Introduction
Colorectal carcinoma (CRC) is the third most common
form of cancer in the world, and the rectum exhibits common
internal malignancies (1). In
China, CRC ranks 5th among cancer deaths, with increasing the
incidence annually (2). Although
there are treatment options including radiotherapeutic,
chemotherapeutic regimens and surgical regimens available for the
clinical management of CRC, outcomes of such strategies are limited
by associated high probability of cancer recurrence, obvious
toxicity on the human body, affecting neurotoxicity,
gastrointestinal reaction, kidney failure, and cardiotoxicity
(3,4). Chemoprevention is a strategy that was
first proposed by Sporn et al (5). It is a way to reverse, suppress, or
prevent molecular or histologic premalignant lesions from
progressing to invasive cancer by using natural or synthetic agents
(6). To date, many food/plant
derived chemopreventive agents that exhibit strong efficacy against
various cancers in vitro and preclinical models have been
identified (7–10). Colorectal carcinoma is a rationale
cancer to target for chemoprevention studies due to high incidence
of pre-neoplastic lesions and cancerous tumors (11). An ideal chemopreventive compound
should be nontoxic, potent, highly effective, less expensive, and
easily available (4).
Luteolin (3′,4′,5,7-tetrahydroxyflavone), a
flavonoid polyphenolic compound found in many plant types such as
fruits, vegetables, and medicinal herbs. It has been shown
biological activities, such as anti-inflammatory, anti-allergy, and
anticancer activities (12–14).
Recent studies have reported the anticancer effects of luteolin
against lung cancer, head and neck cancer, prostate, breast, colon,
liver, cervical, and skin cancer was associated with inducing
apoptosis, suppressing metastasis, and angiogenesis (15–22).
These results of above studies warrant the further evaluation of
the chemopreventive potential of luteolin in human subjects
(4). However, it has very low
bioavailability after oral administration and it is very difficult
to make intravenous or intraperitoneal administration because of
its poor aqueous solubility. Therefore, there is a clear need to
increase its potential in clinical application (23).
During the past few years, liposomes have drawn much
attention for cancer therapy because of longer blood circulation
time, higher biocompatibility, excellent bioavailability, and
higher tumor-specific delivery (24–27).
Thus, in the present study, we investigated whether liposomes can
be used as delivery system to improve the antitumor efficacy of
luteolin in vitro and in vivo. We used CT26 cell and
mouse tumor model to evaluate the activity of luteolin before and
after encapsulating into liposome. Meanwhie, we established a
liposome-formulated luteolin that is capable of effective
suppressing tumor growth through inducing apoptosis and inhibiting
angiogenesis. It is our hope that understanding of these mechanisms
in detail may provide a basis for novel targeting strategies for
cancers.
Materials and methods
Liposome preparation
Lipo-Lut formulations were prepared by the thin film
hydration method. Briefly, the mixtures of
luteolin/cholesterol/lecithin (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) in 1:2:7 weight ratios were dissolved in
ethanol and were transferred into a round bottom flask. The flask
was then connected to a rotary evaporator at 50 rpm and water bath
with temperature maintained at 40°C. Vacuum was applied to the
flask to evaporate the ethanol and form a homogeneous lipid film on
the flask wall. Then the lipid film was then hydrated in normal
saline by rotating the flask at 37°C until the lipid film was
completely hydrated. At last the luteolin liposome was sonicated
with 50 watts of power for 10 min. The empty liposome without
luteolin was prepared in the same as the Lipo-Lut.
Size distribution and ζ-potential
The mean particle sizes and ζ-potential of the
obtained liposomes were measured by photon correlation spectroscopy
using Malvern Zeta sizer 3000 HS (Malvern Instruments, Malvern, UK)
at 25°C. The form feature of the liposomes was determined by
transmission electron microscope (TEM) (H-600; Hitachi, Tokyo,
Japan) using a negative staining method with 1% sodium
phosphotungstate solution for 2 min at room temperature.
Drug loading and encapsulation
efficiency
The prepared liposomes were solubilized in methanol
[liposomes: methanol=1:9, volume/volume (v/v)]. After using a
cyclomixer to completely extract the drug from lipid to methanol,
the drug content was analyzed by an Agilent 1100 Series HPLC System
(Agilent Technologies, Santa Clara, CA, USA). An octadecylsilyl
column (4.6×250.0 mm, 5 µm) was used for the analysis. The mobile
phase was a mixture of acetonitrile and 0.1% formic acid (30:70,
v/v), and the flow rate was 0.5 ml/min. The UV detection wavelength
of 350 nm was used, the column temperature was 35°C. The amount of
soluble unencapsulated drug was measured by ultrafiltration using
centrifugal filter tubes with a molecular weight cut-off of 300 kDa
(Millipore, Carrigtwohill, Ireland). Drug loading and encapsulation
efficiency were calculated using following equations 1 and 2, respectively. The data were obtained
using three different batches of liposome preparations.
Drug loading(%)=CsClipidx100%
Encapsulation efficiency(%)=CsCtotlax100%
Where Cs represents drug mass in
liposome, Clipid represents total liposome mass, and
Ctotal represents total drug mass.
Drug release
The release of luteolin from liposomes in
vitro was measured with a dialysis method. Lipo-Lut solution
(0.5 ml) and Free-Lut (0.5 ml) in dimethyl sulphoxide (DMSO)
solution were placed in a dialysis bag (MW cut off: 3500 Da). After
that, dialysis bag was placed in a 50 ml PBS supplemented with 0.5%
Tween-20 (PBST). This bottle was introduced in a shaking incubator
with stirring speed of 100 rpm at 37°C. At specific time intervals
(0, 30 min, 1, 2, 4, 6, 8, 12, 24, 48, 72, 96, 120 h), samples were
withdrawn and replaced with an equal volume of medium. The amount
of luteolin released at each time-point was determined using HPLC,
as described earlier. All assays were performed in triplicate.
To measure in vivo pharmacokinetics, BALB/c
mice, weighting 18–20 g were randomly divided into two groups (six
animals per time-point) for treatment with Free-Lut or Lipo-Lut at
a dose of 50 mg/kg body weight via intravenous injection. After
dosing, blood was immediately collected via cardiac puncture at 5,
15, 30, 45, 1, 2, 4, 6, 8, 10, 12, 24 h and centrifuged at 8,000
rpm for 10 min to separate the plasma. The diphenhydramine was
added to plasma samples as the internal standard, and acetonitrile
as protein precipitator. The drug was then extracted from plasma
samples and processed for HPLC analysis to determine luteolin
levels.
MTT assay
The murine CT26 colorectal carcinoma cell line was
obtained from the ATCC, and it was maintained in RPMI-1640
(Sigma-Aldrich; Merck KGaA) supplemented with 10% fetal bovine
serum (Invitrogen Life Technologies, Carlsbad, CA, USA) in a 5%
CO2 incubator at 37°C. The inhibition of luteolin to
CT26 cell was determined by the MTT assay. Briefly, cells in the
logarithmic growth phase (3×103 or
5×103/well) were placed in wells of a 96-well plate at
37°C overnight. Cells were then treated with various concentrations
of Free-Lut or Lipo-Lut and cultured for 24. After an additional 3
h of culture with 0.5 mg/ml MTT at 37°C, 150 µl DMSO was added to
each well to dissolve formazan crystals. The cells which received
only the medium containing 150 µl DMSO served as the control group.
All of samples were analyzed using a microplate reader (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). The test was repeated three
times. The cell viability was assessed as a percentage of the
absorbance present in the drug-treated cells compared to that in
the control cells.
Assessment of apoptosis
The apoptotic rate of CT26 cells were detected by
flow cytometry using the Annexin V-fluorescein isothiocyanate
(FITC) and propidium iodide (PI) (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA). CT26 cells cultured in 6-well plates were
treated with various concentrations of Free-Lut or Lipo-Lut, along
with no drug treatment as control. After incubation for 24 h at
37°C, cells were trypsinized, washed and resuspended in PBS. The
cells were subsequently treated with Annexin V-FITC and PI in the
dark for 15 min. Flow cytometry analysis was performed with an
EpicsXL Coulter flow cytometer (Beckman Coulter, Fullerton, CA,
USA) and repeated thrice.
Mouse tumor model
Female BALB/c mice, 6 weeks old and weighting 18–20
g, was obtained from Beijing HFK Bioscience Co., Ltd. (Beijing,
China). The mice were kept in specific pathogen free (SPF)
conditions for 1 week prior to start of experimental procedures.
All the animal experiments were evaluated and approved by the
Animal and Ethics Review Committee of Xinjiang Medical University
(Urumqi, Xinjiang). The murine tumor models were established by
subcutaneous inoculation in the right flanks of female BALB/c mice
with CT26 cells (1×106/mouse). The growth of the tumor
was monitored, and tumor volumes were calculated from vernier
calipers every 3 days, following the formula of 0.52 × length ×
width2. The drug treatments were initiated when tumors
had reached an average volume of 100 mm3, which occurred
around day 7 post-cell inoculation. The luteolin dose administered
to the mice was 50 mg/kg. The mice were randomly assigned into four
groups (six animals per group). Each group was respectively treated
with normal saline (NS), empty liposome (EM-Lipo), Free-Lut, and
Lipo-Lut via the tail vein every 2 days a total of five times.
After the last treatment, the mice were sacrificed on the day 22,
and the tumors were excised, weighed and fixed in 10% neutral
buffered formalin solution or frozen at −80°C.
Hematoxylin and eosin (H&E)
staining and immunofluorescence staining
CT26 xenograft specimens were fixed in 4%
paraformaldehyde for 12 h and embedded in paraffin. Sections (5-µm
thick) were cut, dewaxed, rehydrated, and stained with H&E. To
observe the inhibitory effect on neovascularization, the frozen
tissues were sectioned (5 µm) and fixed in acetone. The tissues
sections were incubated with monoclonal anti-CD31 antibody (Santa
Cruz Biotechnology, Inc.) at 4°C overnight, flowing stained with a
secondary goat antibody (FITC). The number of microvessels per
high-power field was counted in sections. The immunofluorescence of
Ki-67 expression in tumor was done as follows: Tissue sections were
incubated with monoclonal anti-Ki67 antibody followed by incubation
with FITC labelled goat anti-mouse secondary antibody (Santa Cruz
Biotechnology, Inc.). Tissue apoptotic cells were detected with
TUNEL Detection kit (Promega, Madison, WI, USA), according to the
manufacturer's instructions. All the slides were evaluated by
fluorescence microscope. Five areas were randomly selected from
each slide for analysis.
Statistical analysis
The SPSS statistics 17.0 (SPSS, Inc., Chicago, IL,
USA) were used for statistical analysis. Quantitative data were
expressed as the mean ± standard deviation of three independent
experiments and analyzed by one-way ANOVA. Comparison between the
groups was made by analyzing data with S-N-K method. A P-value
<0.05 was considered to indicate a statistically significant
difference.
Results
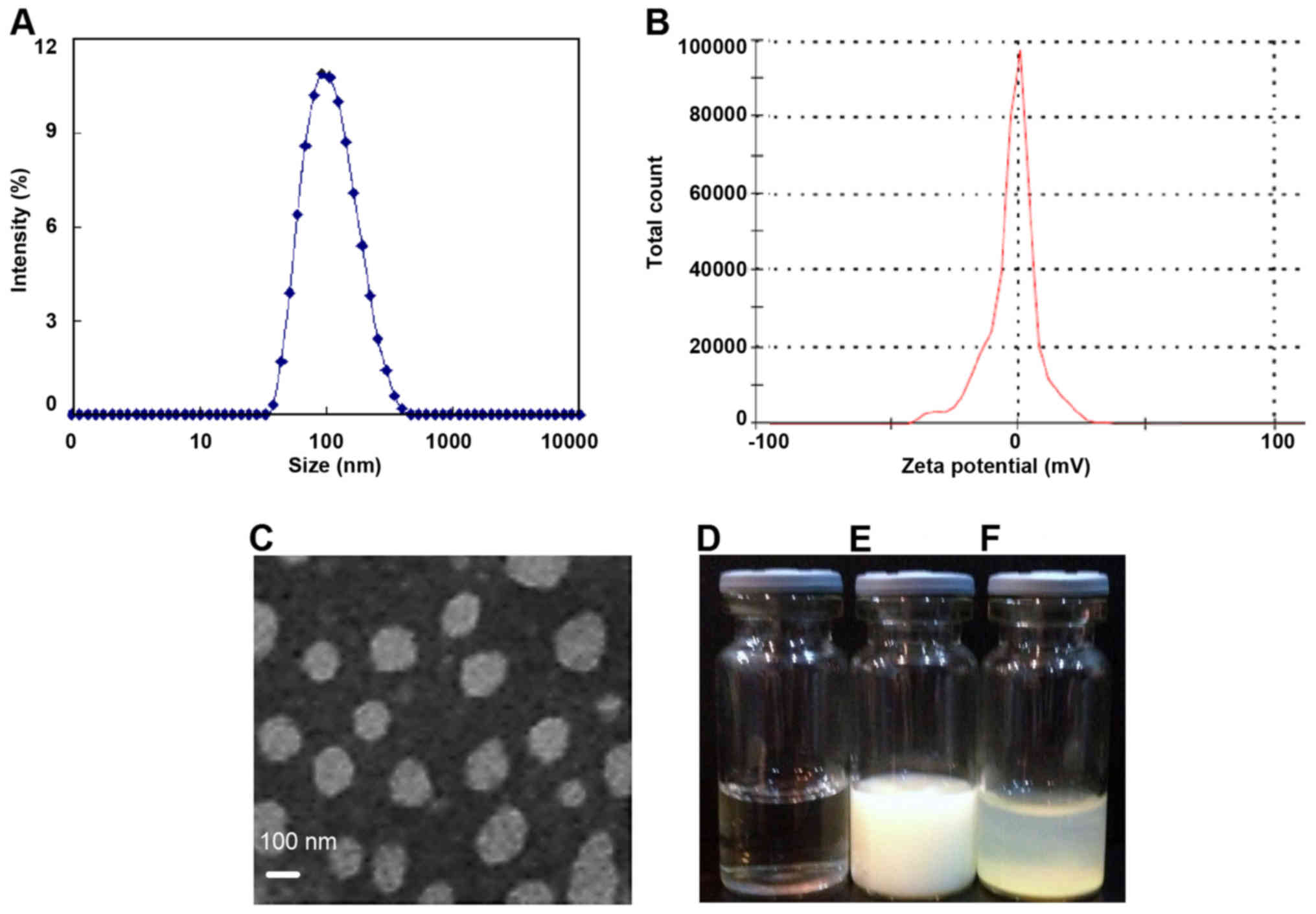
Characterization of Lipo-Lut
Our results demonstrated the successful application
of the thin-film hydration method to formulate the water soluble
Lipo-Lut. As shown in Fig. 1,
dynamic light scattering results showed that the diameter of
Lipo-Lut was around 105 nm. The ζ-potential of Lipo-Lut was 0.12
mv. The morphology of Lipo-Lut was observed using TEM, most
Lipo-Lut was spherical and had a regular shape. Free-Lut appeared
stratified in water, had apparent precipitation in the bottom of
the bottle. Lipo-Lut can be stably suspended in water solution.
Consequently, the drug loading and encapsulation efficiency were
found to be 10 and 90%, for the Lipo-Lut formulation.
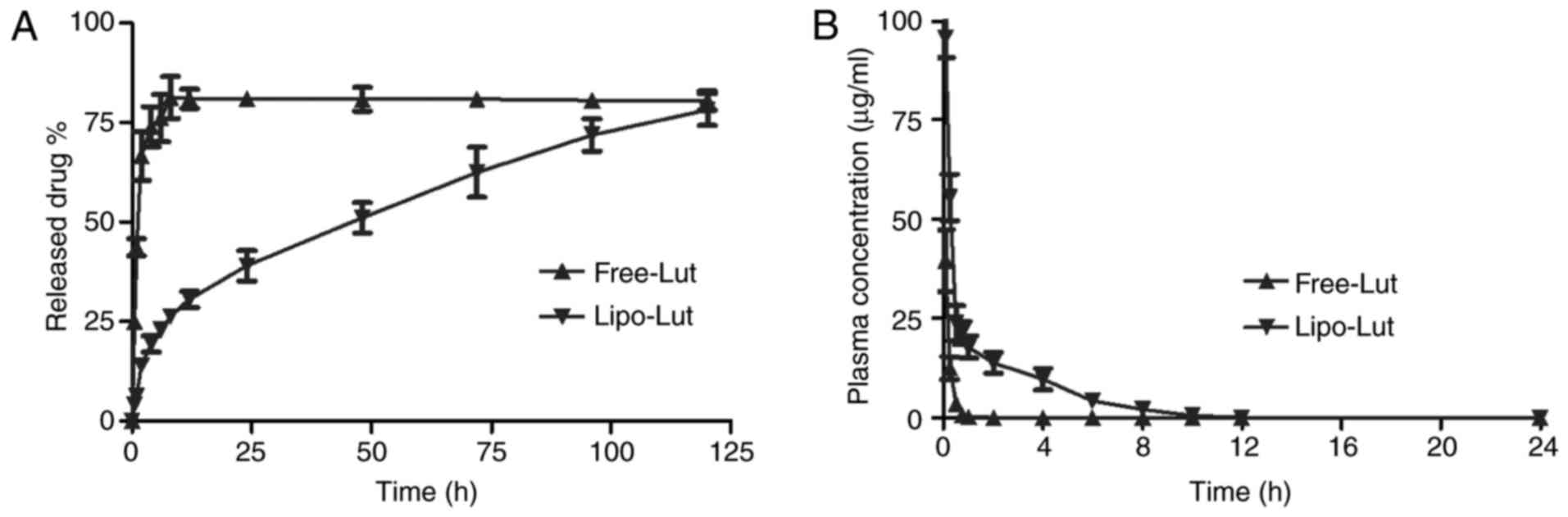
Drug release and Pharmacokinetics of
Lipo-Lut
The in vitro release (Fig. 2A) results showed that the luteolin
can be released slowly from the liposomes and then Free-Lut was
released very quickly. The cumulative percentage release
demonstrated that the amount of drug released from liposomes was
gradually increased over time, and after 120 h there was an
increase of over 80%. The free drug exhibited high level (80%) at 8
h.
To assess whether the liposome improved
bioavailability of poor water soluble drugs, the mean plasma
concentration-time profiles of luteolin after intravenous
administration of free and liposome drug were presented in Fig. 2B. Free-Lut was rapidly cleared and
the plasma levels of luteolin were less than 50% of the injected
dose within 5 min of injection. Compared to free drug, luteolin
concentration in plasma was almost 10-fold higher for Lipo-Lut at 2
h after drug injection, a result that was most evident at
time-points beyond 1 h. The results demonstrated that liposomal
encapsulation reduced drug elimination.
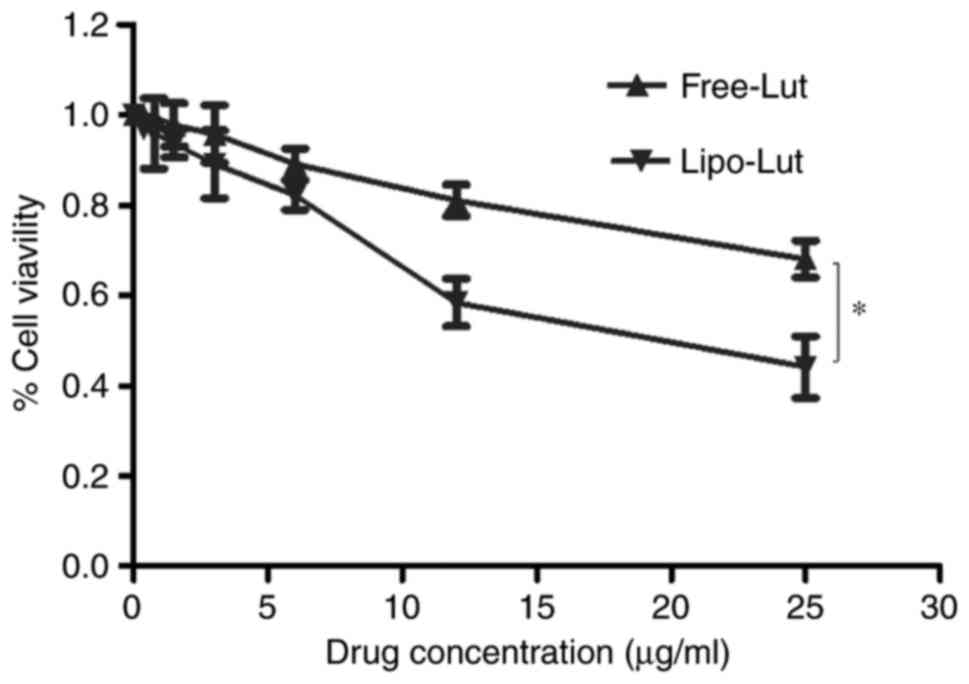
Lipo-Lut demonstrated better
tumoricidal effect on colorectal cancer cells than the
Free-Lut
Lipo-Lut and Free-Lut were evaluated for inhibition
against CT26 cells using MTT assay. The results (Fig. 3) clearly established that Lipo-Lut
exhibited potent inhibitory effect, as similar to Free-Lut.
Further, both drugs showed dose-dependent inhibition of cell
growth. After the incubation of the cells with Lipo-Lut or Free-Lut
for 24 h, the Lipo-Lut showed significantly higher inhibition
compared to the Free-Lut at all the concentrations tested. In
contrast, the empty liposomes did not show any toxicity to the
cells (data not shown). These data indicated that Lipo-Lut
inhibited tumor proliferation in vitro.
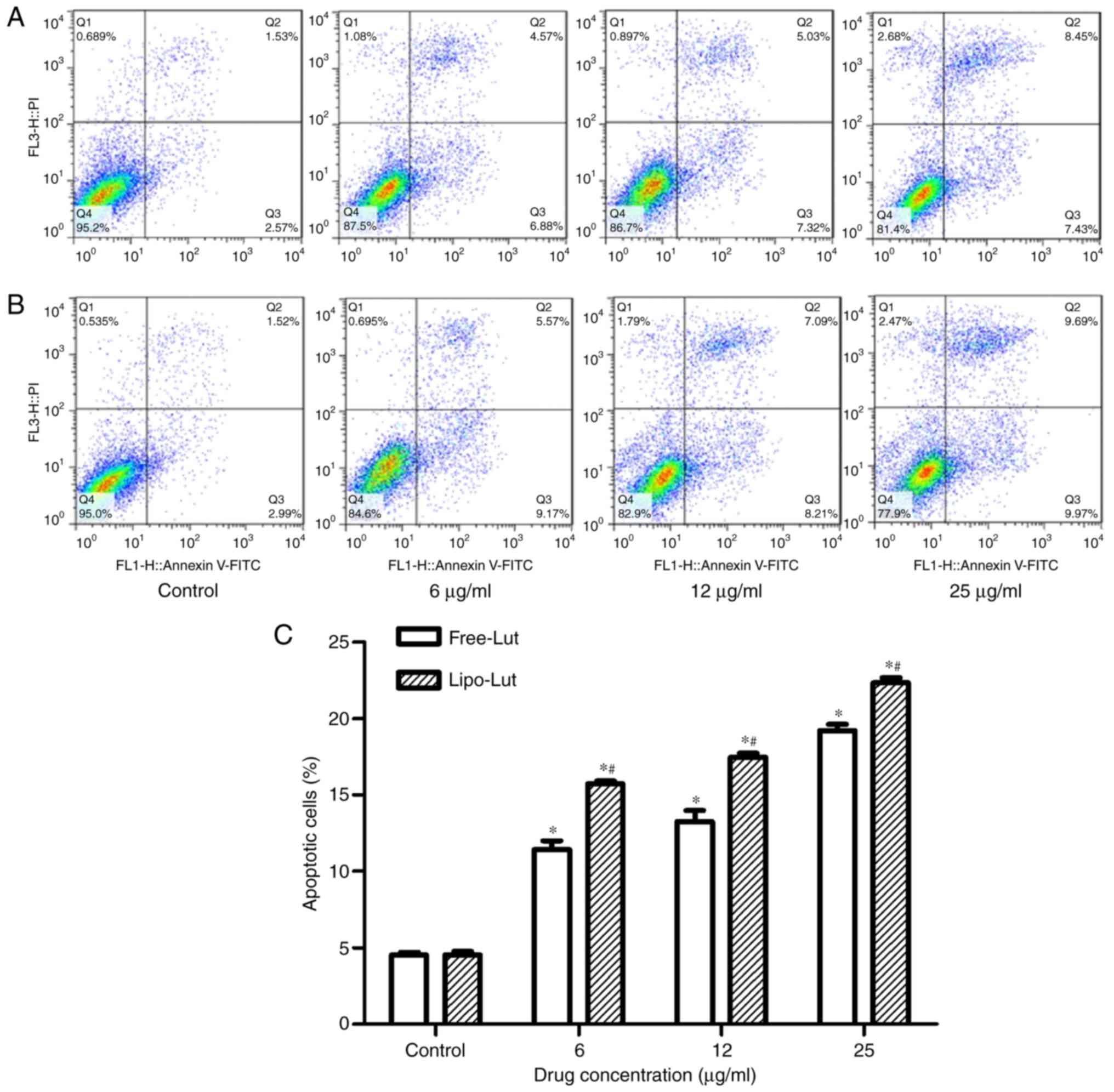
Next, we determined whether the inhibitory effect of
Lipo-Lut involved the initiation of apoptosis. A flow cytometry
analysis of Annexin V staining for phosphatidylserine, an early
apoptosis marker, was performed for evaluation of apoptotic
cells-after incubating CT26 cells with Lipo-Lut or Free-Lut at
various concentrations. The cells were collected and stained with
Annexin V-FITC and PI. When the cells undergoing apoptosis, the
phosphatidylserine normally located in the inner leaflet of the
cellular membrane translocates to the outer leaflet of the plasma
membrane at the early stages of apoptosis, which can be labeled
with Annexin V-FITC. Viable cells with intact membranes exclude PI
whereas the membranes of dead and damaged cells are permeable to
PI. CT26 cells were divided into four groups, ie, necrotic cells
(upper left quadrant, Annexin V−/PI+),
healthy viable cells (lower left quadrant, Annexin
V−/PI−), cells in the early apoptosis stage
(lower right quadrant, Annexin V+/PI−), and
cells that are in late apoptosis or already dead (upper right
quadrant, Annexin V+/PI+). As shown in
Fig. 4, more than 95% of control
CT26 cells (control) were viable whereas all the cells incubated
with Lipo-Lut or Free-Lut displayed evidence of apoptosis. The
percentage of early apoptotic cells and late apoptotic cells in
Lipo-Lut or Free-Lut were showed dose dependence. Meanwhile, the
extent of apoptosis in the Lipo-Lut group was significantly higher
than Free-Lut group.
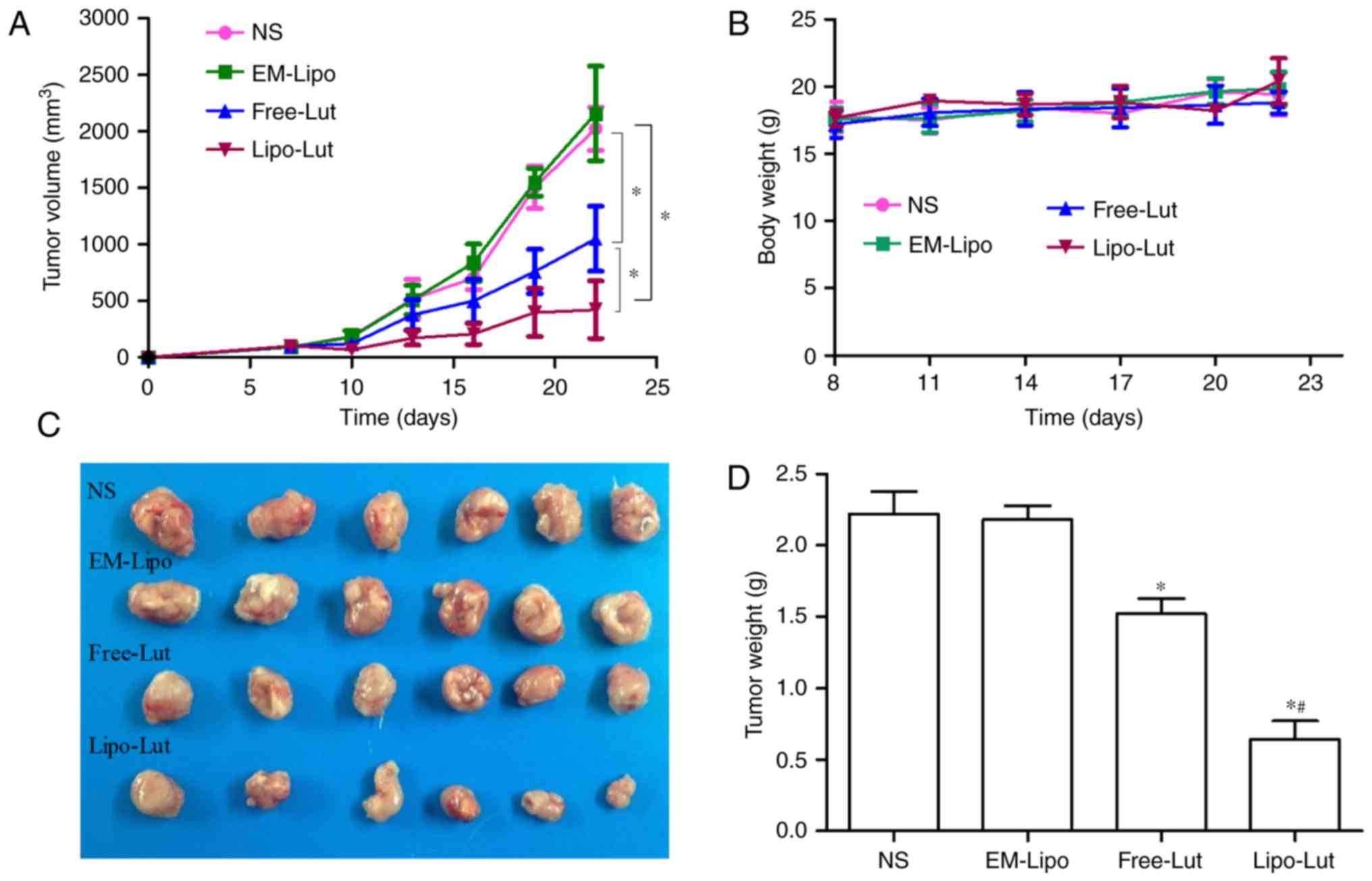
Lipo-Lut demonstrated better
tumoricidal effect on the mouse tumor model than the Free-Lut
To validate antitumor efficiency of Lipo-Lut in
vivo, we used a CT26 colorectal carcinoma graft model in BALB/c
mice (Fig. 5). Fig. 5A displayed the tumor volume of each
group during the 22-day treatment. Compared to the NS and EM-Lipo,
the final tumor volume of mice treated with luteolin was notably
reduced. The Lipo-Lut group exhibited the most significant
inhibitory efficacy compared with the other groups. There was no
significant difference between NS group and EM-Lipo group. At the
end of the treatment, the tumor weights were obtained (Fig. 5D), the results were consistent with
the tumor volumes at the end of the treatment. The smallest tumor
weights were observed in the Lipo-Lut group among the four groups.
These results indicated that the treatment of Lipo-Lut resulted in
a robust efficacy in reducing tumor volume. Body weights were not
significantly different in the four groups throughout the whole
experiment (Fig. 5B).
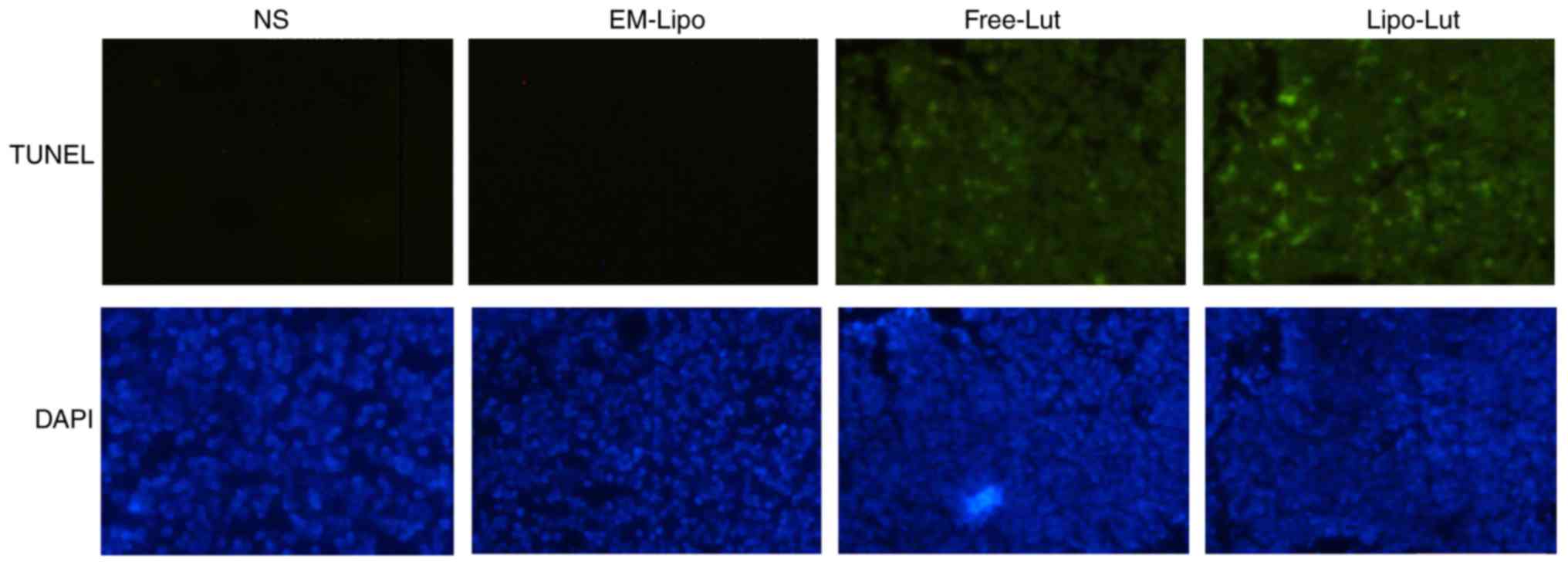
Lipo-Lut induced apoptosis
To further investigated whether the in vivo
antitumor effects of the Lipo-Lut were associated with enhanced
induction of the apoptotic cells, TUNEL assay was applied. As shown
in Fig. 6, a significant number of
apoptotic cells appeared with green fluorescence in the tumor
tissue of luteolin-treated mice. Treatment with Lipo-Lut clearly
produced more pronounced apoptotic cells than treatment with the
Free-Lut. These results suggested that Lipo-Lut inhibited tumor
growth probably through induction of tumor cellular apoptosis.
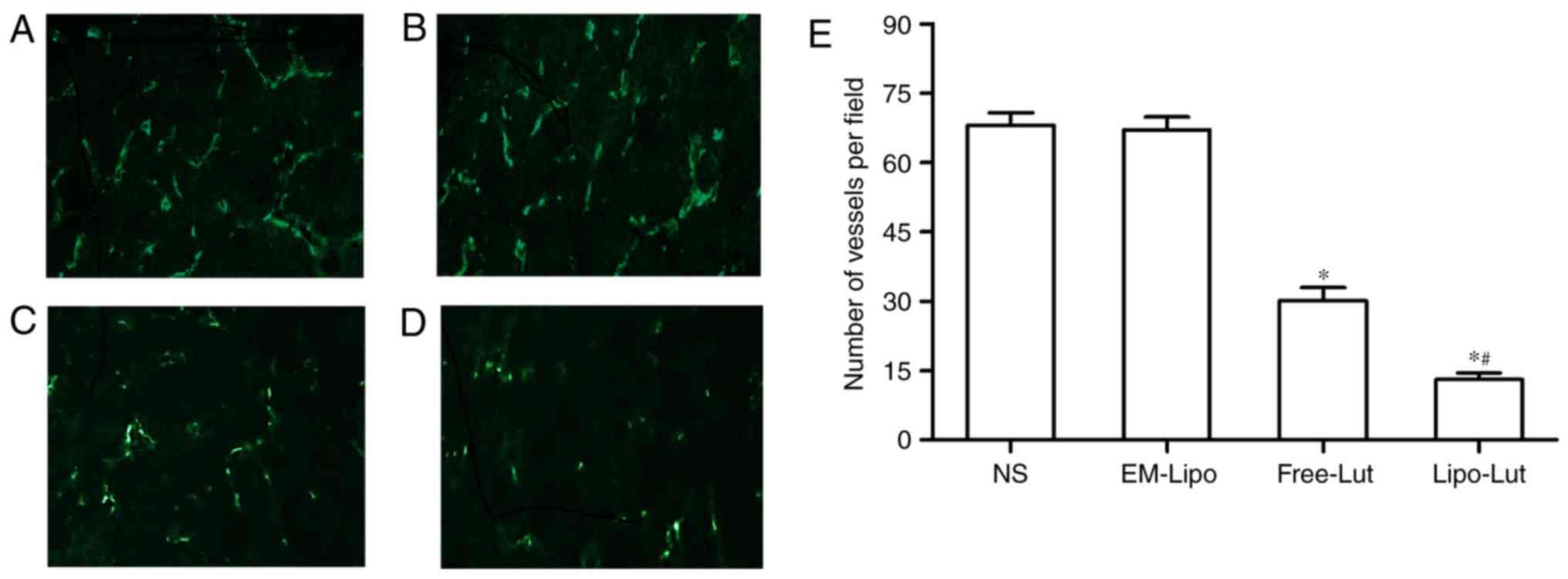
Lipo-Lut inhibited tumor
vascularization
We performed immunofluorescence analysis with
anti-CD31 monoclonal antibody to observe the new vasculature
content in frozen tumor sections (Fig.
7). As shown in Fig. 7D,
CD31-positive endothelial cells in luteolin treated groups had
weaker fluorescence than those of NS group and EM-Lipo group
(Fig. 7A and B). The results from
the determination of microvessel numbers (Fig. 7E) revealed that there were
significantly less number of microvessels present in the Free-Lut
group and Lipo-Lut group compared to the control group (P<0.01).
In addition, Lipo-Lut group reduced number of microvessels more
remarkably compared with Free-Lut groups (P<0.01). No
significant difference was observed between NS group and EM-Lipo
group (P>0.05).
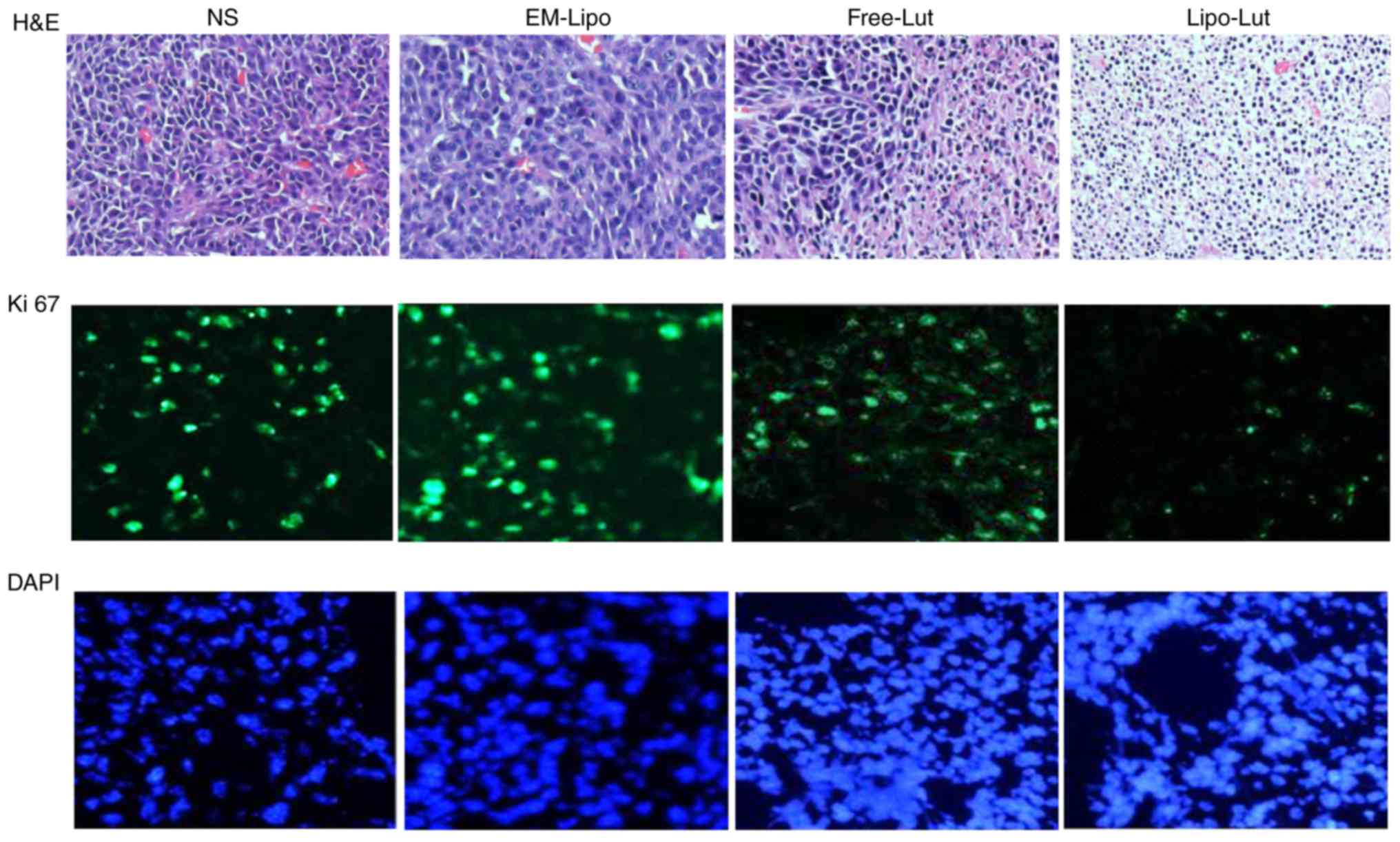
Lipo-Lut decreased Ki-67
expression
The expression of Ki-67 is strictly associated with
tumor cell proliferation and growth, and is widely used in routine
pathological investigation as a proliferation marker and a
diagnosis tool (28).
Immunofluorescence examination of Ki-67 staining revealed a greater
inhibition of proliferating cells in the Lipo-Lut-treated mice than
other groups (Fig. 8).
Discussion
The present study demonstrated that
liposome-encapsulated luteolin exerted stronger tumor
growth-inhibition than free luteolin both in vitro and in
vivo. Liposome have been widely used as potential drug delivery
systems (DDS) for delivery of anti-cancer agents (24,29).
Over the past few decades, several liposome-encapsulated drugs have
been approved for clinic applications. The best-known formulation
is liposome-encapsulated doxorubicin, marketed as Doxil or Caelyx
(30–32), which produced less cardiotoxicity
than free doxorubicin while providing comparable antitumor activity
(33,34). In our study, we have prepared water
soluble Lipo-Lut, sized ~105 nm. According to the cancer type, the
size of the gaps between the endothelial cells of the tumor
capillaries ranges from 100 to 780 nm, as opposed to that in a
typical normal endothelium of 5–10 nm (35,36).
Liposomes of about 100 nm in diameter have been demonstrated to be
optimal for the delivery of anticancer drugs to tumors (24,37),
which readily translocate across the capillary endothelium.
Liposomes are known to be safe and well tolerated delivery system.
Many researchers demonstrated that the actions of some poor water
soluble drugs were evidently enhanced after they were encapsulated
into liposome (38,39). This study showed that Lipo-Lut
could prolong the drug release. Meanwhile, the concentration in
plasma-time profiles indicated that the bioavailability of Lipo-Lut
has been improved.
In order to study the antitumor efficacy of Lipo-Lut
in vitro, we initially compared the efficacy of Free-Lut and
Lipo-Lut on CT26 cell growth inhibition by MTT assay and apoptosis
by flow cytometry. We observed that Lipo-Lut exhibited more
effectively than Free-Lut. This prompted us to further evaluate the
antitumor activities of Lipo-Lut on CT26 cells grafts in BALB/c
mice. The results suggested that luteolin encapsulated into
liposome exerted stronger tumor growth-inhibiting effects,
suppressed angiogenesis, increased apoptosis than Free-Lut.
Angiogenesis plays a important role in the progress of tumor growth
and invasion. The most essential angiogenic factors is vascular
endothelial growth factor (VEGF). It have been reported that
luteolin could significantly inhibit VEGF-stimulated endothelial
cell proliferation, migration, invasion and tumor angiogenesis by
targeting VEGF receptor 2-regulated AKT/ERK/mTOR/P70S69K/MMPs
pathway, leading to the inhibition of tumor growth and tumor
angiogenesis. A study from Norhaizan suggested that luteolin
induced apoptosis in colon cancer by modulating the expressions of
bax, Bcl-2 and caspase 3 in vitro and in vivo. On the
other hand luteolin acted against DNA damage and activated DNA
repair mechanism in Caco-2 colon cancer cells (40–42).
Moreover, Owing to the leaky vasculature of the tumor tissue, these
may provide a channel allowing liposome to more easily target tumor
tissue (1). Meanwhile, solid
tumors usually lack effective lymphatic drainage. All of these
factors lead to the accumulation of liposome in the tumor
microenvironment much more than they do in normal tissues (43,44).
Most likely because of a combination of these advantages, Lipo-Lut
was a marked inhibition of tumor growth over time when compared to
Free-Lut in a mouse tumor model. In fact, the present study also
indicated that Nano-luteolin showed higher efficacy compared to
Free-Lut against lung cancer and head and neck cancer (4). However, Lipo-Lut was unable to
completely inhibit tumor growth. In order to gain better
therapeutic efficacy, it is necessary to optimize liposomal
formations and therapeutic scheme.
We prepared a novel antitumor agent using liposome
as the delivery system to encapsulate luteolin. The Lipo-Lut
inhibited activity of tumor growth more effectively than the
Free-Lut in both CT26 cells and mouse tumor model of colorectal
carcinoma. The mechanisms of action of Lipo-Lut appear
multifaceted. Firstly, it could directly induce apoptosis of tumor
cells. Secondly, tumor angiogenesis were reduced, blocking the
nutrition supply into tumor tissue, which promoted apoptosis of
tumor cells. Third, Lipo-Lut inhibited tumor proliferation. The
results in this study could contribute to the development of
chemotherapy for patients with colorectal carcinoma in future
clinical applications.
Acknowledgements
The study was supported by the National Natural
Science Foundation, People's Republic of China (grant no. 81660480)
and Scientific Research Project of Xinjiang University (grant no.
XJEDU2014I019).
Glossary
Abbreviations
Abbreviations:
|
Lut
|
luteolin
|
|
NS
|
normal saline
|
|
Lipo-Lut
|
liposomal luteolin
|
|
Free-Lut
|
free luteolin
|
|
EM-Lipo
|
empty liposome
|
References
|
1
|
Yang C, Liu HZ, Fu ZX and Lu WD:
Oxaliplatin long-circulating liposomes improved therapeutic index
of colorectal carcinoma. BMC Biotechnol. 11:212011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nobili S, Checcacci D, Filippelli F, Del
Buono S, Mazzocchi V, Mazzei T and Mini E: Bimonthly chemotherapy
with oxaliplatin, irinotecan, infusional 5-fluorouracil/folinic
acid in patients with metastatic colorectal cancer pretreated with
irinotecan- or oxaliplatin-based chemotherapy. J Chemother.
20:622–631. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen AY and Chen YC: A review of the
dietary flavonoid, kaempferol on human health and cancer
chemoprevention. Food Chem. 138:2099–2107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Majumdar D, Jung KH, Zhang H, Nannapaneni
S, Wang X, Amin AR, Chen Z, Chen ZG and Shin DM: Luteolin
nanoparticle in chemoprevention: In vitro and in vivo anticancer
activity. Cancer Prev Res (Phila). 7:65–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sporn MB, Dunlop NM, Newton DL and Smith
JM: Prevention of chemical carcinogenesis by vitamin A and its
synthetic analogs (retinoids). Fed Proc. 35:pp. 1332–1338. 1976;
PubMed/NCBI
|
|
6
|
Madhunapantula SV and Robertson GP:
Chemoprevention of melanoma. Adv Pharmacol. 65:361–398. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mann CD, Neal CP, Garcea G, Manson MM,
Dennison AR and Berry DP: Phytochemicals as potential
chemopreventive and chemotherapeutic agents in
hepatocarcinogenesis. Eur J Cancer Prev. 18:13–25. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khor TO, Yu S and Kong AN: Dietary cancer
chemopreventive agents-targeting inflammation and Nrf2 signaling
pathway. Planta Med. 74:1540–1547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaur M, Singh RP, Gu M, Agarwal R and
Agarwal C: Grape seed extract inhibits in vitro and in vivo growth
of human colorectal carcinoma cells. Clin Cancer Res. 12:6194–6202.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaur M, Velmurugan B, Tyagi A, Agarwal C,
Singh RP and Agarwal R: Silibinin suppresses growth of human
colorectal carcinoma SW480 cells in culture and xenograft through
down-regulation of beta-catenin-dependent signaling. Neoplasia.
12:415–424. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benbrook DM, Guruswamy S, Wang Y, Sun Z,
Mohammed A, Zhang Y, Li Q and Rao CV: Chemoprevention of colon and
small intestinal tumorigenesis in APC(min/+) mice by SHetA2
(NSC721689) without toxicity. Cancer Prev Res (Phila). 6:908–916.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang MY, Wang CJ, Chen NF, Ho WH, Lu FJ
and Tseng TH: Luteolin enhances paclitaxel-induced apoptosis in
human breast cancer MDA-MB-231 cells by blocking STAT3. Chem Biol
Interact. 213:60–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang X, Dai S, Dai J, Xiao Y, Bai Y, Chen
B and Zhou M: Luteolin decreases invasiveness, deactivates STAT3
signaling and reverses interleukin-6 induced epithelial-mesenchymal
transition and matrix metalloproteinase secretion of pancreatic
cancer cells. Onco Targets Ther. 8:2989–3001. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yan M, Liu Z, Yang H, Li C, Chen H, Liu Y,
Zhao M and Zhu Y: Luteolin decreases the UVA-induced autophagy of
human skin fibroblasts by scavenging ROS. Mol Med Rep.
14:1986–1992. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee HZ, Yang WH, Bao BY and Lo PL:
Proteomic analysis reveals ATP-dependent steps and chaperones
involvement in luteolin-induced lung cancer CH27 cell apoptosis.
Eur J Pharmacol. 642:19–27. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ong CS, Zhou J, Ong CN and Shen HM:
Luteolin induces G1 arrest in human nasopharyngeal carcinoma cells
via the Akt-GSK-3β-cyclin D1 pathway. Cancer Lett. 298:167–175.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiu FL and Lin JK: Downregulation of
androgen receptor expression by luteolin causes inhibition of cell
proliferation and induction of apoptosis in human prostate cancer
cells and xenografts. Prostate. 68:61–71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai X, Ye T, Liu C, Lu W, Lu M, Zhang J,
Wang M and Cao P: Luteolin induced G2 phase cell cycle arrest and
apoptosis on non-small cell lung cancer cells. Toxicol In Vitro.
25:1385–1391. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Balyan R, Kudugunti SK, Hamad HA, Yousef
MS and Moridani MY: Bioactivation of luteolin by tyrosinase
selectively inhibits glutathione S-transferase. Chem Biol Interact.
240:208–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu JF, Ma Y, Wang Y, Du ZY, Shen JK and
Peng HL: Reduction of lipid accumulation in HepG2 cells by luteolin
is associated with activation of AMPK and mitigation of oxidative
stress. Phytother Res. 25:588–596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdel Hadi L, Di Vito C, Marfia G,
Ferraretto A, Tringali C, Viani P and Riboni L: Sphingosine kinase
2 and ceramide transport as key targets of the natural flavonoid
luteolinto induce apoptosis in colon cancer cells. PLoS One.
10:e01433842015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wölfle U, Esser PR, Simon-Haarhaus B,
Martin SF, Lademann J and Schempp CM: UVB-induced DNA damage,
generation of reactive oxygen species and inflammation are
effectively attenuated by the flavonoid luteolin in vitro and in
vivo. Free Radic Biol Med. 50:1081–1093. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Wang L, Zhao Y, He M, Zhang X, Niu
M and Feng N: Nanostructured lipid carriers versus microemulsions
for delivery of the poorly water-soluble drug luteolin. Int J
Pharm. 476:169–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yuan Y, Zhao Y, Xin S, Wu N, Wen J, Li S,
Chen L, Wei Y, Yang H and Lin S: A novel PEGylated
liposome-encapsulated SANT75 suppresses tumor growth through
inhibiting hedgehog signaling pathway. PLoS One. 8:e602662013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fetterly GJ, Grasela TH, Sherman JW, Dul
JL, Grahn A, Lecomte D, Fiedler-Kelly J, Damjanov N, Fishman M and
Kane MP: Pharmacokinetic/pharmacodynamic modeling and simulation of
neutropenia during phase I development of liposome-entrapped
paclitaxel. Clin Cancer Res. 14:5856–5863. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gabizon A, Catane R, Uziely B, Kaufman B,
Safra T, Cohen R, Martin F, Huang A and Barenholz Y: Prolonged
circulation time and enhanced accumulation in malignant exudates of
doxorubicin encapsulated in polyethylene-glycol coated liposomes.
Cancer Res. 54:987–992. 1994.PubMed/NCBI
|
|
27
|
Guo L, Fan L, Ren J, Pang Z, Ren Y, Li J,
Wen Z, Qian Y, Zhang L and Ma H: Combination of TRAIL and
actinomycin D liposomes enhances antitumor effect in non-small cell
lung cancer. Int J Nanomedicine. 7:1449–1460. 2012.PubMed/NCBI
|
|
28
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(Review). Mol Med Rep. 11:1566–1572. 2015.PubMed/NCBI
|
|
29
|
Koudelka S, Turanek Knotigova P, Masek J,
Prochazka L, Lukac R, Miller AD, Neuzil J and Turanek J: Liposomal
delivery systems for anti-cancer analogues of vitamin E. J Control
Release. 207:59–69. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barenholz Y: Doxil® - the first
FDA-approved nano-drug: Lessons learned. J Control Release.
160:117–134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schiffelers RM, Metselaar JM, Fens MH,
Janssen AP, Molema G and Storm G: Liposome-encapsulated
prednisolone phosphate inhibits growth of established tumors in
mice. Neoplasia. 7:118–127. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gabizon A and Martin F: Polyethylene
glycol-coated (pegylated) liposomal doxorubicin. Rationale for use
in solid tumours. Drugs. 4 Suppl 54:S15–S21. 1997. View Article : Google Scholar
|
|
33
|
Harris L, Batist G, Belt R, Rovira D,
Navari R, Azarnia N, Welles L and Winer E; TLC D-99 Study Group, :
Liposome-encapsulated doxorubicin compared with conventional
doxorubicin in a randomized multicenter trial as first-line therapy
of metastatic breast carcinoma. Cancer. 94:25–36. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hioki A, Wakasugi A, Kawano K, Hattori Y
and Maitani Y: Development of an in vitro drug release assay of
PEGylated liposome using bovine serum albumin and high temperature.
Biol Pharm Bull. 33:1466–1470. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deshpande PP, Biswas S and Torchilin VP:
Current trends in the use of liposomes for tumor targeting.
Nanomedicine (Lond). 8:1509–1528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Haley B and Frenkel E: Nanoparticles for
drug delivery in cancer treatment. Urol Oncol. 26:57–64. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cukierman E and Khan DR: The benefits and
challenges associated with the use of drug delivery systems in
cancer therapy. Biochem Pharmacol. 80:762–770. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fan Y, Liu J, Wang D, Song X, Hu Y, Zhang
C, Zhao X and Nguyen TL: The preparation optimization and immune
effect of epimedium polysaccharide-propolis flavone liposome.
Carbohydr Polym. 94:24–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhu Y, Wang M, Zhang J, Peng W, Firempong
CK, Deng W, Wang Q, Wang S, Shi F, Yu J, et al: Improved oral
bioavailability of capsaicin via liposomal nanoformulation:
Preparation, in vitro drug release and pharmacokinetics in rats.
Arch Pharm Res. 38:512–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pandurangan AK and Esa NM: Luteolin, a
bioflavonoid inhibits colorectal cancer through modulation of
multiple signaling pathways: A review. Asian Pac J Cancer Prev.
15:5501–5508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang LM, Xie KP, Huo HN, Shang F, Zou W
and Xie MJ: Luteolin inhibits proliferation induced by IGF-1
pathway dependent ERα in human breast cancer MCF-7 cells. Asian Pac
J Cancer Prev. 13:1431–1437. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pratheeshkumar P, Son YO, Budhraja A, Wang
X, Ding S, Wang L, Hitron A, Lee JC, Kim D, Divya SP, et al:
Luteolin inhibits human prostate tumor growth by suppressing
vascular endothelial growth factor receptor 2-mediated
angiogenesis. PLoS One. 7:e522792012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
44
|
Vasey PA, Kaye SB, Morrison R, Twelves C,
Wilson P, Duncan R, Thomson AH, Murray LS, Hilditch TE, Murray T,
et al: Phase I clinical and pharmacokinetic study of PK1
[N-(2-hydroxypropyl)methacrylamide copolymer doxorubicin]: First
member of a new class of chemotherapeutic agents-drug-polymer
conjugates. Cancer research campaign phase I/II committee. Clin
Cancer Res. 5:83–94. 1999.PubMed/NCBI
|