Introduction
Pancreatic cancer is associated with high rates of
morbidity worldwide. Data from the American Cancer Society indicate
that, in 2016, 53,070 patients were diagnosed with pancreatic
cancer, and 41,780 cases were predicted to succumb to this
malignancy in the United States (1). In China, the figures are higher, as
statistical data from 2015 showed that 90,100 individuals suffered
from pancreatic cancer, and the associated rate of mortality was as
high as 79,400 (2). The accepted
therapeutic strategy for pancreatic cancer is combined treatment
including surgery; however, the five-year survival rate remains
poor, which may be attributed to the fact that the molecular
mechanisms underlying the development of pancreatic cancer remain
to be fully elucidated (1,2). The entire process of cancer
development follows a complex etiology, including the abnormal
activation of oncogenes and inactivation of tumor suppressor genes
(2,3). The subsequent dysregulation of
signaling pathways in a closed network of abnormally expressed
genes, which indicates that gene-based prevention and treatment may
be effective in pancreatic cancer (4,5).
P16ink4a, also known as cyclin-dependent
kinase inhibitor 2a, is usually considered a tumor suppressor gene.
The encoded P16 protein can bind and inhibit cyclin-dependent
kinase 4 with high specificity to modulate the cell cycle, which
can inhibit the G1/S transition and result in tumor-suppressing
activity (6). Mutation and
methylation of the p16 gene are important in the development of
tumors, which also show abnormal protein expression of P16, the
clinical staging of pancreatic cancer, and the patient's lifespan
(7). The inactivation and
decreased expression of anti-oncogenes, including
p16ink4a and small mothers against decapentaplegic 4,
and the abnormal activation of oncogenes, including K-ras, have
been reported in pancreatic ductal adenocarcinoma and are also
associated with the degree of malignancy (7).
The Wnt/β-catenin signaling pathway is essential in
cell differentiation and the maintenance of cellular homeostasis,
and decreased activity of this pathway may contribute to the
processes of tumor formation and inflammation (8,9). Wnt
signaling is highly conserved across a wide range of species from
nematode to humans, and the name for this pathway originated from
the genes wingless and int-1 in Drosophila. The core
molecule in this pathway is β-catenin, encoded by CTNNB1, which can
interact with E-cadherin at the cell membrane or shuttle between
the cytoplasm and the nucleus. The Wnt/β-catenin signaling pathway
is closely associated with the development of malignant tumors
(10,11). A previous study showed that vitamin
A was able to inhibit the activation of the Wnt/β-catenin signaling
pathway through astrocytes in pancreatic cancer tissues, and this
finding was attributed to the increased expression of
extracellularsecreted frizzled-related protein 4 and the subsequent
decrease of β-catenin in the nucleus (12). Another study found that the
survival of melanoma cells was largely dependent on the high
concentration of intracellular β-catenin. β-catenin can function as
a regulator of certain genes associated with cell differentiation
and proliferation, including cyclin D1 and c-myc. The knockout of
β-catenin causes a sharp decrease in the expression of
p16ink4a, which may be associated with lymphoid enhancer
factor (LEF)/T cell factor (Tcf), which serve as effectors of
Wnt-β-catenin in the nucleus. β-catenin can also inhibit
transcription as a translation-activating factor (13,14).
The knockout of low-density lipoprotein receptor-related protein 5
(Lrp5) results in increased mRNA levels of p16ink4a in
the breast ductal epithelium and basal cells (15).
In the present study, p16ink4a was
introduced into pancreatic cancer cells using a pc-DNA3.0 plasmid
as the vector. The effect of the overexpression of
p16ink4a on the Wnt/β-catenin signaling pathway was
investigated. The results may provide evidence to support further
investigations into gene therapy targeting p16ink4a.
Materials and methods
Cell lines
The Bxpc-3 and Miapaca-2 human pancreatic cancer
cell lines were purchased from the Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China).
Cell culture and treatment
The Bxpc-3 and Miapaca-2 cells were cultured in 10%
FCS-containing Dulbecco's modified Eagle's medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). The density of the
cells was adjusted to 5×105/ml, and the culture
conditions consisted of 5% CO2 at 37°C. Cells in the
logarithmic growth phase were harvested and then inoculated onto a
6-well plate, and grown until adherence.
Pc-DNA3.0-p16ink4a plasmid
transfection
The Pc-DNA3.0-p16ink4a plasmid
(Invitrogen; Thermo Fisher Scientific, Inc.) was introduced into
and replicated in E. coli, followed by molecular
identification and adjustment of DNA concentration. The cells were
seeded onto 6-well plates at a density of
1×106/cm2 and incubated for 24 h. X-tremeGENE
HP DNA transfection reagent (Roche Diagnostics, Basel,
Switzerland), plasmid DNA was diluted in ddH2O and
incubated for 15 min at room temperature. Based on previous
experiments, the X-tremeGENE HP DNA transfection
reagent:pc-DNA3.0-p16ink4a (1 µg/100 µl) ratio of 3:1
(X:P=3:1) was used as the concentration for transfection.
In terms of treatment groups, mock-treated specimens
were considered as the control group. The specimens treated with an
equal dose of pc-DNA3.0-p16ink4a without transfection
reagent were considered the plasmid group. The samples treated with
X-tremeGENE HP without the plasmid were considered the X-tremeGENE
HP group. The samples transfected with the X:P=3:1 mix were
considered the X:P=3:1 group. Following incubation for 24 h
post-transfection, the cells were harvested. The transfection
procedure was performed based on the manufacturer's protocol of the
X-tremeGENE HP DNA transfection reagent and completed on a clean
bench top.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
RT-qPCR analysis was performed to evaluate the mRNA
levels of p16ink4a, β-catenin, c-myc and cyclin D1. The
total RNA was extracted using MiniBEST Universal RNA Extraction kit
(Takara Bio, Inc., Otsu, Japan). The integrity of the RNA was
determined, following which the RT reaction was performed in
accordance with the manufacturer's protocol
(Primescript™ RT reagent kit; Takara Bio, Inc.). β-actin
was used as the internal control. The cDNA was stored at −2°C. Each
25-µl reaction sample contained 12.5 µl of SYBR® Premix
Ex Taq II (Takara Bio, Inc.), 1 µl of forward primer, 1 µl of
reverse primer, 1.0 µl of ROX Reference, 1 µl of cDNA and 6.0 µl of
ddH2O. The amplification conditions of RT-qPCR were set
as follows: 95°C for 30 sec, 95°C for 5 sec, 60°C for 34 sec, 95°C
for 15 sec and a total of 40 cycles. The primer sequences and
annealing temperatures are summarized in Table I. The PCR products were analyzed by
ABI Prism 7700 Sequence Detection System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) (16). Relative expression levels of mRNA
were normalized to β-actin expression in each sample, and the data
were analyzed according to the comparative threshold cycle
(2−ΔΔCq) method (17).
All experiments were done in triplicate.
 | Table I.Primer sequences of genes. |
Table I.
Primer sequences of genes.
| Primer | Sequence | Annealing temperature
(°C) |
|---|
| p16ink4a
CDKN2a | Forward
5′-CAGACATCCCCGATTGAAAGAAC-3′ | 56 |
|
| Reverse
5′-GGTAGTGGGGGAAGGCATATATCT-3′ |
|
| β-catenin | Forward
5′-GAGTGCTGAAGGTGCTATCTGTCTG-3′ | 56 |
|
| Reverse
5′-GTTCTGAACAAGACGTTGACTTGGA-3′ |
|
| C-myc | Forward
5′-CCTGGTGCTCCATGAGGAGA-3′ | 56 |
|
| Reverse
5′-CTCCAGCAGAAGGTGATCCAGA-3′ |
|
| Cyclin D1 | Forward
5′-ATGTTCGTGGCCTCTAAGATGA-3′ | 57 |
|
| Reverse
5′-CAGGTTCCACTTGAGCTTGTTC-3′ |
|
| β-actin | Forward
5′-TGGCACCCAGCACAATGAA-3′ | 56 |
|
| Reverse
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
|
Western blot analysis
The protein levels of β-catenin, c-myc and cyclin D1
in the p16ink4a-transfected cells were determined using
western blot analysis. RIPA buffer (1% Nonidet P-40), consisting of
50 mM Tris, 150 mM NaCl, 0.25% deoxycholate, 1 mM EGTA and 1 mM
NaF, was precooled prior to use. The sample, with 1 µl of protease
inhibitor cocktail (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany),
was centrifuged at 1,000 × g at 4°C for 10 min following incubation
on ice for 20 min, and the pellet was harvested.
Protein samples (50 µg/lane) were separated by
electrophoresis on 10% SDS-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred onto polyvinylidene difluoride
(PVDF) membrane filters (EMD Millipore, Billerica, MA, USA) in a
wet transfer system (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The PVDF membranes were placed in methanol for 1 min and then
incubated in TBS at room temperature for 15 min. The membranes were
blocked with 2% BSA for 1 h at room temperature, and then incubated
with anti-beta Catenin antibody (cat. no. ab32572; 1:1,000; Abcam,
Cambridge, UK), Rabbit monoclonal anti-c-Myc antibody (cat. no.
ab32072; 1:1,000; Abcam), Mouse monoclonal anti-cyclin D1 antibody
and mouse monoclonal anti-β actin antibody (cat. no. ab6276;
1:1,000; Abcam) at 4°C overnight. Then the membranes were incubated
by horseradish peroxidase-conjugated goat anti-rabbit (cat. no.
ab6721; 1:5,000; Abcam) or goat polyclonal secondary antibody to
mouse (cat. no. ab6789; 1:5,000; Abcam) for 1 h at room
temperature. The relative protein expression of the membranes was
then normalized to the β-actin levels. The samples were developed
using an enriched chemiluminescence system and then visualized
(Hyperfilm ECL; GE Healthcare Life Sciences, Chalfont, England).
The densities of the immunoreactive bands were quantified using a
gel imaging system (VersaDoc4000; Bio-Rad Laboratories, Inc.). The
mean values were obtained from three independent experiments.
Statistical analysis
Measurement data are presented as the mean ±
standard deviation and were processed using SPSS 17.0 (SPSS, Inc.,
Chicago, IL, USA). Student's t-test (paired) was used for
comparison between groups and one-way analysis of variance was used
for comparison among groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
mRNA expression of
p16ink4a
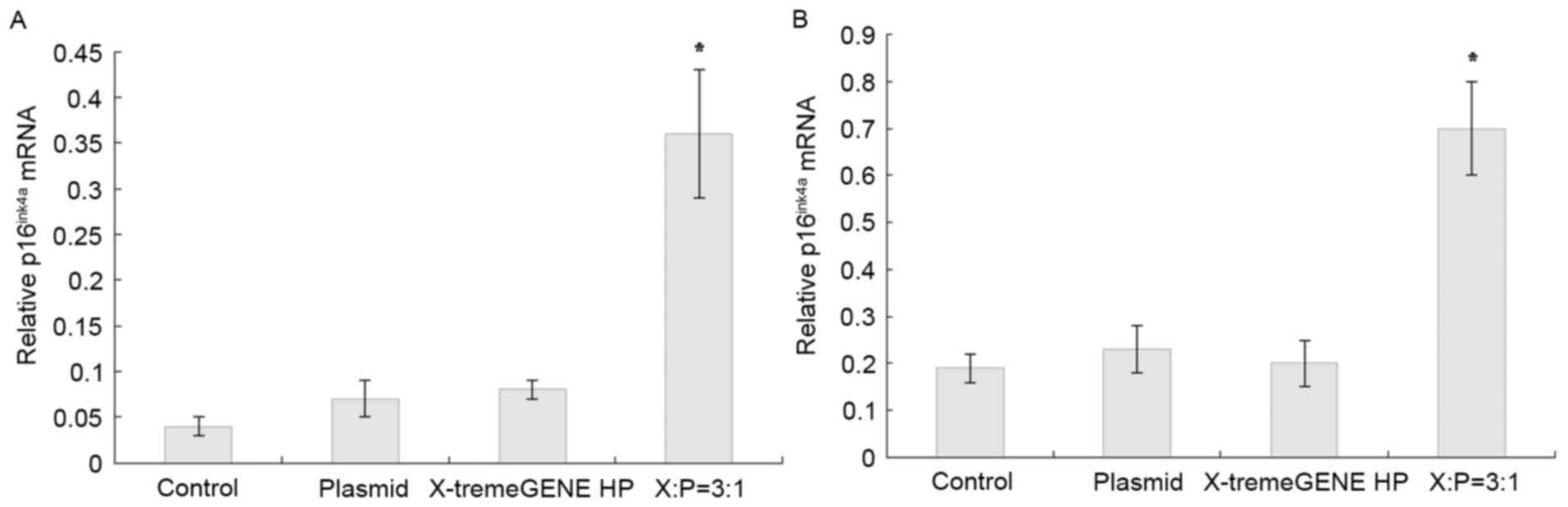
The expression of p16ink4a in the X:P=3:1
group was significantly higher, compared with that in the control,
plasmid and pc-DNA3.0-p16ink4a groups. The lack of
intergroup differences between these three groups (P>0.05)
confirmed successful transfection (Fig. 1).
mRNA expression of key molecules in
the Wnt signaling pathway
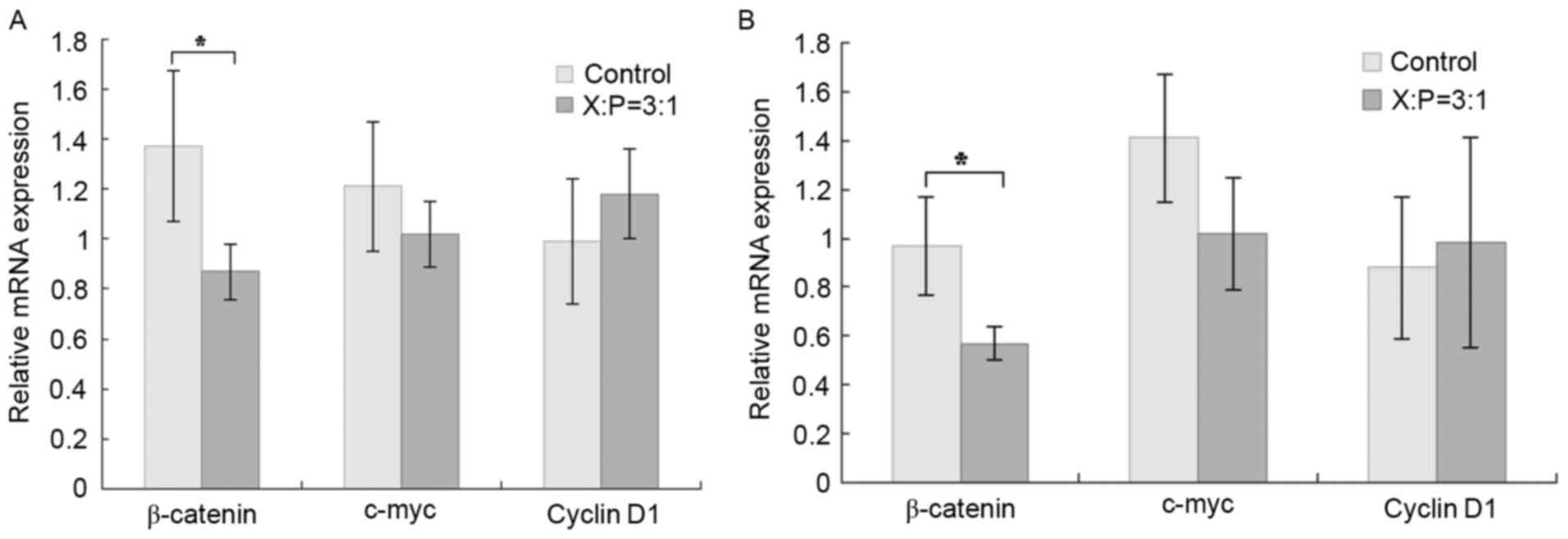
In the cells transfected with p16ink4a,
the expression of β-catenin, a key molecule in the Wnt/β-catenin
signaling pathway, was significantly inhibited (P<0.05), whereas
no significant changes were observed in the mRNA expression levels
of c-myc or cyclin D1 (P>0.05; Fig.
2A and B).
Protein expression of β-catenin, c-myc
and cyclin D1
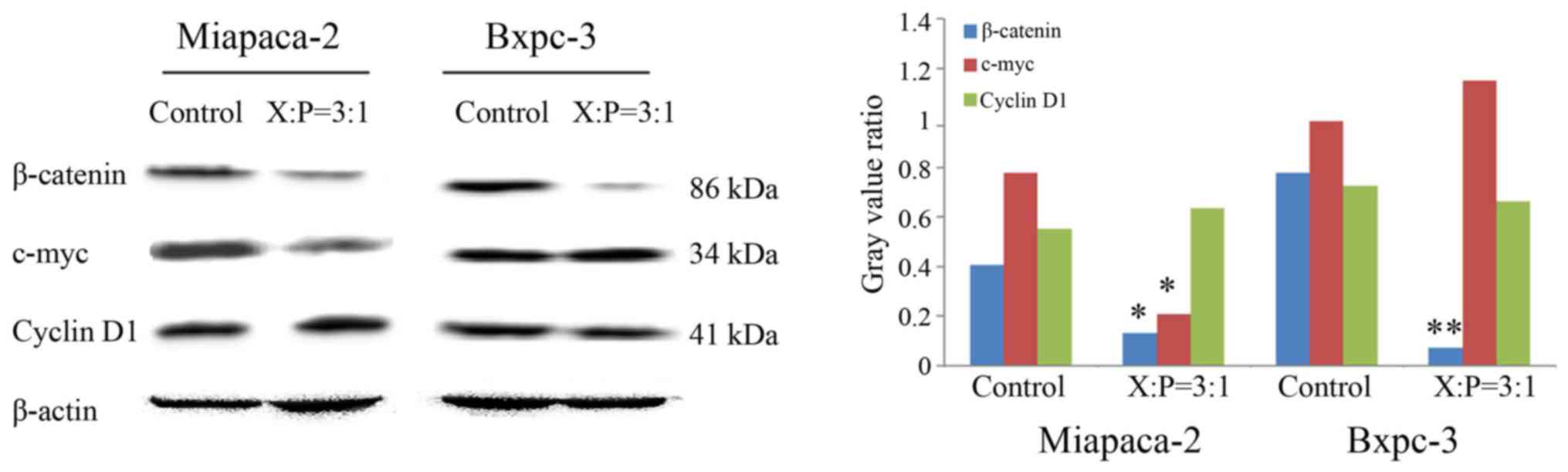
The protein expression levels of β-catenin, a key
molecule in the Wnt signaling pathway, were measured in the
pcDNA3.0-p16ink4a-transfected Bxpc-3 and Miapaca-2 cell
lines. The protein level of c-myc was decreased in the Miapaca-2
cells (P<0.05), however, the level was not altered significantly
in the Bxpc-3 cells (P>0.05). No significant alteration in the
protein level of cyclin D1 was detected in either cell line
(P>0.05; Fig. 3).
Discussion
P16ink4a is a key modulator of the cell
cycle and cell aging, although the correlation between the
P16ink4a protein and the Wnt/β-catenin signaling pathway
in pancreatic cancer remains to be fully elucidated. In the present
study, the overexpression of p16ink4a inhibited the
expression of β-catenin, c-myc and cyclinD, which are key molecules
in the Wnt/β-catenin signaling pathway.
P16ink4a, first identified in 1994, is a
significant tumor suppressor gene, and is involved in cell cycle
modulation, including the inhibition of cell proliferation and
division (18). Therefore, this
molecule is also referred to as a ‘slow brake’ of the cell cycle
(19,20). The p16 gene also modulates the
oxidative stress reaction (21).
P16 gene mutation and inactivation may occur during the early
phases of tumor development, which may inhibit cancer progression.
In several types of malignancy, abnormal activation of the
Wnt/β-catenin signaling pathway can result in the accumulation of
intracellular β-catenin and its subsequent entry into the nucleus
through transmembrane channels. In the nucleus, β-catenin binds to
the transcriptional TCF/LEF, which contributes to the increased
expression of downstream target genes or the silencing of genes
(22–24); the most commonly affected genes are
cyclin D1 and c-myc (24). Certain
medications, including ginsenoside, ginseng extract Rg1 and vitamin
E, can downregulate Wnt/β-catenin signaling, restrict the oxidative
stress response, and reduce the activity of the
p16Ink4a-Rb and p53-p21Cip1/Waf1 pathways, leading to
the amelioration of DNA damage and aging (25). These effects indicate a potential
positive correlation between p16ink4a and Wnt/β-catenin.
In the present study, the overexpression of p16ink4a in
pancreatic cancer cells significantly inhibited the expression of
β-catenin. Of note, no significant changes in the levels of cyclin
D1 and c-myc were observed. Few studies have focused on the
association between p16Ink4a and the Wnt/β-catenin
signaling pathway. The abnormal activation of Wnt/β-catenin and the
associated inhibition of malignant melanoma may be the primary
mechanism of cell immortalization in malignant melanoma. In
addition, the knockout of the Lrp5 gene was reported to result in a
significant increase in the expression of p16Ink4a in
breast ductal epithelium and basal cells (15). Compared with previous studies on
the effects of the Wnt/β-catenin signaling pathway on
p16Ink4a, the present study focused on the effects of
p16Ink4a on the Wnt/β-catenin signaling pathway. The
results showed that the overexpression of p16ink4a in
pancreatic cancer cells significantly inhibited the expression of
β-catenin but did not affect the levels of cyclin D1 or c-myc.
With activation of the K-ras gene, the expression of
cyclin D1 is increased, whereas the expression of
p16ink4a is decreased. However, whether changes in the
expression of p16ink4a are early events or are the
result of pancreatic cancer progression remain to be elucidated
(26). The expression of cyclin D1
and E2F1 has been reported to be regulated by intracellular
p16ink4a in cells from humans and rats (27). In EH2 cells treated with
actinomycin D-IPTG, the protein level of P16 was significantly
increased, leading to a two-fold increase in the protein expression
of cyclin D1 and E2F1, compared with that in the mock-treatment
group; this result indicated the synergistic effect of p16 and
cyclin D1 (27). As a downstream
effector molecule of the Wnt/β-catenin signaling pathway, the
activity of c-myc differs from that of p16. However, previous
studies have found that the overexpression of c-myc induced cell
aging, suggesting a potential mechanism for c-myc-associated DNA
damage and increased reactive oxygen species (26,28).
In the present study, the increase in the expression of
p16ink4a significantly inhibited the expression of
β-catenin, a key molecule in the Wnt/β-catenin signaling pathway,
whereas no significant changes were observed in the expression of
cyclin D1 or c-myc in the pancreatic cancer cell lines
examined.
In terms of the limitations of the present study,
the results were derived only from in vitro experiments, and
the external environment may have introduced bias into the results.
In the development of pancreatic cancer, the abnormal expression of
genes is an early event accompanied with a series of changes,
including the increased expression of oncogenes and decreased
expression of anti-oncogenes. The results of the present study
showed that high levels of p16ink4a significantly
inhibited the expression of β-catenin but did not affect the
expression of cyclin D1 or c-myc. The mechanism responsible for
these results may involve the positive regulation of other
compensatory pathways, and further investigations are required with
a focus on Rb, a downstream molecule of p16ink4a.
In conclusion, the present study demonstrated that
the overexpression of p16ink4a inhibited the expression
of β-catenin but did not affect the expression of downstream
proteins c-myc and cyclin D1. The interrelation between
p16ink4a, c-myc and cyclin D1 suggests a dual-direction
regulatory mechanism involving multiple pathways.
Acknowledgements
This study was supported by the Gansu Provincial
Natural Science Funds (grant no. 1606RJZA139).
References
|
1
|
Ilic M and Ilic I: Epidemiology of
pancreatic cancer World. J Gastroenterol. 22:1–9705. 2016.
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Buscail L, Bournet B, Dufresne M,
Torrisani J and Cordelier P: Advance in the biology of pancreatic
of cancer. Bull Cancer. 102 6 Suppl 1:S53–S61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bykov VJ and Wiman KG: Mutant p53
reactivation by small molecules makes its way to the clinic. FEBS
Lett. 588:2622–2627. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang Z, Ju H, Ling J, Zhuang Z, Li Z,
Wang H, Fleming JB, Freeman JW, Yu D, Huang P and Chiao PJ:
Cooperativity of oncogenic K-ras and downregulated p16/ink4a in
human pancreatic tumorigenesis. PLoS One. 9:e1014522014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hosotani R, Miyamoto Y, Fujimoto K, Doi R,
Otaka A, Fujii N and Imamura M: Trojan p16 peptide suppresses
pancreatic cancer growth and prolongs survival in mice. Clin Cancer
Res. 8:1271–1276. 2002.PubMed/NCBI
|
|
8
|
Kozak G, Blanco FF and Brody JR: Novel
targets in pancreatic cancer research. Semin Oncol. 42:177–187.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vilchez V, Turcios L, Marti F and Gedaly
R: Targeting Wnt/β-catenin pathway in hepatocellular carcinoma
treatment. World J Gastroenterol. 22:823–832. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signaling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McCubrey JA, Rakus D, Gizak A, Steelman
LS, Abrams SL, Lertpiriyapong K, Fitzgerald TL, Yang LV, Montalto
G, Cervello M, et al: Effects of mutations in Wnt/β-catenin,
hedgehog, Notch and PI3K pathways on GSK-3 activity-Diverse effects
on cell growth, metabolism and cancer. Biochim Biophys Acta.
1863:2942–2976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Froeling FE, Feig C, Chelala C, Dobson R,
Mein CE, Tuveson DA, Clevers H, Hart IR and Kocher HM: Retinoic
acid-induced pancreatic stellate cell quiescence reduces paracrine
Wnt-β-catenin signaling to slow tumor progression.
Gastroenterology. 141:1486–1497. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fargnoli MC, Gandini S, Peris K,
Maisonneuve P and Raimondi S: MC1R variants increase melanoma risk
in families with CDKN2A mutations: A meta-analysis. Eur J Cancer.
46:1413–1420. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goldstein AM, Chan M, Harland M,
Gillanders EM, Hayward NK, Avril MF, Azizi E, Bianchi-Scarra G,
Bishop DT, Bressac-de Paillerets B, et al: High-risk melanoma
susceptibility genes and pancreatic cancer, neural system tumors,
and uveal melanoma across GenoMEL. Cancer Res. 66:9818–9828. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Badders NM, Goel S, Clark RJ, Klos KS, Kim
S, Bafico A, Lindvall C, Williams BO and Alexander CM: The Wnt
receptor, Lrp5, expressed by mouse mammary stem cells and is
required to maintain the basal lineage. PLoS One. 4:e65942009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Exner MM and Lewinski MA: Sensitivity of
multiplex real-time PCR reactions, using the LightCycler and the
ABI PRISM 7700 sequence detection system, is dependent on the
concentration of the DNA polymerase. Mol Cell Probes. 16:351–357.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamb A: Role of a cell cycle regulator in
hereditary and sporadic cancer. Cold Spring Harb Symp Quant Biol.
59:39–47. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Holst CR, Nuovo GJ, Esteller M, Chew K,
Baylin SB, Herman JG and Tlsty TD: Methylation of p16(INK4a)
promoters occurs in vivo in histologically normal human mammary
epithelia. Cancer Res. 63:1596–1601. 2003.PubMed/NCBI
|
|
20
|
Attri J, Srinivasan R, Majumdar S, Radotra
BD and Wig J: Alterations of tumor suppressor gene p16ink4a in
pancreatic ductal carcinoma. BMC Gastroenterol. 5:222005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Whelan AJ, Bartsch D and Goodfellow PJ:
Brief report: A familial syndrome of pancreatic cancer and melanoma
with a mutation in the CDKN2a tumor-suppressor gene. N Engl J Med.
333:975–977. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Witkiewicz AK, Borja NA, Franco J, Brody
JR, Yeo CJ, Mansour J, Choti MA, McCue P and Knudsen ES: Selective
impact of CDK4/6 suppression on patient-derived models of
pancreatic cancer. Oncotarget. 6:15788–15801. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nusse R: Wnt signaling in disease and in
development. Cell Res. 15:28–32. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
MacDonald BT, Tamai K and He X:
Wnt/beta-catenin signaling: Components, mechanisms, and diseases.
Dev Cell. 17:9–26. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li J, Cai D, Yao X, Zhang Y, Chen L, Jing
P, Wang L and Wang Y: Protective effect of ginsenoside Rg1 on
hematopoietic stem/progenitor cells through attenuating oxidative
stress and the Wnt/β-catenin signaling pathway in a mouse model of
d-galactose-induced aging. Int J Mol Sci. 17:pii: E8492016.
View Article : Google Scholar
|
|
26
|
Campaner S, Doni M, Hydbring P, Verrecchia
A, Bianchi L, Sardella D, Schleker T, Perna D, Tronnersjö S, Murga
M, et al: Cdk2 suppresses cellular senescence induced by the c-myc
oncogene. Nat Cell Biol. 12:54–59. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Khalaf HH, Colak D, Al-Saif M,
Al-Bakheet A, Hendrayani SF, Al-Yousef N, Kaya N, Khabar KS and
Aboussekhra A: p16(INK4a) positively regulates cyclin D1 and E2F1
through negative control of AUF1. PLoS One. 6:e211112011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ray S, Atkuri KR, Deb-Basu D, Adler AS,
Chang HY, Herzenberg LA and Felsher DW: Myc can induce DNA breaks
in vivo and in vitro independent of reactive oxygen species. Cancer
Res. 66:6598–6605. 2006. View Article : Google Scholar : PubMed/NCBI
|