Introduction
Chronic inflammation is one of the well-established
causative conditions for the development and progression of
atherosclerosis, whereas inciting inflammation in the artery wall
remains largely unknown. Monocyte-derived macrophages in late
atherogenesis are regarded as primary inflammatory stimuli through
either synthesis or secretion of numerous substances, such as
interleukin (IL)-1β and −18 (1,2). A
clinical report has demonstrated that increased expression levels
of IL-1β are associated with clinical severity in patients with
coronary artery disease (CAD) (3).
Additionally, targeting IL-1β with a monoclonal antibody can impede
the progression of atherosclerosis in ApoE−/− mice
(4).
The nucleotide-binding domain,
leucine-rich-containing family, pyrin domain-containing-3 (NLRP-3)
inflammasome is formed around NLRP3, which also contains the
adaptor molecules; apoptosis-associated speck-like protein
containing a CARD (ASC) and pro-caspase-1. The NLRP3 inflammasome
has been demonstrated to be associated with chronic inflammation,
resulting in IL-1β and IL-18 maturation (5). Previously, aberrantly elevated NLRP3
inflammasome levels have been reported to be associated with
vascular inflammation and endothelial dysfunction in
atherosclerotic pig aorta (6).
Furthermore, gene silencing of NLRP3 has prevented plaque
progression increasing atherosclerotic plaque stability by
inhibiting proinflammatory cytokines (7). In a previous study, NLRP3
inflammasome levels were higher in the CAD group compared with the
non-CAD group, with a positive association between NLRP3
inflammasome and IL-1β and IL-18 levels (8). Based on these observations,
dysregulated NLRP3 inflammasome activation may have been involved
in the pathogenesis of atherosclerosis, and the NLRP3 inflammasome
might serve as a potential candidate for ameliorating the
development of atherosclerosis.
Toll-like receptor 4 (TLR4), an intensively
investigated member of the TLR family, serves a critical role in
initiating inflammation (9) and
participates in mediating inflammatory cell formation (10). As the downstream effectors of TLR4
(11), myeloid differentiation
primary response gene 88 (MyD88) and nuclear factor (NF)-κB
regulate the expression of many inflammatory genes and participate
in the development of several diseases, such as cancer (12), inflammatory bowel disease and
atherosclerosis (13–15). Additionally, studies have
demonstrated that NF-κB induces NLRP3 expression and NLRP3
inflammasome activity (16,17).
In the present study, the role of the TLR4/Myd88/NF-κB signaling
pathway was investigated in phorbol 12-myristate 13-acetate
(PMA)-induced macrophages. Furthermore, the effects of isoquinoline
alkaloid berberine on this signaling pathway were also studied.
Berberine, a botanical alkaloid, is isolated from
medicinal herbs, such as Rhizoma coptidis (Chinese name,
Huanglian) and Cortex phellodendri (Chinese name, Huangbai)
(18). Berberine has been reported
to inhibit N-acetyltransferase activity in several tumor cells in a
dose-dependent manner, including human bladder tumor (carcinoma)
cells (T24) (19). Additionally,
berberine has been reported to have an anti-obesity effect by
inhibiting adipocyte differentiation and lipid accumulation in
3T3L-1-cells (20). Furthermore,
increasing evidence has demonstrated that berberine has an
anti-atherosclerosis effect in cardiovascular disease (21–23).
Therefore, the present study investigated the effect of berberine
on NLRP3 inflammasome activation in PMA-induced macrophages.
Materials and methods
Reagents
RPMI 1640 medium, fetal bovine serum (FBS), and
penicillin/streptomycin (pen/strep, 10,000 U/ml each) were
purchased from Gibco; Thermo Fisher Scientific, Inc. (Waltham, MA,
USA). Dimethyl sulfoxide (DMSO), PMA, and berberine were obtained
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). BAY 11–7082
and nuclear and cytoplasmic extraction reagents were obtained from
Beyotime Institute of Biotechnology (Shanghai, China). TRIzol
reagent for RNA isolation was purchased from Invitrogen; Thermo
Fisher Scientific, Inc. Omniscript reverse transcriptase for
first-strand cDNA synthesis was obtained from Qiagen GmbH (Hilden,
Germany). Anti-IL-1β (cat. no. 12703), anti-NLRP3 (cat. no. 13158),
anti-ASC (cat. no. 13833), anti-p65 (cat. no. 8242), anti-p-IκB-α
(cat. no. 9246), anti-lamin B (cat. no. 13435) and anti-GAPDH (cat.
no. 5174) were purchased from Cell Signaling Technology, Inc.
(Danvers, MA, USA). Anti-TLR4 (cat. no. ab22048) and anti-Myd88
(cat. no. ab2064) were produced by Abcam (Cambridge, UK).
Anti-caspase-1 (cat. no. sc-56036) was obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Rabbit anti-mouse
conjugated-horseradish peroxidase (HRP) (cat. no. A16166) and goat
anti-rabbit conjugated-HRP (cat. no. A10547) secondary antibodies
were from Invitrogen; Thermo Fisher Scientific, Inc. A
Bicinchoninic Acid protein assay kit was produced by Pierce; Thermo
Fisher Scientific, Inc. Polyvinylidene difluoride membranes were
obtained from EMD Millipore (Billerica, MA, USA).
Cell culture
The THP-1 human monocyte cell line was purchased
from the American Type Culture Collection (Manassas, MD, USA) and
maintained at a density of 106/ml in RPMI 1640 medium
containing 10% FBS and 1% penicillin/streptomycin solution at 37°C
in a 5% CO2 incubator. Cells at a density of
5×105/ml per well were cultured in 6-well plates for 48
h in the presence of 100 nM PMA except the control group, which
allowed them to differentiate into adherent macrophages (24). Berberine was dissolved in DMSO at a
final concentration of 5, 10, 25 or 50 µM in Berberine-treated
groups 1 h prior to adding PMA. THP-1 cells that were treated with
DMSO (1:1,000 in culture medium) only in PMA-treated group served
as the control.
RNA isolation, cDNA synthesis, and
Taqman reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
THP-1 cells were homogenized in TRIzol for
extraction of RNA according to the manufacturer's protocol. RT-qPCR
was carried out using a Superscript III cDNA synthesis kit
(Invitrogen; Thermo Fisher Scientific, Inc.) and SYBR-Green reagent
kits (Invitrogen; Thermo Fisher Scientific, Inc.). Relative
expression data was analyzed using the 2-ΔΔCq method (25). The specific primers of genes were
synthesized from Invitrogen; Thermo Fisher Scientific, Inc. The
following primers were used: i) IL-1β forward,
5′-ACTGCCTGCCTTAGGGTAG-3′ and reverse, 5′-GTGGGAGCGAATGACAGAG-3′;
ii) NLRP3 forward, 5′-CTGGAGGATGTGGACTTG-3′ and reverse,
5′-GTCTGCCTTCTCTGTCTG-3′; and iii) GAPDH forward,
5′-CACCCACTCCTCCACCTTTG-3′ and reverse, 5′-CCACCACCCTGTTGCTGTAG-3′.
GAPDH was used as an internal housekeeping control.
Protein isolation and western blot
analysis
Protein extracts from all treatment groups were
isolated from the cytoplasm and the nucleus using nuclear protein
and cytoplasmic protein extraction kit (cat. no. P0027; Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. Protein isolation and Western blot analysis of the cell
lysates were performed as previously described (26). Lysates (50 µg) micrograms were
separated by 10% SDS-PAGE and electrotransferred to a PVDF
membrane. Each membrane was pre-incubated for 1.5 h at room
temperature in Tris-buffered saline, pH 7.6, containing 0.05%
Tween-20 and 5% non-fat milk (Sangon Biotech Co., Ltd., Shanghai,
China). Each membrane was incubated with primary IL-1β, NLRP3, ASC,
p65, p-IκB-α, lamin B, TLR4, Myd88, GAPDH antibodies diluted
1:1,000 and caspase-1 diluted 1:200 at 4°C overnight. Bands were
then detected by incubating with a secondary antibody (diluted
1:1,000) conjugated with HRP at room temperature for 2 h and
visualizing using enhanced chemiluminescence reagents (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Results were then analyzed
using Image J analysis software (version no. 2.1.4.7; National
Institutes of Health, Bethesda, MD, USA) and normalized to GAPDH
and lamin B.
ELISA assays of proinflammatory
cytokines
For the determination of IL-1β levels in cell
medium, a cytokine-specific ELISA kit (cat. no. DLB50; R&D
Systems Europe Ltd., Abingdon, UK) was used according to the
manufacturer's protocol.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Results were analyzed by one-way analysis of variance (ANOVA) with
Student-Newman-Kewls and Dunnettis methods as post hoc tests using
SPSS (version 18.0; SPSS, Inc., Chicago, IL, USA). All experiments
were performed at least three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
IL-1β level and NLRP3 inflammasomes
are activated in PMA-induced macrophages in a time-dependent
manner
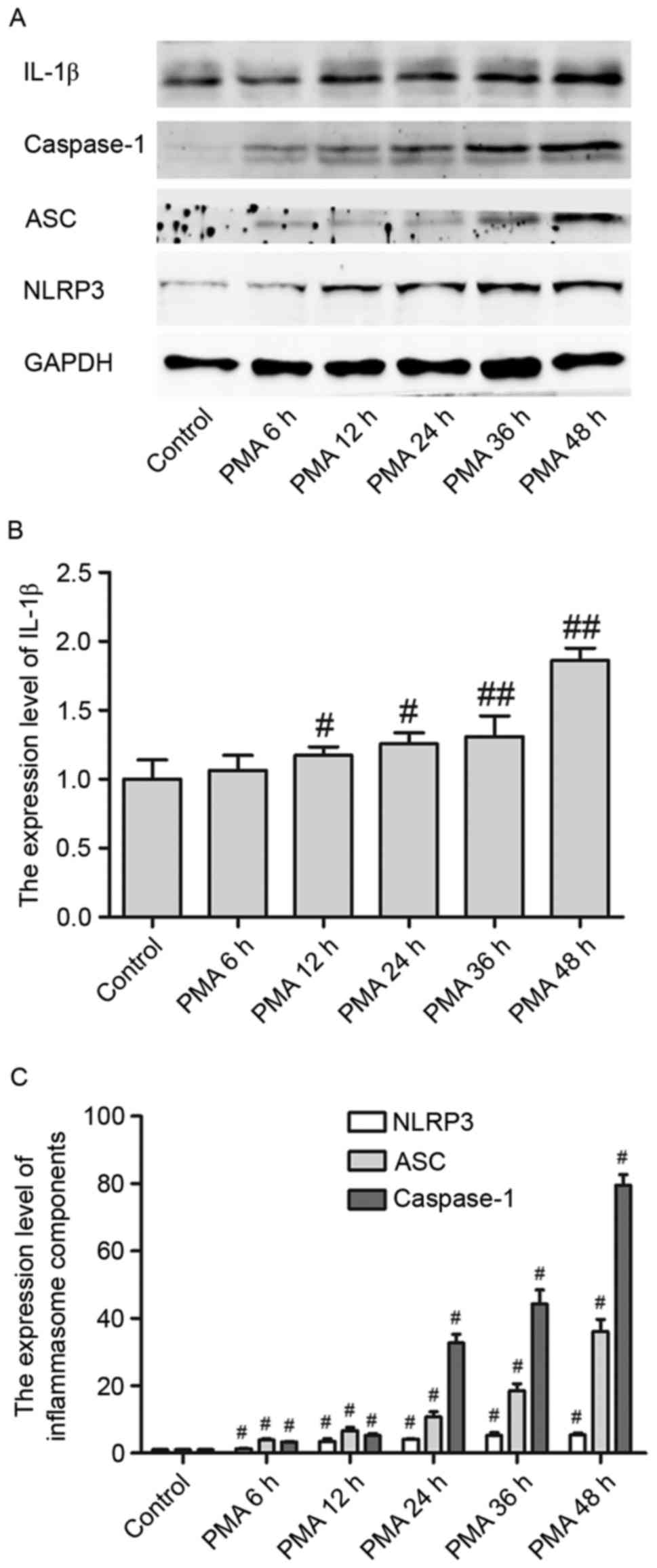
IL-1β protein expression levels in PMA-induced
macrophages were upregulated in a time-dependent manner from 6 to
48 h, reaching a peak at 48 h (Fig. 1A
and B). Additionally, NLPR3 inflammasome expression levels were
upregulated when macrophages were treated with PMA in a
time-dependent manner (Fig. 1A).
Furthermore, as a critical component of inflammasomes, NLPR3
activates caspase-1, which is a (p20/p10) tetramer necessary and
sufficient for the cleavage of IL-1β precursor, through its adaptor
ASC. In the present study, both ASC and pro-caspase-1 were
activated in PMA-induced macrophages in a time-dependent manner
(Fig. 1A and C). These experiments
indicated that NLRP3 inflammasomes and its downstream signaling
cascade may be involved in the differentiation process of
macrophages when treated by PMA.
Reduced IL-1β and NLRP3 inflammasome
levels by berberine, in a dose-dependent manner in PMA-induced
macrophages
Our previous study has demonstrated that berberine
doses ranging from 5 to 75 µM caused no significant reduction
(~5–10%) in cell viability, and doses ranging from 5 to 50 µM were
used in subsequent experiments (27). In the present study, ELISA was
performed to investigate the effects of berberine on the secretion
of IL-1β in the supernatant. THP-1 cells were pretreated with the
indicated concentration of berberine for 1 h, followed by treatment
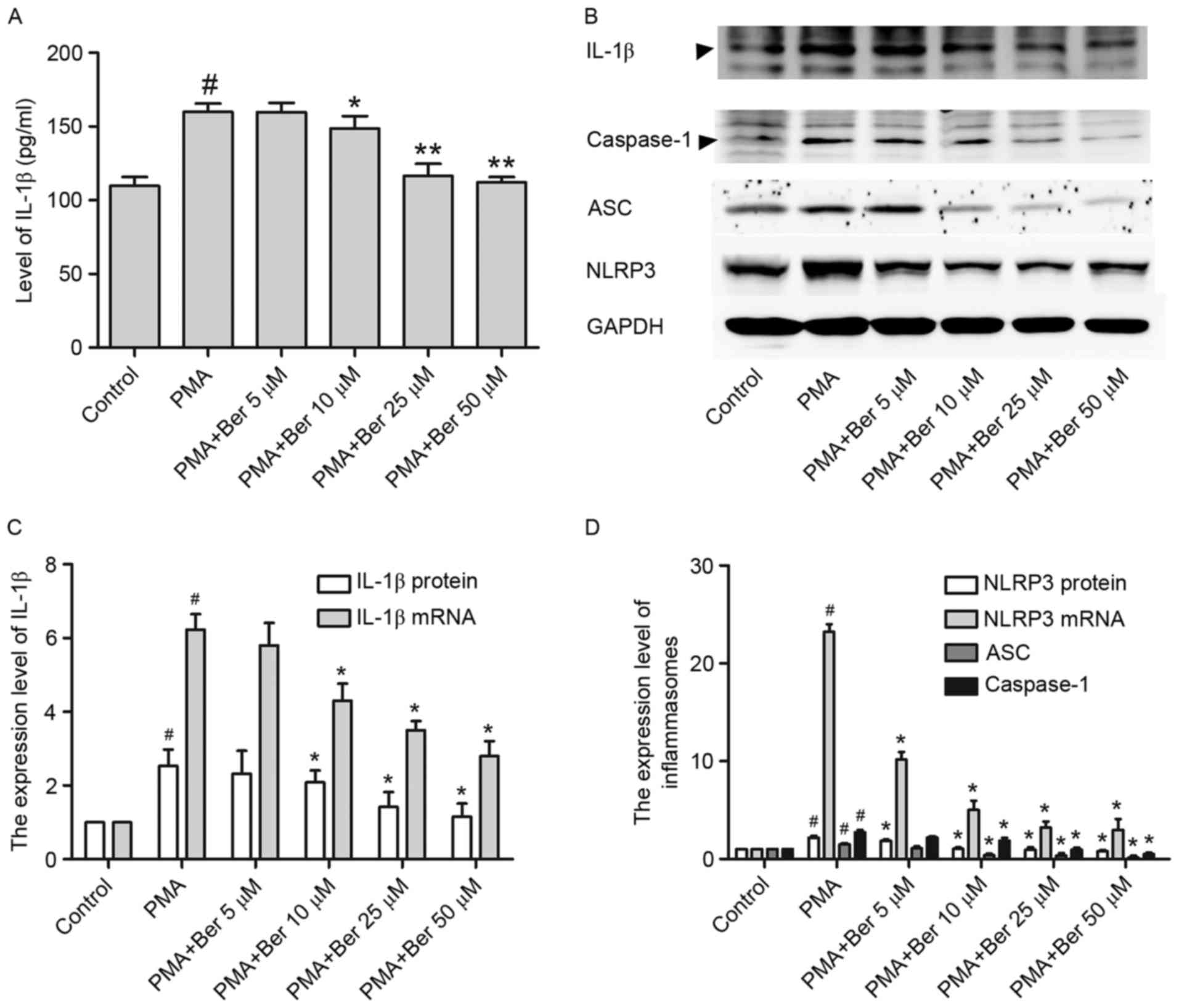
with PMA (100 nM) for 48 h. As illustrated in Fig. 2A, IL-1β was sharply increased in
the supernatant of PMA-treated group, whereas berberine treatment
significantly reduced its secretion in a dose-dependent manner.
Similar results were observed in THP-1 cells at the protein
(Fig. 2B and C) and mRNA level
(Fig. 2C), as detected by western
blotting and RT-qPCR, respectively. Thus, IL-1β expression is
reduced by berberine, at both transcription and translation level
in PMA-induced macrophages.
Given that IL-1β expression is positively regulated
by NLRP3 inflammasomes (28) and
berberine was demonstrated to reduce IL-1β expression in
PMA-induced macrophages, the present study then investigated
whether the inhibitory effect of berberine on IL-1β expression
might be a consequence of the inhibition of NLRP3 inflammasome
expression in PMA-induced macrophages. Results consistently
demonstrated that berberine had a similar effect on NLRP3 at both
the mRNA and protein level in PMA-induced macrophages (Fig. 2B and D). Furthermore, the
expression of ASC and activated caspase-1 were both significantly
inhibited in the berberine-treated group (Fig. 2B and D). This downregulation of
NLRP3 inflammasomes by berberine may be partly responsible for the
reduction of IL-1β expression in PMA-induced macrophages.
TLR4/Myd88/NF-κB pathway is involved
in the activation of NLRP3 inflammation in PMA-induced
macrophages
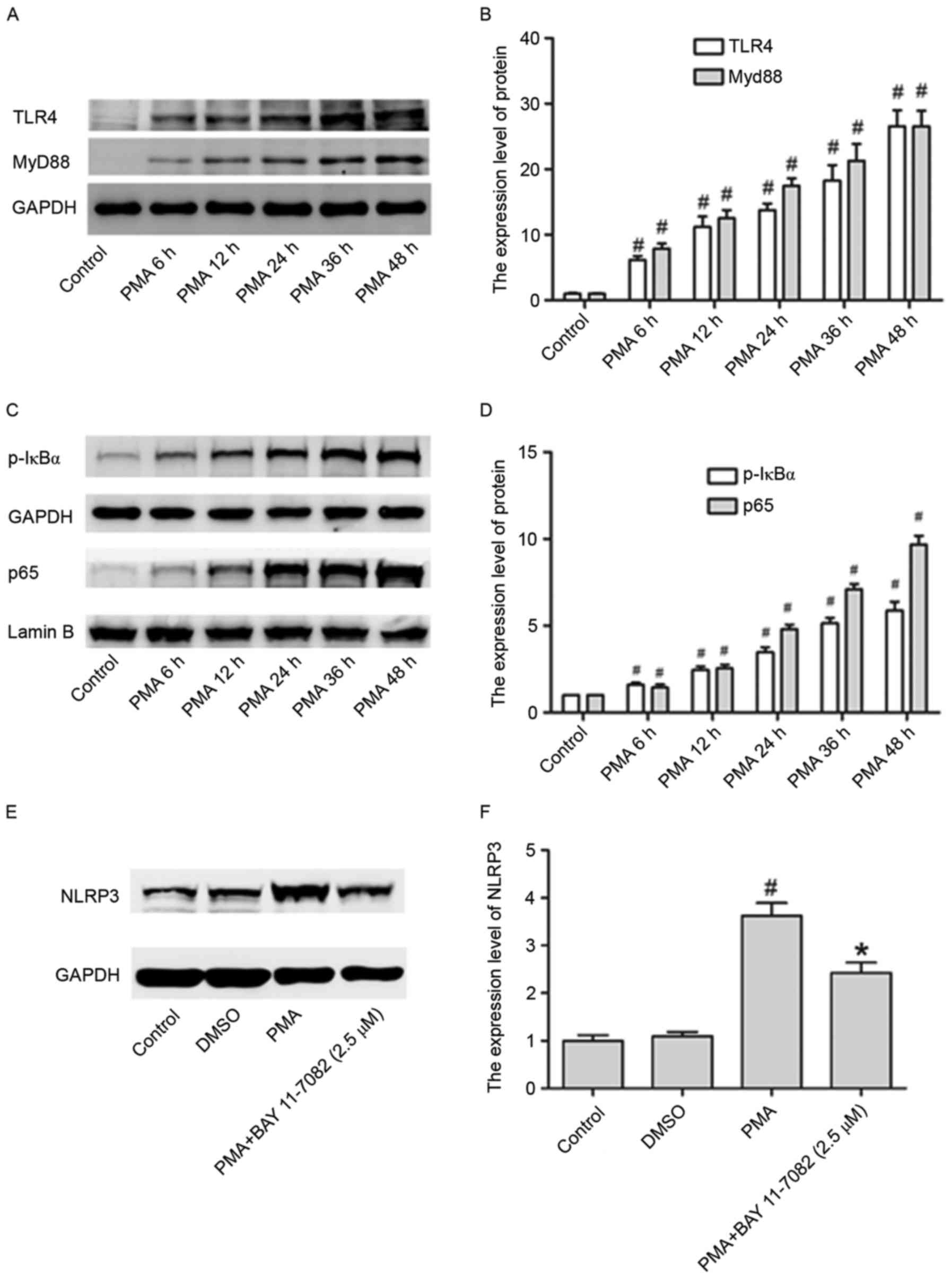
In the present study, in order to assess whether the
TLR4/Myd88/NF-κB signaling pathway is stimulated by PMA, its
expression pattern at different time points (6, 12, 24, 24, 36 and
48 h) was investigated, in THP-1 cells. The expression of TLR4,
Myd88, p-IκBα and p65 in cell nuclei were all upregulated by PMA in
a time dependent manner (Fig.
3A-D).
Whether this pathway is also involved in the
upregulation of NLRP3 inflammasome expression in PMA-induced
macrophages was investigated. Fig. 3E
and F illustrate that the NLRP3 increased levels were partly
abrogated in PMA-induced macrophages by treating cells with BAY
11–7082 (NF-κB inhibitor), suggesting that the activation of NF-κB
signaling pathway may be involved in the activation of NLRP3
inflammasomes.
TLR4/Myd88/NF-κB signaling pathway is
required for the inhibitory effect of berberine on NLRP3
inflammasome expression in PMA-induced macrophages
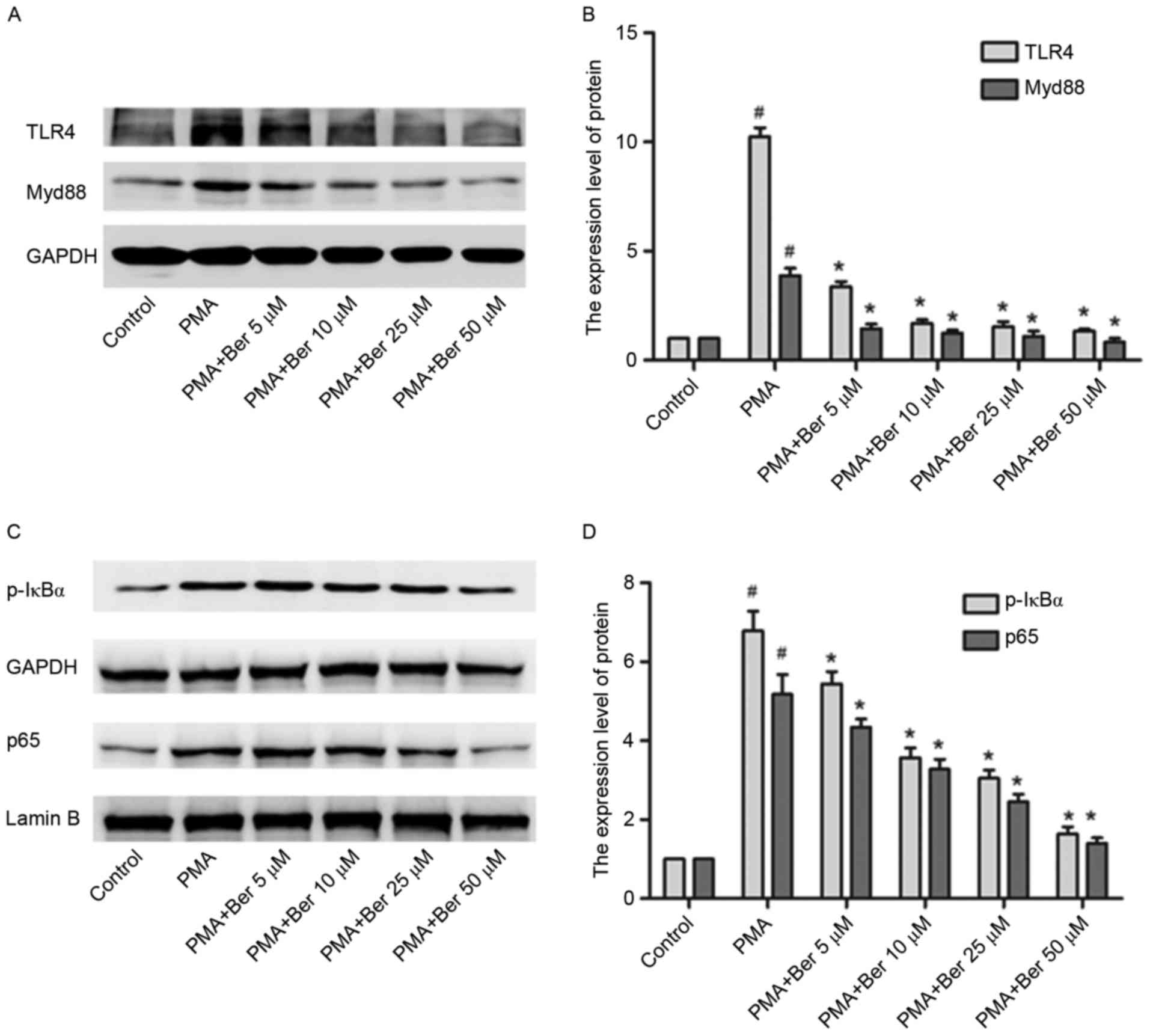
Given that berberine may lead to downregulation of
NLRP3 expression and that activation of the TLR4/Myd88/NF-κB
signaling pathway is involved in activating NLRP3 in PMA-induced
macrophages, whether the inhibitory effect of berberine on NLRP3
expression was associated with the TLR4/Myd88/NF-κB signaling
pathway was investigated. THP-1 cells in berbeirne-treated groups
were pretreated with berberine at the indicated concentration (5 to
50 µM) for 1 h and were subsequently cultured with PMA for 48 h.
Berberine significantly inhibited the protein expression of TLR4,
Myd88, p-IκBα and p65 induced by PMA in a dose-dependent manner
(Fig. 4). By inhibiting the
activation of the TLR4/Myd88/NF-κB signaling pathway, berberine
conclusively downregulates NLRP3 expression in PMA-induced THP-1
macrophages.
Discussion
The main findings of the present study were the
following: i) Expression levels of NLRP3 (both at mRNA and protein
level), ASC and caspase-1 were all upregulated in a time-dependent
manner, leading to IL-1β maturation during the conversion of THP-1
cells to macrophages by PMA; ii) berberine effectively suppressed
mRNA and protein expression levels of NLRP3 inflammasomes in a
dose-dependent manner in PMA-induced macrophages; and iii) the
TLR4/Myd88/NF-κB signaling pathway was partly involved in the
inhibition of NLRP3 inflammasome by berberine.
Atherosclerosis is considered as a chronic
inflammation process and is influenced by inflammatory mediators. A
recent study has suggested the causal role of IL-1β in the
development and progression of atherosclerotic vascular disease
(29). Studies in mouse models
have demonstrated that mice with IL-1β mutation/repression have low
atherosclerosis risk (30),
whereas mice over-expressing IL-1β can develop atherogenesis
(31). Furthermore, genetic
deletion of IL-1 receptor type I in atherosclerosis-prone mice
leads to a reduction of advanced atherosclerotic plaques size in
the aortic root (32). In the
present study, both mRNA and protein expression levels of IL-1β
were increased in PMA-induced macrophages, in a time-dependent
manner. Furthermore, an increase of IL-1β expression in the
supernatant of the PMA-induced group was demonstrated by ELISA,
indicating IL-1β as a key factor in the course of atherosclerosis.
Additionally, berberine significantly inhibited IL-1β levels in
PMA-induced macrophages, reducing its secretion.
IL-1β is involved in at least two separate signaling
cascades (33,34). In the first cascade, pattern
recognition and cytokine receptors in host cells control pro-IL-1β
transcription (33). In the second
cascade, the protein complex known as the inflammasome regulates
the proteolytic process of pro-IL-1β into a mature IL-1β (28). In the present study, focus was
given on the NLRP3 inflammasome functions and the NLRP3
inflammasome was illustrated to be activated in PMA-induced
macrophages, in a time-dependent manner. The NLRP3 inflammasome
consists of NLRP3, ASC and pro-caspase-1. Inflammation induces
NLRP3 inflammasome oligomerization leading to the recruitment of
ASC, which controls the activation of caspase-1. Activated p10 and
p20 caspase-1 are able to cleave 117 amino acids of pro-IL-1β
N-terminus leading to the bioactive form of 17 kD (35,36).
The NLRP3 inflammasome has recently been reported to be activated
by several endogenous molecules, such as glucose (37), oxidized low density lipoprotein
(LDL) (38) and cholesterol
crystals (38), which are
recognized as a hallmark in atherosclerotic lesions. NLRP3- or
ASC-deficient mice fed with a high-cholesterol diet exhibit
markedly decreased atherosclerosis and inflammasome-dependent
proinflammatory cytokine levels (38). Furthermore, Vandanmagsar et
al (39) demonstrated that
ablation of NLRP3 in mice fed a high-fat diet prevents inflammasome
activation and reduces production of IL-1β. Furthermore, increasing
evidence has demonstrated that the activation of the NLRP3
inflammasome is involved in atherosclerosis-associated inflammation
(7,8,40).
In the current study, the expression of the NLRP3 inflammasome,
which regulates the activated forms of ACS and pro-caspase-1, were
strongly upregulated in PMA-induced macrophages. Berberine markedly
inhibited NLRP3 and ACS expression and suppressed pro-caspase-1
activation in a dose-dependent manner. Given the vital roles of the
NLRP3 inflammasome in the development of atherosclerosis, and
considering the inhibitory effects of berberine on NLRP3
inflammasomes, berberine may exert beneficial properties by
ameliorating chronic inflammation caused by macrophages.
Berberine is a common drug used for treating
diarrhea and gastrointestinal disorders (41). Increasing evidence has demonstrated
that berberine has protective effects against atherosclerotic
diseases. Berberine can counteract high-fat diet-elicited
hyperhomocysteinemia and hyperlipidemia by upregulating LDL
receptor and apolipoprotein E mRNA levels and by suppressing
3-hydroxy-3-methylglutaryl-CoA reductase gene expression.
Furthermore, no atherosclerotic lesions were developed in
berberine-treated rats for 16 weeks (21). Berberine abrogates the formation of
foam cells, which serve a critical role in the progression of
atherosclerosis, by enhancing LXRalpha-ABCA1-dependent cholesterol
efflux (42). Furthermore,
berberine reduces oxidative stress and vascular inflammation, and
suppresses atherogenesis via stimulation of AMPK-dependent UCP2
expression (42). Our previous
study has illustrated that berberine exerts anti-atherogenic
effects by inhibiting matrix metalloproteinase-9 and extracellular
matrix metalloproteinase inducer (27). This provides some evidence that
supports the multiple functions of berberine in the prevention of
atherosclerosis. In the present study, berberine suppressed NLRP3
inflammasome expression and decreased the production and secretion
of IL-1β. These results illustrate some of the anti-atherogenic
effects of berberine.
Few studies have demonstrated that the
TLR4/Myd88/NF-κB signaling pathway is involved in regulatng NLRP3
inflammasome expression (16,43,44).
In the present study, the expression levels of TLR4, Myd88 and
NF-κB were all increased in PMA-induced macrophages. The expression
of the NLRP3 inflammasome was partly abrogated by BAY 11–7082 (a
recognized NF-κB inhibitor). Additionally, berberine significantly
inhibited activation of the TLR4/Myd88/NF-κB signaling pathway.
Berberine was observed to suppress the activation of NLRP3
inflammasome via the inhibition of the TLR4/Myd88/NF-κB signaling
pathway.
In conclusion, berberine significantly inhibited the
upregulation of the NLRP3 inflammasome, decreasing the production
and secretion of IL-1β via inhibition of the TLR4/Myd88/NF-κB
signaling pathway in PMA-induced macrophages. Considering the vital
role of the IL-1β and NLRP3 inflammasome in chronic inflammatory
responses, these results provide novel insights of how berberine
may mitigate atherogenic progression, and further confirm its
anti-inflammatory effects in atherosclerotic disease. However,
further studies are required to confirm if the above effects of
berberine also occur in vivo.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81102837 and
81370224), the Natural Science Foundation of Zhejiang Province
(grant nos. LY13H280004 and LQ15H020005) and the Key Construction
Academic Subject (Traditional Chinese Medicine) of Zhejiang
Province (grant no. 2012-XK-A28).
References
|
1
|
Kawamura A, Baitsch D, Telgmann R,
Feuerborn R, Weissen-Plenz G, Hagedorn C, Saku K, Brand-Herrmann
SM, von Eckardstein A, Assmann G and Nofer JR: Apolipoprotein E
interrupts interleukin-1beta signaling in vascular smooth muscle
cells. Arterioscler Thromb Vasc Biol. 27:1610–1017. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yearley JH, Xia D, Pearson CB, Carville A,
Shannon RP and Mansfield KG: Interleukin-18 predicts
atherosclerosis progression in SIV-infected and uninfected rhesus
monkeys (Macaca mulatta) on a high-fat/high-cholesterol diet. Lab
Invest. 89:657–667. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galea J, Armstrong J, Gadsdon P, Holden H,
Francis SE and Holt CM: Interleukin-1 beta in coronary arteries of
patients with ischemic heart disease. Arterioscler Thromb Vasc
Biol. 16:1000–1016. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhaskar V, Yin J, Mirza AM, Phan D,
Vanegas S, Issafras H, Michelson K, Hunter JJ and Kantak SS:
Monoclonal antibodies targeting IL-1 beta reduce biomarkers of
atherosclerosis in vitro and inhibit atherosclerotic plaque
formation in apolipoprotein E-deficient mice. Atherosclerosis.
216:313–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
De Nardo D and Latz E: NLRP3 inflammasomes
link inflammation and metabolic disease. Trends Immunol.
32:373–379. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li Y, Xu S, Jiang B, Cohen RA and Zang M:
Activation of sterol regulatory element binding protein and NLRP3
inflammasome in atherosclerotic lesion development in diabetic
pigs. PLoS One. 8:e675322013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng F, Xing S, Gong Z, Mu W and Xing Q:
Silence of NLRP3 suppresses atherosclerosis and stabilizes plaques
in apolipoprotein E-deficient mice. Mediators Inflamm.
2014:5072082014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Satoh M, Tabuchi T, Itoh T and Nakamura M:
NLRP3 inflammasome activation in coronary artery disease: Results
from prospective and randomized study of treatment with
atorvastatin or rosuvastatin. Clin Sci (Lond). 126:233–241. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang K, Zhang XJ, Cao LJ, Liu XH, Liu ZH,
Wang XQ, Chen QJ, Lu L, Shen WF and Liu Y: Toll-like receptor 4
mediates inflammatory cytokine secretion in smooth muscle cells
induced by oxidized low-density lipoprotein. PLoS One.
9:e959352014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin YW, Liao SQ, Zhang MJ, Liu Y, Li BH,
Zhou Y, Chen L, Gao CY, Li JC and Zhang LL: TLR4-mediated
inflammation promotes foam cell formation of vascular smooth muscle
cell by upregulatng ACAT1 expression. Cell Death Dis. 6:16592015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barton GM and Medzhitov R: Toll-like
receptor signaling pathways. Science. 300:1524–1525. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang JM, Zhang GN, Shi Y, Zha X, Zhu Y,
Wang MM, Lin Q, Wang W, Lu HY, Ma SQ, et al: Atractylenolide-I
sensitizes human ovarian cancer cells to paclitaxel by blocking
activation of TLR4/MyD88-dependent pathway. Sci Rep. 4:38402014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hu ZP, Fang XL, Fang N, Wang XB, Qian HY,
Cao Z, Cheng Y, Wang BN and Wang Y: Melatonin ameliorates vascular
endothelial dysfunction, inflammation, and atherosclerosis by
suppressing the TLR4/NF-κB system in high-fat-fed rabbits. J Pineal
Res. 55:388–398. 2013.PubMed/NCBI
|
|
14
|
Shang T, Ran F, Qiao Q, Liu Z and Liu CJ:
Tanshinone IIA attenuates elastase-induced AAA in rats via
inhibition of MyD88-dependent TLR-4 signaling. Vasa. 43:39–46.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang G, Bao P, Zhang L, Lyu Z, Zhou B,
Chen K, Peng S, Wang Y, Yao L, Zhou Y and Li Y: Critical role of
myeloid differentiation factor 88 in necrotizing enterocolitis.
Pediatr Res. 75:707–715. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bauernfeind FG, Horvath G, Stutz A,
Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks
BG, Fitzgerald KA, et al: Cutting edge: NF-kappaB activating
pattern recognition and cytokine receptors license NLRP3
inflammasome activation by regulatng NLRP3 expression. J Immunol.
183:787–791. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bauernfeind F, Bartok E, Rieger A, Franchi
L, Núñez G and Hornung V: Cutting edge: Reactive oxygen species
inhibitors block priming, but not activation, of the NLRP3
inflammasome. J Immunol. 187:613–617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ikram M: A review on the chemical and
pharmacological aspects of genus Berberis. Planta Med. 28:353–358.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung JG, Wu LT, Chu CB, Jan JY, Ho CC,
Tsou MF, Lu HF, Chen GW, Lin JG and Wang TF: Effects of berberine
on arylamine N-acetyltransferase activity in human bladder tumour
cells. Food Chem Toxicol. 37:319–326. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang C, Zhang Y, Gong Z, Sheng X, Li Z,
Zhang W and Qin Y: Berberine inhibits 3T3-L1 adipocyte
differentiation through the PPARgamma pathway. Biochem Biophys Res
Commun. 348:571–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang XX, Yan HM, Xu Q, Xia MF, Bian H,
Zhu TF and Gao X: The effects of berberine on hyperhomocysteinemia
and hyperlipidemia in rats fed with a long-term high-fat diet.
Lipids Health Dis. 11:862012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huang Z, Meng S, Wang L, Wang Y, Chen T
and Wang C: Suppression of oxLDL-induced MMP-9 and EMMPRIN
expression by berberine via inhibition of NF-κB activation in human
THP-1 macrophages. Anat Rec (Hoboken). 295:78–86. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Z, Dong F, Li S, Chu M, Zhou H, Lu Z
and Huang W: Berberine-induced inhibition of adipocyte
enhancer-binding protein 1 attenuates oxidized low-density
lipoprotein accumulation and foam cell formation in phorbol
12-myristate 13-acetate-induced macrophages. Eur J Pharmacol.
690:164–169. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsuchiya S, Kobayashi Y, Goto Y, Okumura
H, Nakae S, Konno T and Tada K: Induction of maturation in cultured
human monocytic leukemia cells by a phorbol diester. Cancer Res.
42:1530–1536. 1982.PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Z, Wang C, Wei L, Wang J, Fan Y,
Wang L, Wang Y and Chen T: Resveratrol inhibits EMMPRIN expression
via P38 and ERK1/2 pathways in PMA-induced THP-1 cells. Biochem
Biophys Res Commun. 374:517–521. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang Z, Wang L, Meng S, Wang Y, Chen T
and Wang C: Berberine reduces both MMP-9 and EMMPRIN expression
through prevention of p38 pathway activation in PMA-induced
macrophages. Int J Cardiol. 146:153–158. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Takeuchi O and Akira S: Pattern
recognition receptors and inflammation. Cell. 140:805–820. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ridker PM, Everett BM, Thuren T, MacFadyen
JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker
SD, et al: Antiinflammatory therapy with canakinumab for
atherosclerotic disease. N Engl J Med. 377:1119–1131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kirii H, Niwa T, Yamada Y, Wada H, Saito
K, Iwakura Y, Asano M, Moriwaki H and Seishima M: Lack of
interleukin-1beta decreases the severity of atherosclerosis in
ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 23:656–660.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Merhi-Soussi F, Kwak BR, Magne D,
Chadjichristos C, Berti M, Pelli G, James RW, Mach F and Gabay C:
Interleukin-1 plays a major role in vascular inflammation and
atherosclerosis in male apolipoprotein E-knockout mice. Cardiovasc
Res. 66:583–593. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alexander MR, Moehle CW, Johnson JL, Yang
Z, Lee JK, Jackson CL and Owens GK: Genetic inactivation of IL-1
signaling enhances atherosclerotic plaque instability and reduces
outward vessel remodeling in advanced atherosclerosis in mice. J
Clin Invest. 122:70–79. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eder C: Mechanisms of interleukin-1beta
release. Immunobiology. 214:543–553. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lopez-Castejon G and Brough D:
Understanding the mechanism of IL-1β secretion. Cytokine Growth
Factor Rev. 22:189–195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dinarello CA: Immunological and
inflammatory functions of the interleukin-1 family. Annu Rev
Immunol. 27:519–550. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wilson KP, Black JA, Thomson JA, Kim EE,
Griffith JP, Navia MA, Murcko MA, Chambers SP, Aldape RA, Raybuck
SA, et al: Structure and mechanism of interleukin-1 beta converting
enzyme. Nature. 370:270–275. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou R, Tardivel A, Thorens B, Choi I and
Tschopp J: Thioredoxin-interacting protein links oxidative stress
to inflammasome activation. Nat Immunol. 11:136–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duewell P, Kono H, Rayner KJ, Sirois CM,
Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nuñez G, Schnurr
M, et al: NLRP3 inflammasomes are required for atherogenesis and
activated by cholesterol crystals. Nature. 464:1357–1361. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vandanmagsar B, Youm YH, Ravussin A,
Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM and Dixit
VD: The NLRP3 inflammasome instigates obesity-induced inflammation
and insulin resistance. Nat Med. 17:179–188. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Leemans JC, Cassel SL and Sutterwala FS:
Sensing damage by the NLRP3 inflammasome. Immunol Rev. 243:152–162.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi SH, Cho SK, Kang SS, Bae CS, Bai YH,
Lee SH and Pak SC: Effect of apitherapy in piglets with preweaning
diarrhea. Am J Chin Med. 31:321–326. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Q, Zhang M, Liang B, Shirwany N, Zhu
Y and Zou MH: Activation of AMP-activated protein kinase is
required for berberine-induced reduction of atherosclerosis in
mice: The role of uncoupling protein 2. PLoS One. 6:e254362011.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Palová-Jelínková L, Dáňová K, Drašarová H,
Dvořák M, Funda DP, Fundová P, Kotrbová-Kozak A, Černá M, Kamanová
J, Martin SF, et al: Pepsin digest of wheat gliadin fraction
increases production of IL-1β via TLR4/MyD88/TRIF/MAPK/NF-kB
signaling pathway and an NLRP3 inflammasome activation. PLoS One.
8:e624262013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Segovia J, Sabbah A, Mgbemena V, Tsai SY,
Chang TH, Berton MT, Morris IR, Allen IC, Ting JP and Bose S:
TLR2/MyD88/NF-kB pathway, reactive oxygen species, potassium efflux
activates NLRP3/ASC inflammasome during respiratory syncytial virus
infection. PLoS One. 7:e296952012. View Article : Google Scholar : PubMed/NCBI
|