Introduction
According to the spleen-stomach theory in
traditional Chinese medicine (TCM), the spleen is not anatomically,
physiologically or pathophysiologically synonymous with one organ
as it is in western medicine (1,2). In
TCM, the term spleen is used to describe the digestive system,
including its vegetative nervous system, immunity, hemopoiesis,
muscle metabolism, endocrine function, hepatic metabolic function,
and protein, nucleotide, energy, water and salt metabolism
(1,2). Spleen deficiency is characterized by
symptoms including epigastralgia, flatulence, lack of appetite,
wilted complexion, loose stools, lassitude and fatigue. Spleen
deficiency is one of the most common digestive diseases in China
and is frequently associated with imbalances in the
gastrointestinal microflora (3,4).
Therefore, clinical studies are being conducted in order to
identify an association between TCM and intestinal microbiota and
to contribute to the treatment of spleen deficiency (1,5,6).
Jianweixiaoshi tablets were approved by the Chinese
Ministry of Health as one of the national protected traditional
medicines in 1995 and were listed as the first class of
over-the-counter drugs in 1999. Jianweixiaoshi tablets, containing
hawthorn, malt, tangerine peel, Radix Pseudostellariae and
yam, are used for treatment of indigestion, anorexia and abdominal
distension, via invigoration of the stomach and tonification the
spleen. Jianweixiaoshi tablets have been demonstrated to promote
gastrointestinal peristalsis and gastric secretion of digestive
juices, and enhance the activity of pepsin, general physique and
immune function (7). In China,
sales of Jianweixiaoshi tablets generate >1.2 billion renminbi
in income each year, although they also produce ~100,000 tons of
herb residues which are primarily used in food additives (7,8).
Herb residues are by-products of TCM materials
extracted by water or ethanol, and ~30–50% of the medically active
ingredients remain inaccessible (9). The microorganism fermentation theory
in TCM suggests that digestive enzymes, including cellulase,
protease, pectinase, lignin and lipase, produced by microorganisms
may effectively degrade plant cell walls, expand the intercellular
region and improve the yield of extraction of active ingredients
(10). In addition, microorganisms
may degrade macromolecules to smaller molecules, making them
accessible for direct absorption by the human body, reduce the side
effects of drugs by degrading toxic substances and introduce novel
therapeutic effects by biological modification. In addition,
probiotics have been defined as ‘live microorganisms that, when
administered in adequate amounts, confer a health benefit on the
host’, including anti-carcinogenic and anti-mutagenic properties,
immune stimulation and lowering of serum cholesterol (11,12).
Probiotics may be used for the prevention and treatment of certain
pathological conditions (13,14).
In the present study, herb residues of
Jianweixiaoshi tablets were reused via probiotics, a
spleen-deficient mouse model was established and the therapeutic
effect of the herb residue fermentation supernatant on spleen
deficiency was evaluated.
Materials and methods
Preparation of herb residue extract
and fermentation supernatant
The herb residue of Jianweixiaoshi tablets was
obtained from Jiangsu Yangtze River Pharmaceutical Group Company,
Ltd. (Taizhou, China) and homogenized for 2 h using a pulper.
Bacillus subtilis (isolated from Douchi and stored in
Professor Chen's laboratory at Nanchang University), Aspergillus
oryzae (isolated from Douchi and stored in Professor Chen's
laboratory as above) and Lactobacillus plantarum M3
(isolated from sourdough and stored in in Professor Chen's
laboratory as above) bacteria (all 108 colony forming
U/ml) were used as the inoculum for the preparation of the herb
residue fermentation supernatant. B. subtilis and A.
oryzae were added to the herb residue for 24 h, and L.
plantarum M3 was added for 24–36 h at 37°C. Fermented products
were subsequently centrifuged for 30 min at 1,000 × g at 4°C
in a refrigerated centrifuge (Hunan Xiangyi Laboratory Instrument
Development Co., Ltd., Changsha, China) to obtain the fermentation
supernatant.
Spleen-deficient mouse model and
treatment
The present study was approved by the Ethical
Committee of the Second Affiliated Hospital of Nanchang University
(Nanchang, China) and all methods were performed in accordance with
the approved guidelines.
A total of 30 specific pathogen free 6–8-week-old
male C57BL/6 mice weighting 20–30 g were housed and fed a
commercial diet with water ad libitum. All mice were
purchased from the Hebei Center for Disease Control and Prevention
(Hebei, China), housed five per cage, on a 12-h light/dark cycle at
23±2°C and at 50±10% relative humidity. To establish the
spleen-deficient mice model, 20 ml/kg/day 100% rhubarb decoction
(supplied by Jiangxi Provincial Hospital of Traditional Chinese
Medicine, Jiangxi, China) was administered intragastrically to mice
for 7 days. All control animals were treated with the equivalent
volume of PBS. Subsequently, model mice were divided into 3 groups:
i) The modeling group (n=10), in which mice were only administered
PBS; ii) the probiotics + drug residues group (n=10), in which mice
were administered herb residue fermentation supernatant (0.1 ml/20
g); and iii) the Jianweixiaoshi tablets group (n=10), in which mice
were administered Jianweixiaoshi tablets (0.1 ml/20 g). Mouse feces
were collected at four time-points: i) During the control stage, on
day 0, prior to treatment; ii) during the modeling stage, on day 7,
following treatment with rhubarb decoction; iii) during the
treatment stage, on day 14, following drug treatment; and iv)
during the recovery stage, on day 21. In each group, feces of three
mice from the modeling, probiotics + drug residue and
Jianweixiaoshi tablets groups were randomly selected and used for
denaturing gradient gel electrophoresis (DGGE) analysis.
Determination of immune indices and
inflammatory factors
A total of 24 h following the final drug
administration, five animals in each group were sacrificed by
decapitation. Tail vein method was used to obtain the whole blood,
and serum was obtained by the centrifugation of clotted blood at
2,500 × g for 15 min at 4°C, and interleukin (IL)-2, IL-4 and
interferon-γ (IFN-γ) were determined using ELISA kits for IL-2
(88-7024-88; Mouse IL-2 ELISA Ready-SET-Go!), IL-4 (88-7044-22;
Mouse IL-4 ELISA Ready-SET-Go!) and IFN-γ (BMS606; Mouse IFN-γ
Platinum ELISA; all from eBioscience; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) (15).
Spleen and thymus were harvested from mice and weighed immediately.
Thymus and spleen indices were calculated according to the
following formula: Thymus or spleen index = [(weight of thymus or
spleen)/body weight] × 100.
Viable cell count
Fresh fecal samples were subjected to treatment
within 2 h following collection. All samples were serially diluted
10-fold with saline solution and 300-µl solutions resulting from
each dilution were separately plated on a brain-heart infusion agar
supplemented with 10% sterile skimmed milk (Beijing Land Bridge
Technology Co., Ltd., Beijing, China) for total anaerobic bacteria,
and incubated anaerobically at 37°C for 36 h. De Man, Rogosa and
Sharpe agar (Oxoid; Thermo Fisher Scientific, Inc.) was used for
anaerobic culture of Lactobacilli at 37°C for 24 h.
Enterococci were aerobically cultured at 37°C for 24 h on
Slanetz-Bartley medium agar and MacConkey agar (both from Oxoid;
Thermo Fisher Scientific, Inc.) was used for aerobic culture of
Enterobacteria at 37°C for 24 h (16–18).
DGGE and statistical analysis
DNA was isolated using bead-beating method (19). Following phenol-chloroform
extraction, DNA was precipitated with 75% ethanol and resuspended
in 50 µl TE buffer (10 mM Tris-Cl, 1 mM EDTA; pH 7.6). Primers,
including 357 forward (5′-TACGGGAGGCAGCAG-3′) and 519 reverse
(5′-ATTACCGCGGCTGCTGG-3′), were used to amplify total bacterial
DNA, and Lac1 (5′-AGCAGTAGGGAATCTTCCA-3′) and Lac2
(5′-ATTYCACCGCTACACATG-3′) were used to amplify DNA from
Lactobacillus species. GC clam-in primers were selected to
generate GC-enriched polymerase chain reaction (PCR) products
suitable for separation by DGGE. Subsequently, a PCR was performed
using the Taq DNA Polymerase kit (Takara Biotechnology Co., Ltd.,
Dalian, China) in a Biosci PCR system, with 30 cycles of 94°C for
30 sec, 56°C for 30 sec, and 72°C for 60 sec. Amplicons of 16S
ribosomal RNA were used for sequence-separation by DGGE, as
previously described (20,21). DGGE was performed on 8%
polyacrylamide gels containing acrylamide, bisacrylamide, formamide
and a gradient of 35–65% urea. Tris-HCl (40 mM; pH 8.0) was used as
the electrophoresis buffer in a Bio-Rad DGGE system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Electrophoresis was
initiated by a pre-run for 5 min at 220 V followed by run at a
fixed voltage of 85 V for 16 h at 60°C. Gels were stained with
AgNO3 and developed following electrophoresis.
Subsequently, gels were covered with cellophane membranes and dried
overnight at 4°C. DGGE patterns were subsequently normalized and
analyzed using Bionumeric software (version 2.0; Applied Maths,
Sint-Martens-Latem, Belgium). During the processing, lanes were
defined and the background was subtracted. In the process of
normalization, differences in the intensity of the lanes were
compensated and the association matrix was calculated. Clustering
was performed using the Pearson correlation and unweighted pair
group method with arithmetic mean (UPGMA) methods.
Sequencing of DGGE bands
Bands of interest were excised from the gel using a
sterile blade and incubated overnight at 4°C in Tris-EDTA buffer
(pH 8.0) to allow for DNA diffusion out of the polyacrylamide
matrix. The solution was used directly for further amplifications.
For sequencing, eluted DNA was amplified using the same primer
pairs and conditions as described above, without the GC clamp. PCR
products for sequencing were purified using a QIAquick PCR
purification kit (Qiagen GmbH, Hilden, Germany). PCR products were
subcloned using the pMD18-T vector system I (Takara Biotechnology
Co., Ltd.) according to the manufacturer's instructions. E.
coli DH5α cells (Beijing Tiangen Biochemical Technology Co.,
Ltd., Beijing, China) were subjected to electrotransformation with
recombinant plasmids. Selection of transformants was performed on
lysogeny broth agar (Oxoid; Thermo Fisher Scientific, Inc.)
containing 100 mg/ml ampicillin. Transformants were randomly
selected and sequenced by Invitrogen (Thermo Fisher Scientific,
Inc.) (22–24).
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were analyzed using SPSS software (version 13.0; SPSS Inc.,
Chicago, IL, USA) using one-way analysis of variance with the least
significant difference post hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Treatment with fermentation
supernatant increases body weight, thymus and spleen indices in
mice
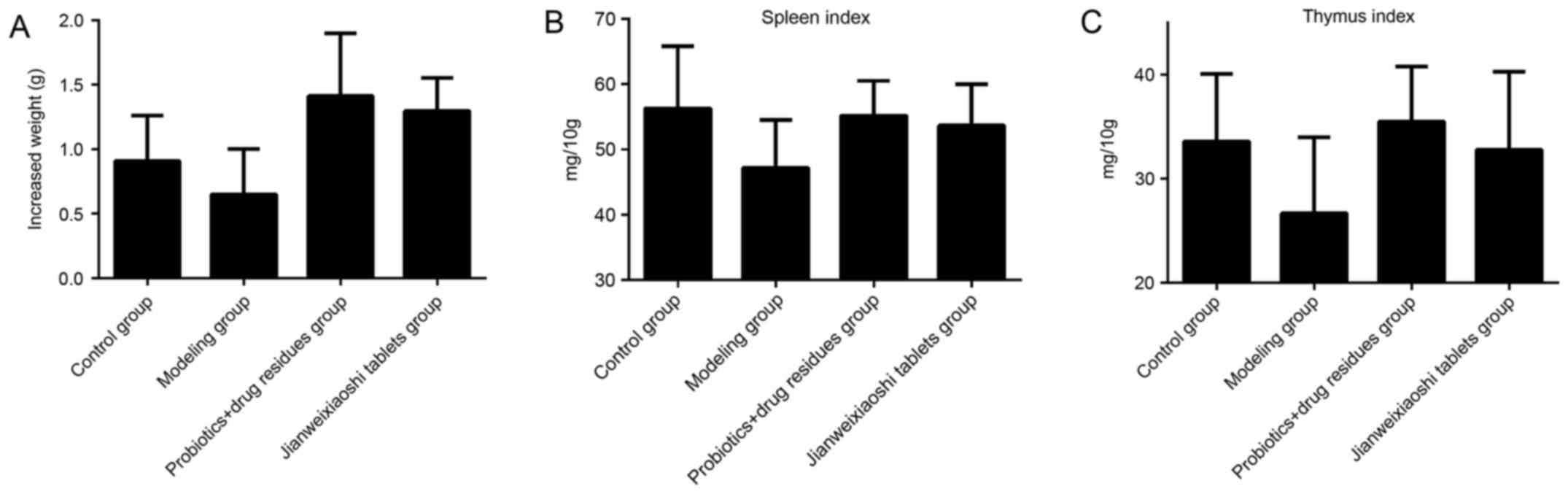
Compared with the control group, treatment with
rhubarb reduced body weight, spleen index and thymus index
(Fig. 1) in the modeling group,
and presented depressive behavior, constipation, unkempt fur and
poor appetite. Treatment with herb residue fermentation supernatant
and Jianweixioashi tablets resulted in an increase in body weight,
spleen index and thymus index, although these differences were not
statistically significant.
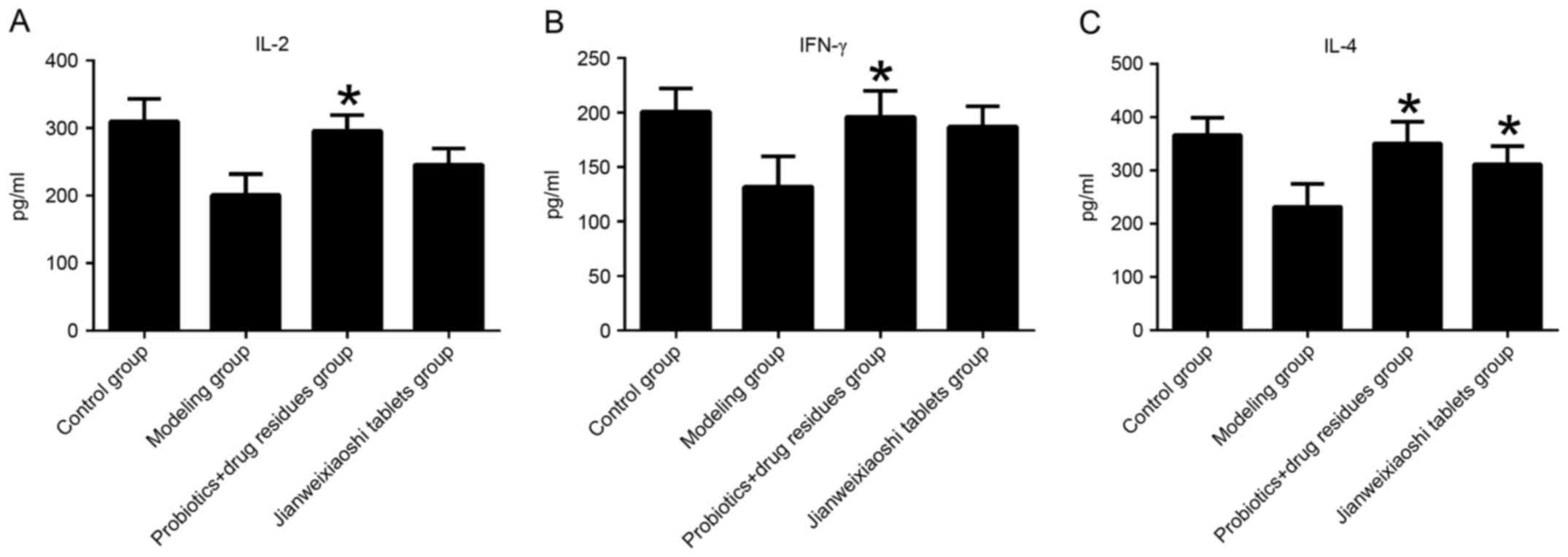
Levels of IL-2, IL-4 and IFN-γ in
serum
Treatment with rhubarb decreased the levels of IL-2,
IL-4 and IFN-γ in the modeling group, compared with the control
group. Administration of probiotics with herb residues
significantly increased levels of IL-2, −4 and IFN-γ, compared with
the modeling group (all P<0.05; Fig. 2). Jianweixioashi tablets
significantly increased levels of IL-4, compared with the modeling
group (P<0.05).
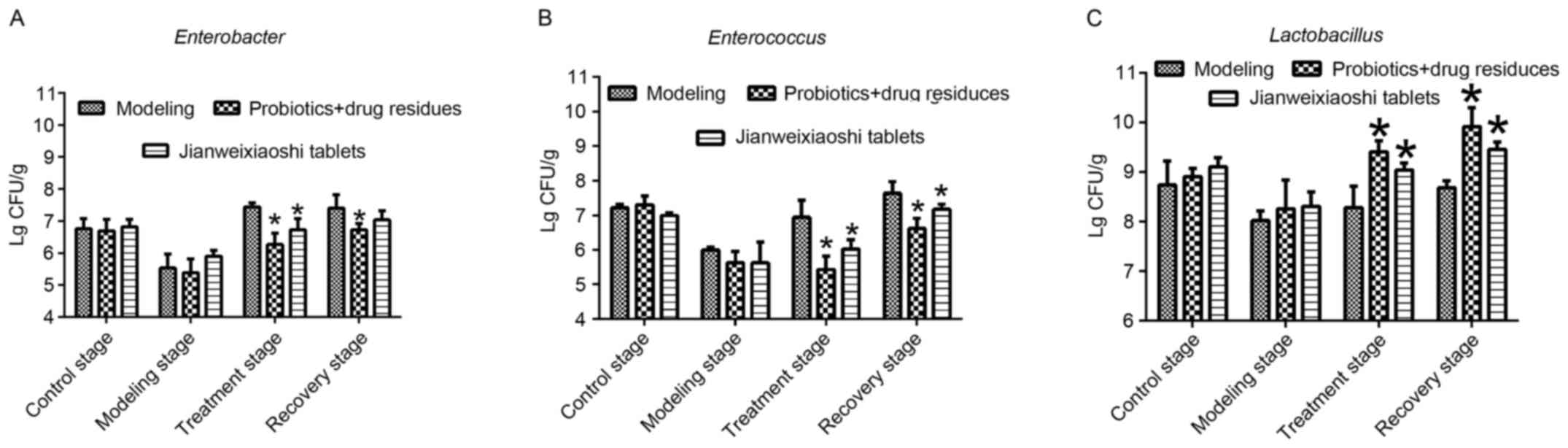
Effects of fermentation supernatant on
microbial number in vivo
The effects of the fermentation supernatant on the
viable number of Enterococci, Enterobacteria and
Lactobacilli were determined in the present study. Viable
cell counts indicated that, during the treatment stage,
fermentation supernatant and Jianweixioashi tablets increased the
number of Lactobacilli, and reduced the number of
Enterococci and Enterobacteria, compared with the
modeling group (all P<0.05; Fig.
3). During the recovery stage, fermentation supernatant
significantly inhibited the growth of Enterococci and
Enterobacteria, and promoted the growth of
Lactobacilli, compared with the modeling group
(P<0.05).
Effects of the herb residue
fermentation supernatant on bacterial diversity in vivo
The results of the DGGE analysis indicated that an
uncultured bacterium, L. murinus, and Enterococcus
sp. were the dominant bacteria in all groups throughout the
experiments. In addition, the DGGE profile indicated that treatment
with rhubarb markedly reduced the band number, while the
administration of fermentation supernatant and Jianweixiaoshi
tablets enhanced the bacterial diversity in the treatment and
recovery stages (Fig. 4; Table IA). However, the results of the
UPGMA analysis demonstrated low levels of similarity between
bacteria in the control, treatment and recovery stages in the
probiotics + drug residues and Jianweixiaoshi tablets treatment
groups.
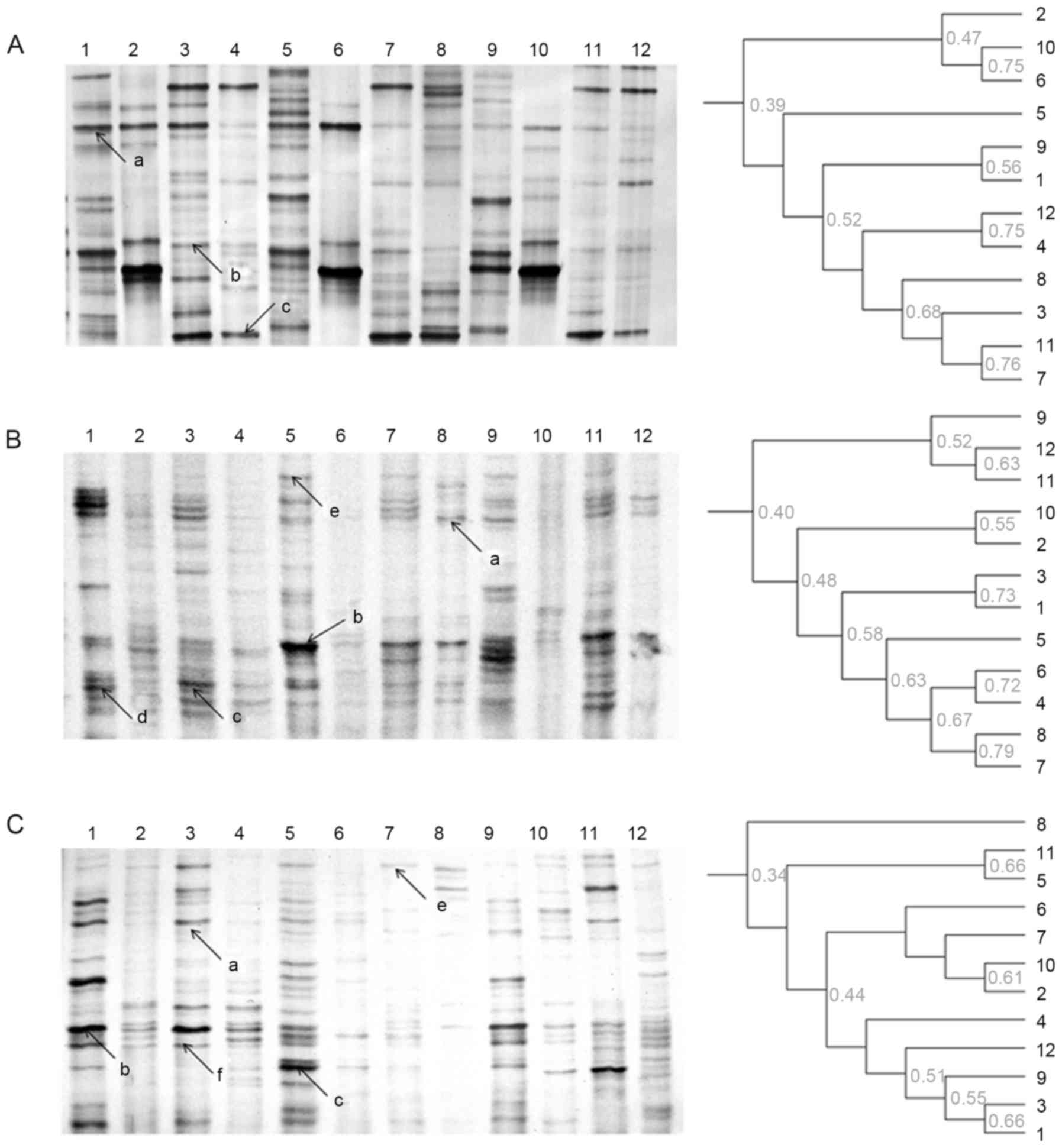 | Figure 4.DGGE profile and unweighted pair
group method with arithmetic mean analysis of fecal microbiota
using bacterial primers. DGGE profile of (A) the modeling group,
(B) the probiotics + drug residues group and (C) the Jianweixiaoshi
tablets group. Lanes 1–3, control stage samples; lanes 4–6,
modeling stage samples; lanes, 7–9 treatment stage samples; and
lanes 10–12, recovery stage samples. a, uncultured bacterium; b,
Lactobacillus murinus; c, Enterococcus sp.; d,
Clostridium sp.; e, uncultured bacterium; f, uncultured
bacterium. DGGE, denaturing gradient gel electrophoresis. |
 | Table I.Strains identified from mouse
intestines by denaturing gradient gel electrophoresis using
bacterial primers and Bacillus primers. |
Table I.
Strains identified from mouse
intestines by denaturing gradient gel electrophoresis using
bacterial primers and Bacillus primers.
| A, Bacterial
primers |
|---|
|
|---|
| Strain no. | Closest
relatives | Similarity, % | GenBank no. |
|---|
|
|---|
| a | Uncultured
bacterium | 100 | HQ321493.1 |
| b | Lactobacillus
murinus | 100 | HQ668465.1 |
| c | Enterococcus
sp. | 100 | JF910016.1 |
| d | Clostridium
sp. | 100 | JF813180.1 |
| e | Uncultured
bacterium | 100 | GU606372.1 |
| f | Uncultured
bacterium | 100 | JF837882.1 |
|
| B,
Bacillus primers |
|
| Strain
no. | Closest
relatives | Similarity,
% | GenBank
no. |
|
| a | Uncultured
bacterium | 99 | HM363549.1 |
| b | Uncultured
bacterium | 100 | EU491355.1 |
| c | Lactobacillus
murinus | 100 | HQ668465.1 |
| d | Uncultured
bacterium | 100 | HM363550.1 |
| e | Uncultured
bacterium | 100 | EU475615.1 |
| f | Uncultured
bacterium | 100 | EU006313.1 |
| g | Escherichia
fergusonii | 100 | HQ259962.1 |
| h | Uncultured
bacterium | 100 | FJ881122.1 |
Effects of the herb residue
fermentation supernatant on Bacillus diversity in vivo
As demonstrated by Bacillus DGGE profiles,
treatment with rhubarb induced an effect on L. murinus, and
uncultured bacteria d and f (Fig.
5). L. murinus was the dominant bacterium in all groups
and during all stages. In addition, treatment with rhubarb
eliminated uncultured bacterium d during the modeling stage in the
probiotics + drug residue treatment group, while treatment with
fermentation supernatant recovered the growth of uncultured
bacterium d during the treatment and recovery stages (Fig. 5; Table
IB).
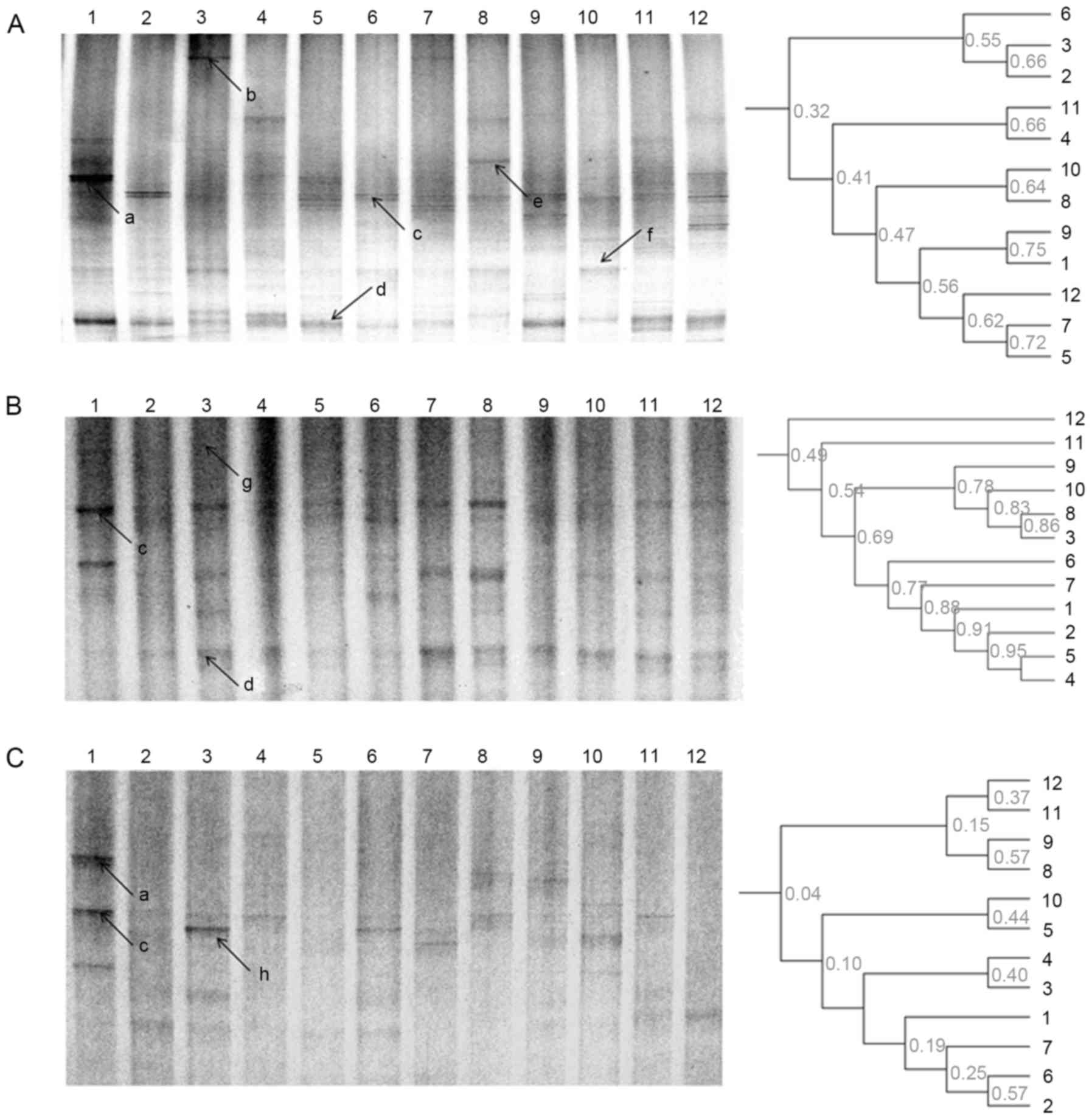 | Figure 5.DGGE profile and unweighted pair
group method with arithmetic mean analysis of fecal microbiota
using Bacillus primers. DGGE profile of (A) the modeling
group, (B) the probiotics + drug residues group and (C) the
Jianweixiaoshi tablets group. Lanes 1–3, control stage samples;
lanes 4–6, modeling stage samples; lanes, 7–9 treatment stage
samples; and lanes 10–12, recovery stage samples. a, uncultured
bacterium; b, uncultured bacterium; c, Lactobacillus
murinus; d, uncultured bacterium; e, uncultured bacterium; f,
uncultured bacterium; g, Escherichia fergusonii; h,
uncultured bacterium. DGGE, denaturing gradient gel
electrophoresis. |
Discussion
Large amounts of microbes have been identified in
human intestines, particularly lactic acid bacteria, forming a
complex ecological community that influences physiological
homeostasis and serves a role in maintaining human health,
including protection against entero-pathogens, extraction of
nutrients and energy, and maintenance of normal immune functions
(25–28). Jianweixiaoshi tablets have been
demonstrated to alleviate various spleen-stomach diseases
associated with intestinal bacteria, and the active ingredients
identified in herb residues demonstrated a therapeutic effect on
these diseases (29,30). Therefore, the combination of
probiotics and herb residues may exert positive effects on
spleen-stomach diseases.
In the present study, a spleen-deficient mouse model
was established, which demonstrated symptoms comprising depressive
behavior, constipation, unkempt fur and poor appetite (5,6).
When treated with the herb residue fermentation supernatant, the
mice exhibited a minor increase in body weight, spleen index and
thymus index, suggesting that the fermentation supernatant was able
to enhance growth and immunity in the mice.
Cytokines serve a role in immune responses. IL-2 and
IFN-γ are classified as T helper 1 cytokines, and stimulate the
proliferation of cytotoxic T lymphocytes, helper T lymphocytes,
natural killer cells, lymphokine activated killer cells and
macrophages (31–34). IL-4 is a T helper 2 cytokine,
serving a role in the stimulation of activated B-cells, the
proliferation of T-cells and the differentiation of B cells into
plasma cells, and it has additionally been implicated in the
regulation of humoral and adaptive immunity (31,32).
Therefore, increased production of IL-2, IL-4 and IFN-γ indicated
that the fermentation supernatant enhanced the immunity of the
spleen-deficient mice.
Certain diseases, including obesity, malnutrition,
inflammatory bowel disease, neurological disorders and cancer, may
result from microbial imbalances (35–39).
Considering the health-promoting effect of TCM and probiotics,
fermentation supernatant may be hypothesized to enhance microbial
diversity, promote the growth of probiotics and inhibit the growth
of pathogens. The results of the viable cell counts in the present
study indicated that the fermentation supernatant markedly enhanced
the number of Lactobacilli and reduced the number of
Enterococci and Enterobacteria during the treatment
stage. The results of the DGGE analysis indicated that the
fermentation supernatant enhanced bacterial diversity during the
treatment and recovery stages. In the present study, low similarity
levels between bacteria in the control, treatment and recovery
stages indicated that a novel microbial balance was established by
treatment with the fermentation supernatant. The diversity of
bacteria in host intestines contributes to more efficient host
defenses from external invasion and, therefore, the reduced number
of bands in the modeling group indicated weak resistance to foreign
pathogens, while enhanced diversity in the fermentation supernatant
group indicated recovery of intestinal health (28,36,37,40).
In conclusion, in the present study, the therapeutic
effects of herb residues from Jianweixiaoshi tablets were used for
the treatment of spleen deficiency in mice in vivo. The
results of the present study indicated that fermentation
supernatant was able to enhance host immunity and inflammatory
responses, by increasing the number and diversity of beneficial
bacteria. Therefore, the present study demonstrated that the
combination of Jianweixiaoshi herb residues and probiotics may, in
the future, provide a novel method for the treatment of spleen
deficiency.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (grant nos. 81503364,
31560264, 31360377, 91639106, 81270202 and 91339113), the National
Basic Research Program of China (grant no. 2013CB531103) and grants
from Jiangxi Province (grant nos. 20171BCB23028 and 20175526).
References
|
1
|
Zhang S, Zhao L, Wang H, Wang C, Huang S,
Shen H, Wei W, Tao L and Zhou T: Efficacy of modified LiuJunZi
decoction on functional dyspepsia of spleen-deficiency and
qi-stagnation syndrome: A randomized controlled trial. BMC
Complement Altern Med. 13:542013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Si FF, Tu JF, Liu TI and Liu ZJ: The
effects of superfine powder on the total anti-oxidative ability and
no of sijunzi decoction in spleen-deficient mice. Prog Vet Med.
27:R28–R58. 2006.
|
|
3
|
Wu XN: Current concept of Spleen-Stomach
theory and Spleen deficiency syndrome in TCM. World J
Gastroenterol. 4:2–6. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peng Y, Wang Z, Lu Y, Wu CF, Yang JY and
Li XB: Intestinal microflora molecular markers of spleen-deficient
rats and evaluation of traditional Chinese drugs. World J
Gastroenterol. 15:2220–2227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao N, Zhang W, Guo Y, Jia H, Zha Q, Liu
Z, Xu S and Lu A: Effects on neuroendocrinoimmune network of
Lizhong Pill in the reserpine induced rats with spleen deficiency
in traditional Chinese medicine. J Ethnopharmacol. 133:454–459.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li LS, Qu RY, Wang W and Guo H:
Significance of changes of gastrointestinal peptides in blood and
ileum of experimental spleen deficiency rats. World J Gastroentero.
9:553–556. 2003. View Article : Google Scholar
|
|
7
|
Chen T, Xiong S, Jiang S, Wang M, Wu Q and
Wei H: Effects of traditional Chinese medicines on intestinal
bacteria: A review. Indian J Tradit Know. 11:401–407. 2012.
|
|
8
|
Meng F, Yang S, Wang X, Chen T, Wang X,
Tang X, Zhang R and Shen L: Reclamation of Chinese herb residues
using probiotics and evaluation of their beneficial effect on
pathogen infection. J Infect Public Health. 10:749–754. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Selvam A and Wong JW: Effect of
Chinese medicinal herbal residues on microbial community succession
and anti-pathogenic properties during co-composting with food
waste. Bioresour Technol. 217:190–199. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Himmel ME, Ding SY, Johnson DK, Adney WS,
Nimlos MR, Brady JW and Foust TD: Biomass recalcitrance:
Engineering plants and enzymes for biofuels production. Science.
315:804–807. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Diplock AT, Aggett PJ, Ashwell M, Bornet
F, Fern EB and Roberfroid MB: Scientific concepts in functional
foods in Europe: Consensus document. Br J Nutr. 81:S1–S27. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hill C, Guarner F, Reid G, Gibson GR,
Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S,
et al: Expert consensus document: The international scientific
association for probiotics and prebiotics consensus statement on
the scope and appropriate use of the term probiotic. Nat Rev
Gastroenterol Hepatol. 11:506–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang M, Deng K, Jiang C, Fu M, Guo C,
Wang X, Wang X, Meng F, Yang S, Deng K, et al: Evaluation of the
antioxidative, antibacterial, and anti-inflammatory effects of the
aloe fermentation supernatant containing lactobacillus plantarum
HM218749.1. Mediators Inflamm. 2016:29456502016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu D, Jiang XY, Zhou LS, Song JH and
Zhang X: Effects of probiotics on intestinal mucosa barrier in
patients with colorectal cancer after operation: Meta-analysis of
randomized controlled trials. Medicine (Baltimore). 95:e33422016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo F, He H, Fu ZC, Huang S, Chen T,
Papasian CJ, Morse LR, Xu Y, Battaglino RA, Yang XF, et al:
Adipocyte-derived PAMM suppresses macrophage inflammation by
inhibiting MAPK signalling. Biochem J. 472:309–318. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X, Wu Q, Deng K, Wei Q, Hu P, He J,
Liu H, Zheng Y, Wei H, Shah NP and Chen T: A novel method for
screening of potential probiotics for high adhesion capability. J
Dairy Sci. 98:4310–4317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng K, Chen T, Wu Q, Xin H, Wei Q, Hu P,
Wang X, Wang X, Wei H and Shah NP: In vitro and in vivo examination
of anticolonization of pathogens by Lactobacillus paracasei
FJ861111.1. J Dairy Sci. 98:6759–6766. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li S, Chen T, Dong S, Xiong Y, Wei H and
Xu F: The effects of rebaudioside A on microbial diversity in mouse
intestine. Food Sci And Technol Res. 20:459–467. 2014. View Article : Google Scholar
|
|
19
|
Chen T, Xiong S, Jiang S, Wang M, Wu Q and
Wei H: Molecular identification of microbial community in Chinese
douchi during post-fermentation process. Food Sci Biotechnol.
20:1633–1638. 2011. View Article : Google Scholar
|
|
20
|
Chen T, Wu Q, Li S, Xiong S, Jiang S, Tan
Q, Zhang Z, Zhu D and Wei H: Microbiological quality and
characteristics of probiotic products in China. J Sci Food Agric.
94:131–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen T, Wang M, Li S, Wu Q and Wei H:
Molecular identification of microbial community in surface and
undersurface douchi during postfermentation. J Food Sci.
79:M653–M658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen TT, Wang MJ, Li SJ, Wu QL and Wei H:
Molecular Identification of Microbial Community in Surface and
Undersurface Douchi During Postfermentation. J Food Sci.
79:M653–M658. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen T, Wang M, Jiang S, Xiong S and Wei
H: The application of polymerase chain reaction-denaturing gradient
gel electrophoresis (PCR-DGGE) method in microbial screening. Afr J
Biotechnol. 10:9387–9395. 2011. View Article : Google Scholar
|
|
24
|
Chen T, Tan Q, Wang M, Xiong S, Jiang S,
Li S, Luo C and Wei H: Identification of bacterial strains in viili
by molecular taxonomy and their synergistic effects on milk curd
and exopolysaccharides production. Afr J Biotechnol.
10:16969–16975. 2011.
|
|
25
|
Hooper LV, Littman DR and Macpherson AJ:
Interactions between the microbiota and the immune system. Science.
336:1268–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nelson MH, Diven MA, Huff LW and Paulos
CM: Harnessing the microbiome to enhance cancer immunotherapy. J
Immunol Res. 2015:3687362015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sivan A, Corrales L, Hubert N, Williams
JB, Aquino-Michaels K, Earley ZM, Benyamin FW, Lei YM, Jabri B,
Alegre ML, et al: Commensal bifidobacterium promotes antitumor
immunity and facilitates anti-PD-L1 efficacy. Science.
350:1084–1089. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhernakova A, Kurilshikov A, Bonder MJ,
Tigchelaar EF, Schirmer M, Vatanen T, Mujagic Z, Vila AV, Falony G,
Vieira-Silva S, et al: Population-based metagenomics analysis
reveals markers for gut microbiome composition and diversity.
Science. 352:565–569. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang ZB, Peiy Y and Liu W: Jianweixiaoshi
tablets determination of hesperidin content tesr. J Pract Tradit
Chin Intern Med. 4:0152011.(In Chinese).
|
|
30
|
Min Y, Jing L, Jinjin M and Ting X:
Effects of oridonin on activities of rabbit's intestinal smooth
muscle. China Pharm. 14:0152012.
|
|
31
|
Liao W, Lin JX and Leonard WJ: IL-2 family
cytokines: New insights into the complex roles of IL-2 as a broad
regulator of T helper cell differentiation. Curr Opin Immunol.
23:598–604. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gaffen SL and Liu KD: Overview of
interleukin-2 function, production and clinical applications.
Cytokine. 28:109–123. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wheelock EF: Interferon-like
virus-inhibitor induced in human leukocytes by phytohemagglutinin.
Science. 149:310–311. 1965. View Article : Google Scholar
|
|
34
|
Green JA, Cooperband SR and Kibrick S:
Immune specific induction of interferon production in cultures of
human blood lymphocytes. Science. 164:1415–1417. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Browne HP, Forster SC, Anonye BO, Kumar N,
Neville BA, Stares MD, Goulding D and Lawley TD: Culturing of
‘unculturable’ human microbiota reveals novel taxa and extensive
sporulation. Nature. 533:543–546. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Derrien M and van Hylckama Vlieg JE: Fate,
activity, and impact of ingested bacteria within the human gut
microbiota. Trends Microbiol. 23:354–366. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kaeberlein T, Lewis K and Epstein SS:
Isolating ‘uncultivable’ microorganisms in pure culture in a
simulated natural environment. Science. 296:1127–1129. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu
X, Zeng L, Chen J, Fan S, Du X, et al: Gut microbiome remodeling
induces depressive-like behaviors through a pathway mediated by the
host's metabolism. Mol Psychiatry. 21:786–796. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Forsythe P, Kunze W and Bienenstock J:
Moody microbes or fecal phrenology: What do we know about the
microbiota-gut-brain axis? BMC Med. 14:582016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Karst SM: The influence of commensal
bacteria on infection with enteric viruses. Nat Rev Microbiol.
14:197–204. 2016. View Article : Google Scholar : PubMed/NCBI
|