Introduction
Gastric cancer (GC) is a main prevalent malignancy
in the world. It is considered the second most widely diagnosed
malignancy causing 12% of cancer-associated deaths worldwide every
year (1). The cancer incidence is
extremely high in eastern Asia countries, such as China, Japan and
South Korea. (2). GC mortality is
on the top of the list of malignancies in China. Most of GC
patients are diagnosed at late stages that missing the best chance
to recover, due to the poor diagnosis in early stage (3). The metastasis ratio is more than 50%,
which causes difficulty to cure it. The comprehensive treatment,
giving priority to chemotherapy is the main therapeutic schedule
for the late stage GC. But the effect is not so good that it calls
for better treatments relying on the deeper understanding of the
cancer's molecular mechanism. As tumor proliferation and inhibited
apoptosis are both very important reasons for malignant
progression, it is essential and possible to discover new
therapeutic methods and related functional molecules.
The Hippo pathway plays an important role in
organism development, and its dysregulation can result in
tumorigenesis. WW-domain containing transcription regulator 1
(WWTR1) is also called TAZ, transcriptional co-activator with PDZ
binding motif. It acts downstream of the Hippo pathway as a
transcription co-activator (4).
WWTR1 can activate transcriptional factors and play an important
role in tissue development. WWTR1 is also regarded as a
cancer-related gene and increased WWTR1 protein levels have been
found in many cancers (5).
Previous studies have demonstrated that higher WWTR1 mRNA/protein
levels were associated with the onset of gastric, lung, colorectal
and breast cancers. The knockdown of WWTR1 in colorectal cells was
reported to inhibit cell proliferation by blocking cell cycle or
promoting the apoptosis (6–8).
Wang et al (9) verified
that WWTR1 promoted non-small cell lung cancer cell growth and
inhibited apoptosis by Cyclin A and C transforming growth factor
(TGF) regulation. Recent research indicated that high expression of
WWTR1 existed in GC tissue, related to tumor TNM staging and lymph
node transferance (10).
WWTR1 interacted with other regulation factors
except being regulated by Hippo pathway. TGF-β signal pathway,
important in cell growth and development, has a close relationship
with tumor development and progression. It inhibites tumor occuring
in physiological status, but promotes tumor invasion and metastasis
in tumor development process. Attisano and Wrana reported that,
WWTR1 interacted with Smads in TGF-β pathway, and Hippo activation
prevented Smad nuclear accumulation and transcriptional activity
(11).
Function of WWTR1 on the downstrean regulation
pathway like TGF-β pathway in GC is of significant value for GC
treatment.
Although previous studies have shown that WWTR1
functioned as an oncogene in GC, the mechanism of WWTR1 modulating
GC cell proliferation and apoptosis is still unclear. In order to
illuminate the mechanism, we detected WWTR1 expression in GC tissue
and cell lines. We examined the silencing role of siWWTR1 on the
cell cycle progression and apoptosis of GC cell SGC7901 which
expressed WWTR1 notably, and explored the potential mechanism to
provide new thoughts for the treatment of GC.
Patients and methods
Patients and tissue samples
Informed consent was obtained before the study. A
total of 52 patients aged from 38–75 years old (median age, 58
years old) with GC admitted to Hospital were enrolled, consisting
of 36 males and 16 females. Among all patients there were 42 cases
of gastric adenomas, and the pathological grades were: 13 cases of
high differentiation, and 39 cases of low/media differentiation,
while the pathological stages were: 15 cases of I+II stage, and 37
cases of III+IV stages. While 38 cases with lymphatic metastasis
and 14 cases without lymphatic metastasis. No patient had received
radiotherapy or chemotherapy prior to surgery. Matched adjacent
normal gastric tissues were collected as negative controls
Preoperative clinical and pathological follow-up data were
completed by all patients. All tissue-samples of patients were
collected according to the procedures approved by the institutional
review board of the independent Ethics Committee, Hospital.
Cell culture
Human gastric mucosa epithelia cell GES1 and GC cell
lines (SGC7901, BGC823, HGC-27, MGC-803, MKN45) purchased from ATCC
(Manassas, VA, USA) were cultured in RPMI 1640 medium (Gibco, Grand
Island, NY, USA) containing 10% fetal bovine serum (FBS; Gibco)
with 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA, USA) at
37°C, 5% CO2 atmosphere. Cells of logarithm phase were
used in our research.
siRNA transfection
siRNA-WWTR1 was designed and synthesized by
GenePharma (Shanghai, China). siRNA sequence, GGT ACT TCC TCA ATC
ACA T; and control sequence, TTC TCC GAA CGT GTC ACG T. GC cell
SGC7901 was selected for transfection and the follow-up experiments
due to high-expression WWTR1. For siRNA transfection, cells were
seeded onto 12-well cell culture plates at an initial density of
6×104 cells/well. When cells were 70% confluent, they
were transfected with the WWTR1 siRNA and nonspecific siRNA
(forming mock groups as negative control) according to the
manufacturer's protocol (Qiagen, Chatsworth, CA, USA). Reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting were used to measure the interference efficiency
after 48 h transfection.
Cell viability assay
The effect of siRNA-WWTR1 on SGC7901 cell viability
was detected by Cell Counting Kit-8 (CCK-8) assay. Briefly,
following 12, 24, 48 h transfection, the cells were seeded in
96-well plates at a density of 5×103 cells/well and
incubated for the indicated times. CCK-8 (20 µl) was then added to
each well of the plate. Then the plate was incubated for another 1
h. The optical density (OD) values were read at 450 nm by a
microplate reader (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Data were presented as relative proportion of viable cells to
control cells.
Cell cycle analysis
Cell cycle was evaluated by propidium iodide (PI)
staining after siRNA-WWTR1 transfection. Cells were trypsinized,
washed twice using phosphate-buffered saline (PBS) and fixed
overnight at 4°C in ice-cold 70% ethanol. After being washed twice
with PBS, cells were incubated in 50 µg/ml PI and 100 µg/ml RNAase
for 30 min at room temperature. Thereafter, analysis was
immediately performed with a FACS flow cytometer (BD Pharmingen,
San Diego, CA, USA). The proportion of cells in
G0/G1, S and G2/M phases was
detected.
Apoptosis detection
The apoptosis status was measured by Annexin V/PI
double-stain assay, according to the manufacturer's protocol
(BioVision, Mountain View, CA, USA). Briefly, after the
interfection of siRNA-WWTR1, cells were collected and resuspended
by 500 µl binding buffer including 5 µl Annexin V-fluorescein
isothiocyanate and 5 µl PI. After the incubation for 5 min in the
dark at room temperature, cells were analyzed by a flow cytometer
(BD Pharmingen). Cell Quest software was used to analyze the
apoptosis rate.
Independent experiments
RT-qPCR
Total RNA was extracted from transfected cells, mock
cells and non-transfected cells respectively and reversely
transcribed to cDNA with a first strand cDNA kit (Sigma-Aldrich,
Munich, Germany), according to the protocol provided by the
manufacturer. PCR amplification was performed for 30 sec at 95°C,
followed by 40 cycles: Denaturation at 95°C for 5 sec,
annealing/extension at 60°C for 30 sec in ABI 7300 Thermocycler
(Applied Biosystems, Foster City, CA, USA) using the SYBR Premix Ex
Taq kit (Takara, Dalian, China). The primer sequences were
displayed in Table I.
 | Table I.Primers used in RT-PCR analysis. |
Table I.
Primers used in RT-PCR analysis.
| Name | Sequence (5′-3′) |
|---|
| WWTR1 |
|
|
Forward |
CAGCCAAATCTCGTGATGAATC |
|
Reverse |
GGTTCTGCTGGCTCAGGGT |
| Cyclin A |
|
|
Forward |
GCAGAGGCCGAAGACGAGA |
|
Reverse |
TCCAAGGAGGAACGGTGACA |
| Cyclin D1 |
|
|
Forward |
GCTGGAGGTCTGCGAGGA |
|
Reverse |
ACAGGAAGCGGTCCAGGTAGT |
| Cyclin E |
|
|
Forward |
AGCCAGCCTTGGGACAATAAT |
|
Reverse |
GAGCCTCTGGATGGTGCAAT |
| c-Myc |
|
|
Forward |
GCCACGTCTCCACACATCAG |
|
Reverse |
TGGTGCATTTTCGGTTGTTG |
| Bcl-2 |
|
|
Forward |
ACGGTGGTGGAGGAGCTCTT |
|
Reverse |
CGGTTGACGCTCTCCACAC |
| XIAP |
|
|
Forward |
GGCACGAGCAGGGTTTCTT |
|
Reverse |
TCCAACTGCTGAGTCTCCATATTG |
| Bax |
|
|
Forward |
TGCTTCAGGGTTTCATCCA |
|
Reverse |
GGCCTTGAGCACCAGTTT |
| ASNS |
|
|
Forward |
ACGCTGACCCACTACAAG |
|
Reverse |
ACCCAAGTTAGCCTGAGTT |
| ID1 |
|
|
Forward |
AGAGACTTTAGGGGGTGGGA |
|
Reverse |
TGAGAAGCACCAAACGTGAC |
| SMAD3 |
|
|
Forward |
TTCACTGGTGCTGGGGTTAG |
|
Reverse |
GGCGGCAGTAGATGACATGA |
| BTC |
|
|
Forward |
GAAATGGAAACTCTGGGT |
|
Reverse |
CAAATGAGCAAGGCACT |
| GAPDH |
|
|
Forward |
TGACTTCAACAGCGACACCCA |
|
Reverse |
CACCCTGTTGCTGTAGCCAAA |
Western blot analysis
The concentrations of proteins were determined by
BCA assay (Beyotime, Nantong, China). Then proteins were subjected
to sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE) and electroblotted onto a polyvinylidene fluoride
membrane (PVDF; Amersham Pharmacia Biotech, Amersham, UK).
Following blockage with 5% non-fat dry milk in PBS for 1 h, the
blotting membranes were probed overnight respectively at 4°C, with
the following primary antibodies: Rabbit anti-WWTR1 (ab84927,
1:500), mouse anti-Cyclin D1 (ab6152, 1:1,000), mouse anti-cancer
Myc (c-Myc; ab17356, 1:1,000), mouse anti-B cell
lymphoma/leukemia-2 (Bcl-2; ab692, 1:1,000), mouse anti-Bcl-2
associated X protein (Bax; ab77566, 1:1,000); mouse anti-asparagine
synthetase (ASNS; ab171800, 1:1,000), mouse anti-inhibitor of DNA
binding 1, HLH protein (ID1; ab168256, 1:1,000), mouse anti-mothers
against decapentaplegic homolog family member 3 (SMAD3; ab55480,
1:500), mouse anti-betacellulin (BTC; ab89156, 1:500), and mouse
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; ab9484,
1:5,000; all from Abcam, Cambridge, UK), then they were probed with
the appropriate HRP-conjugated secondary antibodies: rabbit anti
mouse IgG H+L (HRP) (ab6728, 1:5,000), goat anti rabbit IgG H+L
(HRP) (ab6721, 1:5,000; both from Abcam). The PVDF membrane was
exposed to X-ray film and immunoreactive bands were detected by
reaction with Enhanced Chemiluminescense Detection system reagents
(Amersham, Arlington Heights, IL, USA). For loading control, the
membrane was probed with a monoclonal antibody for GAPDH. Lab Works
Image Acquisition and Analysis Software (UVP, Inc., Upland,. CA,
USA) were used to quantify band intensities.
Statistical analysis
All results were presented as mean ± SD of three
independent experiments. Statistical analysis was performed using a
SPSS 13.0 statistical package and data were subjected to one-way
analysis of variance, followed by Dunnett's test. P<0.05 was
considered significant, P<0.01 was considered especially
significant.
Results
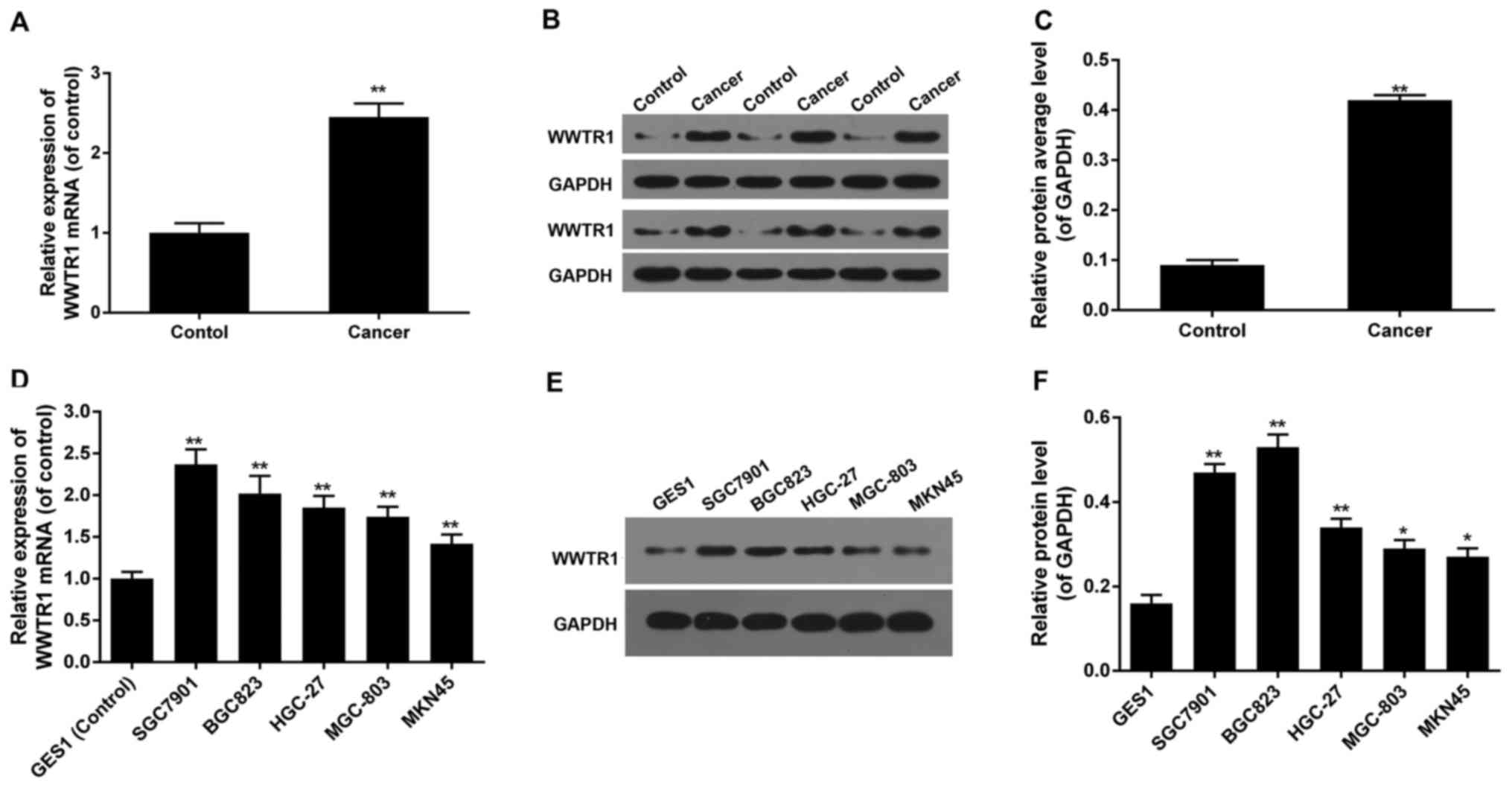
High expression of WWTR1 in GC
tissue
To verify the biological role of WWTR1 in GC, the
expression levels of WWTR1 in GC tissues were detected by RT-PCR
and western blotting. It showed that the mRNA expression of WWTR1
was much higher in GC tissues, and the protein expression of WWTR1
was much higher in most of GC tissues than that of adjacent normal
tissues (P<0.01), we choose some representative blots to diaplay
in Fig. 1. It indicated that the
over-expression of WWTR1 may be involved in the initiation and/or
progression of GC (Fig. 1A-C).
WWTR1 expression in GC cell lines
Based on the evident difference in WWTR1 expression
between GC tissues and normal tissues, the mRNA and protein
expression of WWTR1 in normal gastric mucosa epithelia cell GES1
and GC cell lines including SGC7901, BGC823, HGC-27 MGC-803 and
MKN45 were detected by RT-PCR and western blotting. The mRNA
expression of WWTR1 was remarkably higher in SGC7901 cell than in
any other cell lines, twice of GES1 cells (Fig. 1D). And the protein level of WWTR1
in SGC7901 was the second highest among all cell lines. Protein
level of WWTR1 in BGC823 was a little higher than that of SGC7901,
but of no significant difference. And both mRNA and protein levels
of SGC7901 increased remarkably compared with GES1 (control group)
(Fig. 1E and F). While SGC7901 is
used in more study, of representative meaning, it was chosen to be
used in our study.
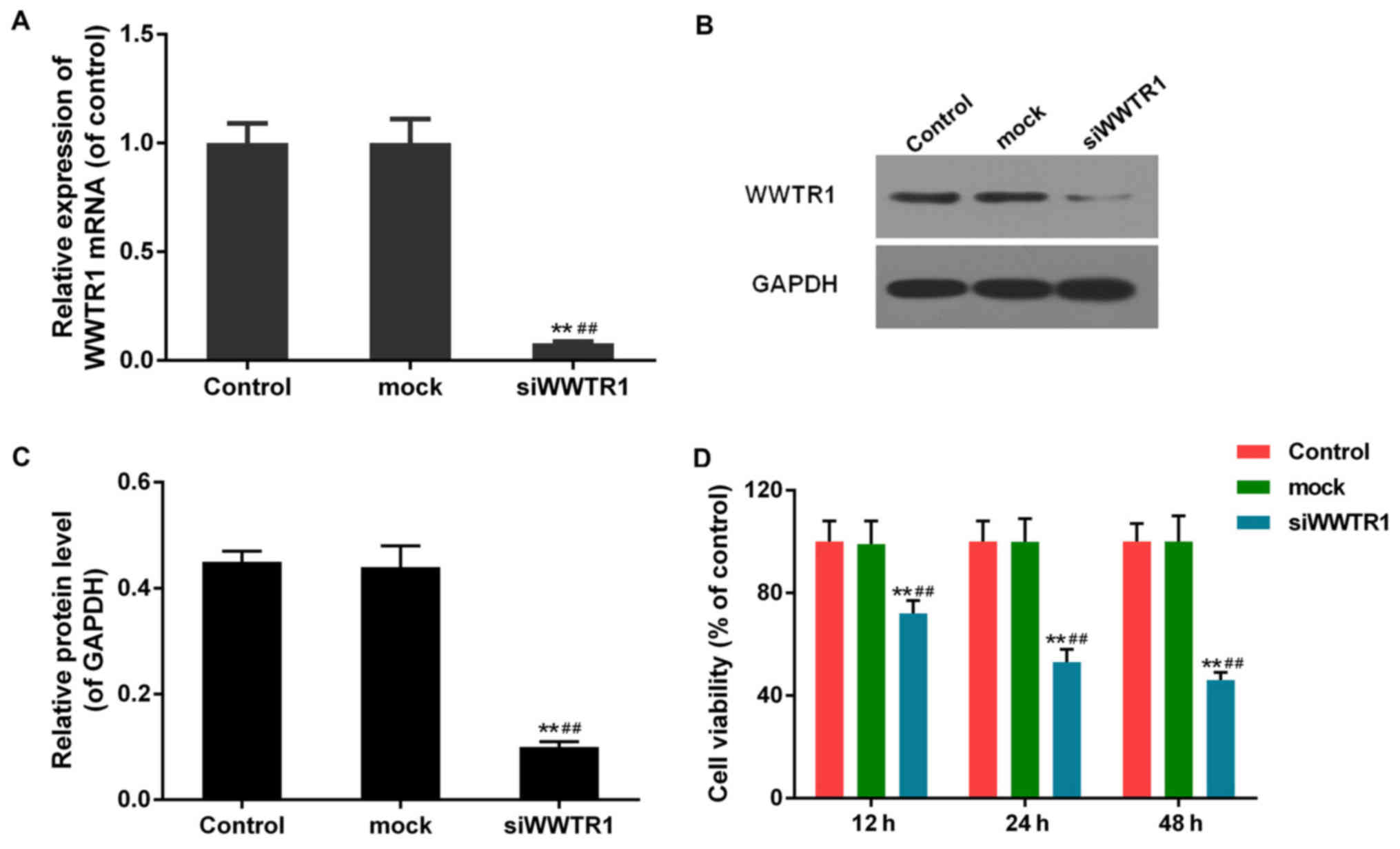
Effect of siRNA-WWTR1 on SGC7901 cell
viability
WWTR1 mRNA was interfered in SGC7901 cell line as
previously described. The interference efficience was then
identified by means of RT-PCR and western blotting. As displayed in
(Fig. 2A-C), transfection of
siRNA-WWTR1 resulted in dramatic decline in WWTR1 mRNA and protein
levels in siRNA-WWTR1 group compared with the control group and
mock group, which confirmed that siRNA-WWTR1 was effective in
silencing WWTR1 expression.
The effect of siRNA-WWTR1 on SGC7901 cell viability
measured by CCK-8 assay was shown in Fig. 2D. The cell viability was
significantly suppressed in siRNA-WWTR1 group 24 and 48 h post
transfection compared with that of control and mock cells.
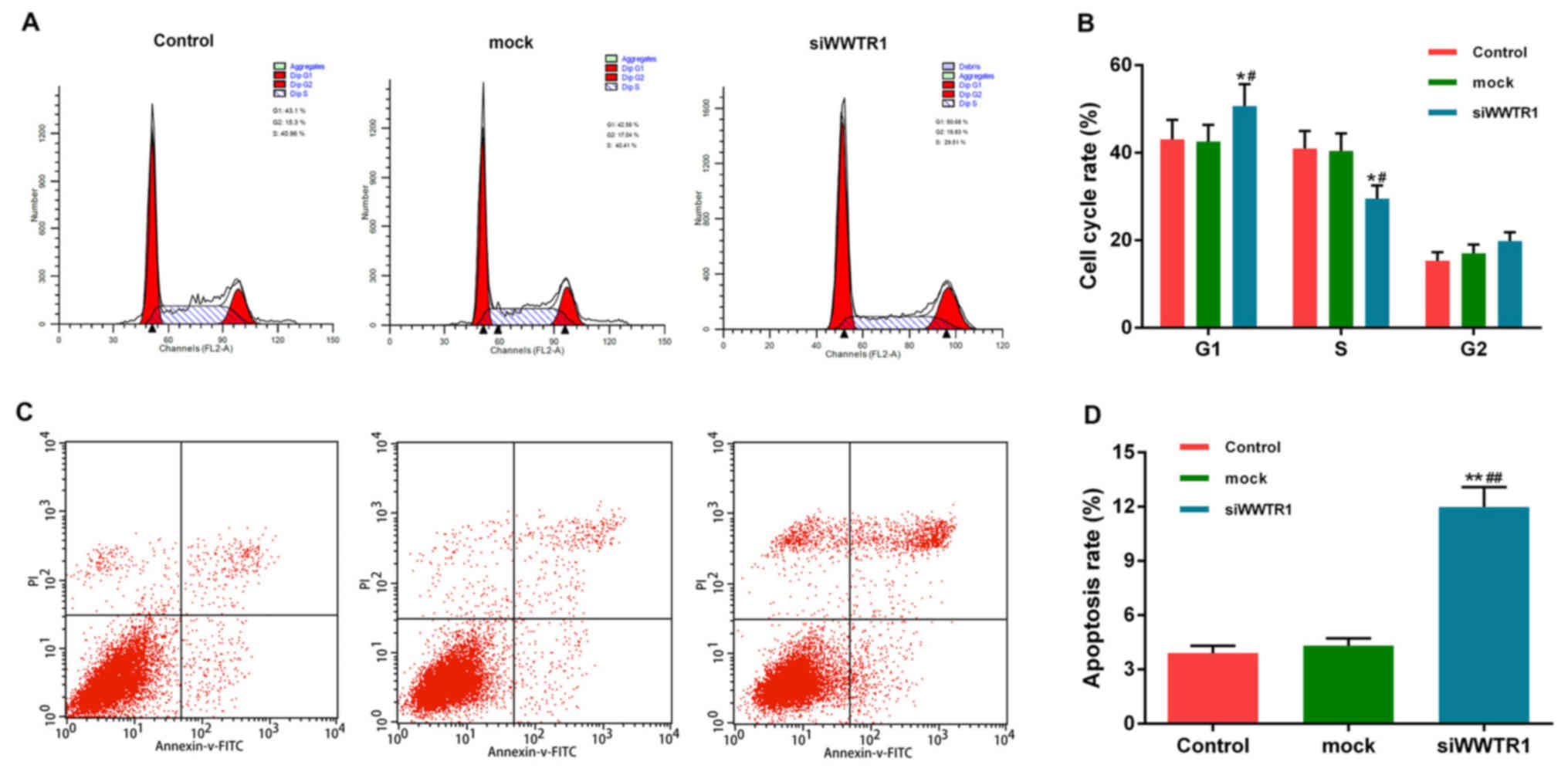
Effect of siRNA-WWTR1 on SGC7901 cell
cycle arrest and apoptosis
As we can see, cell viability was decreased in
siRNA-WWTR1 group, we examined the effect of WWTR1 on cell cycle
progression and apoptosis. As displayed in (Fig. 3A and B), pretreatment of GC cells
with siRNA-WWTR1 blocked the cell cycle transition from G1 to S
phase. The percentage of cells in G0/G1-phases increased from 43.10
to 50.68%, while S-phase fraction decreased from 40.96 to
29.51%.
We performed Annexin V/PI double-staining to
quantify apoptotic cell death, and used flow cytometry for analysis
(Fig. 3C and D). At 48 h after
transfection with siWWTR1, apoptosis increased as the
phosphatidylserine (PS) extrution to the outer leaflet of the
plasma membrane increased, which was detected by Annexin V binding
to the surface of cells. The apoptosis of SGC7901 cell increased
3-fold, compared with control and mock groups.
Effect of siRNA-WWTR1 on cell cycle
and apoptosis related factors in SGC7901
The critical role of siRNA-WWTR1 in SGC7901 cell
cycle blocking and induction of apoptosis promoted us to further
explore the potential mechanism by measuring the relevant genes and
proteins. The RNA levels of Cyclin D1, c-Myc, Bcl-2 were decreased
dramatically, and Bax increased significantly, while Cyclin A,
Cyclin E and X-linked inhibitor of apoptosis (XIAP) changed little
in siWWRT1-interferring cells compared to the control and mock
cells (Fig. 4A). Western blotting
was performed to measure the protein expression of Cyclin D1,
c-Myc, Bcl-2 and Bax, for the mRNA expression of them changed
significantly. The protein level of Cyclin D1, c-Myc, Bcl-2 were
decreased and Bax increased significantly in siWWRT1-interferring
cells compared with control and mock cells, being consistent with
mRNA variation (Fig. 4B and C).
The results indicated regulation of WWTR1 on cell proliferation and
apoptosis may rely on the expression of Cyclin D1, c-Myc, Bcl-2 and
Bax.
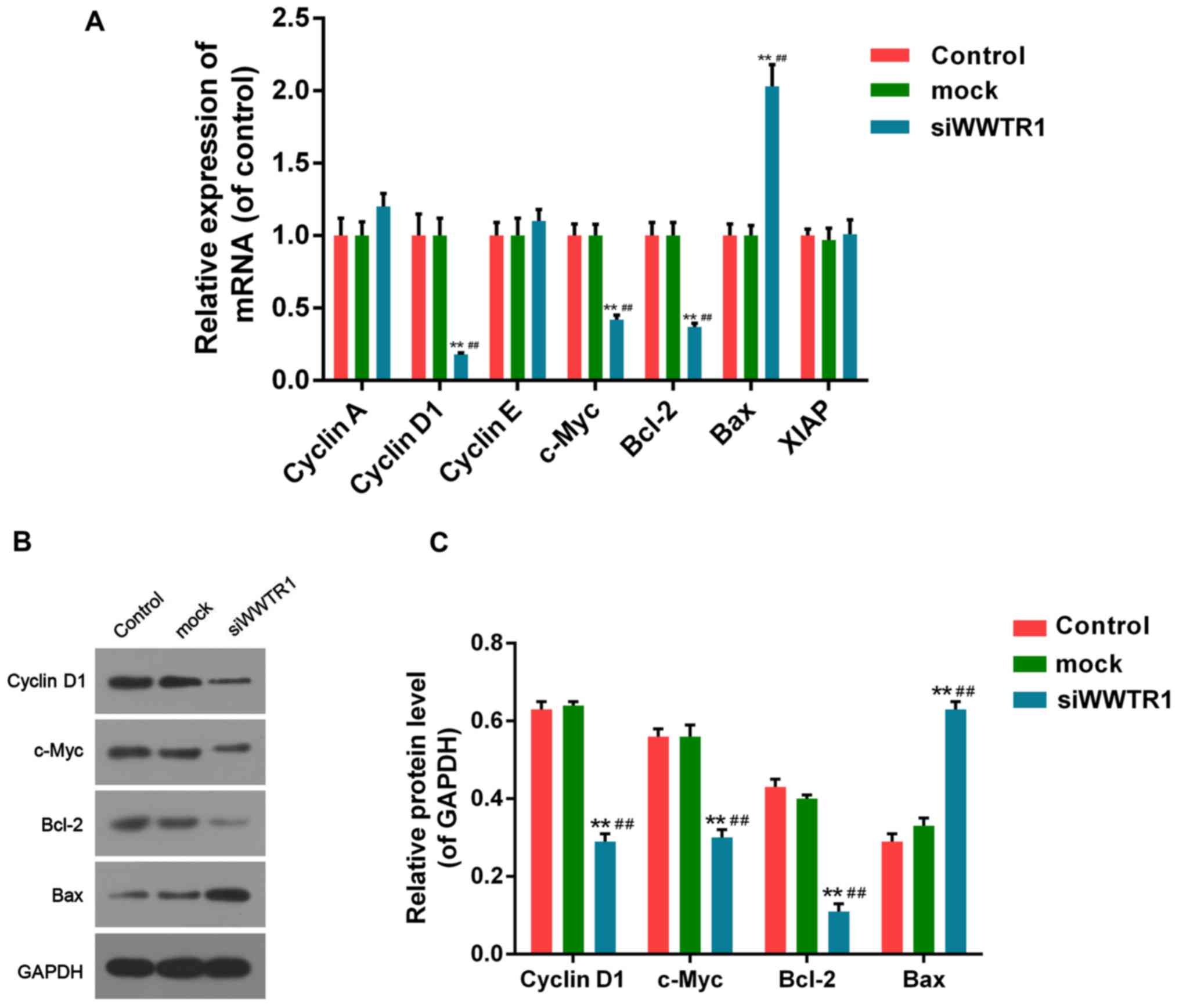 | Figure 4.Effect of siRNA-WWTR1 on expression of
cell cycle and apoptosis related factors. (A) RT-PCR was conducted
for mRNA expression detection of cell cycle factors such as Cyclin
A, Cyclin D1, Cyclin E, c-Myc, and apoptosis related factors such
as Bcl-2, XIAP and Bax, in SGC7901 cells after siRNA-WWTR1
transfection for 48 h. (B and C) The protein levels of Cyclin D1,
c-Myc, Bcl-2 and Bax in SGC7901 cells were analyzed by western
blotting after 48 h of siRNA-WWTR1 treatment. GAPDH was detected as
the control of sample loading. Data were presented as mean ± SD,
n=6, **P<0.01 vs. control; ##P<0.01 vs. mock.
WWTR1, WW-domain containing transcription regulator 1; Bcl-2, B
cell lymphoma/leukemia-2; XIAP, X-linked inhibitor of apoptosis;
Bax, Bcl-2 associated X protein; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase; c-Myc, cancer Myc. |
Effect of siRNA-WWTR1 on WWTR1
downstream effectors in SGC7901
Regardless of Hippo pathway, we explored the role of
WWTR1 on the downstream effectors in other signaling pathways like
TGF-β/Smad pathway and so on. RT-PCR and western blotting were
performed to assess the expression of WWTR1 downstream factors such
as ASNS, ID1, SMAD3 and BTC, reported important in tumors. The
expression of ASNS was decreased notably, while ID1, SMAD3 and BTC
increased significantly in siWWRT1-interferring cells compared with
control and mock cells, both in RNA and protein manners (Fig. 5).
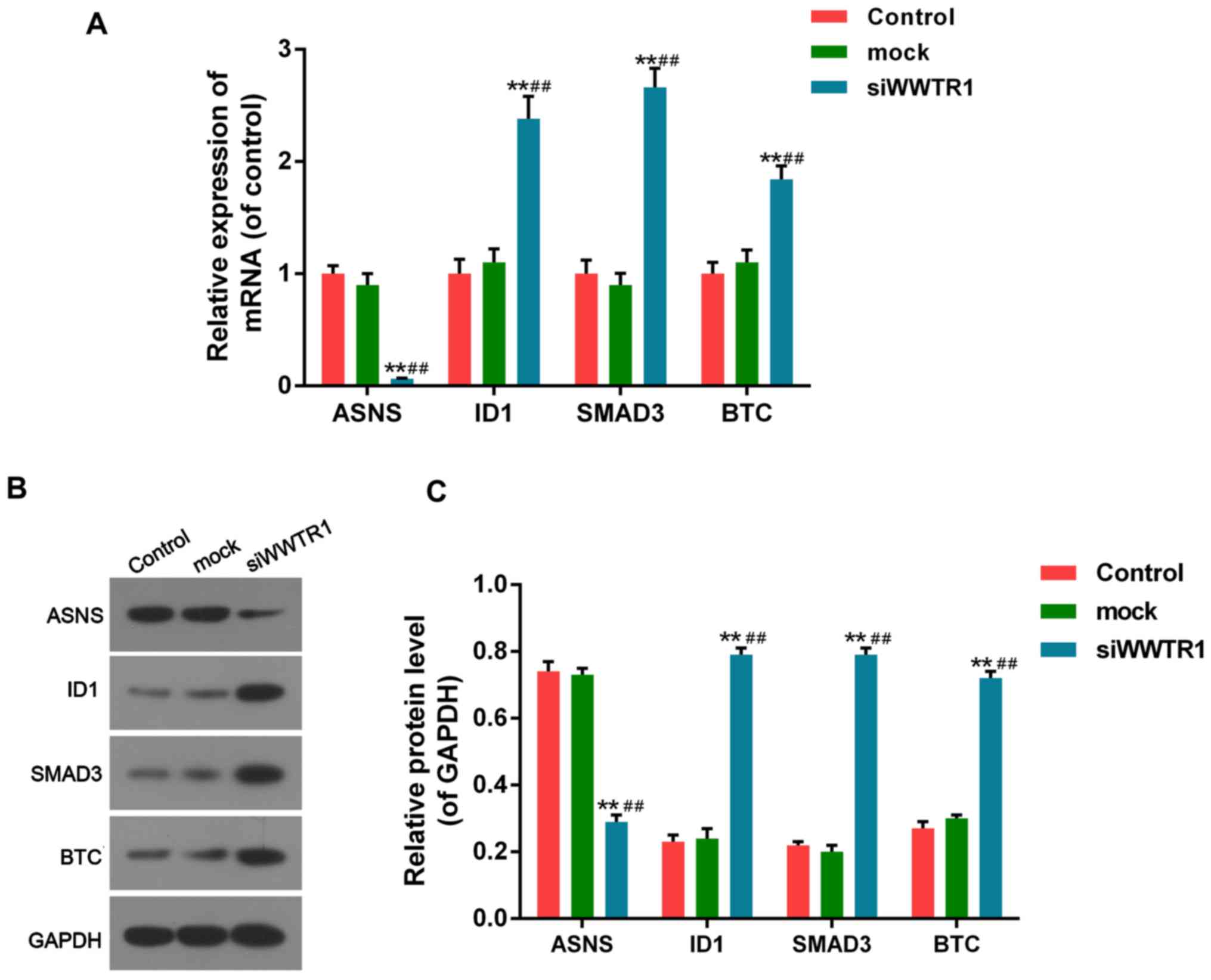 | Figure 5.Effect of siRNA-WWTR1 on expression of
WWTR1 downstream factors. (A) After siRNA-WWTR1 transfection for 48
h, the mRNA expression of ASNS, ID1, SMAD3 and BTC were measured by
RT-PCR. (B and C) ASNS, ID1, SMAD3 and BTC protein expression were
determined by western blotting after siRNA-WWTR1 transfection for
48 h. GAPDH was also detected as the control of sample loading.
Data were presented as mean ± SD, n=6, **P<0.01 vs. control;
##P<0.01 vs. mock. WWTR1, WW-domain containing
transcription regulator 1; ASNS, asparagine synthetase
(glutamine-hydrolyzing); ID1, inhibitor of DNA binding 1, HLH
protein; SMAD3, mothers against decapentaplegic homolog family
member 3; BTC, betacellulin; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
Discussion
Being the second widely diagnosed malignancy in the
world, most of GCs are diagnosed at late stage due to the poor
diagnosis in early stage. The development and metastasis of tumor
is a multi-step process including lots of genetic variations.
Apoptosis is a process of programmed cell death that occurs in
multicellular organisms (12).
Decreased apoptosis causes uncontrolled cell proliferation, such as
tumor (13). As tumor
proliferation and inhibited apoptosis are main causes of malignant
progression, it is essential and possible to discover new
therapeutic methods and related functional molecules.
WWTR1 mainly acts downstream of the Hippo pathway as
a transcription co-activator (14). It is also regarded as a
cancer-related gene and increased WWTR1 protein levels have been
found in some cancers (15).
Although previous studies have shown that WWTR1 functioned as an
oncogene in GC, the mechanism of WWTR1 modulating GC cell
proliferation and apoptosis is still unclear.
In the present study, we explored that the
expression of WWTR1 in most of GC tissues and cells exactly
increased notably. SGC7901 was selected for further research for
the significant increasing of WWTR1 mRNA and protein compared with
GES1 (control group). Certainly the study on BGC823 which presented
highest protein level maybe also an interesting research project,
we would keep an eye on it and investigate the molecular mechanism
in it in future. For the elevated levels of WWTR1, we chose
silencing as the strategy to study more. As WWTR1 levels improved
not too much in some cell lines like MKN45, maybe we could also do
more research on it by over-exprssion strategy next time.
As results demonstrated, siRNA-WWTR1 effectively
inhibited the vitality of the cells. Then we explored the function
of siWWTR1 on cell cycle progression and apoptosis, finding siWWTR1
inhibiting cell proliferation, blocking cell cycle transition from
G1 to S phase, and promoting apoptosis significantly. We also
provided evidence that the mechanism underlying the above effects
was related to the adjustment of cell cycle and apoptosis-related
factors.
Cyclin D1 is an important factor in cell G1
progressing (16). C-Myc encodes
phosphorylated proteins, which facilitate cell proliferation and
differentiation (17). Bcl-2
activates caspase pathway, facilitates cell mitosis and inhibits
apoptosis by dissolving protein structures, resulting in tumor
proliferation. On the contrary (18), Bax can facilitate apoptosis by
preventing function of Bcl-2 as forming heterodimer with it. Our
research found that siWWTR1 suppressed expression of Cyclin D1,
c-Myc, Bcl-2 and increased expression of Bax, which could lead to
interference of cell proliferation, cell cycle blocking and
apoptosis promoting. Wang et al (9) found that Cyclin A and connect tissure
growth factor (CTGF) levels were inhibited by siWWTR1 in non-small
cell lung cancer cells, but the levels of Cyclin D1, Cyclin E,
c-Myc, Bcl-2, Bax and XIAP did not change too much. Though a little
different with our results, it is reasonable for different
diseases.
Regardless of Hippo pathway, we explored the role of
WWTR1 on the downstream effectors in other signaling pathways like
TGF-β/Smad and so on in SGC7901. The family of TGF-β can adjust
cell proliferation or differentiation, apoptosis and migration. The
biological function depends on the normal signaling pathway. The
dysregulation of them can result in tumor initiation and
development. Smad is a critical factor in TGF-β pathway (19,20),
Researches before had proved the genetic defect of it can induce
ubiquitylation-mediated degradation increase, related to colorectal
cancer (21,22). Id1 can promote cell proliferation
by inhibiting the expression of P21, the inhibitor of
cyclin-dependent kinase and promoting cell cycle transition from G1
to S phase (23,24). In our present study, we found that
the expression of ID1, SMAD3 increased remarkably in
siWWTR1-interferring cells compared to the control and mock cells,
both in RNA and protein manners. It indicated that WWTR1
participated in TGF-β pathway to enhance tumor proliferation by
inhibiting the expression of SMAD3 and ID1.
BTC is a-member of epidermal growth factor (EGF)
family. BTC can inhibit cell apoptosis by promoting the
phosphorylation of EGR receptor (EGFR) (25). EGFR is an important binding site of
targeted drug for cancers. In our present study, we found that the
expression of BTC increased significantly in siWWTR1-interferring
cells compared to the control and mock cells, both in RNA and
protein manners. The phenomenon occurred maybe due to the
inhibition of EGFR phosphorylation or blocking of EGFR downstream
pathway, which made BTC function impossible. Though it needs
proving furthermore, the result would supply novel basis for EGFR
targeting treatment.
ASNS is a member of aminotransferase family
(26). ASNS is related to G1-S
phase transition, promoting cell mitosis and proliferation. In our
study, the expression of ASNS was decreased notably in
siWWRT1-interferring cells compared to the control and mock cells
in RNA and protein manners, indicating WWTR1 may positive regulate
ASNS expression which related to cell proliferation and tumor
development. It indicated that WWTR1 may regulate cell
proliferation not only by Hippo pathway, but also related to TGF-β
pathway including SMAD3 and ID1, and factors in other pathways like
ASNS and BTC.
In conclusion, our study showed that WWTR1 was
highly expressed in human GC tissues/cells. Furthermore,
siRNA-WWTR1 can notably inhibit cell proliferation, block cell
cycle, and promote apoptosis of SGC7901 cells by regulating
expression of cycle and apoptosis-related factors such as Cyclin
D1, c-Myc, Bcl-2 and Bax. And the role of WWTR1 could be related to
TGF-β pathway including SMAD3 and ID1, and factors in other
pathways like ASNS and BTC. It may provide novel effective
therapeutic strategy for GC.
References
|
1
|
Hamashima C: Current issues and future
perspectives of gastric cancer screening. World J Gastroentero.
20:13767–13774. 2014. View Article : Google Scholar
|
|
2
|
Hamashima C, Sasazuki S, Inoue M and
Tsugane S: JPHC Study Group: Receiver operating characteristic
analysis of prediction for gastric cancer development using serum
pepsinogen and Helicobacter pylori antibody tests. BMC Cancer.
17:1832017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): A phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang S, Ji M, Zhang L, Chen Y, Wennmann
DO, Kremerskothen J and Dong J: Phosphorylation of KIBRA by the
extracellular signal-regulated kinase (ERK)-ribosomal S6 kinase
(RSK) cascade modulates cell proliferation and migration. Cell
Signal. 26:343–351. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujiwara S, Ida H, Yoshioka Y, Yoshida H
and Yamaguchi M: The warts gene as a novel target of the Drosophila
DRE/DREF transcription pathway. Am J Cancer Res. 2:36–44.
2012.PubMed/NCBI
|
|
6
|
Dong J, Feldmann G, Huang J, Wu S, Zhang
N, Comerford SA, Gayyed MF, Anders RA, Maitra A and Pan D:
Elucidation of a universal size-control mechanism in Drosophila and
mammals. Cell. 130:1120–1133. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu S, Huang J, Dong J and Pan D: Hippo
encodes a Ste-20 family protein kinase that restricts cell
proliferation and promotes apoptosis in conjunction with salvador
and warts. Cell. 114:445–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu
J, Lin JD, Wang CY, Chinnaiyan AM, et al: TEAD mediates
YAP-dependent gene induction and growth control. Genes Dev.
22:1962–1971. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Chen Z, Wang Y, Chang D, Su L, Guo
Y and Liu C: TR1 promotes cell proliferation and inhibits apoptosis
through cyclin A and CTGF regulation in non-small cell lung cancer.
Tumour Biol. 35:463–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Justice RW, Zilian O, Woods DF, Noll M and
Bryant PJ: The Drosophila tumor suppressor gene warts encodes a
homolog of human myotonic dystrophy kinase and is required for the
control of cell shape and proliferation. Genes Dev. 9:534–546.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Attisano L and Wrana JL: Signal
integration in TGF-β, WNT, and Hippo pathways. F1000Prime Rep.
5:172013. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Böhm I and Schild H: Apoptosis: The
complex scenario for a silent cell death. Mol Imaging Biol. 5:2–14.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhu M, Li W, Dong X, Chen Y, Lu Y, Lin B,
Guo J and Li M: Benzyl-isothiocyanate induces apoptosis and
inhibits migration and invasion of hepatocellular carcinoma cells
in vitro. J Cancer. 8:240–248. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang Y, Xue C, Cui H and Huang Z: High
expression of TAZ indicates a poor prognosis in retinoblastoma.
Diagn Pathol. 10:1872015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Edgar BA: From cell structure to
transcription: Hippo forges a new path. Cell. 124:267–273. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang P, Liu S, Cheng B, Wu XZ, Ding SS, Xu
L, Liu Y, Duan L and Sun SZ: Promoting effect of cyclin D1
overexpression on proliferation and epithelial mesenchymal
transition of cervical squamous cell carcinoma SiHa cells. Zhonghua
Bing Li Xue Za Zhi. 46:187–192. 2017.(In Chinese). PubMed/NCBI
|
|
17
|
Zhou L, Wu F, Jin W, Yan B, Chen X, He Y,
Yang W, Du W, Zhang Q and Guo Y: Theabrownin inhibits cell cycle
progression and tumor growth of lung carcinoma through
c-myc-related mechanism. Front Pharmacol. 8:752017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sims EK, Lakhter AJ, Anderson-Baucum E,
Kono T, Tong X and Evans-Molina C: MicroRNA 21 targets BCL2 mRNA to
increase apoptosis in rat and human beta cells. Diabetologia.
60:1057–1065. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luwor RB, Hakmana D, Iaria J, Nheu TV,
Simpson RJ and Zhu HJ: Single live cell TGF-β signalling imaging:
Breast cancer cell motility and migration is driven by
sub-populations of cells with dynamic TGF-β-Smad3 activity. Mol
Cancer. 14:502015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai F, Lin X, Chang C and Feng XH: Nuclear
export of Smad2 and Smad3 by RanBP3 facilitates termination of
TGF-beta signaling. Dev Cell. 16:345–357. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu J and Attisano L: Mutations in the
tumor suppressors Smad2 and Smad4 inactivate transforming growth
factor beta signaling by targeting Smads to the
ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 97:pp.
4820–4825. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Petersen M, Pardali E, van der Horst G,
Cheung H, van den Hoogen C, van der Pluijm G and Ten Dijke P: Smad2
and Smad3 have opposing roles in breast cancer bone metastasis by
differentially affecting tumor angiogenesis. Oncogene.
29:1351–1361. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu Y, Liang Y, Yin C, Liu X, Su Y, Zhang L
and Wang H: Inhibitor of DNA-binding 1 promotes endothelial
progenitor cell proliferation and migration by suppressing E2-2
through the helix-loop-helix domain. Int J Mol Med. 38:1549–1557.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao Y, Luo A, Li S, Zhang W, Chen H, Li
Y, Ding F, Huang F and Liu Z: Inhibitor of differentiation/DNA
binding 1 (ID1) inhibits etoposide-induced apoptosis in a
c-Jun/c-Fos-dependent manner. J Biol Chem. 291:6831–6842. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Singh B, Carpenter G and Coffey RJ: EGF
receptor ligands: Recent advances. F1000Res. 5:pii: F1000 Faculty
Rev–2270. 2016. View Article : Google Scholar
|
|
26
|
Xu Y, Lv F, Zhu X, Wu Y and Shen X: Loss
of asparagine synthetase suppresses the growth of human lung cancer
cells by arresting cell cycle at G0/G1 phase. Cancer Gene Ther.
23:287–294. 2016. View Article : Google Scholar : PubMed/NCBI
|