Introduction
Urothelium of lower urinary tract, which is composed
of bladder and urethra, is often subjected to various pathologies
including inflammatory lesions, trauma, congenital anomalies and
cancers (1). The damage is
associated to urinary retention, incontinence or upper tract (e.g.,
kidney) impairment, and often needs surgical intervention for the
repair (1,2). But clinically, the limited amount of
substitution tissues, mainly of autologous epithelium, is a main
cause of the high rates of repair failures (3). Human mesenchymal stem cells (hMSCs)
have been regarded as a potential alternative for tissue repair due
to their ability to differentiate into diverse cell types. hMSCs
are multipotent progenitor cells, which predominantly exist in bone
marrow and adipose tissues, respectively termed as bone
marrow-derived mesenchymal stem cells (BMSCs) and adipose-derived
mesenchymal stem cells (ADSCs) (4). ASCs have the advantage of being
harvested in abundant quantity and causing little trauma to donor
site (3). ASCs can differentiate
toward cells with characteristics of urothelial cells under the
synergistic stimulation of contributing factors (3). However, mechanisms underlying the
difference are not fully understood, and application of ASCs for
urothelium repair still has some drawbacks, such as the
low-efficient or incomplete differentiation and the lost of
epithelial phenotype after the implantation in vivo. These
reasons prevent their clinical use.
Wnt/β-catenin pathway and transforming growth
factor-β (TGF-β)/TGF-β receptor (TGFR) are important in modulating
differentiation of mesenchymal stem cells (4). Canonical Wnt signaling is mediated by
β-catenin. Activated Wnt results in β-catenin accumulates in the
nucleus, where β-catenin forms a transcriptional complex with
DNA-binding T-cell factors and drives transactivation of the Wnt
signaling-targeted genes (4). The
signal transduction by TGF-β is initiated by binding to the
transmembrane receptors, TGFRs, and then activates Smad2 and Smad3.
The phosphorylated Smad2 and Smad3 recruit Smad4, and translocate
it into the cell nucleus. Smad4 cooperates with DNA-binding
transcription factors influencing the targeted gene transcription
(5). Extensive evidence manifest
that Wnt/β-catenin and TGF-β signaling pathways are involved in
differentiation of stem cells towards endothelial cells,
hepatocytes, neurons, and chondrocytes under various stimulation
conditions (4,6–8).
The miRNAs are a group of noncoding RNA molecules
that control the expression of their target genes at the
post-transcriptional level by binding to the 3′ untranslated region
and inducing the mRNA degradation (9). miRNAs are implicated in multiple
biological functions through modulating genes related to the
functions. miR-33 is considered as an important regulator of cell
differentiation. A study has revealed that miR-33 through targeting
Hmga2 promotes osteoblast differentiation in response to specific
environment stimulus like microgravity and fluid shear stress
(10). In addition, miR-33 is
associated to adipose tissue differentiation and development of
gastrointestinal tract (11,12).
Nevertheless, the potential effect of miR-33 on the differentiation
of ASCs towards urothelial cells has not been investigated to
date.
In this study, we found that miR-33 blocked ASCs
differentiation towards urothelial cells through targeting
β-catenin and TGFR1. This novel finding suggests that miR-33 may be
an important target in tissue engineering for urothelium repair and
reconstruction.
Materials and methods
Donor specification
All experiments were approved by the Ethics
Committee of Xiangya School of Medicine (Changsha, China). Each
human subject signed a consent form. Human subcutaneous adipose
tissue from the abdomen was obtained from six females healthy women
undergoing cosmetic liposuction surgery. The age range of the women
was 28–40 years (average age 34±6 years). Adipose samples were
washed with Hank's balanced salt solution (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and used for ASCs isolation.
Isolation of ADSCs
ADSCs isolation was performed as described by
Naaijkens (13). Briefly, the
fresh adipose tissue was minced and then digested with 1 mg/ml of
collagenase type 1A (Sigma-Aldrich; Merck KGaA) under shaking at
37°C for approximately 60 min until the detachment of the cells
from tissues. The adipose suspension was diluted with equal volume
of Dulbecco's modified Eagle's medium media (DMEM; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.) to
neutralize the collagenase. After centrifugation at 400 × g for 10
min, cell pellets at the bottom of the centrifugal tube were
dispersed in DMEM media containing 10% FBS, 1%
antibiotic-antimycotic solution (Thermo Fisher Scientific, Inc.)
and cultured in humidified incubator at 37°C with 5%
CO2. The non-attached cells were eliminated on the next
day by changing the medium and replaced with the fresh medium. The
cells of passage 3 were collected for flow cytometry analysis
(Becton Dickinson, San Jose, CA, USA) to detect the surface
markers, including CD29, CD90, and CD34 for ADSCs
identification.
Flow cytometry
ASCs were detached by brief trypsinization followed
by washing steps with PBS (Sigma-Aldrich; Merck KGaA). Cells were
incubated for 45 min with APC- or PE-labeled antibodies against
CD29, CD90, and CD34 (Abcam, Cambridge, UK). Cells were washed and
surface expression was analyzed using a FACSCalibur (Becton
Dickinson).
Induction of ADSCs for the urothelium
phenotype differentiation
Induction of ADSCs for the urothelium phenotype
differentiation was done as described by Zhao (14). A conditioned medium is prepared by
supplementation of DMEM media with 2% FBS, 2.5 µM all-trans
retinoic acid, 20 ng/ml epidermal growth factor, 10 ng/ml
hepatocyte growth factor, 10 ng/ml keratinocyte growth factor, and
0.5 µg/ml hydrocortisone. Except for DMEM media and FBS, there
agents were purchased from Sigma-Aldrich (Merck KGaA). ADSCs were
incubated in the conditioned medium for 14 d. The medium was
exchanged every 2 d. The un-induced ADSCs were used as the negative
control.
Cell viability assay
Cell viability was evaluated by using CCK-8 reagent
(Dojindo, Kumamoto, Japan). 10 µl of the CCK-8 reagent was added to
each well of 96-well plates. Absorbance at 450 nm was detected with
a microplate reader (Multiskan MK3; Thermo Lab systems, Shanghai,
China), after the incubation at 37°C for 2–4 h. The obtained values
were normalized to those from control cells.
Quantitative polymerase chain reaction
(qPCR)
Total RNA, including small RNA, was extracted using
a mirVana miRNA isolation kit (Thermo Fisher Scientific, Inc.). The
High Capacity RNA-to-cDNA Master Mix (Thermo Fisher Scientific,
Inc.) was used to synthesize cDNA. Primer sequences were as follow:
miR-33a-5p, forward: 5′-GTGTGCATTGTAGTTGC-3′, and reverse:
5′-TTTGGCACTAGCACATT-3′; miR-33b-5p, forward:
5′-TTGTGCATTGCTGTTG-3′, and reverse: 5′-TTTGGCACTAGCACATT-3′, as
well as U6, forward: 5′-CTCGCTTCGGCAGCACA-3′, and reverse:
5′-AACGCTTCACGAATTTGCGT-3′. qPCR was performed using the ABI PRISM
7500 DNA Sequence Detection System (Thermo Fisher Scientific,
Inc.). The relative expression levels were determined using the
comparative quantification cycle (2−ΔΔCq). U6 RNA
expression level was used as internal (house keeping) control.
Western blot assay
Whole cell extracts were prepared with a cell lysis
reagent (Sigma-Aldrich; Merck KGaA) according to the manual, and
the protein was quantified by a BCA assay (Pierce; Thermo Fisher
Scientific, Inc.). Protein extracts were separated using
SDS/polyacrylamide gel electrophoresis and transferred to
nitrocellulose membranes. The membranes were hybridized with
anti-β-catenin antibody (dilution 1:500, ab32572; Abcam), TGFR1
(dilution 1:2,000, ab31013; Abcam), CK7 (dilution 1:500, ab9021;
Abcam), CK20 (dilution 1:1,000, ab76126; Abcam), UPIII (dilution
1:800, ab78196; Abcam) and anti-β-actin antibody (dilution 1:800,
sc-47778; Santa Cruz Biotechnology, Inc., Dallas, TX, USA), at 4°C
overnight. After extensive washing three times for 10 min each in
TBS-T (Sigma-Aldrich; Merck KGaA), secondary biotin-conjugated
antibodies were added for 1 h at 22°C. Blots were again washed
three times for 10 min each in TBS-T, and immunoreactive bands were
developed by an avidin/biotin/peroxidase complex (Vectastain ABC
Elite kit; Vector Laboratories lnc., Burlingame, CA, USA) and
substrate (Vector NovaRED, Vectastain; Vector Laboratories
lnc.).
Immunofluorescence (IF) assays
Cells were fixed with 4% paraformaldehyde for 10 min
at room temperature and with methanol at −20°C for 20 min. Normal
goat serum (10%; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) was added to cells for 30 min to block nonspecific binding
sites. The fixed cells were immunostained with primary antibodies
targeting CK7 (dilution 1:500, ab9021; Abcam), CK20 (dilution
1:300, ab76126; Abcam) and UPIII (dilution 1:500, ab78196; Abcam)
overnight at 4°C and the Alexa Fluor 488-conjugated secondary
antibody (1:500 dilution, SP-9000; ZSGB-BIO, Beijing, China) for 1
h at 37°C. The coverslips were stained with DAPI (1:1,000, SC-3598;
Santa Cruz Biotechnology, Inc.) for 2 min and mounted on slides
using anti-fade mounting medium. Images were acquired with a
high-resolution CoolSNAP™ CCD camera (Photometrics Inc., Tucson,
AZ, USA) under the control of a computer using Leica FW4000
software version 1.2 (Leica Microsystems, Ltd., Milton Keynes,
UK).
Luciferase reporter assay
Luciferase reporter assay was performed to
identified that β-catenin and TGFR1 are targets of miR-33a. The
predicted miR-33a-binding site sequence (miRNA response element,
MRE) on the 3′ untranslated regions (3′UTR) of β-catenin and TGFR1
mRNA were subcloned into pmirGLO (including XhoI and NotI
restriction enzyme sites; Promega, Madison, WI, USA) respectively
to construct miR-33a/β-catenin and miR-33a/TGFR1 MRE luciferase
reporters. These reporters were transfected into ADSCs alone or in
combination with miR-33a mimics, using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.). Luciferase and
renilla signals were measured 48 h after transfection using the
Dual Luciferase Reporter Assay System (Promega Corporation,
Madison, WI, USA). The activity of firefly luciferase was
normalized to that of renilla luciferase.
Transfections
miR-33a mimics (5′-GUGCAUUGUAGUUGCAUUGCA-3′),
miR-33a inhibitors (5′-UGCAAUGCAACUACAAUGCAC-3′) and appropriate
negative control molecules were purchased from RiboBio Corporation
(Guangzhou, China). These RNA oligoribonucleotides were transfected
into ADSCs using X-tremeGENE siRNA Transfection Reagent (Roche
Diagnostics GmbH, Mannheim, Germany) according to the
manufacturer's protocol. The final concentration was 125 nM for
miRNA mimics and 250 nM for miRNA inhibitors. Transfections were
repeated per two days during the ADSCs towards the urothelium
phenotype differentiation. miR-33a expression in ADSCs was detected
by qPCR.
Statistical analysis
Results were presented as means ± standard
deviation. Statistical analysis was performed by SPSS 11.0 (SPSS,
Inc., Chicago, IL, USA). Means of experimental groups were compared
with those of other experimental groups or controls using the
Student's t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
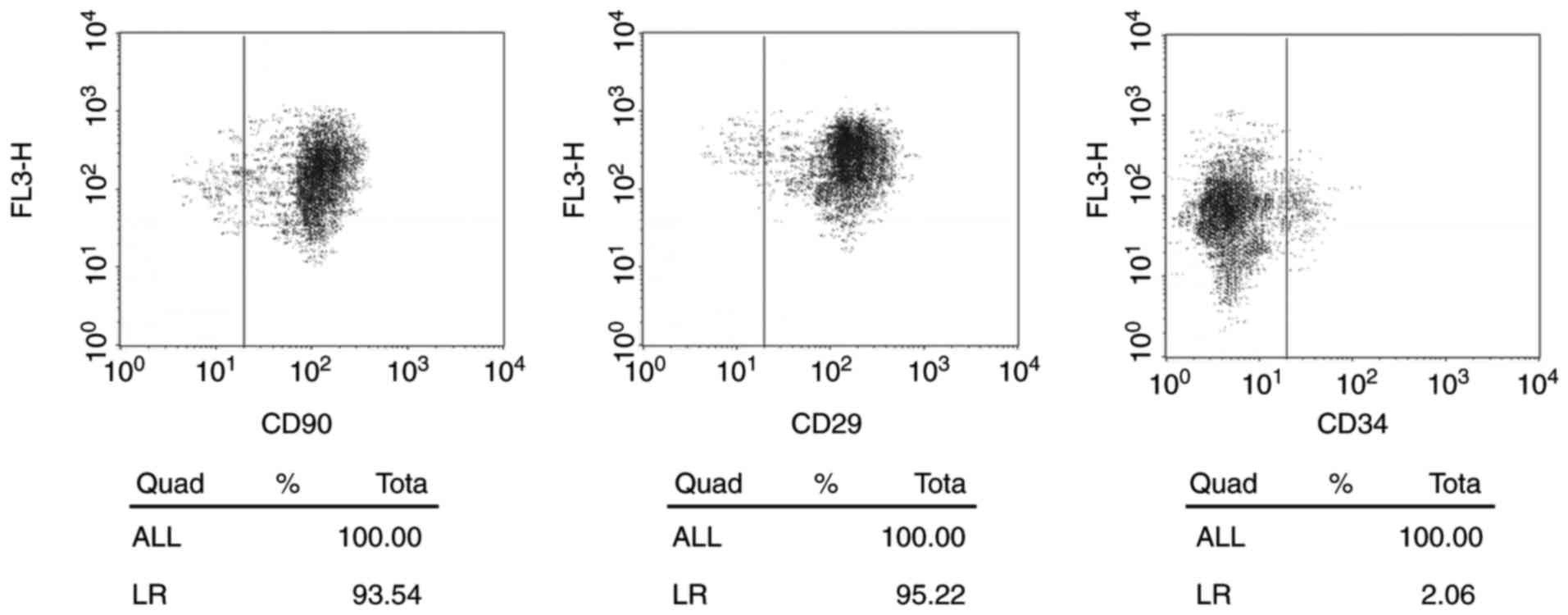
ADSCs identification
In the present study, human ADSCs were isolated from
abdominal adipose tissues. ADSCs showed a spindle-like morphology
with large nucleus. Flow cytometry identified the positive
expression of surface markers of ADSCs, including CD29 (93.54%) and
CD90 (95.22%) and negative expression of a negative marker, CD33
(2.06%) (Fig. 1).
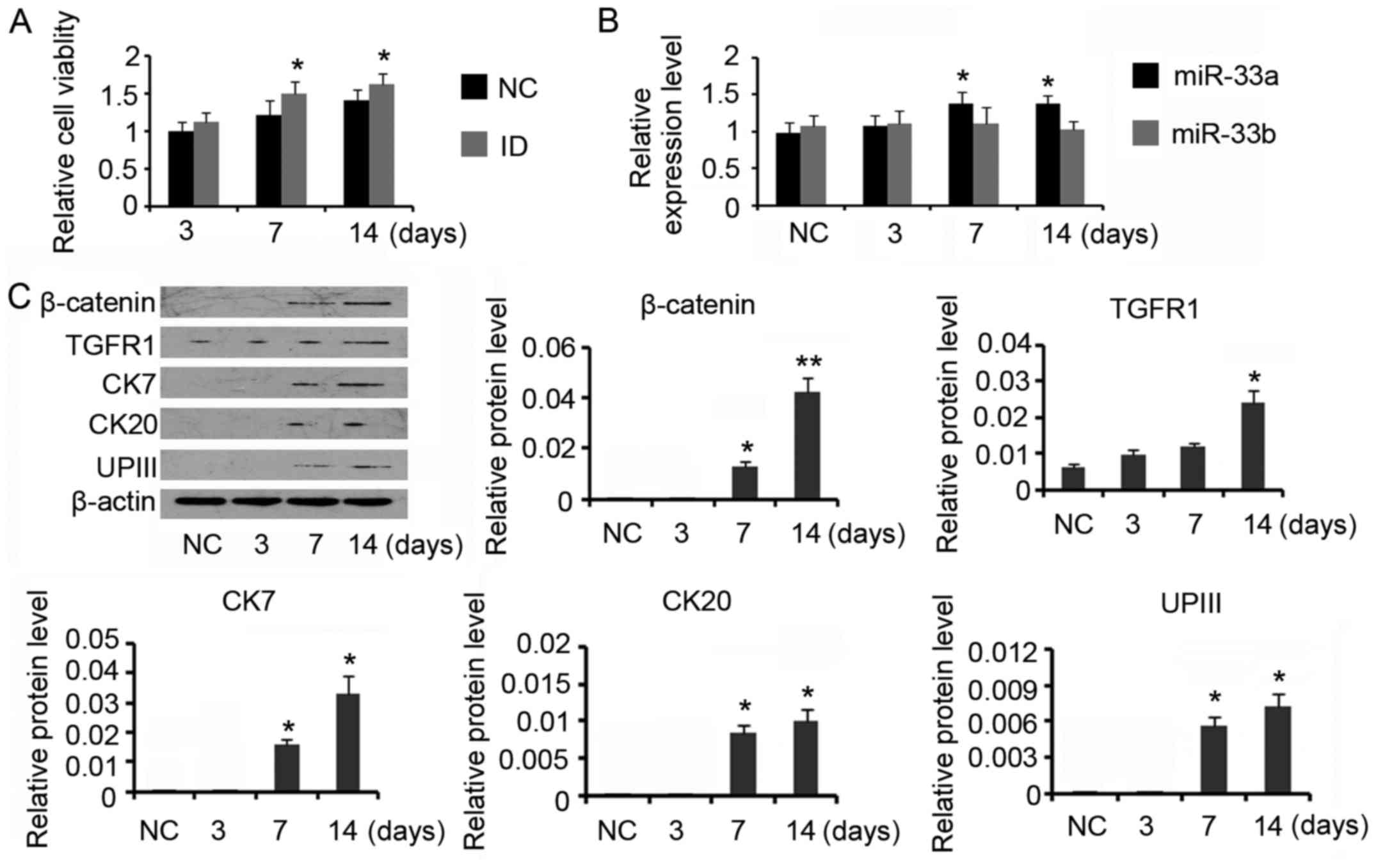
Induction of ADSCs towards the
urothelium phenotype differentiation increased miR-33a
expression
For urothelial differentiation, ADSCs were incubated
in the conditioned medium for 14 days. The induction increased the
cell viability compared with control (P<0.05 on day 7 and day
14, Fig. 2A). PCR assay revealed
that miR-33a expression was gradually increased during the
differentiation processes (P<0.05 on day 7 and day 14, Fig. 2B), but miR-33b expression level was
not significantly affected. Data from western blotting indicated
that β-catenin (P<0.05 on day 7 and P<0.01 on day 14) and
TGFR (P<0.05 on day 14) expression levels were increased after
the induction (Fig. 2C). CK7, CK20
and UPIII are urothelial specific proteins, which are commonly used
as biomarker for urothelium cells. Inducing ADSCs for the
urothelium phenotype differentiation increased CK7 expression to 2-
and 3-folds on day 7 and day 14 respectively (both P<0.05). CK20
and UPIII expression levels were also increased after 7 days'
induction (P<0.05 on day 7), but their increased degrees were
much lower than that of CK7. Prolonging the incubation time to 14
days conferred moderate effect on further increasing CK20 and UPIII
expression. CK20 expression was not detectable during the
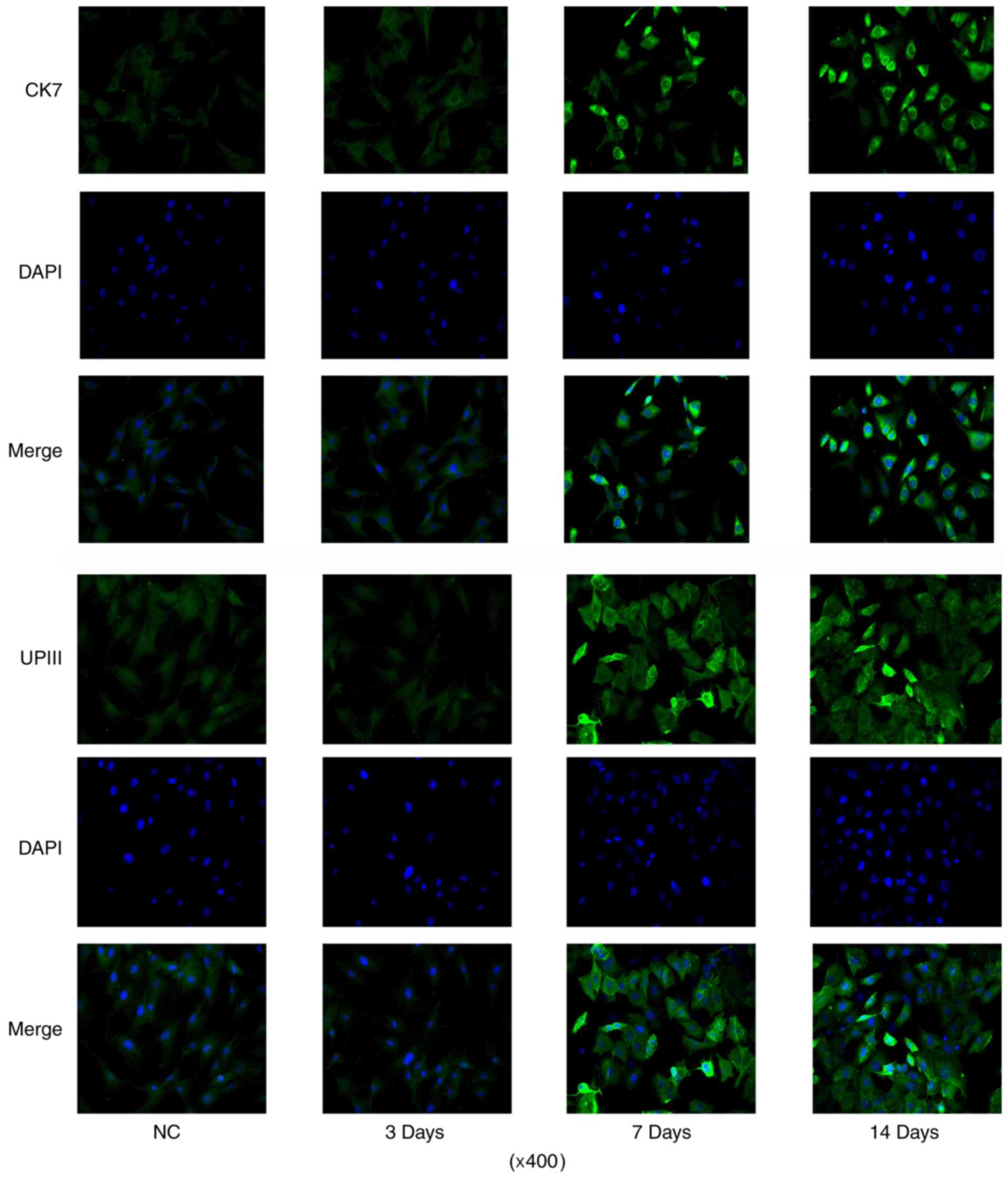
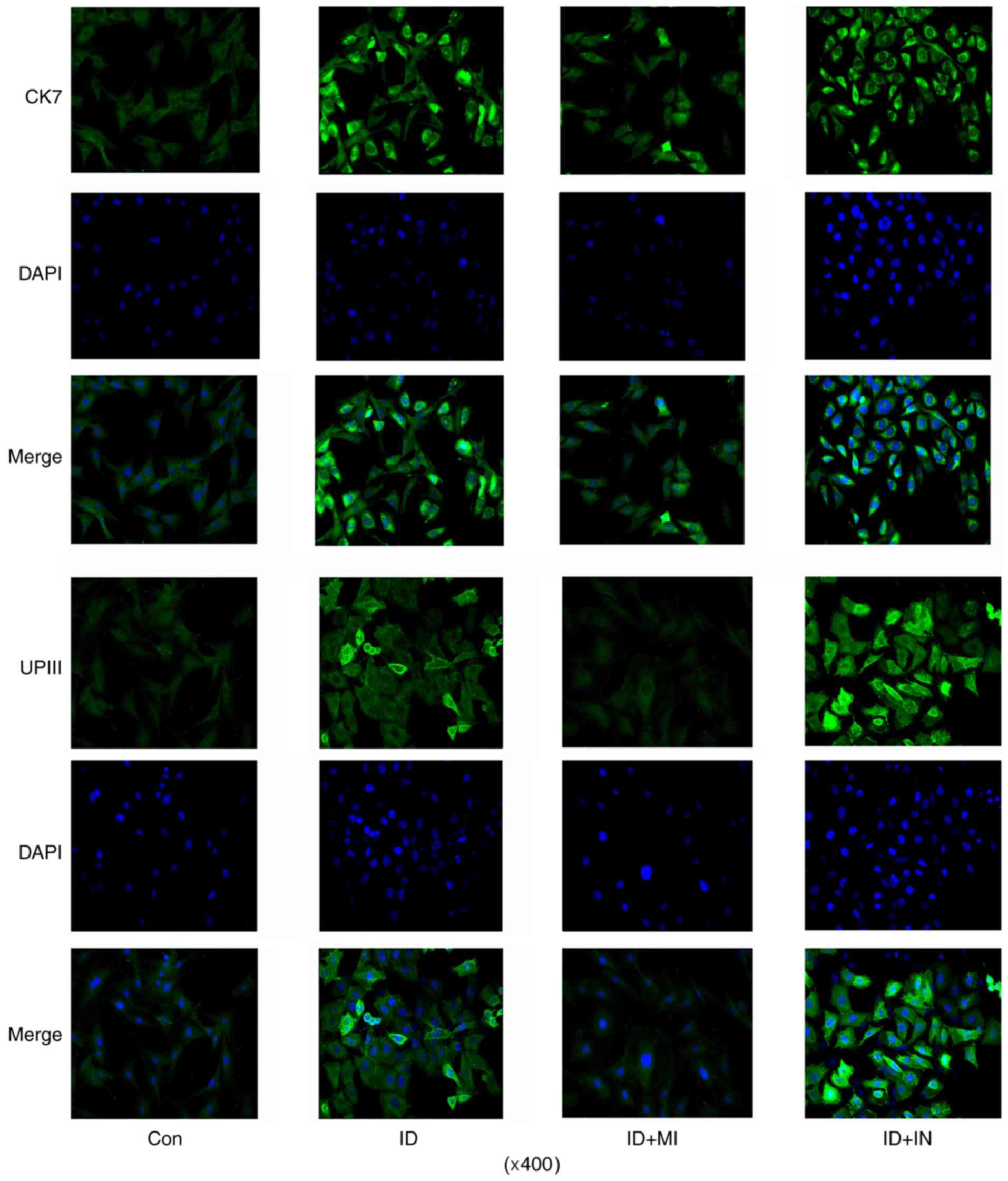
differentiation processes by IF assay (data not shown). CK7 IF
signal was increased after 7 days' induction, with the maximum on
day 14 (Fig. 3). And the number of
cells with positive IF staining were also increased with the
incubation. The number of cells with UPIII IF staining was
increased after 7 days' induction when compared to control. But
prolonging the incubation time to 14 days did not further increased
the number of cells with UPIII IF staining.
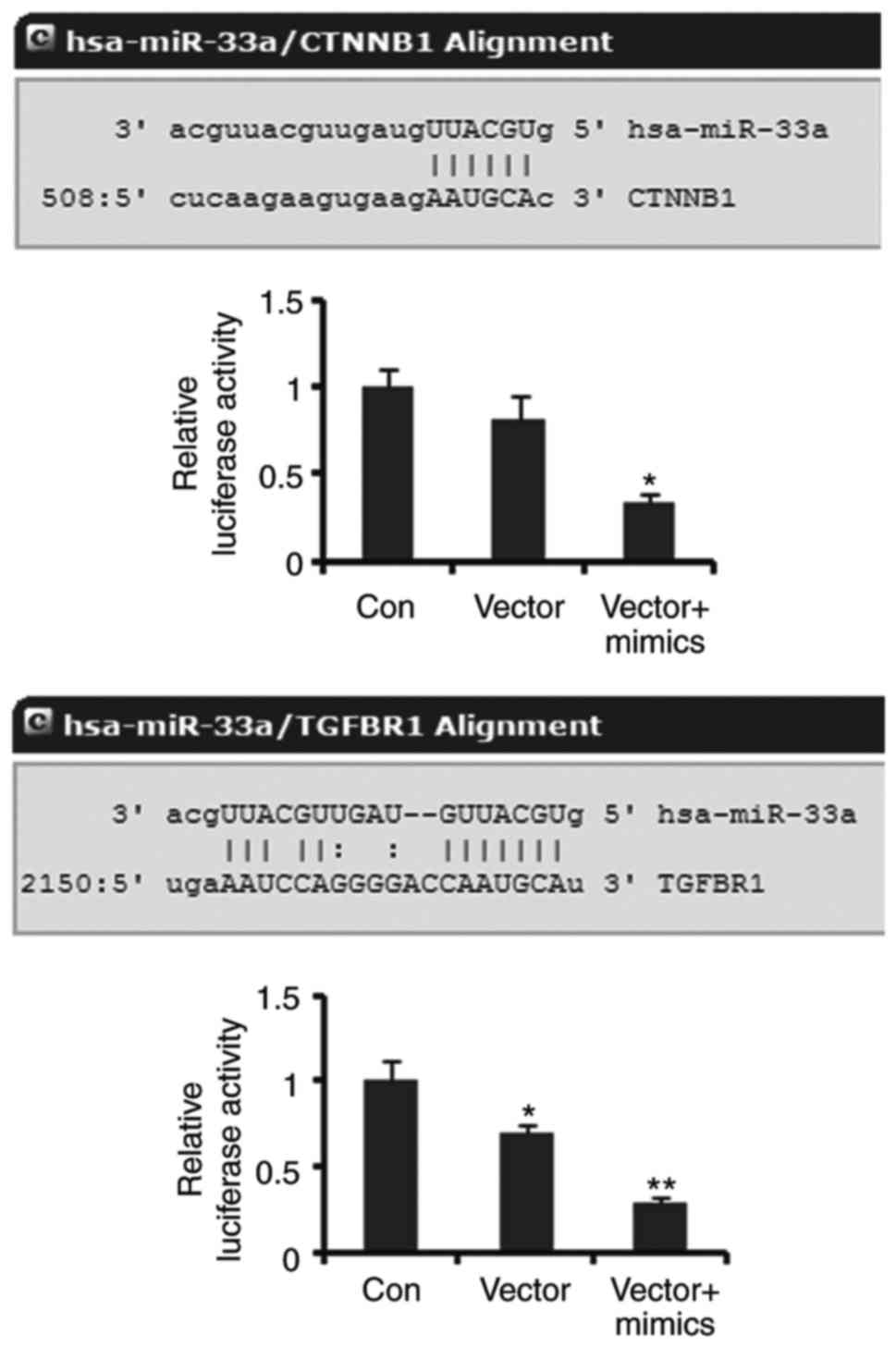
β-catenin and TGFR are targets of
miR-33a
Luciferase reporter assay was performed to elucidate
the correlation of β-catenin and TGFR with miR-33a. Luciferase
reporters containing the sequence of miR-33a binding 3′UTR of
β-catenin showed decreased luciferase activity compared to the
empty vector (control), but the decrease did not reach to
significant difference. Co-transfection with miR-33a mimics notably
decreased the luciferase activity of the miR-33a/β-catenin MRE
luciferase reporters (P<0.05, Fig.
4). The luciferase activity of miR-33a/TGFR1 MRE luciferase
reporters was dramatically lower than that of empty vector
(P<0.05). Co-transfection with miR-33a further reduced the
luciferase activity of miR-33a/TGFR1 MRE luciferase reporters
(P<0.01 vs. control). These data demonstrated that β-catenin and
TGFR are targets of miR-33a.
miR-33a blocks ADSCs towards the
urothelium phenotype differentiation
To get insight into the role of miR-33a in the
urothelial differentiation of ADSCs, miR-33a expression in the
ADSCs was downregulated by miR-33a inhibitors, or increased by
miR-33a mimics, during the differentiation. Although cell viability
was increased after 14 days' incubation in the conditioned medium
(P<0.05, Fig. 5A), continuous
transfection with miR-33a mimics abolished the increase in cell
viability. In the contrast, the increased cell viability was no
influenced by the transfection with miR-33a inhibitors.
Transfection with miR-33a inhibitors reversed miR-33a expression
that was increased by the conditioned medium (P<0.01 vs.
control, Fig. 5B), while miR-33a
mimics further increased the miR-33a expression (P<0.01 vs.
control). A Western blot assay showed that miR-33a mimics impaired
the increase in β-catenin and TGFR expression during the ADSCs
differentiation (P<0.05 vs. the induction group, Fig. 5C), whereas miR-33a inhibitors
further increased the β-catenin and TGFR expression (P<0.05 vs.
the induction group). Increasing miR-33a expression by mimics also
reversed CK7, CK20 and UPIII expression levels that were increased
during the ADSCs differentiation (P<0.05 vs. the induction
group). miR-33a expression reduction did not significantly
influenced CK7 expression, but notably increased CK20 and UPIII
expression (P<0.05 and P<0.01, respectively). A IF assay
showed that the number of cells with strong CK7 IF signal was
increased by the transfection with miR-33a inhibitor, but decreased
by the transfection with miR-33a mimics (Fig. 6). Similarly, the transfection with
miR-33a inhibitors increased the number of cells with strong UPIII
IF signal, while the transfection with miR-33a mimics decreased the
number of cells.
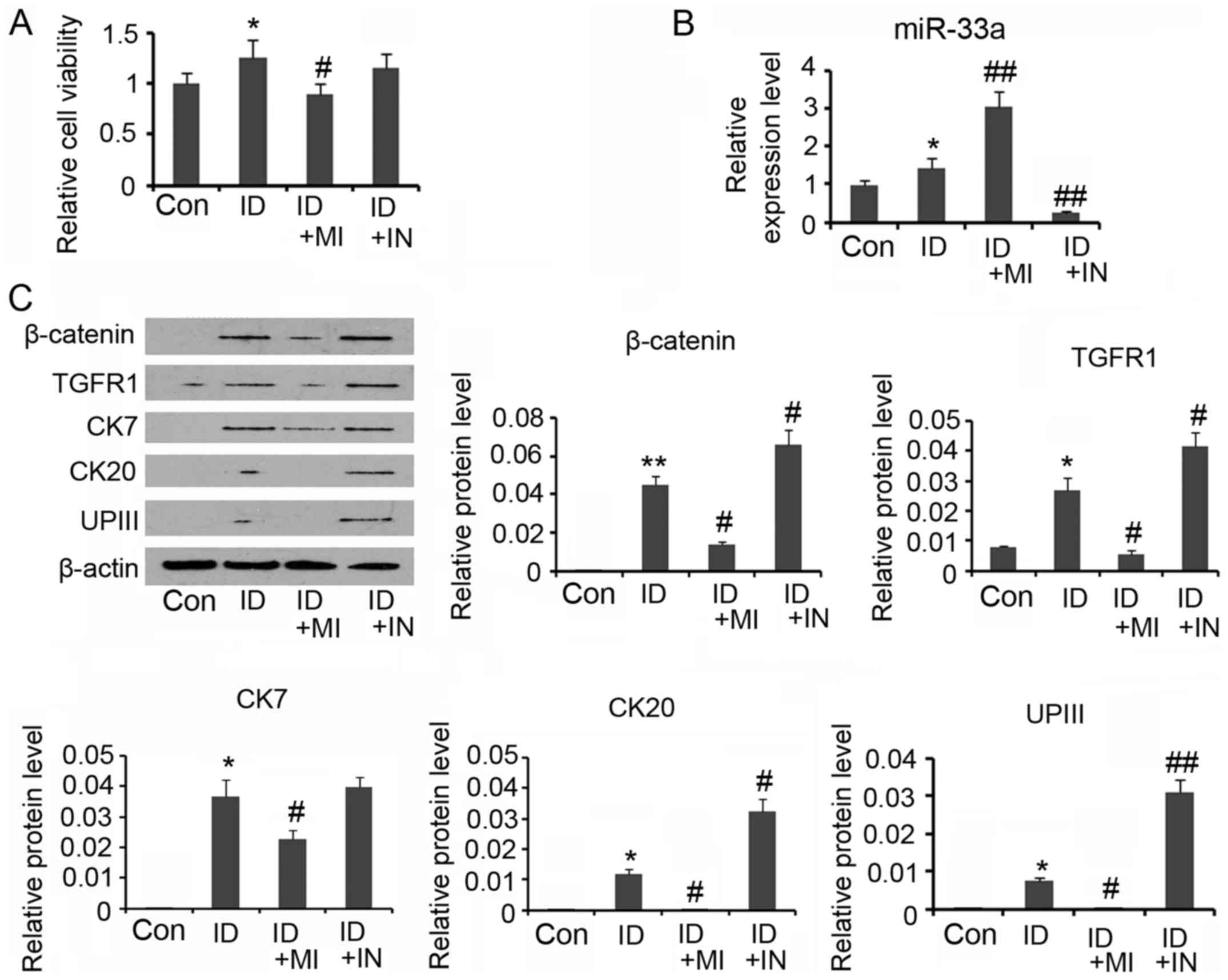 | Figure 5.miR-33a blocks urothelial
differentiation of adipose-derived mesenchymal stem cells (ADSCs).
For urothelial differentiation, ADSCs were incubated in the
conditioned medium for 14 days. To determine the role of miR-33a in
the differentiation, this study used synthetic miRNAs to mimic or
inhibit the action of miR-33a. (A) Cell viability was evaluated
during the differentiation. (B) PCR assay was conducted to evaluate
miR-33a and miR-33b expression. (C) Western blot assay was
performed to detect levels of β-catenin, TGFR, CK7, CK20 and UPIII
expression. *P<0.05 and **P<0.01 vs. control. *P<0.05 and
**P<0.01 vs. control, #P<0.05 and
##P<0.01 vs. the ID group. Con, Control; ID,
induction of urothelial differentiation; MI, miR-33a mimics; IN,
miR-33a inhibitors. |
Discussion
Tissue engineering represents a promising strategy
to repair damaged urothelium caused by various diseases of the
genitourinary system. ADSCs are a rich source of stem cells, which
can be easily obtained from fat tissues. ADSCs, with multilineage
potential, has been used in tissue engineering for the management
of skin wounds, orthopedic injuries, neurological dysfunctions and
cardiovascular disease, and many encouraging achievements have been
made (3,14). However, inducing ADSCs
differentiation towards the urothelium is challenging, because
urothelial cells derived from the endoderm, whereas ADSCs are
derived from the mesoderm. The cross-mesoderm differentiation of
urothelial cells from ADSCs is relatively difficult (14). It has been reported that the
co-culture with urothelial cells drives ADSCs differentiation
towards urothelium-like cells, which is most likely related to
cytokines released by urothelial cells (15). Previous study have found a
urothelial differentiation of human umbilical cord-derived
mesenchymal stromal cells when cultured in media that have cultured
urothelial cells (16). It has
been reported that cytokines, like epidermal growth factor,
hepatocyte growth factor, and keratinocyte growth factor, have
critical role in the differentiation of ADSCs (14). Thus, these cytokines has been
suggested for ADSCs differentiation towards urothelial cells in
vitro. In the present study, these cytokines had been added to
culture medium to induce ADSCs differentiation. Results showed that
expression levels of urothelial specific marks, like CK7, CK20 and
UPIII were increased after seven days' induction, suggesting the
urothelial phenotype differentiation. However, through the
microscopic observation, cells with strong CK7 and UPIII IF signal
were very scarce. Moreover, except for CK7, prolonging the
incubation time conferred moderate effect on further increasing
CK20 and UPIII expression in the urothelial lineage cells, neither
the number of cells with strong UPIII IF staining. CK7 is regarded
as an early marker of urothelial cells, but CK20 and UPIII belongs
to urothelial specific proteins occurring in the intermediate or
terminal stages of urothelial cell differentiation (14). Therefore, the current method
remained low-efficient or incomplete for the urothelial
differentiation of ADSCs.
Previous studies report that miR-33 exerts important
effects on osteoblast and adipose tissue differentiations (10,12).
The present study found that miR-33a expression was increased in
the urothelial differentiation. Interference of the miR-33a
expression with inhibitors promoted the urothelial differentiation,
as indicated by further increased expression of CK20 and UPIII in
cells and percentage of cells with strong UPIII IF staining. But
using synthetic miRNAs to mimic the action of miR-33a blocked ADSCs
towards the urothelium phenotype differentiation. These data
suggest that miR-33a plays negative role in urothelial
differentiation. It is currently unclear why miR-33a expression was
increased during the urothelial differentiation. Understanding the
underlying mechanisms may contribute to the development of a new
strategy to inhibit miR-33a expression and consequently improve the
efficiency of the differentiation.
Wnt/β-catenin and TGF-β/TGFR signaling pathways are
critical for stem cell differentiation. In particular,
Wnt/β-catenin signaling is involved in the differentiation of human
pluripotent stem cells towards endothelial cells (17–19).
TGFR was reported to regulate urothelial cells' renewal and
regeneration by inducing the differentiation of basal uroepithelial
stem and the early progenitor cells (USCs) (20). An induced ablation of TGFR blocks
USC differentiation into transitional epithelium of the bladder
(20). In addition, TGF-β
participates in molecular signals that drive differentiation of
endoderm-derived stem cells to prostate epithelia (21). In the present study, we confirmed
that β-catenin and TGFR are targets of miR-33a by the luciferase
reporter assay. Using synthetic miRNAs to mimic or inhibit the
action of miR-33a respectively reduced and increased β-catenin and
TGFR expression. This suggests that the increase in miR-33a
expression in the urothelial differentiation may impair the effects
of β-catenin and TGFR on the cell differentiation. In the present
study, protein levels of β-catenin and TGFR1 in ADSCs were
increased when cultured in the conditioned medium. This suggests
that some components in the conditioned medium promotes β-catenin
and TGFR1 expression. Interestingly, miR-33a was also increased
during the incubation. As β-catenin and TGFR1 are target of
miR-33a, the increased miR-33a, to some extent, hindered the
increase in β-catenin and TGFR1 expression. Indeed, when miR-33a
expression was suppressed by the inhibitors, β-catenin and TGFR1
showed more notable increase during the incubation in the
conditioned medium, and ADSCs differentiation towards the
urothelium phenotype cells was more remarkably promoted. This study
for the first time revealed that miR-33 hinders the differentiation
of ADSCs towards urothelium phenotype in the conditioned medium.
The inhibitory effect is likely associated to the negative
regulation of β-catenin and TGFR expression. This novel founding
suggests that miR-33 may be an important target in tissue
engineering for urothelium repair and reconstruction.
Acknowledgements
This study was supported by the Young fund of Hunan
Natural Science Foundation of China (no. 14JJ3151).
References
|
1
|
Orabi H, Bouhout S, Morissette A, Rousseau
A, Chabaud S and Bolduc S: Tissue engineering of urinary bladder
and urethra: Advances from bench to patients.
ScientificWorldJournal. 2013:1545642013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Steins A, Dik P, Müller WH, Vervoort SJ,
Reimers K, Kuhbier JW, Vogt PM, van Apeldoorn AA, Coffer PJ and
Schepers K: In vitro evaluation of spider silk meshes as a
potential biomaterial for bladder reconstruction. PLoS One.
10:e01452402015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li H, Xu Y, Xie H, Li C, Song L, Feng C,
Zhang Q, Xie M, Wang Y and Lv X: Epithelial-differentiated
adipose-derived stem cells seeded bladder acellular matrix grafts
for urethral reconstruction: An animal model. Tissue Eng Part A.
20:774–784. 2014.PubMed/NCBI
|
|
4
|
Tanthaisong P, Imsoonthornruksa S,
Ngernsoungnern A, Ngernsoungnern P, Ketudat-Cairns M and Parnpai R:
Enhanced chondrogenic differentiation of human umbilical cord
wharton's jelly derived mesenchymal stem cells by GSK-3 inhibitors.
PLoS One. 12:e01680592017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ni J, Shi Y, Li L, Chen J, Li L, Li M, Zhu
J, Zhu Y and Fan G: Cardioprotection against heart failure by
shenfu injection via TGF-β/Smads signaling pathway. Evid Based
Complement Alternat Med. 2017:70830162017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Touboul T, Chen S, To CC, Mora-Castilla S,
Sabatini K, Tukey RH and Laurent LC: Stage-specific regulation of
the WNT/β-catenin pathway enhances differentiation of hESCs into
hepatocytes. J Hepatol. 64:1315–1326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chuang JH, Tung LC and Lin Y: Neural
differentiation from embryonic stem cells in vitro: An overview of
the signaling pathways. World J Stem Cells. 7:437–447. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heck BE, Park JJ, Makani V, Kim EC and Kim
DH: PPAR-δ agonist with mesenchymal stem cells induces type II
collagen-producing chondrocytes in human arthritic synovial fluid.
Cell Transplant. 26:1405–1417. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ventayol M, Viñas JL, Sola A, Jung M,
Brüne B, Pi F, Mastora C and Hotter G: miRNA let-7e targeting MMP9
is involved in adipose-derived stem cell differentiation toward
epithelia. Cell Death Dis. 5:e10482014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang H, Sun Z, Wang Y, Hu Z, Zhou H, Zhang
L, Hong B, Zhang S and Cao X: miR-33-5p, a novel mechano-sensitive
microRNA promotes osteoblast differentiation by targeting Hmga2.
Sci Rep. 6:231702016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liang G, Malmuthuge N, McFadden TB, Bao H,
Griebel PJ, Stothard P and Guan le L: Potential regulatory role of
microRNAs in the development of bovine gastrointestinal tract
during early life. PLoS One. 9:e925922014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Price NL and Fernández-Hernando C: miRNA
regulation of white and brown adipose tissue differentiation and
function. Biochim Biophys Acta. 1861:2104–2110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naaijkens BA, Niessen HW, Prins HJ,
Krijnen PA, Kokhuis TJ, de Jong N, van Hinsbergh VW, Kamp O, Helder
MN, Musters RJ, et al: Human platelet lysate as a fetal bovine
serum substitute improves human adipose-derived stromal cell
culture for future cardiac repair applications. Cell Tissue Res.
348:119–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Z, Yu H, Fan C, Kong Q, Liu D and
Meng L: Differentiate into urothelium and smooth muscle cells from
adipose tissue-derived stem cells for ureter reconstruction in a
rabbit model. Am J Transl Res. 8:3757–3768. 2016.PubMed/NCBI
|
|
15
|
Shi JG, Fu WJ, Wang XX, Xu YD, Li G, Hong
BF, Hu K, Cui FZ, Wang Y and Zhang X: Transdifferentiation of human
adipose-derived stem cells into urothelial cells: Potential for
urinary tract tissue engineering. Cell Tissue Res. 347:737–746.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu S, Cheng Z, Liu G, Zhao X, Zhong L, Zhu
Y and Zhu J: Urothelial differentiation of human umbilical
cord-derived mesenchymal stromal cells in vitro. Anal Cell Pathol
(Amst). 36:63–69. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith Q, Chan XY, Carmo AM, Trempel M,
Saunders M and Gerecht S: Compliant substratum guides endothelial
commitment from human pluripotent stem cells. Sci Adv.
3:e16028832017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leach LL, Buchholz DE, Nadar VP,
Lowenstein SE and Clegg DO: Canonical/β-catenin Wnt pathway
activation improves retinal pigmented epithelium derivation from
human embryonic stem cells. Invest Ophthalmol Vis Sci.
56:1002–1013. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lian X, Bao X, Al-Ahmad A, Liu J, Wu Y,
Dong W, Dunn KK, Shusta EV and Palecek SP: Efficient
differentiation of human pluripotent stem cells to endothelial
progenitors via small-molecule activation of WNT signaling. Stem
Cell Reports. 3:804–816. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mysorekar IU, Isaacson-Schmid M, Walker
JN, Mills JC and Hultgren SJ: Bone morphogenetic protein 4
signaling regulates epithelial renewal in the urinary tract in
response to uropathogenic infection. Cell Host Microbe. 5:463–475.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li X, Wang Y, Sharif-Afshar AR, Uwamariya
C, Yi A, Ishii K, Hayward SW, Matusik RJ and Bhowmick NA:
Urothelial transdifferentiation to prostate epithelia is mediated
by paracrine TGF-beta signaling. Differentiation. 77:95–102. 2009.
View Article : Google Scholar : PubMed/NCBI
|