal disease, due to the rapid
growth, high local aggressiveness, and metastasizing potential
(20). Numerous DEGs and
regulatory relationships between transcription factors and DEGs in
OS have been identified using microarray data (12). Additionally, susceptibility genes
associated with OS were also reported by analyzing SNP/CNV arrays
(11). However, the underlying
molecular mechanism of OS remains to be elucidated. In the present
study, 1,601 DEGs were identified, including 784 upregulated and
817 downregulated DEGs and CNVs in 678 genes (239 duplications and
439 deletions) were observed in MET-HOBs samples when compared with
controls. By analyzing the transcriptional profile and SNP/CNV
arrays, CDH18, LAMA1, SPTB, CROCCP2,
B4GALNT, GPRIN1, GFI1, EHBP1L1, CTSZ,
PRKWNK1, GSTM2 and MGST1 were identified as CNV-driven
DEGs.
The DEGs obtained in the current study suggested
that several genes such as E2F transcription factor 1 (E2F1)
and 2 (E2F2), retinoblastoma 1 (RB1) and cyclin D1
(CCND1) were involved in various pathways. E2F1 and
E2F2, members of the E2F family of transcription factors,
were upregulated in the MET-HOBs samples. E2F proteins regulate the
transcription of genes required for DNA synthesis (21). The E2F family has an important role
in cell cycle regulation and action of tumor suppressor proteins,
and is also a target of the transforming proteins of small DNA
tumor viruses (22,23). Additionally, the RB protein has
been previously identified to bind to E2F transcription factors
(24). It is evident that the
RB/E2F pathway is very important in regulating the initiation of
DNA replication and the pathway is disrupted in the majority of
human cancers (25). CCND1, is
part of the highly conserved cyclin family, is a nuclear protein
required for cell cycle progression in G1 phase (26). It has been previously reported that
CCND1 has an important role in the regulation of OS cell
proliferation (26). Consistently,
the findings of the present study revealed that those genes were
involved in several cell cycle-associated GO terms, such as cell
cycle, cell cycle phase, regulation of cell cycle and cell
division, and cancer-associated KEGG pathways, including pathways
in cancer, small cell lung cancer and melanoma. Therefore, the
present study is reliable and may suggest that the screened DEGs
such as E2F1, E2F2, RB1 and CCND1 are
closely associated with the cell cycle and cell division of OS.
CNVs such as deletions, duplications and
amplifications across the whole genome may contribute to OS
tumorigenesis (27). It is of note
that CNVs in cyclin-dependent kinase inhibitor 2A (CDKN2A),
sex determining region Y-box 6 (SOX6) and phosphatase and
tensin homolog (PTEN) were associated with Ewing sarcoma
(28). The trail of
CDKN2A/B locus was detected in OS cell lines (29), whereas two SNPs in the SOX6 gene
were identified to be associated with both hip bone mineral density
and body mass index in Caucasians (30). In addition, copy number losses in
PTEN were common events in OS (31). In the present study, further
analysis identified 12 CNV-driven genes in MET-HOBs samples, such
as CDH18 (upregulated) and LAMA1 (downregulated),
which were associated with cell adhesion. Since the 1990s, many
cadherins and cadherin-associated proteins had been identified and
implicated in cancers as candidate tumor suppressors or
proto-oncogenes (32).
Deregulation of cadherin-catenin complexes may contribute to tumor
development by influencing the adhesion of epithelial cells
(33). CDH18 is a member of
the cadherin superfamily that mediates calcium-dependent cell-cell
adhesion (34). Although, no
previous studies have not identified a direct association between
CDH18 and OS, it has been revealed that an Exon 2
deletion of CDH18 may be associated with human colorectal
cancer and CDH18 may act as novel candidate gene involved in
colorectal cancer predisposition (35). In the current study, CDH18
was upregulated and CNVs were detected in CDH18 of MET-HOBs
samples; therefore, CDH18 may have a role in cell-cell
adhesion of OS. Additionally, LAMA1, also termed EHS
laminin, was downregulated in MET-HOBs samples when compared with
controls. Laminin is a complex glycoprotein and is considered to
control the attachment, migration and organization of cells during
embryonic development by interacting with other ECM components
(36,37). Additionally, it has been previously
reported that metadherin, as a laminin receptor, has an important
role in controlling tumorigenesis and metastasis in many human
cancers (38–40). Metadherin, a type II membrane
protein in OS cells, may enhance cell invasion by regulating cell
adhesion to the ECM through interaction with laminin (41). Therefore, LAMA1 may be
involved in cell adhesion and cell-cell interactions in OS.
It is of note that there were some limitations in
the present study. Only the OS-associated CNVs and DEGs were
identified, whereas the transcription factors and protein-protein
interaction network remain to be determined. The current findings
were obtained by bioinformatics analysis and the corresponding
validations were not performed. Therefore, future studies should
involve in performing experiments such as reverse
transcription-quantitative polymerase reaction and western blotting
to validate the CNVs and DEGs identified.
In conclusion, a series of DEGs were identified to
be associated with cell cycle and cell division of human OS,
specifically E2F1, E2F2, RB1 and CCND1.
Additionally, 12 CNV-driven DEGs were obtained and the cell
adhesion-associated genes such as CDH18 and LAMA1 may
contribute to OS cell-cell adhesion. These genes may act as
alternative diagnosis and/or therapeutic markers for patients with
OS. The present study developed the current understanding about the
etiology of OS and provided the foundation for the development of
novel treatment strategies for OS. However, further experiments are
required to confirm these findings.
References
|
1
|
Heymann D and Rédini F: Targeted therapies
for bone sarcomas. Bonekey Rep. 2:3782013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li C, Cheng Q, Liu J, Wang B, Chen D and
Liu Y: Potent growth-inhibitory effect of TRAIL therapy mediated by
double-regulated oncolytic adenovirus on osteosarcoma. Mol Cell
Biochem. 364:337–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He JP, Hao Y, Wang XL, Yang XJ, Shao JF,
Guo FJ and Feng JX: Review of the molecular pathogenesis of
osteosarcoma. Asian Pac J Cancer Prev. 15:5967–5976. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xiong Y, Wu S, Du Q, Wang A and Wang Z:
Integrated analysis of gene expression and genomic aberration data
in osteosarcoma (OS). Cancer Gene Ther. 22:524–529. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Molyneux SD, Di Grappa MA, Beristain AG,
McKee TD, Wai DH, Paderova J, Kashyap M, Hu P, Maiuri T, Narala SR,
et al: Prkar1a is an osteosarcoma tumor suppressor that defines a
molecular subclass in mice. J Clin Invest. 120:3310–3325. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tang N, Song WX, Luo J, Haydon RC and He
TC: Osteosarcoma development and stem cell differentiation. Clin
Orthop Relat Res. 466:2114–2130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Walkley CR, Qudsi R, Sankaran VG, Perry
JA, Gostissa M, Roth SI, Rodda SJ, Snay E, Dunning P, Fahey FH, et
al: Conditional mouse osteosarcoma, dependent on p53 loss and
potentiated by loss of Rb, mimics the human disease. Genes Dev.
22:1662–1676. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kirov G, Rees E, Walters JT, Escott-Price
V, Georgieva L, Richards AL, Chambert KD, Davies G, Legge SE, Moran
JL, et al: The penetrance of copy number variations for
schizophrenia and developmental delay. Biol Psychiatry. 75:378–385.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Riccardi VM and Lupski JR: Duplications,
deletions, and single-nucleotide variations: The complexity of
genetic arithmetic. Genet Med. 15:172–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Porat RM, Pasic I, Shlien A, Golgoz N,
Andrulis I, Wunder JS and Malkin D: Genome-wide copy number
analysis reveals two novel loci for susceptibility to sporadic
osteosarcoma. Cancer Res. 71 8 Suppl:S53342011. View Article : Google Scholar
|
|
12
|
Luo Y, Deng Z and Chen J: Pivotal
regulatory network and genes in osteosarcoma. Arch Med Sci.
9:569–575. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
MacEwen EG, Kutzke J, Carew J, Pastor J,
Schmidt JA, Tsan R, Thamm DH and Radinsky R: c-Met tyrosine kinase
receptor expression and function in human and canine osteosarcoma
cells. Clin Exp Metastasis. 20:421–430. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferracini R, Angelini P, Cagliero E,
Linari A, Martano M, Wunder J and Buracco P: MET oncogene aberrant
expression in canine osteosarcoma. J Orthop Res. 18:253–256. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coltella N, Manara MC, Cerisano V,
Trusolino L, Di Renzo MF, Scotlandi K and Ferracini R: Role of the
MET/HGF receptor in proliferation and invasive behavior of
osteosarcoma. FASEB J. 17:1162–1164. 2003.PubMed/NCBI
|
|
16
|
Naka T, Iwamoto Y, Shinohara N, Ushijima
M, Chuman H and Tsuneyoshi M: Expression of c-met proto-oncogene
product (c-MET) in benign and malignant bone tumors. Mod Pathol.
10:832–838. 1997.PubMed/NCBI
|
|
17
|
Dani N, Olivero M, Mareschi K, van Duist
MM, Miretti S, Cuvertino S, Patanè S, Calogero R, Ferracini R,
Scotlandi K, et al: The MET oncogene transforms human primary
bone-derived cells into osteosarcomas by targeting committed
osteo-progenitors. J Bone Miner Res. 27:1322–1334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang DW, Sherman BT, Tan Q, Collins JR,
Alvord WG, Roayaei J, Stephens R, Baseler MW, Lane HC and Lempicki
RA: The DAVID gene functional classification tool: A novel
biological module-centric algorithm to functionally analyze large
gene lists. Genome Biol. 8:R1832007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kanehisa M and Goto S: KEGG: Kyoto
encyclopedia of genes and genomes. Nucleic Acids Res. 28:27–30.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohseny AB, Tieken C, van der Velden PA,
Szuhai K, de Andrea C, Hogendoorn PC and Cleton-Jansen AM: Small
deletions but not methylation underlie CDKN2A/p16 loss of
expression in conventional osteosarcoma. Genes Chromosomes Cancer.
49:1095–1103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lim JH, Chang YC, Park YB, Park JW and
Kwon TK: Transcriptional repression of E2F gene by proteasome
inhibitors in human osteosarcoma cells. Biochem Biophys Res Commun.
318:868–872. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsantoulis PK and Gorgoulis VG:
Involvement of E2F transcription factor family in cancer. Eur J
Cancer. 41:2403–2414. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Helt AM and Galloway DA: Mechanisms by
which DNA tumor virus oncoproteins target the Rb family of pocket
proteins. Carcinogenesis. 24:159–169. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lees JA, Saito M, Vidal M, Valentine M,
Look T, Harlow E, Dyson N and Helin K: The retinoblastoma protein
binds to a family of E2F transcription factors. Mol Cell Biol.
13:7813–7825. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nevins JR: The Rb/E2F pathway and cancer.
Hum Mol Genet. 10:699–703. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gokgoz N, Wunder JS and Andrulis IL:
Genome-wide analysis of DNA copy number variations in osteosarcoma.
Cancer Res. 72 8 Suppl:S50752012. View Article : Google Scholar
|
|
28
|
Lynn M, Wang Y, Slater J, Shah N, Conroy
J, Ennis S, Morris T, Betts DR, Fletcher JA and O'Sullivan MJ:
High-resolution genome-wide copy-number analyses identify localized
copy-number alterations in Ewing sarcoma. Diagn Mol Pathol.
22:76–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ottaviano L, Schaefer KL, Gajewski M,
Huckenbeck W, Baldus S, Rogel U, Mackintosh C, de Alava E,
Myklebost O, Kresse SH, et al: Molecular characterization of
commonly used cell lines for bone tumor research: A trans-European
EuroBoNet effort. Genes Chromosomes Cancer. 49:40–51. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu YZ, Pei YF, Liu JF, Yang F, Guo Y,
Zhang L, Liu XG, Yan H, Wang L, Zhang YP, et al: Powerful bivariate
genome-wide association analyses suggest the SOX6 gene influencing
both obesity and osteoporosis phenotypes in males. PLoS One.
4:e68272009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Freeman SS, Allen SW, Ganti R, Wu J, Ma J,
Su X, Neale G, Dome JS, Daw NC and Khoury JD: Copy number gains in
EGFR and copy number losses in PTEN are common events in
osteosarcoma tumors. Cancer. 113:1453–1461. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Berx G and Van Roy F: Involvement of
members of the cadherin superfamily in cancer. Cold Spring Harb
Perspect Biol. 1:a0031292009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Goss KH and Groden J: Biology of the
adenomatous polyposis coli tumor suppressor. J Clin Oncol.
18:1967–1979. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yagi T and Takeichi M: Cadherin
superfamily genes: Functions, genomic organization, and neurologic
diversity. Genes Dev. 14:1169–1180. 2000.PubMed/NCBI
|
|
35
|
Venkatachalam R, Verwiel ET, Kamping EJ,
Hoenselaar E, Görgens H, Schackert HK, Van Krieken JH, Ligtenberg
MJ, Hoogerbrugge N, van Kessel AG and Kuiper RP: Identification of
candidate predisposing copy number variants in familial and
early-onset colorectal cancer patients. Int J Cancer.
129:1635–1642. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Dziadek M: Role of laminin-nidogen
complexes in basement membrane formation during embryonic
development. Experientia. 51:901–913. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kleinman HK, Cannon FB, Laurie GW, Hassell
JR, Aumailley M, Terranova VP, Martin GR and DuBois-Dalcq M:
Biological activities of laminin. J Cell Biochem. 27:317–325. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li X, Kong X, Huo Q, Guo H, Yan S, Yuan C,
Moran MS, Shao C and Yang Q: Metadherin enhances the invasiveness
of breast cancer cells by inducing epithelial to mesenchymal
transition. Cancer Sci. 102:1151–1157. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu K, Dai Z, Pan Q, Wang Z, Yang GH, Yu
L, Ding ZB, Shi GM, Ke AW, Yang XR, et al: Metadherin promotes
hepatocellular carcinoma metastasis through induction of
epithelial-mesenchymal transition. Clin Cancer Res. 17:7294–7302.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wei Y, Hu G and Kang Y: Metadherin as a
link between metastasis and chemoresistance. Cell Cycle.
8:2132–2137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhu L, Zhang P, Yang Y, Buford AS, Wang
WL, Thomas DG and Hughes DP: Abstract A53: Metadherin functions as
a laminin receptor that is essential for metastasis and is
associated with poor survival in osteosarcoma. Cancer Res. 74 20
Suppl:A532014. View Article : Google Scholar
|