Introduction
Ischemic cerebrovascular disease (ICD) refers to a
group of clinical syndromes arising from cerebral circulatory
dysfunction of various causes, and brain damage and neurological
dysfunction of patients caused by cerebral ischemia, hypoxia and
necrosis at the associated blood vessel zone (1). The incidence rate of ICD among
cerebrovascular diseases is ~70% in China (2). It has a serious effect on the health
and life of patients, is a burden to the family and society, and
inflicts substantial social and economic costs (3). According to the data of one survey,
it possesses the highest disability and mortality rate in China
(4). The incidence rate is rising
at an annual growth rate of 8.7%, reflecting China's aging
population (1).
ICD originates from a blockage in the local cerebral
blood supply following a vascular occlusion, leading to necrosis
due to brain tissue ischemia and hypoxia, and the formation of an
ischemic penumbra around the necrotic zone (5). A previous study demonstrated that if
the blood supply of the ischemic penumbra is recovered in a timely
manner at the early stage of cerebral ischemia, the moribund
cerebral ischemia tissue may be saved and the damage to the nerve
cells of the ischemic region reversed (6). In recent years, in-depth studies on
ICD have been performed. By observing numerous clinical records, it
may be noted that different levels of increases in microvascular
density are observed in the cerebral ischemia tissue of patients
with cerebral ischemic injury (7).
A study on angiogenesis have demonstrated that the scope and degree
of capillary proliferation at the ischemic region are directly
associated with reperfusion of the ischemic region, and affect the
recovery of neural functions and improve the prognosis of patients
(7). These studies are important
for the investigation of angiogenic factors, and for the treatment
and recovery of patients with ICD (8).
Vascular endothelial growth factor (VEGF) is the
specific mitogen that acts directly on vascular endothelial cells.
It is the most powerful angiogenic growth factor (9) and promotes angiogenesis via the VEGF
receptor (VEGFR). A study demonstrated that the combination of VEGF
and VEGFR promotes reclamation of blood vessels at ischemic
penumbra, is conducive to the establishment of collateral
circulation and serves an important function in the recovery of
cellular function following cerebral ischemia (10). At present, a study investigating
the involvement of the VEGF/VEGFR system in the promotion of ICD
micro-angiogenesis has been performed (11).
The mechanism of ICD involves inflammatory
reactions, changes in gene expression, activation of free radicals,
protease activation, mitochondrial dysfunction and disorder in the
Ca2+ balance (12).
With the development of molecular biology, inflammatory reactions
have received increased attention. Studies have reported that
inflammatory reactions and cell apoptosis are important causes in
the aggravation of cerebral ischemia-reperfusion injury.
Inflammatory reactions inhibit neuron reclamation and functional
rehabilitation following cerebral apoplexy (12,13).
At the early stage of cerebral ischemia-reperfusion, the
blood-brain barrier is damaged, leading to an increase in vascular
endothelium permeability, and the exudation of albumin and other
macromolecular proteins into the blood, which subsequently cause
local edema (14).
Nuclear factor (NF)-κB promotes adherence, the
transmembrane migration of neutrophil granulocytes, leukocyte
infiltration, the release of cytokines and chemokines, and leads to
the activation of a wide range of brain cells and leukocyte
infiltration, which cause further damage to the blood-brain
barrier. It also promotes encephaledema aggravation, the apoptosis
of neurons, glial and vascular endothelium cells, and serves an
important role in reperfusion injury (15,16).
ICD may lead to an inflammation cascade reaction and further
aggravate cerebral tissue damage (17).
Human source microRNA (miR)-132 is located in
chromosome 17pl3.3 and is rich in brain tissues, with a
particularly high expression in the hippocampus (18). miR-132 expression is downregulated
in a number of diseases associated with the nervous system or in
animal models, including Alzheimer's disease, anencephaly,
Huntington's disease, schizophrenia and bipolar affective disorder,
and Parkinson's disease (19,20).
In addition, miR-132 affects the expression of synapse-associated
proteins, including NF-κB and VEGF (21,22).
The present study investigated the role of miR-132 in modifying
angiogenesis in patients with ICD and the underlying mechanism.
Materials and methods
Ethical statement
Study protocols were approved by the Institution
Review Board of Beijing Luhe Hospital, Capital Medical University
(Beijing, China). Written informed consent was obtained from all
participants. Patients with ICD (n=6; 3 male and 3 female;
47.7±12.78 years of age) and healthy volunteers (n=6; 3 male and 3
female; 45.23±8.12 years of age) were recruited from the Department
of Neurology, Beijing Luhe Hospital, Capital Medical University
between March 2016 and April 2016. Between 8:00 and 9:00 a.m.,
cerebrospinal fluid (CSF) of all patients and volunteers was
collected at L3/L4 or L4/L5 interspace by lumbar puncture.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from CSF samples (0.5 ml) was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's protocol. An
iScript cDNA Synthesis kit (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) was used to transcribe to cDNA. A StepOnePlus™
Real-Time PCR System and SYBR Green PCR Master Mix (Applied
Biosystems; Thermo Fisher Scientific, Inc.) were used for qPCR
according to the manufacturer's protocols. PCR amplification
conditions were as follows: 95°C for 30 sec, followed by 40 cycles
at 95°C for 15 sec, 60°C for 30 sec, 72°C for 30 sec and 4°C for 1
min. The following primers were used: U6, CTC GCT TCG GCA GCA CA
(forward) and AAC GCT TCA CGA ATT TGC GT (reverse); miR-132, GCC
CGT AAC AGT CTA CAG CCA T (forward) and GCA GGG TCC GAG GTA TTC
(reverse). The expression of miR-132 was quantified using
2−ΔΔCq method (n=3) (23).
Cell culture and transfection
PC12 cells were purchased from the Cell Bank of the
Shanghai Institute of Cell Biology at the Chinese Academy of
Sciences (Shanghai, China) and were cultured in Dulbecco's modified
Eagle medium (Hyclone; GE Healthcare Life Sciences, Logan, UT, USA)
supplemented with 10% fetal bovine serum (Hyclone; GE Healthcare
Life Sciences) at 37°C and 5% CO2. miR-132 mimics
(5′-CCGCCCCCGCGUCUCCAGGGCAACCGUGGCUUUCGAUUGUUACUGUGGGAACUGGAGGUAACAGUCUACAGCCAUGGUCGCCCCGCAGCACGCCCACGCGC-3′)
and negative mimics (5′-CCGCCCCCCCGCCCCC-3′) were purchased from
Beijing Zoman Biotechnology Co., Ltd. (Beijing, China). A total of
100 nM miR-132 mimics and negative mimics were used to transfect
into PC12 cells using Lipofectamine 2000 (Thermo Fisher Scientific,
Inc.). Following transfection for 48 h, Lipofectamine was replaced
with Earle's balanced salt solution (Hyclone; GE Healthcare Life
Sciences) and placed in an incubator (ThermoForma 3111; Thermo
Scientific, Inc.) containing 5% O2 and 95% N2
at 37°C for 6 h to create an oxygen glucose deprivation (OGD)
model.
ELISA
Total protein from PC12 cells was extracted using
radioimmunoprecipitation assay (RIPA) buffer (BioTeke Corporation,
Beijing, China) and quantified using a BCA protein assay kit
(BioTeke Corporation). Total protein (~10 µg per well) was measured
to determine the levels of tumor necrosis factor (TNF)-α (cat no.
PT516; Beyotime Institute of Biotechnology, Haimen, China),
interleukin (IL)-1β (cat no. PI303; Beyotime Institute of
Biotechnology), IL-6 (cat no. PI328; Beyotime Institute of
Biotechnology), IL-8 (cat no. H008; Nanjing Jiancheng
Bioengineering Institute, Nanjing, China), caspase-3 (cat no.
C1116; Beyotime Institute of Biotechnology) and capase-9 (cat no.
C1158; Beyotime Institute of Biotechnology) using ELISA kits
(RayBiotech, Inc., Norcross, GA, USA).
Western blotting
Total protein from PC12 cells was extracted using
RIPA buffer and quantified using a BCA protein assay kit. Total
protein (~50 µg per well) was loaded on a 10% sodium dodecyl
sulfate-polyacrylamide gel and transferred to a polyvinylidene
fluoride membrane (Thermo Fisher Scientific, Inc.). Membranes were
initially blocked with 5% skimmed milk powder at 37°C for 1 h in
0.1% TBS-Tween-20 and incubated with cyclooxygenase-2 (Cox-2; cat
no. sc-7951; 1:500), Bcl-2 (cat no. sc-783; 1:500),
Bcl-2-associated X (Bax; cat no. sc-6236; 1:500), NF-κB p65 (cat
no. sc-372; 1:500), matrix metalloproteinase (MMP-9; cat no.
sc-10737; 1:500), vascular cell adhesion molecule-1 (VCAM-1; cat
no. sc-8304; 1:500), inducible nitric oxide synthase (iNOS; cat no.
sc-649; 1:1,000), VEGF (cat no. sc-13083; 1:500) and GAPDH (cat no.
sc-25778; 1:1,000) primary antibodies, which were all purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA) at 4°C
overnight. Membranes were washed with PBS three times and probed
with anti-rabbit horseradish peroxidase-conjugated secondary
antibodies (cat no. sc-2004; 1:5,000; Santa Cruz Biotechnology,
Inc.) for 1 h at 37°C, developed with a Luminol chemiluminescence
detection kit (cat no. 12015218001; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) and analyzed using sodium Image_Lab version 3.0
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Data are presented as the mean ± standard error of
the mean using SPSS software version 20.0 (IBM Corp., Armonk, NY,
USA). Statistical significance was determined by one-way analysis
of variance with Dunnett's post-hoc test. P<0.05 was considered
to indicate a statistically significant difference.
Results
miR-132 expression in patients with
ICD
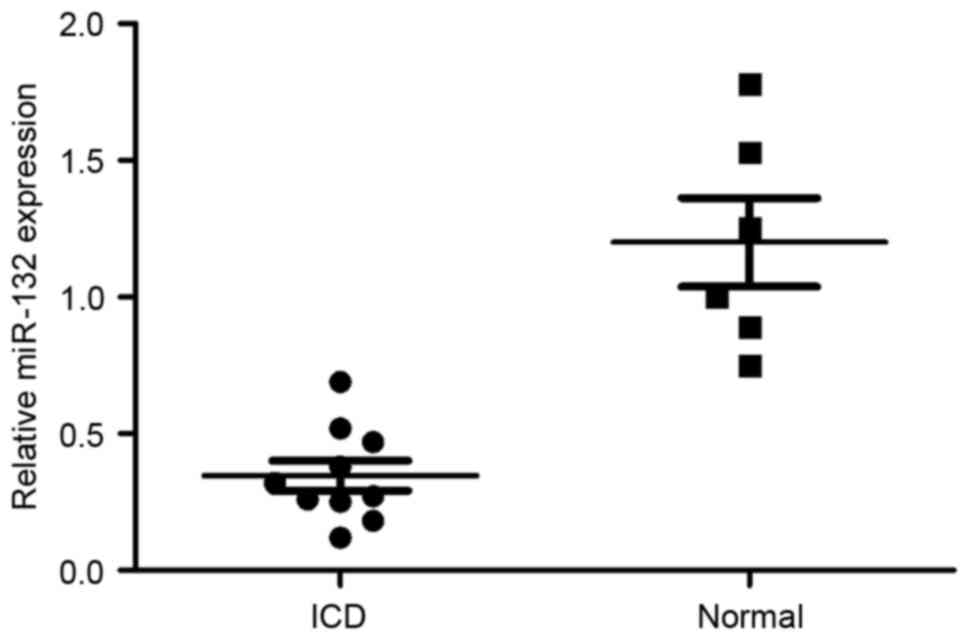
To investigate whether miR-132 may be
neuroprotective, the expression of miR-132 in patients with ICD was
determined. As demonstrated in Fig.
1, miR-132 expression in patients with ICD was lower compared
with the normal group.
Effects of miR-132 overexpression on
inflammation
To evaluate the effect of miR-132 expression on
ICD-induced inflammation in vitro, OGD-injured PC12 cells
were established. As demonstrated in Fig. 2, ELISA results demonstrated that
there were significant increases in the levels of TNF-α, IL-1β,
IL-6 and IL-8 in OGD-injured PC12 cells. Furthermore, it was
observed that miR-132 overexpression significantly reduced
OGD-induced TNF-α, IL-1β, IL-6 and IL-8 levels in OGD-injured PC12
cells (Fig. 2).
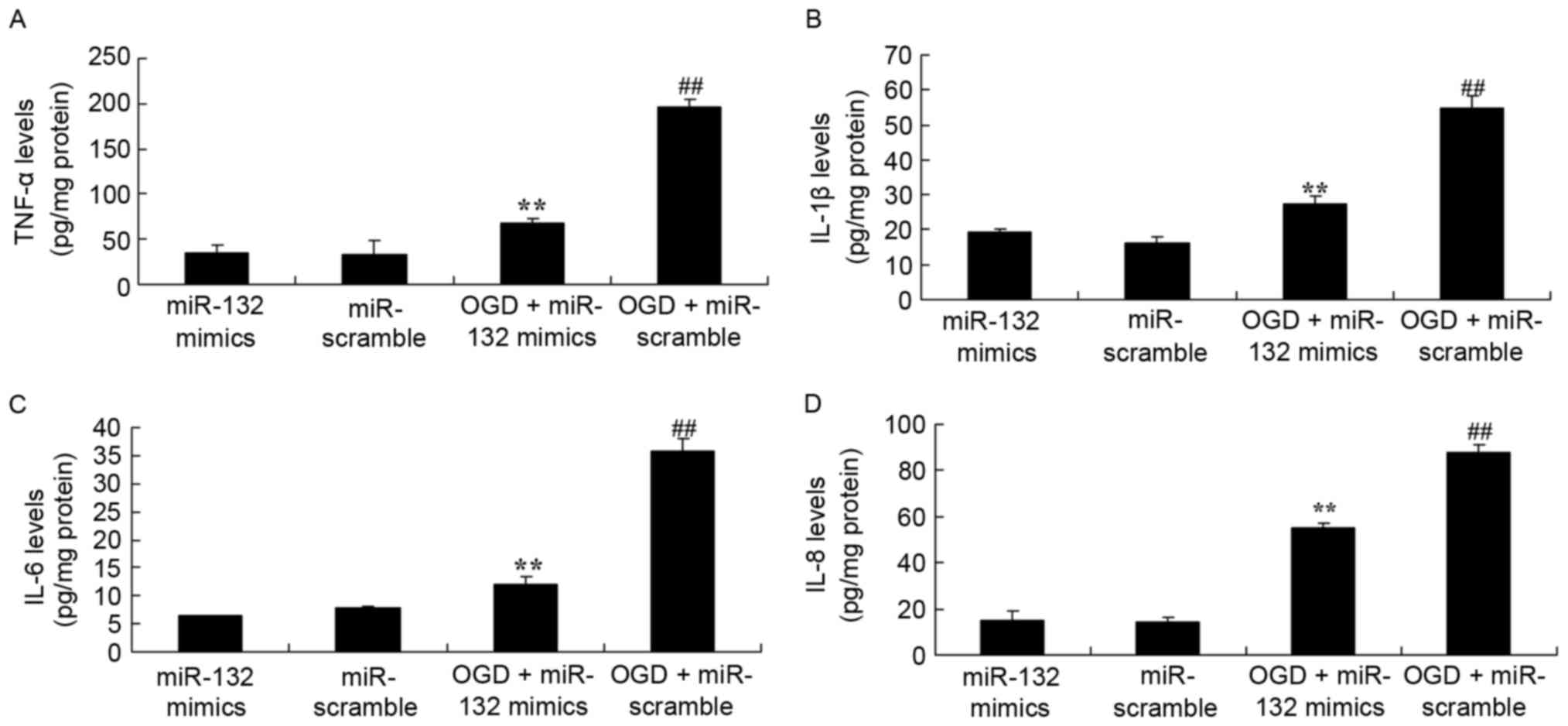 | Figure 2.Effects of miR-132 overexpression on
inflammation. miR-132 overexpression exhibited effects on (A)
TNF-α, (B) IL-1β, (C) IL-6 and (D) IL-8 levels in PC12 cells.
**P<0.01 vs. miR-132 mimics-only group and
##P<0.01 vs. OGD + miR-132 mimics group. miR,
microRNA; TNF, tumor necrosis factor; IL, interleukin; OGD, oxygen
glucose deprivation; miR-132 mimics, miR-132 overexpression group;
miR-scramble, miR-scramble control group; OGD + miR-132 mimics, OGD
+ miR-132 overexpression group; OGD + miR-scramble, OGD +
miR-scramble control group. |
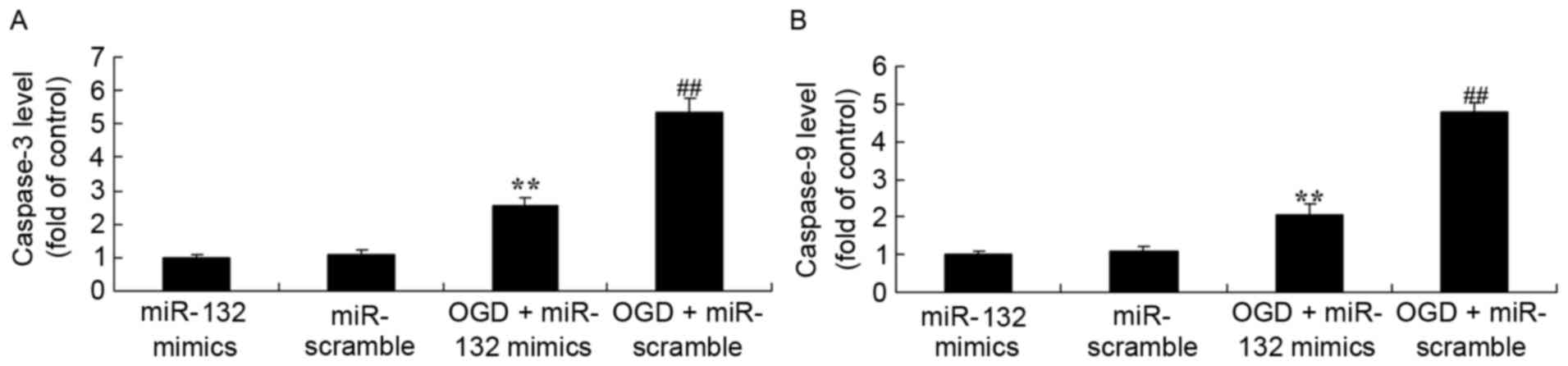
Effects of miR-132 overexpression on
caspase-3 and caspase-9 levels
The effects of the expression of miR-132 on
apoptosis were determined by measuring caspase-3 and caspase-9
levels. As demonstrated in Fig. 3,
ELISA results demonstrated that caspase-3 and caspase-9 levels were
significantly increased in OGD-injured PC12 cells. Overexpression
of miR-132 significantly inhibited OGD-induced increases in
caspase-3 and caspase-9 levels in OGD-injured PC12 cells.
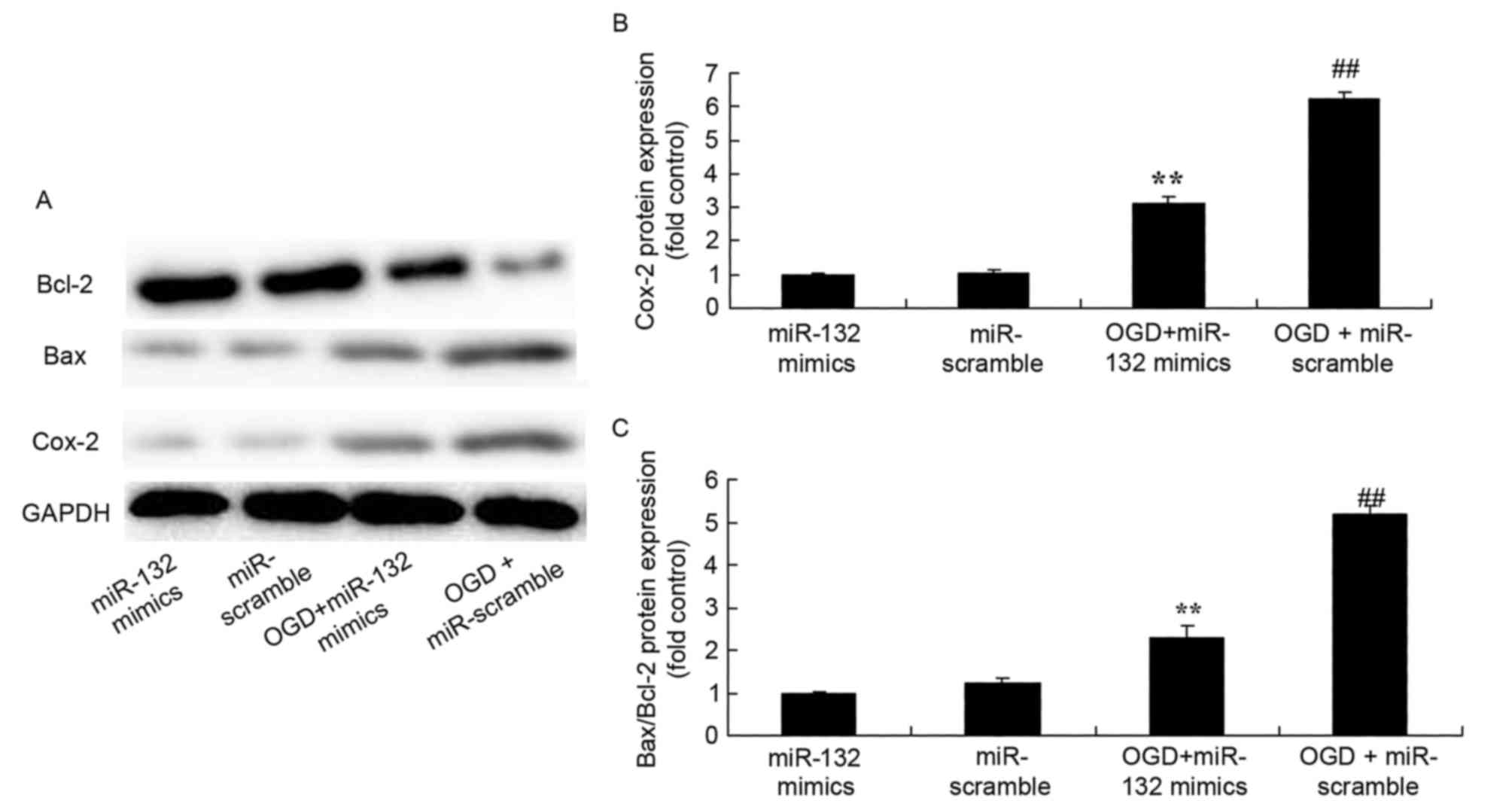
Effects of miR-132 overexpression on
Cox-2 and Bax/Bcl-2 expression
To evaluate the effect of miR-132 expression on
Cox-2 and Bax/Bcl-2 expression protein expression in vitro,
Cox-2 and Bax/Bcl-2 protein expression was measured by western
blotting. As demonstrated in Fig.
4, there was a significant increase of Cox-2 and Bax/Bcl-2
protein expression in OGD-injured PC12 cells compared with the
control group. However, overexpression of miR-132 significantly
suppressed Cox-2 and Bax/Bcl-2 protein expression in OGD-injured
PC12 cells (Fig. 4).
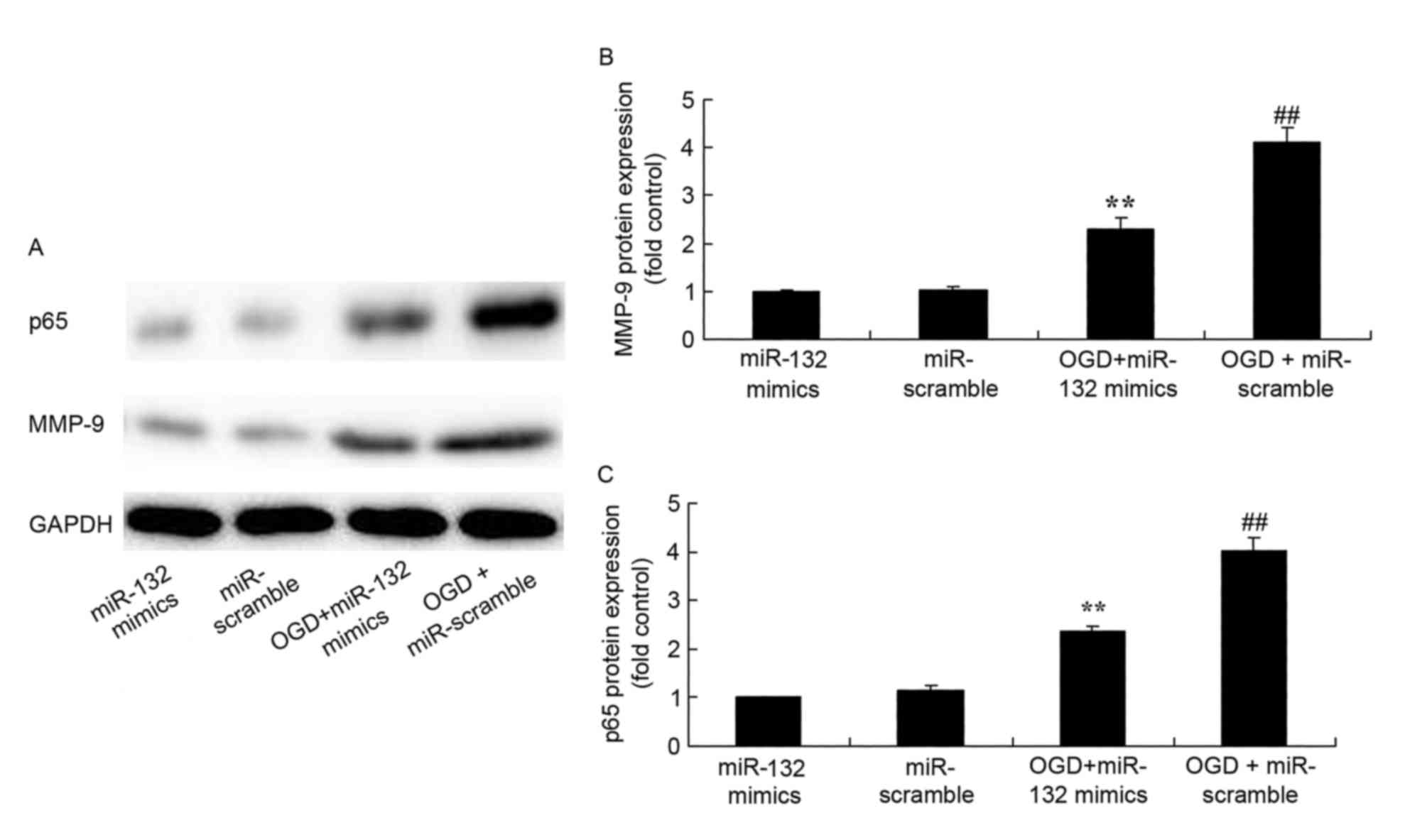
Effects of miR-132 overexpression on
MMP-9 and NF-κB protein expression
Western blotting was performed to analyze MMP-9 and
NF-κB protein expression in OGD-injured PC12 cells. As expected,
MMP-9 and NF-κB protein expression were significantly increased in
OGD-injured PC12 cells compared with control cells (Fig. 5). However, overexpression of
miR-132 significantly suppressed MMP-9 and NF-κB protein expression
in OGD-injured PC12 cells (Fig.
5).
Effects of miR-132 overexpression on
VCAM-1, iNOS and VEGF protein expression levels
VCAM-1, iNOS and VEGF protein expression in
OGD-injured PC12 cells were also determined by western blotting. In
OGD-injured PC12 cells overexpressing miR-132, VCAM-1 and iNOS
protein expression were significantly induced compared with non-OGD
cells, and VEGF protein expression was significantly reduced
(Fig. 6). However, overexpression
of miR-132 significantly suppressed VCAM-1 and iNOS protein
expression, and induced VEGF protein expression in OGD-injured PC12
cells (Fig. 6).
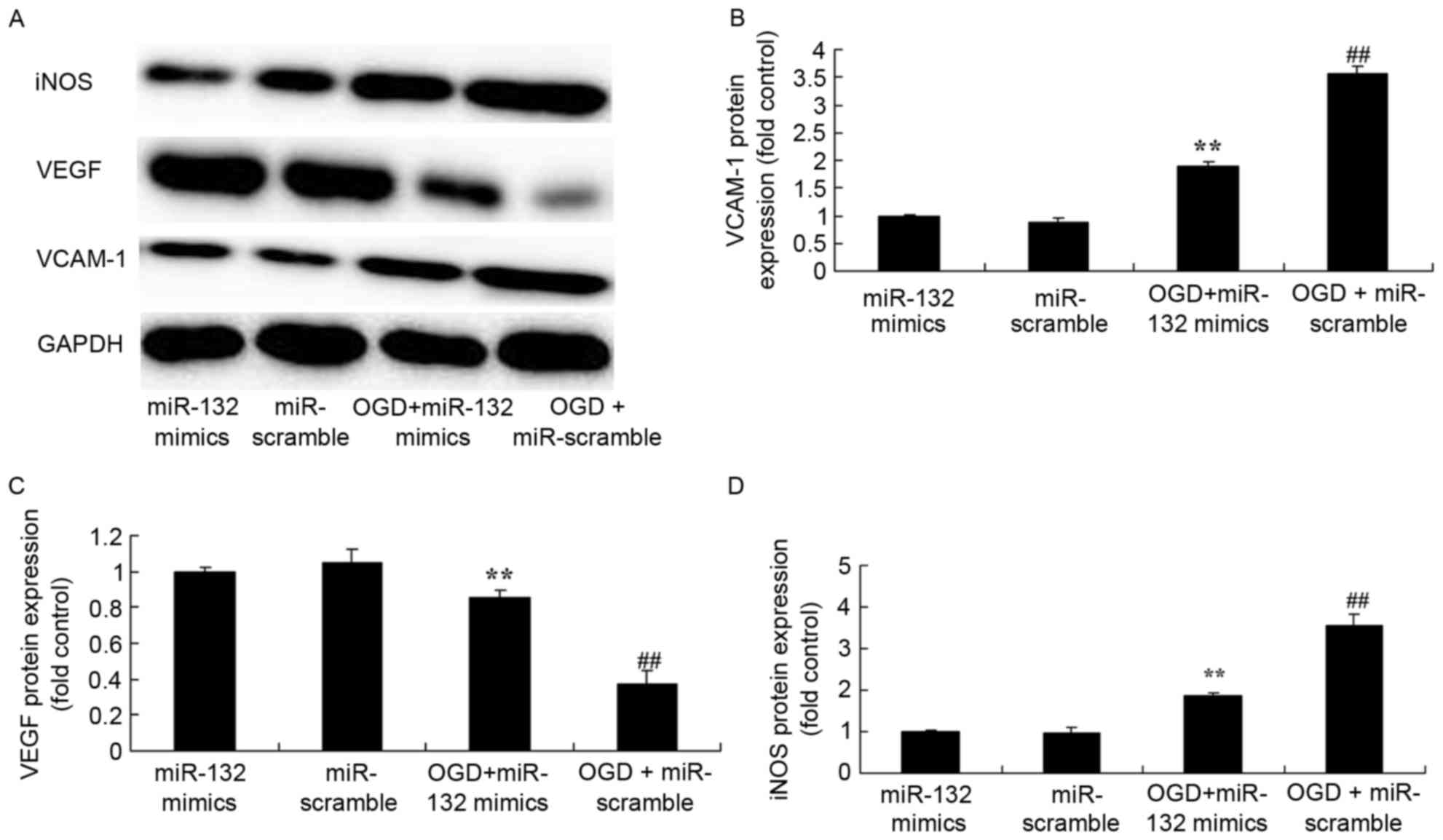 | Figure 6.Effects of miR-132 overexpression on
iNOS, VEGF and VCAM-1 protein expression levels. (A) Effects of
miR-132 overexpression on iNOS, VEGF and VCAM-1 protein expression
were determined by western blotting. Densitometric analysis was
performed to quantify protein expression of (B) VCAM-1, (C) VEGF
and (D) iNOS. **P<0.01 vs. miR-132 mimics-only group and
##P<0.01 vs. OGD + miR-132 mimics group. miR,
microRNA; iNOS, inducible nitric oxide synthase; VEGF, vascular
endothelial growth factor; VCAM, vascular cell adhesion molecule;
OGD, oxygen glucose deprivation; miR-132 mimics, miR-132
overexpression group; miR-scramble, miR-scramble control group; OGD
+ miR-132 mimics, OGD + miR-132 overexpression group; OGD +
miR-scramble, OGD + miR-scramble control group. |
Discussion
In the 21st century, stroke has become the third
highest cause of mortality in the majority of developed countries,
and a major cause of mortality in China (24). It affects productivity and is a
heavy burden for the family of patients and for society. The
fibrinolytic method has been considered as an effective therapeutic
method for ICD at present (25).
However, <50% of patients are treated with this method.
Circumstances have not improved in spite of research efforts and
numerous clinical trials aiming to reduce the rates of disability
and mortality (3). Thus far,
pharmaceutical research has focused on ion channel (Ca2+
and Na+) blockers, oxygen free radical scavengers, and
excitability and toxicity neurotransmitter blockers (primarily
glutamate and glycine receptors) (26). However, the majority of the
clinical trials involving such targets have failed due to low
efficacy, the number of side effects or study difficulties
(26).
Inflammatory reactions encompass the transfer of
liquid and cells from blood vessels to tissue, and protection and
repair of tissue. (14). It is
caused by the interaction of different cells at injured tissues,
including neurons, astrocytes, myofibroblasts, smooth muscle cells,
endothelial cells and hemocytes (27). The most typical infarction center
is an excitatory neurotransmitter released by the damaged neurons,
successively followed by Ca2+ overload, lipid
peroxidation, karyoclasis and neuron death (27). Ischemia and reperfusion have been
reported to induce inflammatory reactions (28). Inflammatory reactions are
characterized by the expression of local inflammatory cytokines,
including TNF-α and IL-1β, the release of chemotactic factors and
upregulation of cell adhesion molecules (29). The results of the present study
revealed that miR-132 overexpression significantly reduced
OGD-induced TNF-α, IL-1β, IL-6 and IL-8 levels in OGD-injured PC12
cells. Kim et al (30)
previously demonstrated that miR-132 is a critical mediator in the
pathogenesis of inflammatory bowel disease by negatively regulating
Forkhead box O3a to enhance the expression of inflammatory
cytokines.
The proliferation and migration of endothelial cells
induced by VEGF promotes the formation of new blood vessels
(11). Increased vasopermeability
serves an important regulatory role in angiogenesis. In the case of
cerebral ischemia and hypoxia, VEGF was reported to increase the
vasopermeability and promote the exosmosis of plasma fibrinogen,
vasoconstriction factors and blood coagulation factors (31). Macromolecular substances in the
blood, such as fibrinogen, enter the extracellular matrix and form
fibrin glue. Extracellular protein fiber, as a temporary substrate,
allows and supports the introversion growth of new blood vessels
and stromal cells, and is conducive to angiopoiesis (32). The increase in permeability
destroys the connective structure of endothelial cells, leading to
an imbalance in the dynamic equilibrium of the blood-brain barrier
with low permeability. This, in turn, may cause microvascular
structural damage to the diseased tissue and plasma extravasation,
which aggravates encephaledema following ischemia damage (33). The results of the present study
demonstrated that miR-132 overexpression suppressed caspase-3 and
caspase-9 levels, reduced the Bax/Bcl-2 ratio and increased VEGF
protein expression in OGD-injured PC12 cells. Mulik et al
(34) reported that miR-132
inhibited angiogenic Ras activity in corneal CD31-enriched cells
via VEGF and VEGF-A.
The promotion of Cox-2 is part of the development of
neuronal apoptosis in brain ischemia and neurodegenerative diseases
(35). NF-κB exists in almost all
types of cells. In the nervous system, it is extensively
distributed in nerve cells, astrocytes and microglial cells. In
addition, vascular endothelial cells include an NF-κB locus that
serves important roles in the pathological process of cerebral
ischemia-reperfusion (16).
Following ICD, NF-κB is one of the transcription factors that
regulate inflammatory gene expression. NF-κB is involved in body
defense functions and inflammatory response-associated gene
expression. It is an important transcription factor that binds to
the promoters of inflammatory genes, including TNF-α, Cox-2, IL-1β,
IL-6, IL-8, VCAM-1 and iNOS (29).
NF-κB is bound to inhibiting factors in its inactive state
(36). Under the effects of a
stimulating factor, NF-κB in neuronal cells, endothelial cells,
neurogliocyte and inflammatory cells around the blood vessel is
activated (37). NF-κB
subsequently participates in inflammation and immune reaction, and
serves a vital role in the pathological process of
ischemia-reperfusion injury (38).
Li et al (21) indicated
that miR-132 enhanced the transition from inflammation to
proliferation during wound healing by suppressing the NF-κB
pathway. The present study hypothesized that miR-132 may affect
Cox-2 expression in ICD, and the results demonstrated that miR-132
overexpression suppressed Cox-2, iNOS, NF-κB and VCAM-1 protein
expression in OGD-injured PC12 cells.
In conclusion, the present study demonstrated that
miR-132 may adjust angiogenesis in patients with ICD by suppressing
the NF-κB pathway and promoting the VEGF pathway. These findings
may provide novel evidence to support the use of miR-132 therapies
for ICD.
References
|
1
|
Ma X, Wang J, Liu J, Mo Q, Yan X, Ma D and
Duan H: Targeting CD146 in combination with vorinostat for the
treatment of ovarian cancer cells. Oncol Lett. 13:1681–1687.
2017.PubMed/NCBI
|
|
2
|
Nakamura K, Terai Y, Tanabe A, Ono YJ,
Hayashi M, Maeda K, Fujiwara S, Ashihara K, Nakamura M, Tanaka Y,
et al: CD24 expression is a marker for predicting clinical outcome
and regulates the epithelial-mesenchymal transition in ovarian
cancer via both the Akt and ERK pathways. Oncol Rep. 37:3189–3200.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ding F, Liu T, Yu N, Li S, Zhang X, Zheng
G, Lv C, Mou K, Xu J, Li B, et al: Nitidine chloride inhibits
proliferation, induces apoptosis via the Akt pathway and exhibits a
synergistic effect with doxorubicin in ovarian cancer cells. Mol
Med Rep. 14:2853–2859. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim BR, Lee SH, Park MS, Seo SH, Park YM,
Kwon YJ and Rho SB: MARCKSL1 exhibits anti-angiogenic effects
through suppression of VEGFR-2-dependent Akt/PDK-1/mTOR
phosphorylation. Oncol Rep. 35:1041–1048. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ding M, Lei J, Han H, Li W, Qu Y, Fu E, Fu
F and Wang X: SIRT1 protects against myocardial
ischemia-reperfusion injury via activating eNOS in diabetic rats.
Cardiovasc Diabetol. 14:1432015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen DL and Yang KY: Berberine alleviates
oxidative stress in islets of diabetic mice by inhibiting miR-106b
expression and up-regulating SIRT1. J Cell Biochem. 118:4349–4357.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang X, Chen J, Zhang C, Zhang Z, Tan Y,
Feng W, Skibba M, Xin Y and Cai L: The protective effect of FGF21
on diabetes-induced male germ cell apoptosis is associated with
up-regulated testicular AKT and AMPK/Sirt1/PGC-1α signaling.
Endocrinology. 156:1156–1170. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu H, Sheng M, Liu Y, Wang P, Chen Y,
Chen L, Wang W and Li B: Expression of SIRT1 and oxidative stress
in diabetic dry eye. Int J Clin Exp Pathol. 8:7644–7653.
2015.PubMed/NCBI
|
|
9
|
Law NC, White MF and Hunzicker-Dunn ME: G
protein-coupled receptors (GPCRs) that signal via protein kinase A
(PKA) cross-talk at insulin receptor substrate 1 (IRS1) to activate
the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. J Biol Chem.
291:27160–27169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Onyechi KC, Eseadi C, Okere AU, Onuigbo
LN, Umoke PC, Anyaegbunam NJ, Otu MS and Ugorji NJ: Effects of
cognitive behavioral coaching on depressive symptoms in a sample of
type 2 diabetic inpatients in Nigeria. Medicine (Baltimore).
95:e44442016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cukierman-Yaffe T, Bosch J, Diaz R, Dyal
L, Hancu N, Hildebrandt P, Lanas F, Lewis BS, Marre M, Yale JF, et
al: Effects of basal insulin glargine and omega-3 fatty acid on
cognitive decline and probable cognitive impairment in people with
dysglycaemia: A substudy of the ORIGIN trial. Lancet Diabetes
Endocrinol. 2:562–572. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yerra VG, Kalvala AK and Kumar A:
Isoliquiritigenin reduces oxidative damage and alleviates
mitochondrial impairment by SIRT1 activation in experimental
diabetic neuropathy. J Nutr Biochem. 47:41–52. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Yan H and Lou MF: Does oxidative
stress play any role in diabetic cataract formation? Re-evaluation
using a thioltransferase gene knockout mouse model. Exp Eye Res.
161:36–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Coskun ZM, Koyuturk M, Karabulut S and
Bolkent S: CB-1R and GLP-1R gene expressions and oxidative stress
in the liver of diabetic rats treated with sitagliptin. Pharmacol
Rep. 69:822–829. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yacoub R, Lee K and He JC: The role of
SIRT1 in diabetic kidney disease. Front Endocrinol (Lausanne).
5:1662014.PubMed/NCBI
|
|
16
|
Lucas T, Schäfer F, Müller P, Eming SA,
Heckel A and Dimmeler S: Light-inducible antimiR-92a as a
therapeutic strategy to promote skin repair in healing-impaired
diabetic mice. Nat Commun. 8:151622017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang L, Li R, He J, Yang Q, Wu Y, Huang J
and Wu B: Co-expression analysis among microRNAs, long non-coding
RNAs, and messenger RNAs to understand the pathogenesis and
progression of diabetic kidney disease at the genetic level.
Methods. 124:46–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fu W, Tao T, Qi M, Wang L, Hu J, Li X,
Xing N, Du R and Han B: MicroRNA-132/212 upregulation inhibits
TGF-β-mediated epithelial-mesenchymal transition of prostate cancer
cells by targeting SOX4. Prostate. 76:1560–1570. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Tang W, Li R, He R, Gan T, Luo Y,
Chen G and Rong M: Downregulation of microRNA-132 indicates
progression in hepatocellular carcinoma. Exp Ther Med.
12:2095–2101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong HK, Veremeyko T, Patel N, Lemere CA,
Walsh DM, Esau C, Vanderburg C and Krichevsky AM: De-repression of
FOXO3a death axis by microRNA-132 and −212 causes neuronal
apoptosis in Alzheimer's disease. Hum Mol Genet. 22:3077–3092.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li D, Wang A, Liu X, Meisgen F, Grünler J,
Botusan IR, Narayanan S, Erikci E, Li X, Blomqvist L, et al:
MicroRNA-132 enhances transition from inflammation to proliferation
during wound healing. J Clin Invest. 125:3008–3026. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang X, Tang W, Chen G, Ren F, Liang H,
Dang Y and Rong M: An encapsulation of gene signatures for
hepatocellular carcinoma, MicroRNA-132 predicted target genes and
the corresponding overlaps. PLoS One. 11:e01594982016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He Z, Chen AY, Rojanasakul Y, Rankin GO
and Chen YC: Gallic acid, a phenolic compound, exerts
anti-angiogenic effects via the PTEN/AKT/HIF-1α/VEGF signaling
pathway in ovarian cancer cells. Oncol Rep. 35:291–297. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai H, Li H, Li W, Gui T, Yang J, Cao D
and Shen K: The PI3K/AKT/mTOR pathway is a potential predictor of
distinct invasive and migratory capacities in human ovarian cancer
cell lines. Oncotarget. 6:25520–25532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ding Q, Chen Y, Zhang Q, Guo Y, Huang Z,
Dai L and Cao S: 8-bromo-7-methoxychrysin induces apoptosis by
regulating Akt/FOXO3a pathway in cisplatin-sensitive and resistant
ovarian cancer cells. Mol Med Rep. 12:5100–5108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKie EA, Reid JL, Mistry PC, DeWall SL,
Abberley L, Ambery PD and Gil-Extremera B: A study to investigate
the efficacy and safety of an anti-interleukin-18 monoclonal
antibody in the treatment of type 2 diabetes mellitus. PLoS One.
11:e01500182016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu W, Mu Y, Zhao J, Zhu D, Ji Q, Zhou Z,
Yao B, Mao A, Engel SS, Zhao B, et al: Efficacy and safety of
metformin and sitagliptin based triple antihyperglycemic therapy
(STRATEGY): A multicenter, randomized, controlled, non-inferiority
clinical trial. Sci China Life Sci. 60:225–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kamenov Z, Fileva S, Kalinov K and Jannini
EA: Evaluation of the efficacy and safety of Tribulus terrestris in
male sexual dysfunction-A prospective, randomized, double-blind,
placebo-controlled clinical trial. Maturitas. 99:20–26. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim HY, Kwon HY, Ha Thi HT, et al:
MicroRNA-132 and microRNA-223 control positive feedback circuit by
regulating FOXO3a in inflammatory bowel disease. J Gastroenterol
Hepatol. 31:1727–1735. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hermanns N, Schmitt A, Gahr A, Herder C,
Nowotny B, Roden M, Ohmann C, Kruse J, Haak T and Kulzer B: The
effect of a Diabetes-Specific Cognitive Behavioral Treatment
Program (DIAMOS) for patients with diabetes and subclinical
depression: Results of a randomized controlled trial. Diabetes
Care. 38:551–560. 2015.PubMed/NCBI
|
|
32
|
Fall E, Roche B, Izaute M, Batisse M,
Tauveron I and Chakroun N: A brief psychological intervention to
improve adherence in type 2 diabetes. Diabetes Metab. 39:432–438.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Derosa G, D'Angelo A, Romano D and
Maffioli P: Evaluation of the effects of mesoglycan on some markers
of endothelial damage and walking distance in diabetic patients
with peripheral arterial disease. Int J Mol Sci. 18:pii: E5722017.
View Article : Google Scholar
|
|
34
|
Mulik S, Xu J, Reddy PB, et al: Role of
miR-132 in angiogenesis after ocular infection with herpes simplex
virus. Am J Pathol. 181:525–534. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu L, Yang B, Wang J, Zhao L, Luo W, Jiang
Q and Yang J: Time course change of COX2-PGI2/TXA2 following global
cerebral ischemia reperfusion injury in rat hippocampus. Behav
Brain Funct. 10:422014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng Z, Guan M, Jia Y, Wang D, Pang R, Lv
F, Xiao Z, Wang L, Zhang H and Xue Y: The coordinated roles of
miR-26a and miR-30c in regulating TGFβ1-induced
epithelial-to-mesenchymal transition in diabetic nephropathy. Sci
Rep. 6:374922016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang Y, Wang JH, Zhang YY, Wang YZ, Wang
J, Zhao Y, Jin XX, Xue GL, Li PH, Sun YL, et al: Deletion of
interleukin-6 alleviated interstitial fibrosis in
streptozotocin-induced diabetic cardiomyopathy of mice through
affecting TGFβ1 and miR-29 pathways. Sci Rep. 6:230102016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Cui YX, Hua YZ, Wang N, Chen X, Wang F,
Liu JY, Wang LL, Yan CY, Ma YG, Cao YH and Zhang XH: miR-24
suppression of POZ/BTB and AT-hook-containing zinc finger protein 1
(PATZ1) protects endothelial cell from diabetic damage. Biochem
Biophys Res Commun. 480:682–689. 2016. View Article : Google Scholar : PubMed/NCBI
|