Introduction
Methotrexate {MTX;
(2S)-2-[[4-[(2,4-diaminopteridin-6-yl)
methyl-methylamino]benzoyl]amino]pentanedioic acid} is a structural
analogue of folic acid and one of the most widely used
antimetabolites in cancer chemotherapy (1). At low doses (up to 25 mg/week) it is
also part of the established treatment of many autoimmune
inflammatory disorders, most notable of which is rheumatoid
arthritis (RA) where it has nowadays become the standard of care
(2).
Folic acid is essential for the synthesis of
deoxyribonucleic acids (DNA), since it is a required co-factor for
the synthesis of thymidylate by the enzyme thymidylate synthetase
(TS), but also plays a key role in purine metabolism, being a
co-factor for the enzyme 5-aminoimidazole-4-carboxamide
ribonucleotide (AICAR) transformylase. In order to be used as
co-factor, folate needs to be converted to its active form by a
two-step reaction that reduces folate first to dihydrofolate and
subsequently to tetrahydrofolate (FH4). This last reaction is
catalysed by the enzyme dihydrofolate reductase (DHFR) (3,4).
MTX inhibits DHFR, thus, it inhibits both TS and
AICAR transformylase. In this way, MTX interferes with DNA
synthesis, repair, and cellular replication, ultimately causing
limitation of the high turnover of inflammatory cells. On the other
hand, MTX-polyglutamates (MTX-glu) are long-lived metabolites of
MTX, which reside in a variety of tissues, such as liver,
erythrocytes and adipose tissue. They persist for weeks to months,
and this is considered to be the key factor behind the slow onset
and prolonged duration of the anti-inflammatory effects of MTX. In
fact, it takes about a week for the onset of the anti-inflammatory
effects, while MTX is almost undetectable 24 h after administration
(3).
MTX-glu causes accumulation of AICAR, due to the
AICAR trasformylase inhibition, and its metabolites, which are
inhibitors of adenosine deaminase and AMP deaminase, ultimately
leading to elevated levels of both intracellular and extracellular
adenosine. When MTX is administered at low doses, it induces
elevated levels of extracellular adenosine, which mainly binds to
A2 receptors and leads to increased intracellular cAMP, thus,
leading to immunosuppression via inhibition of phagocytosis,
lymphocyte proliferation and of the secretion of various cytokines
(4,5).
Other proposed actions of MTX in the treatment of RA
include reduction of various cell adhesion molecules' expression,
indirect inhibition of osteoclast formation and probably some
indirect anti-angiogenetic effects (6).
MTX has been studied as a tumor-diagnostic agent in
a number of published studies, by either direct labelling with
technetium-99m (99mTc) (7–9) or
as a mercaptoacetyltriglycine-MTX (MAG3-MTX) conjugate labelled
with 99mTc (10). The
purpose of this study is to present the possible use of
99mTc-labelled MTX as a radiotracer for the
identification of inflammatory target sites, which are associated
with RA in joints, bones and tissues.
Materials and methods
All chemicals employed for this research were of
analytical grade. Stannous chloride, ascorbic acid and sodium
bicarbonate were purchased from Aldrich, USA. The
99mTc-generator was purchased from GE Healthcare. MTX
was purchased from Pfizer (Athens, Greece). Saline and water for
injection were purchased from Demo (Athens, Greece).
High-performance liquid chromatography (HPLC)
analyses were performed on a Waters µ-Bondapack C18 (3.9 mm
i.d. ×300 mm) cartridge column (Waters GmbH, Eschborn, Germany).
The gradient system employed is described below. Solvents for HPLC
were of analytical grade, and were filtered through 0.22 µm
membrane filters (EMD Millipore, Billerica, MA, USA) and degassed.
Radioactivity measurements were recorded on an automated well-type
γ-counter NaI(Tl) crystal (Packard).
Animal experiments were carried out according to
European and National regulations. Biodistribution studies were
performed using female normal Swiss mice (20±2 g) of the same
colony and age, purchased from the Breeding Facilities of the
Institute of Biosciences and Applications, NCSR ‘Demokritos’.
Radiolabeling and radiochemical purity
analysis
The labelling of MTX was performed by ligand
exchange from a 99mTc(v)O-gluconate precursor. Sodium
gluconate acts as an intermediate exchange ligand for
99mTc, with stannous chloride as the reducing agent
(11). Briefly, a solid mixture of
1 g sodium gluconate, 2 g of sodium bicarbonate and 15 mg of
stannous chloride was homogenized and kept under anhydrous
conditions. 3 mg of this mixture were dissolved in 1 ml of a sodium
pertechnetate solution (Na99mTcO4) containing
296 MBq/8 mCi 99mTc. 10 mg of MTX were added, and the
mixture was stirred for 30 min at room temperature. The pH of the
final solution was 7.
Radiochemical control of the 99mTc-MTX
complex was carried out with Instant Thin Layer
Chromatography-Silica Gel (ITLC-SG) in saline and Whatman 3 mm
chromatography paper (PC) in acetone. Briefly, 2 µl of the
reaction mixture were applied on Silica Gel (ITLC-SG) and Whatman
3-mm strips. The radiochromatographs were developed in saline (0.9%
NaCl) and acetone, respectively, over a distance of 10 cm. After
drying, the strips were cut in 1-cm pieces and their radioactivity
was counted in a well-type scintillation counter. Furthermore,
Reversed-Phase HPLC (RP-HPLC) analysis was performed on an aliquot
of the reaction solution, by applying the following linear gradient
system: From 0 to 80% solvent B (1–20 min), 80% solvent B (20–23
min), 80 to 0% solvent B (23–25 min) and 0% solvent B (25–30 min),
at a 0.8 ml/min flow rate (solvent A: 0.1% Trifluoroacetic Acid
(TFA) in H2O; solvent B: 0.1% TFA in Acetonitrile
(AcCN).
In vitro stability and protein
binding
In vitro stability of 99mTc-MTX at
room temperature was assessed up to 24 h after preparation of the
sample. Plasma stability was carried out in fresh human plasma at
37°C. For preparation of human plasma, a blood sample from healthy
donors was collected in heparinised polypropylene tubes and was
immediately centrifuged at 2,000 × g for 10 min. The supernatant
was collected and used for the stability study. 100 µl
(~29.6MBq/0.8mCi) of 99mTc-MTX was incubated with 900 µl
of plasma at 37°C. At 2 and 4 h, 100-µl aliquots were treated with
a two-fold excess of ethanol and centrifuged at 1,000 × g for 15
min. The remaining pellet was washed thrice with 1 ml EtOH, and
these ethanolic washes were combined with the supernatant and
counted in a γ well counter. This activity was compared to the
activity in the pellet, to give the percentage of
99mTc-MTX not bound to proteins. The supernatant was
analysed by paper chromatography and ITLC, as described above.
Determination of partition
coefficient
The apparent partition coefficient for
99mTc-MTX was determined by mixing aliquots of the
technetium complex with 1-octanol and phosphate buffer (0.125 M, pH
7.4).
In a centrifuge tube, containing 2 ml of each phase,
100 µl of the 99mTc complex solution was added, and the
mixture was agitated on a Vortex mixer for approximately 1 min and
finally centrifuged at 5,000 × g for 5 min. Three samples (0.2 ml
each) from each layer were counted in a γ counter. The
partition coefficient was calculated as the mean value of each
cpm/ml of octanol layer divided by that of the buffer. A sample
(1.0 ml) from the octanol layer was subsequently repartitioned in
octanol/buffer until constant values were obtained. This was
achieved with the third repartition.
Biodistribution studies
All applicable institutional and/or national
guidelines for the care and use of animals were followed. These
studies were approved by the Ethics Committee of the National
Center for Scientific Research of ‘Demokritos’ (Athens, Greece) and
animal care and procedures followed are in accordance with
institutional guidelines and licenses issued by the Department of
Agriculture and Veterinary Policies of the Prefecture of Attiki
(Registration nos. EL 25 BIO 022 and EL 25 BIO 021). Mice were
housed under constant environmental conditions with 12 h light-dark
cycles and had free access to food and water. To induce
inflammation, animals were inoculated with 50 µl of pure turpentine
oil subcutaneously in the left thigh muscle under slight ether
anaesthesia (11,12). All animals developed an oedema 18
to 24 h after turpentine inoculation, which was visible to the
naked eye.
Ex vivo animal experiments were performed in
Swiss Albino mice with experimentally-induced inflammation (20±2 g,
n=3 animals per time-point), by injecting 100 µl (3.7 MBq/0.1 mCi)
of the radiotracer via the tail vein. Animals were sacrificed by
cardiectomy under slight ether anaesthesia at 2, 4 and 24 h post
injection, and the main tissues and organs (blood, heart, liver,
stomach, intestines, spleen, lungs, pancreas and bones) were
excised, blotted dry and weighed. The inflamed thigh was excised
and trimmed of the neighboring subcutaneous tissue. The muscle of
the non-inflamed right thigh was also excised, for reasons of
comparison. Samples were counted in a gamma counter (NaI gamma
counter; Packard, Downers Grove, IL, USA). Standards were prepared
from the injected material and were counted each time
simultaneously with the tissues excised, allowing for calculations
to be corrected for physical decay of the radioisotope.
Radiolabeled MTX distribution over time was expressed as injected
dose per gram (%ID/g).
Imaging system
The imaging system employed is a compact,
Anger-type, γ-ray camera developed at the Center for Gamma-Ray
Imaging of the University of Arizona. Details of the system can be
found elsewhere (13–15). Briefly, the system comprises a 5 mm
thick NaI(Tl) scintillation crystal, a 12 mm thick quartz light
guide, a 3×3 array of 1.5 inch diameter photomultiplier tubes
(PMTs), and a 40-mm thick lead parallel-hole collimator with
hexagonal holes of 1-mm in diameter. The system achieves a spatial
resolution of approximately 2.5 mm at the collimator face and
degrades linearly with distance. The field-of-view of the camera is
4.5 in × 4.5 in, enough to image a whole mouse without axially
moving the camera or the mouse.
Image acquisition
A Swiss mouse, with experimentally-induced
inflammation for 24 h in the left hind limb area, was anesthetized
with an intraperitoneal (IP) injection of ketamine (75 mg/kg) and
xylazine (5 mg/kg) and placed on the camera face in the prone
position. The animal was injected with 100 µl (25.9 MBq/0.7 µCi) of
99mTc-MTX. Dynamic planar scintigraphy was performed by
collecting 10 consecutive two-minute images, for a total period of
20 min. Two h after tracer injection, additional anaesthesia was
applied and a 5-min static image was collected with the animal in
the same position. Furthermore, 24 h after injection the animal was
euthanized and a 1 h image was acquired.
Binding studies on hydroxyapatite
Hydroxyapatite binding studies were performed in
vitro to simulate the binding of the radiotracer under
investigation to bone structure. For this purpose, hydroxyapatite
(Hap; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) was suspended
in isotonic saline at 20 mg/ml and then incubated for 24 h at room
temperature. The following day, 50 µl (0.444MBq/0.012mCi) of
99mTc-MTX were added to the Hap fractions. After a 10s
vortex, the sample was incubated, under agitation, for 10 min at
room temperature and centrifuged. The supernatant was then removed
and the Hap fraction was washed twice with saline. The
radioactivity of the Hap fraction and the saline washes were then
measured with a well-type gamma counter. Control experiments were
performed using 99mTc-MTX, without Hap.
99mTc-MTX binding to Hap was determined as percent of
absorbed onto Hap [Hydroxyapatite binding (%)=(radioactivity of Hap
fraction of each sample/total radioactivity)x100].
Results
Radiolabeling and radiochemical purity
analysis
Radiolabeling of MTX was achieved by the
preconjugation approach, via the precursor
99mTc-gluconate. Radiochemical purity was assessed by
paper chromatography (Whatman 3 MM) and ITLC-SG. With ITLC-SG,
99mTc-MTX and the free pertechnetate
99mTcO4-appeared at Rf=0.9–1.0, while
99mTcO2 was detected at Rf=0.0–0.1. In
acetone the free 99mTcO4 had an Rf of 0.9–1.0
while the 99mTc-MTX and the
hydrolysed99mTcO2 appeared at Rf=0.0–0.1. The
radiochemical purity of 99mTc-MTX was found to be
95–98%. Under the specific HPLC conditions, there was one product
peak of 99mTc-MTX moving with the solvent front, with a
retention time of approximately 20 min, while the retention times
of 99mTcO4− and
99mTc-gluconate were <5 min (9).
In vitro stability and protein
binding
The stability of 99mTc-MTX was determined
by paper chromatography and ITLC at different time points, as
described above. The radiolabeled complex remained stable (up to
90%) at room temperature, for up to 24 h post-labelling. Plasma
stability studies showed that 94.9±1.2% of 99mTc-MTX
remained intact at 2 h, dropping to 85.8±2.7% at 4 h
post-incubation.
Before performing ex vivo biodistribution
studies in mice, the in vitro binding of
99mTc-MTX was assessed in human plasma. Protein binding
in human plasma was found to be 48.1±1.9% at 2 h, remaining
practically stable at 4 h post-incubation (49.1±2.2%).
Determination of partition
coefficient
Regarding lipophilicity, the partition coefficient
indicated that 99mTc-MTX had maximum extraction in
phosphate-buffered saline (PBS), pH 7.4 (hydrophilic medium), while
a negligible amount of activity was observed in octanol (lipophilic
medium), thus suggesting that radiolabeled drug was hydrophilic in
nature. The logP value was estimated to be −2.28±0.03.
Biodistribution studies
The biodistribution of 99mTc-MTX was
assessed on Swiss Albino mice with experimentally-induced
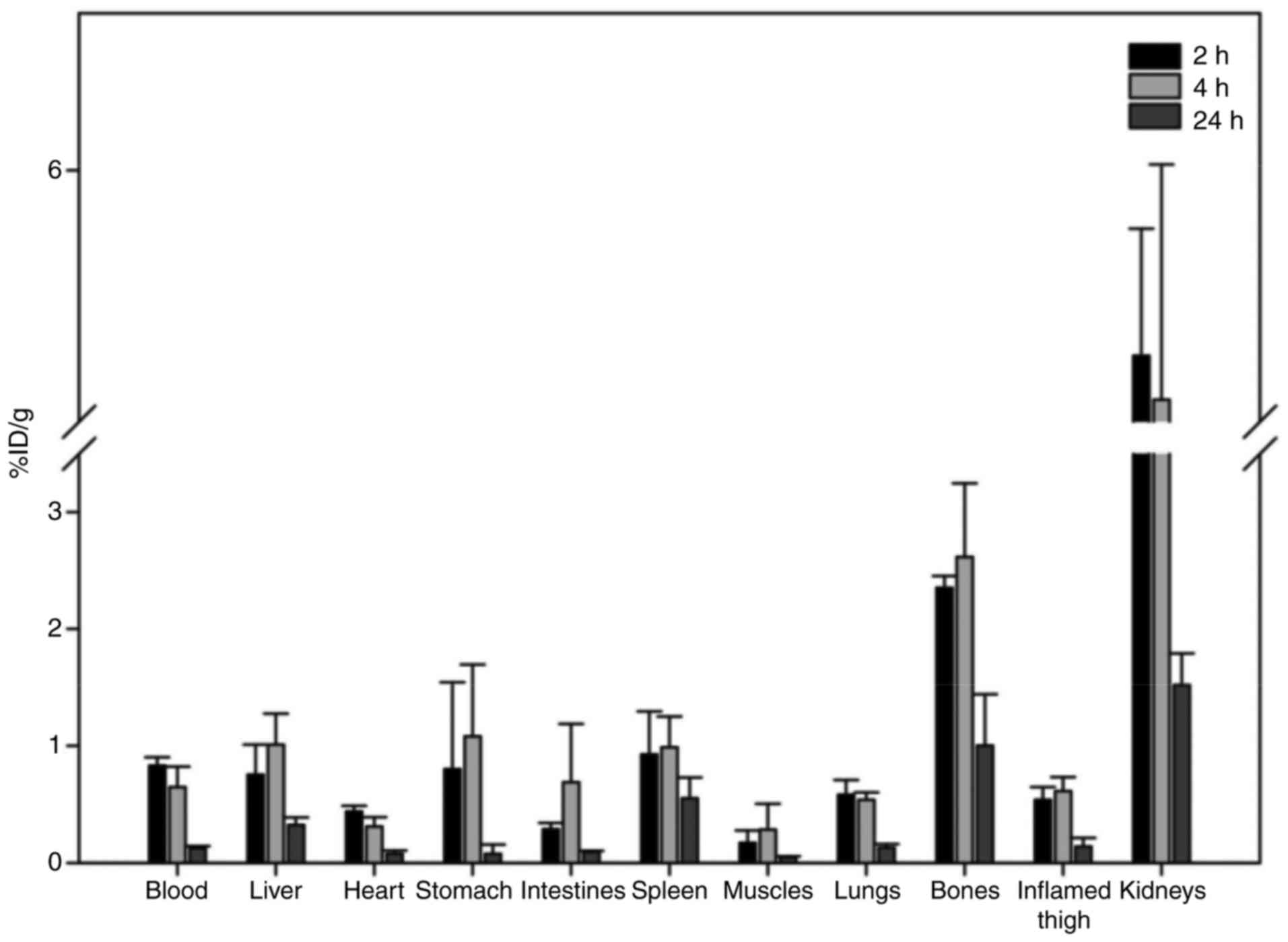
inflammation at 2 and 24 h post injection (Fig. 1). Rapid clearance from blood and
predominant excretion via the urinary system was observed for the
radiotracer. Uptake in the stomach and spleen was low, providing
evidence for the in vivo stability of the tracer. With
regard to the other organs, apart from the kidneys, no major uptake
was observed in all analysed tissues (≤1.0% ID/g from 2 h post
injection). An important observation was the increased uptake of
the radiotracer in the inflamed muscle, in comparison to the
contralateral normal muscle tissue (0.54±0.11 vs. 0.17±0.10;
0.61±0.12 vs. 0.28±0.22; and 0.14±0.07 vs. 0.05±0.01 at 2, 4 and 24
h post injection, respectively), with the inflammation-to-normal
muscle ratio remaining practically stable up to 24 h post
injection. Furthermore, an unexpected observation was the
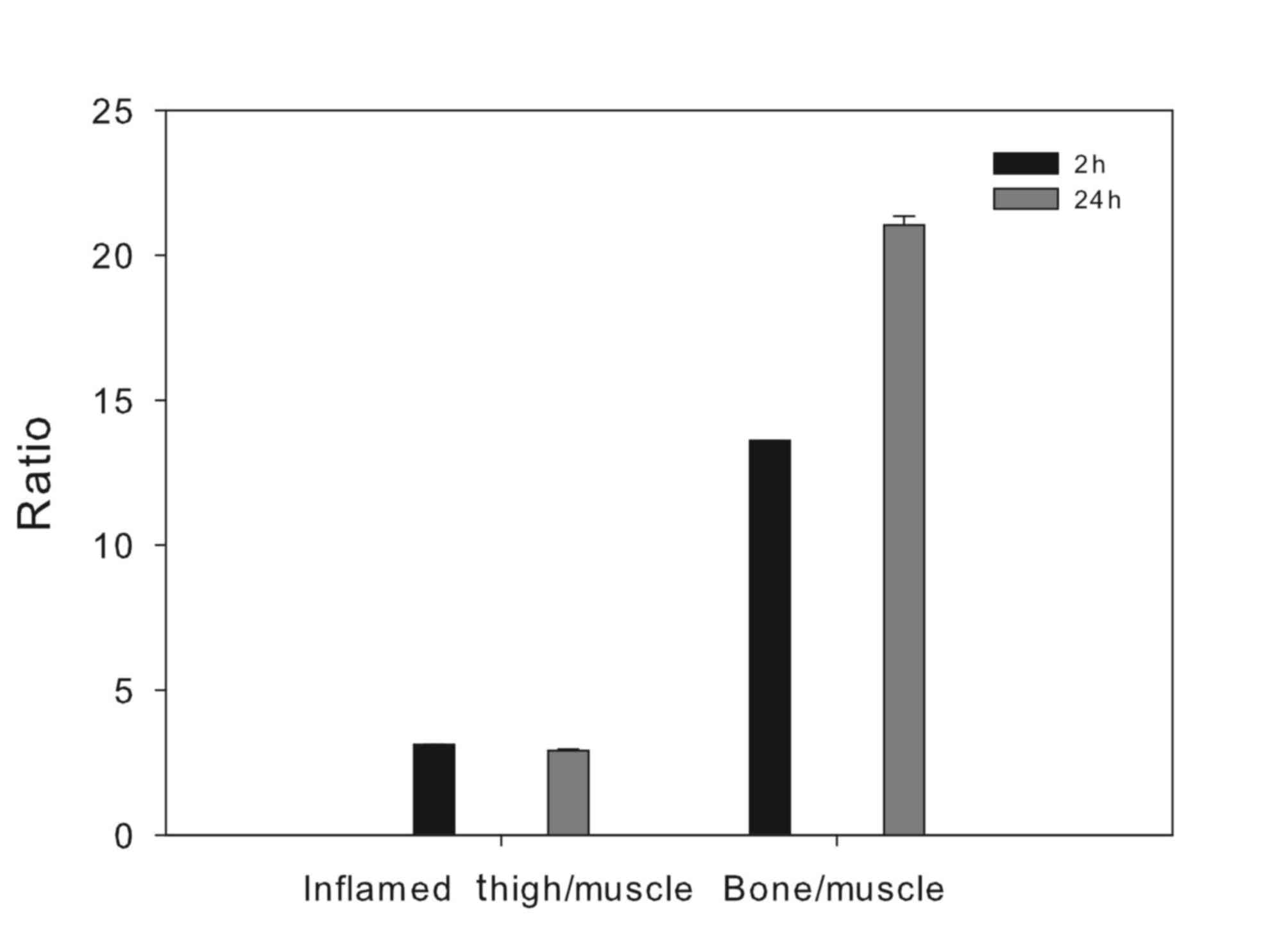
significant bone uptake observed, which led to a pronounced
differentiation between bone and non-osseous tissue, especially at
24 h post injection (Fig. 2).
Imaging studies
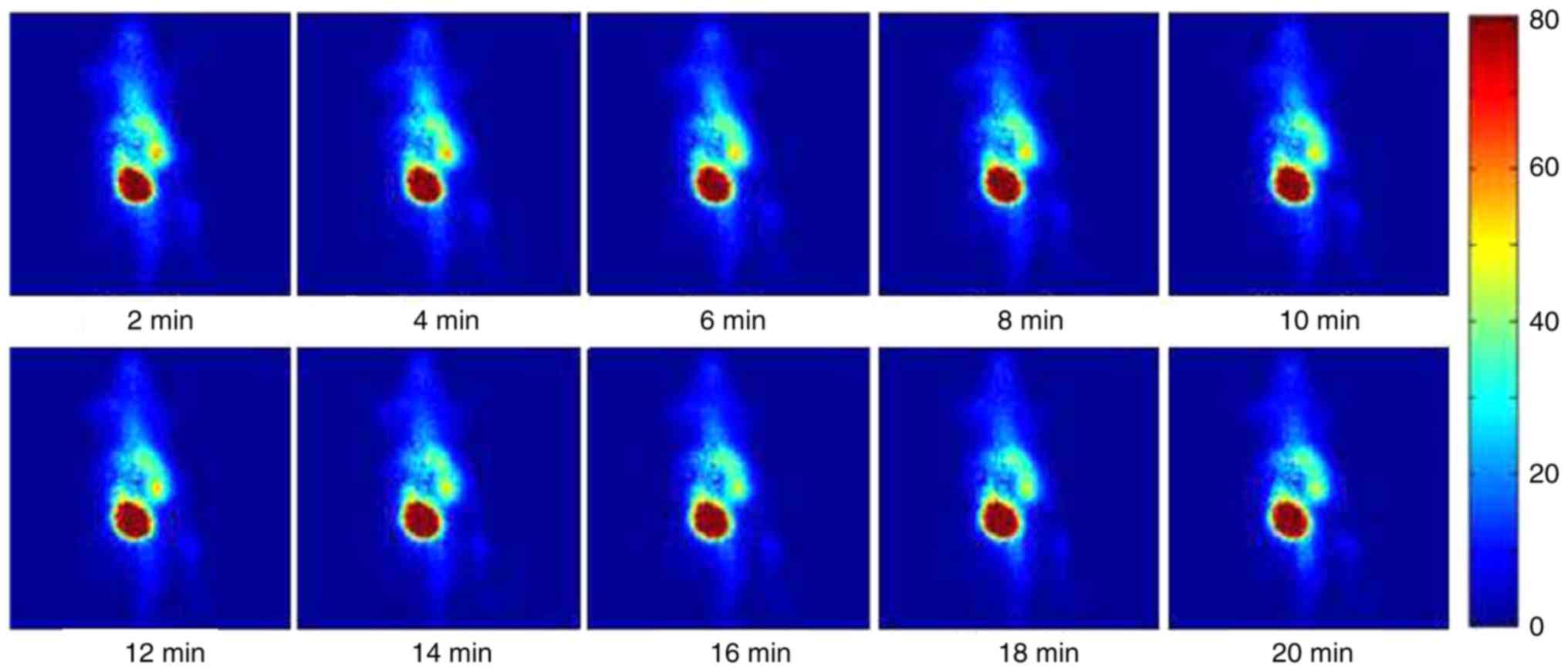
A dynamic sequence of γ-ray images of an
inflammation-induced mouse injected with 25.9 MBq (0.7 mCi) of
99mTc-MTX at consecutive time points is presented in
Fig. 3. All images are 2-min
acquisitions and they are displayed on the same colour scale. The
colour intensity represents the magnitude of the deposited
radiotracer. The inflammation site, in the left side of the animal,
is clearly identified by visual inspection immediately after
injection. Liver uptake of the tracer is also observed at a lower
concentration than the inflammation site.
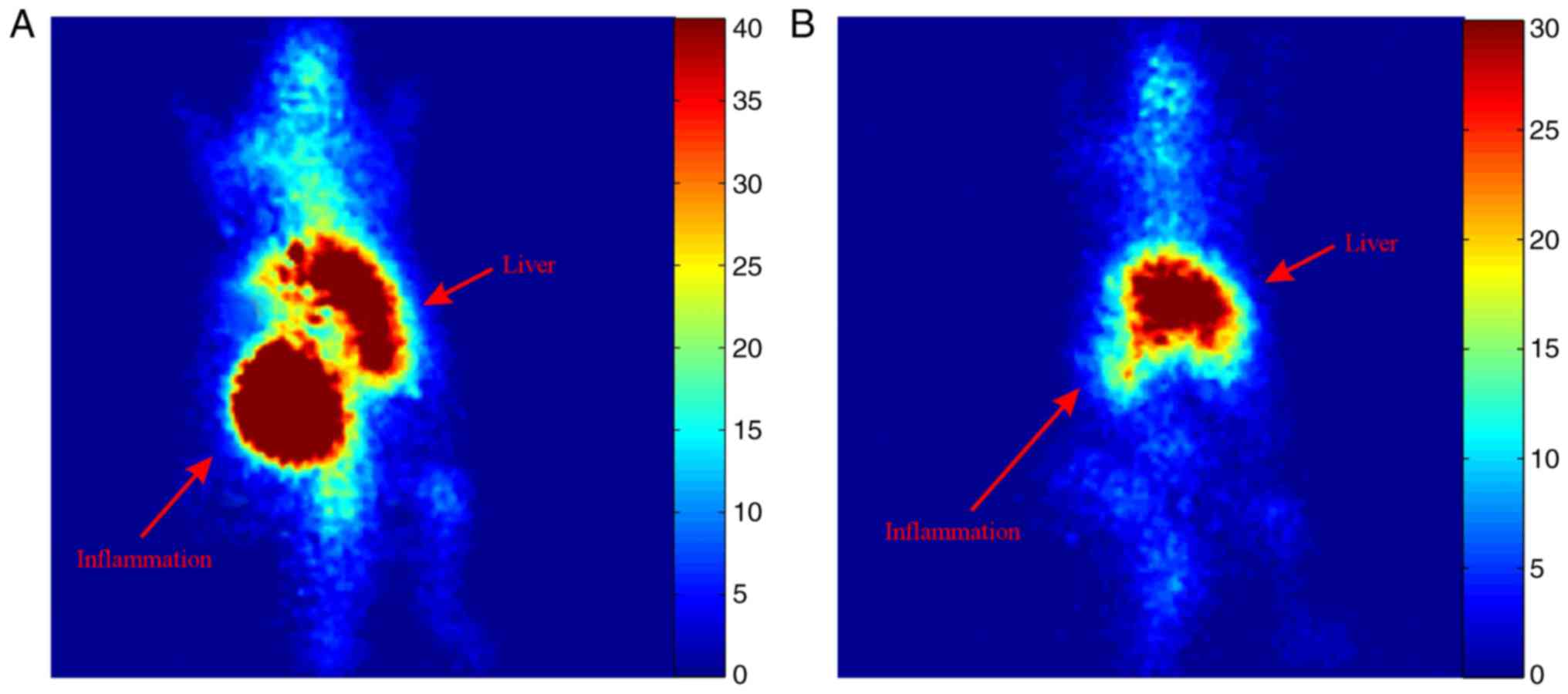
Static planar scintigraphic images of the mouse at 2
and 24 h post injection are shown in Fig. 4. Increased uptake of the tracer can
be visually identified in the inflammation site as well as the
liver 2 h post injection. At 24 h post injection most of the tracer
remains at the liver with very small uptake at the inflammation
site. However, some uptake at the joints and spine is also observed
both at 2 and 24 h after injection.
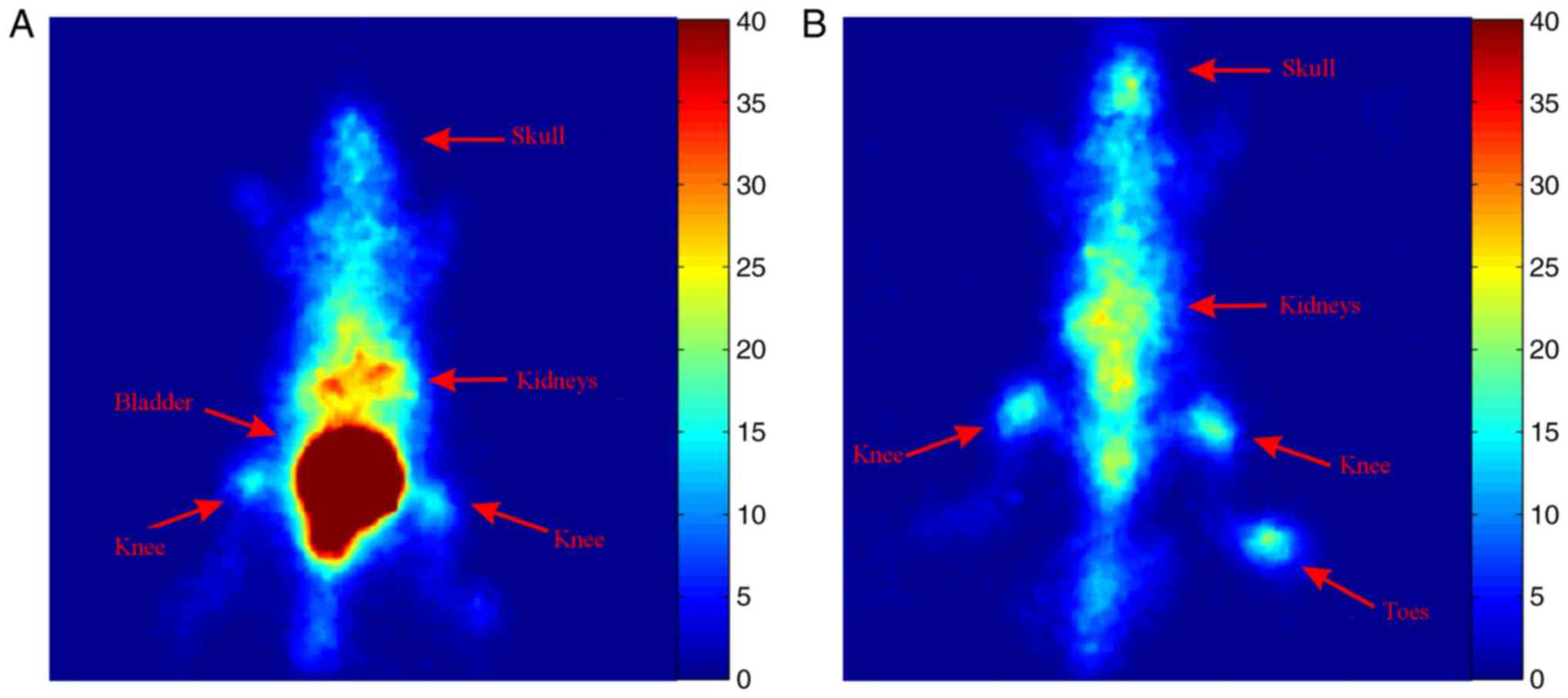
To assess the uptake of 99mTc-MTX at the
bones and joints, a healthy mouse was injected with 0.7 mCi of
99mTc-MTX and planar images were acquired 2 and 24 h
later. Increased uptake of the tracer was observed at the spine,
the knees and the toes of the hind-limbs at both time points
(Fig. 5).
Hydroxyapatite-binding assay
Hydroxyapatite binding of 99mTc-MTX was
determined on a 20 mg/ml saline sample of Hap, and was found to be
44.9±1.3%, at 10 min post incubation. In the control experiments,
we confirmed that the radioactivity adsorbed to the vials was less
than 0.1%.
Discussion
MTX was first developed as a cancer treatment drug
in the 1940, but won FDA approval for treating RA in the late
1980s. Since then, MTX has become the treatment of choice for
people with this condition, as well as for other forms of
inflammatory arthritis. In the present work, MTX radiolabeled with
technetium-99m has been used to assess its capacity in imaging
experimentally-induced inflammation in mice. Radiolabeling of MTX
with Technetium-99m using gluconate as the transfer ligand gave
comparable results to the work described by other groups (7,10),
in terms of radiolabeling yield and stability of the radiolabeled
product. With regard to the preparation of 99mTc, our
method is much more facile and straightforward (10). Stability studies were performed to
evaluate whether 99mTc-MTX is stable enough in plasma to
ensure sufficient delivery of radioactivity to the site of
interest, as only free (i.e., non-protein bound) radiotracer is
available to diffuse out of the vasculature and localize in the
organism. The stability of 99mTc-MTX was determined
in vitro in human plasma, where it was shown that it
remained intact up to 85% after 4 h. Plasma protein binding was
found to be 48.1±1.9% at 2 h, remaining practically stable at 4 h
post-incubation (49.1±2.2%).
The lipophilicity of 99mTc-MTX was
determined by measuring its distribution between n-octanol
and PBS, pH 7.4, and resulted in a logP value of −2.28±0.03, which
is comparable to results of other groups. Indicatively we would
like to refer to the work of Okarvi et al (10), who showed logP values of −2.01 and
−1.90, demonstrating a low lipophilicity of their
99mTc-MTX compounds. Our results showed that
99mTc-MTX is hydrophilic in nature, thus rapidly
reaching the target area and exhibiting satisfactory clearance
characteristics.
Biodistribution studies showed fast blood clearance,
low hepatobiliary uptake and excretion via the urinary tract. The
inflamed thigh showed higher radiotracer accumulation than the
contralateral normal tissue, with an inflammation/muscle ratio of
3.12±0.003 at 2 h post injection, slightly dropping to 2.92±0.05 at
24 h post injection.
Planar imaging concurred with our biodistribution
studies, with increased uptake at the inflammation area. However,
increased uptake was also observed in the joints and spine, both
areas with a relatively high remodelling activity. This prompted us
to perform hydroxyapatite binding studies and a new planar
scintigraphic study on a healthy mouse (without inflammation) after
this pronounced uptake of 99mTc-MTX in the bone. The
hydroxyapatite binding studies showed 45% binding of the tracer to
the synthetic material, thus confirming the results of our
biodistribution studies. Furthermore, the imaging study confirmed
increased uptake of 99mTc-MTX in the spine and knee
joints both at 2 and 24 h after injection (Fig. 5). These results combined with
scintigraphic imaging results in the literature (16) might support the hypothesis that MTX
has a diphosphonate-like behavior at least in the first 24 h, but
further experiments are needed for clarification of these
findings.
The interesting findings of our study have prompted
us to further investigate 99mTc-MTX as a radiotracer for
RA, as well as to demonstrate the efficacy of MTX before it is
prescribed as a therapeutic agent for RA. If RA lesions accumulate
radiolabeled MTX, these patients could be candidates for MTX
therapy, while if the lesions are not addressed, the physician may
proceed to the next line of treatment, without delays due to
ineffective treatment. It is clear that a test which could predict
adequate candidates for MTX treatment and response to MTX therapy
would be welcomed by the rheumatology community. This might be of
greater interest now that RA has been recognized as a major adverse
event of immune checkpoint-inhibitor treatments for cancer
(17).
MTX has been successfully labelled with
99mTc, with a radiochemical purity of >95%. Stability
was assessed in plasma, where it remained intact up to 85% at 4 h
post-incubation. Preclinical ex vivo biodistribution studies
as well as in vivo imaging studies have shown that
99mTc-MTX accumulates in inflammatory sites, as well as
in the spine, the joints and bones, areas with relatively high
remodelling activity.
To the best of our knowledge, this study is the
first to report direct evidence of hydroxyapatite binding of
99mTc-MTX. This information might prove useful in the
current research for bone-targeted drug delivery (18,19).
The results are promising and set the stage for further study on
the development and application of 99mTc-MTX as a tool
for early detection and imaging of inflammation in RA.
Acknowledgements
The publication of this article was funded by the
Onassis Scholars' Association of the ‘Alexander S. Onassis’ Public
Benefit Foundation. Dr L. Furenlid was partially supported by The
National Institutes of Health/National Institute of Biomedical
Imaging and Bioengineering (grant no. P41-EB002035).
Glossary
Abbreviations
Abbreviations:
|
MTX
|
methotrexate
|
|
RA
|
rheumatoid arthritis
|
References
|
1
|
Rang HP, Dale MM, Ritter JM, Flower RH and
Henderson G: Anticancer drugsPharmacology. 7th. Rang HP and Dale
MM: Churchill Livingstone Elsevier; London: pp. 673–688. 2011
|
|
2
|
Weinblatt ME: Methotrexate in rheumatoid
arthritis: A quarter century of development. Trans Am Clin Climatol
Assoc. 124:16–25. 2013.PubMed/NCBI
|
|
3
|
Chan ES and Cronstein BN: Mechanisms of
action of methotrexate. Bull Hosp Jt Dis. 71 Suppl 1:S5–S8.
2013.
|
|
4
|
Cutolo M, Sulli A, Pizzorni C, Seriolo B
and Straub RH: Anti-inflammatory mechanisms of methotrexate in
rheumatoid arthritis. Ann Rheum Dis. 60:729–735. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tian H and Cronstein BN: Understanding the
mechanisms of action of methotrexate: Implications for the
treatment of rheumatoid arthritis. Bull NYU Hosp Jt Dis.
65:168–173. 2007.PubMed/NCBI
|
|
6
|
Wessels JA, Huizinga TW and Guchelaar HJ:
Recent insights in the pharmacological actions of methotrexate in
the treatment of rheumatoid arthritis. Rheumatology (Oxford).
47:249–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dar UK, Khan IU, Javed M, Ahmad F, Ali M
and Hyder SW: Preparation and biodistribution in mice of a new
radiopharmaceutical-technetium-99m labeled methotrexate, as a tumor
diagnostic agent. Hell J Nucl Med. 15:120–124. 2012.PubMed/NCBI
|
|
8
|
Rasheed R, Javed M, Ahmad F, Sohail A,
Murad S, Masood M and Rasheed S and Rasheed S: Preparation of
(99m)Tc-labelled methotraxate by a direct labeling technique as a
potential diagnostic agent for breast cancer and preliminary
clinical results. Hell J Nucl Med. 16:33–37. 2013.PubMed/NCBI
|
|
9
|
Ozgenc E, Ekinci M, Ilem-Ozdemir D,
Gundoglu E and Asikoglu M: Radiolabeling and in vitro evaluation of
99mTc-methotrexate on breast cancer cell line. J Radioanal Nucl
Chem. 307:627–633. 2016. View Article : Google Scholar
|
|
10
|
Okarvi SM and Jammaz IA: Preparation and
in vitro and in vivo evaluation of technetium-99m-labeled folate
and methotrexate conjugates as tumor imaging agents. Cancer Biother
Radiopharm. 21:49–60. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mirzaei A, Jalilian AR, Akhlaghi M and
Beiki D: Production of 68Ga-citrate based on a SnO2 generator for
short-term turpentine oil-induced inflammation imaging in rats.
Curr Radiopharm. 9:208–214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rivera S and Ganz T: Animal models of
anemia of inflammation. Semin Hematol. 46:351–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Furenlid LR, Wilson DW, Chen YC, Kim H,
Pietraski PJ, Crawford MJ and Barrett HH: FastSPECT II: A
second-generation high-resolution dynamic SPECT imager. IEEE Trans
Nucl Sci. 51:631–635. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Furenlid LR, Chen YC and Kim H: SPECT
Imager Design and Data-Acquisition SystemsSmall-Animal SPECT
Imaging. Kupinski MA and Barrett HH: Springer US; Boston, MA: pp.
115S–138S. 2005, View Article : Google Scholar
|
|
15
|
Chen YC, Furenlid LR, Wilson DW and
Barrett HH: Calibration of Scintillation Cameras and Pinhole SPECT
Imaging SystemsSmall-Animal SPECT Imaging. Kupinski MA and Barrett
HH: Springer US; Boston, MA: pp. 195–201. 2005, View Article : Google Scholar
|
|
16
|
Rasheed R, Gillani J, Jielani A, Irum F,
Lodhi N and Rasheed S and Rasheed S: Tc99m methotrexate (MTX): A
novel complex for imaging of rheumatoid arthritis (RA): First
clinical trials. Gen Med (Los Angel) Personalized Medicine.
S2132016.
|
|
17
|
Naidoo J, Cappelli LC, Forde PM, Marrone
KA, Lipson EJ, Hammers HJ, Sharfman WH, Le DT, Baer AN, Shah AA, et
al: Inflammatory arthritis: A newly recognized adverse event of
immune checkpoint blockade. Oncologist. 22:627–630. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cole LE, Vargo-Gogola T and Roeder RK:
Targeted delivery to bone and mineral deposits using bisphosphonate
ligands. Adv Drug Deliv Rev. 99:12–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Raichur V, Vemula KD, Bhadri N and Razdan
R: Zolendronic acid-conjugated PLGA ultrasmall nanoparticle loaded
with methotrexate as a supercarrier for bone-targeted drug
delivery. AAPS PharmSciTech. 18:2227–2239. 2017. View Article : Google Scholar : PubMed/NCBI
|