Introduction
The incidence of uremia (urea in the blood) has
exhibited an increasing trend following increasing in living
standards and lifestyle changes. Uremia is often accompanied by
high mortality and complications that severely threaten health
(1–3). Hemodialysis and purification is one
of the main methods for treatment of uremia. Hemodialysis uses
dialysis membrane to exchange or eliminate small molecules from
patient blood and dialysate, to return the blood components of
normal level (4,5). With the improvement of science and
healthcare, hemodialysis and purification technology has
significantly reduced the mortality rate of uremia and improved
patient quality of life. However, hemodialysis and purification
also cause infection, which seriously restricts uremia treatment
(6–8). Therefore, clarification of the
molecular mechanism that are induced in patients with uremia
receiving hemodialysis, and taking effective counter measures, is
urgently required (9).
Previous studies reported that abnormal protein
factors in the serum of patients with uremia may be associated with
cardiovascular complications, malnutrition and high mortality
(10–12). Furthermore, it was revealed that
inflammation was closely associated with uremia occurrence,
development and prognosis (13).
Inflammatory status in uremic patient was improved after
hemodialysis, suggesting that may be associated with serum
inflammatory factors level (14).
Hemodialysis and purification are the important
methods for the treatment of end-stage renal disease. Maintenance
hemodialysis (MHD) increases the survival of patients with uremia
(15–17). However, the incidence of
complications gradually increased following hemodialysis and
purification, suggesting that inflammation may have an important
role in occurrence and development of complications following MHD
(18–20). Inflammation is often accompanied by
abnormal organ functions and changes in hepatic protein levels.
Specifically, C-reactive protein (CRP), interleukin-2 (IL-2)
(21) and tumor necrosis factor-α
(TNF-α) (21–24) are important serum markers of
inflammation.
Thus, the current study detected CRP, IL-2 and TNF-α
levels in patients with uremia receiving hemodialysis, aiming to
improve the effectiveness of hemodialysis and provide valuable
information for to reduce inflammation.
Materials and methods
Experimental subjects and
grouping
Patients with uremia were enrolled according to the
following inclusion (25) and
exclusion (26) criteria: i)
Hemodialysis for 6 months or longer; ii) no blood transfusion or
hemorrhage in 6 months; iii) no infection, trauma, surgery or tumor
in previous 2 months; iv) no other types of blood system disease.
The causes of abnormal renal function and the number of cases were
as follows: 2 refluxnephropathy, 4 gout kidney disease, 4
interstitial nephritis and chronic nephropyelitis, 10 polycystic
kidney, 72 chronic glomerulonephritis, four hypertensive renal
arteriolar sclerosis, 20 diabetic nephropathy, the remaining
patients had ≥one of the above complications. Patients with uremia
(n=200) receiving continuous high throughput blood purification
along with the hospital-acquired infection, as determined by
routine tests in the hospital, in the First Affiliated Hospital of
Henan University of Science and Technology (Luoyang, China) from
August 2013 and August 2015 were enrolled in the study.
Additionally, 200 healthy volunteers were selected as control
group. The basic patient information is listed in Table I. There were 154 male and 46 female
patients with uremia, with mean age 47.4±12.3 (18–70) years old.
The mean age of the control group (healthy volunteers) was
48.3±13.2 (18–70) years old. Experimental protocols were submitted
to and approved by the ethics committee of the First Affiliated
Hospital of Henan University of Science and Technology. Written
informed consent was provided.
 | Table I.Basic patient information. |
Table I.
Basic patient information.
| Group | Number of cases | Male (%) | Mean age |
|---|
| Test | 200 | 77 |
47.4±12.3 |
| Control | 200 | 77 |
48.3±13.2 |
Therapeutic method
In 200 patients receiving hemodialysis, 58 patients
received hemodialysis six times per week (24 h). Regular dialysis
was maintained for 6 months or longer, and Kt/V >1.2. The
treatment was in accordance with the clinical practice guidelines
of renal anemia (27). Human
erythropoietin was injected at 8,000-12,000 IU per week according
to the hemoglobin level. Fresenius (F8) and Baxter dialyzators were
used for hemodialysis. Bicarbonate was the dialysate, flow rate was
600 ml/min and the blood flow was 400–600 ml/min.
Blood sample collection
Blood samples were collected according to the
regular method (28). Fasting
venous blood was extracted from 200 patients with uremia for
detection of serum inflammatory factors. Following hemodialysis and
purification, 5 ml blood was collected and incubated at 37°C for 30
min. Then the supernatant was collected after centrifuged at 1,500
× g for 8 min at 4°C. Part of the serum was used for ELISA
detection, and the other was used for reverse transcription
(RT)-polymerase chain reaction (PCR). All the procedures were
completed within 60 min. Blood was collected on the day prior to
hemodialysis in the F group and 8 months post-hemodialysis in the R
group.
Reagents and primers
IL-2 (cat no. 04-1584), TNF-α (cat no. T8300), CRP
(cat no. C1688) and β-actin (cat no. A2228) antibodies were
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
IL-2, TNF-α, CRP primers were as follows: IL-2, forward
5′-TGTCCAGATGTAAGTAATAAAACAGAACA-3′ and reverse
5′-CAGAATGTAAGTAATGTCAAATCAGAACA-3′; TNF-α, forward
5′-AACACCTCTTTACAGTGACCAATGCCCCA3′ and reverse
5′ACAGTGACTAATTTCCAACACCTGCCCCA3′; CRP, forward
5′-TTACAGTGACCAACACCTCTAATGCCCCA-3′ and reverse
5′CGTGAAACACCTACACTAATTCTGCCCCA3′; β-actin, forward
5′-CACCAACTGGGACGACAT-3′ and reverse 5′-ACAGCCTGGATAGCAACG-3′.
The primers were designed and synthesized by
Shanghai GenePharma Co., Ltd. (Shanghai, China).
ELISA
ELISA was performed to determine the level of serum
inflammatory factors IL-2 (cat no. ab46054; Abcam, Cambridge, UK),
TNF-α (cat no. ab46087; Abcam), CRP (cat no. ab99995; Abcam) in
serum (18). The antibody was
diluted to 5 µg/ml in coating buffer and added to each well at 0.1
ml. After water bathed at 37°C for 2 h, the plate was washed in
washing buffer six times. Subsequently, 0.1 ml sample was added at
37°C for 60 min, then the plate was washed in washing buffer for
six times and the absorbance was measured at 490 nm with a
microplate reader.
RT-PCR
RT-PCR was performed to determine the mRNA levels of
inflammatory factors using standard procedures (17). Total RNA was isolated using TRIzol
reagent followed by reversely transcription into cDNA using a
Universal RT-PCR kit (Beijing Dingguo Biotechnology Co., Ltd.,
Beijing, China). Briefly, the mixture from Universal RT-PCR kit was
added to the isolated RNA and then placed at 25°C for 10 min
followed by incubation at 42°C for 1 h. After that, the cDNA was
denatured at 95°C and placed on ice for PCR analysis. The reaction
system contained 2 µl cDNA solution, 2.5 µl 10X PCR Buffer, 2.5 µl
dNTP mixture (2 mM), 0.5 µl primer 1 (10 µM), 0.5 µl primer 2 (10
µM), 0.5 µl Taq DNA Polymerase, 2.5 µl MgCl2 (25 mM)
(all from Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 14
µl H2O. The reaction conditions were as follows: 94°C
for 7 min, followed by 30 cycles of 94°C for 30 sec, 50°C for 60
sec and 72°C for 30 sec, and a final step of 72°C for 5 min. Image
J software version 1.4 (National Institutes of Health, Bethesda,
MD, USA) was used to semi-quantify the RT-PCR bands. The relative
expression of inflammatory factor genes was calculated by the ratio
of the band grey of the inflammatory factors and actin.
Western blot analysis
Western blot was performed to detect inflammatory
factor protein level in blood samples (16). Briefly, total protein was extracted
using radioimminoprecipitation lysis buffer (Thermo Fisher
Scientific, Inc.) and was quantified using a bicinchoninic acid
assay. A total of 40 µg per lane protein was loaded into 10%
SDS-PAGE, followed by transferring to polyvinylidene difluoride
membrane and then blocked with 5% defatted milk powder for 2 h at
room temperature. Primary antibodies against IL-2 (cat no: EPR2780;
Abcam), TNF-α cat no: ab9635; Abcam, CRP (cat no. ab50861; Abcam)
or β-actin (cat no. ab8227; Abcam) (1:1,000 or 1:2,000 dilutions)
were added overnight at 4°C. After PBST (0.1% Tween-20) washing,
goat anti-rabbit (cat no. ab97051; Abcam) or rabbit anti-mouse
secondary antibodies (cat no. ab6728; Abcam) (1:5,000) were then
added and incubated in the dark at room temperature for 30 min.
Enhanced Chemiluminescence (Thermo Fisher Scientific, Inc.) reagent
was then used to develop the membrane for 1 min, followed by X-ray
exposure and observation. Image J software version 1.4 was used to
analyze western blot bands. Inflammatory factors protein relative
expression was calculated by the ratio of the band grey of
inflammatory factors and actin.
Statistical analysis
SPSS13.0 was used for statistical analysis. All data
was presented as the mean ± standard deviation. One-way analysis of
variance was performed to assess the statistical significance among
multiple treatment groups followed by Least Significant Difference
test as the post hoc test. Pearson correlation was conducted for
the correlation analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
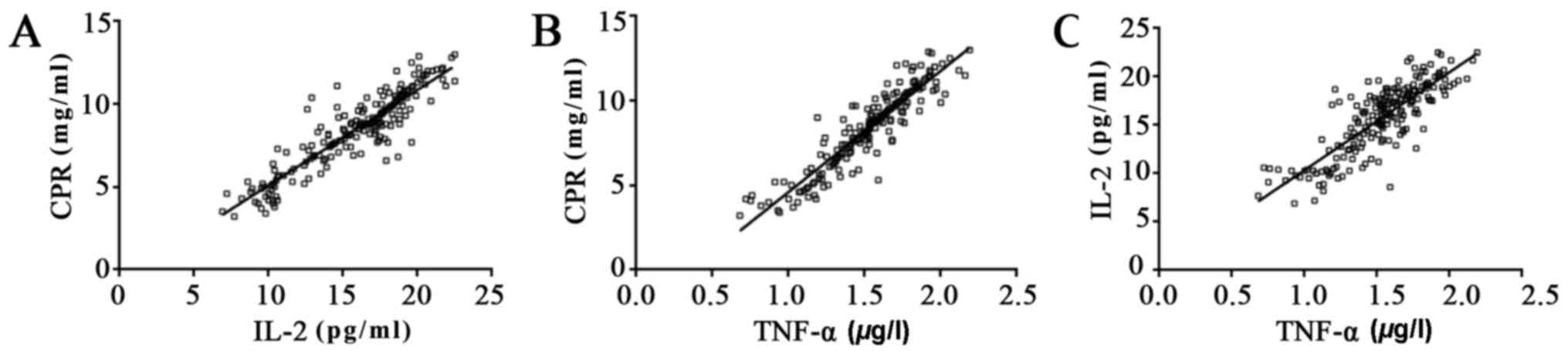
Correlation analysis of IL-2, TNF-α
and CRP
In samples taken from 200 uremia patients prior to
dialysis, ELISA data indicated that the IL-2 level was positively
correlated with CRP (r2=0.8245; P<0.05), TNF-α was
positively correlated with CRP (r2=0.8513; P<0.05)
and TNF-α was positively correlated with IL-2 (r2=0.684;
P<0.05), which indicated that IL-2, TNF-α and CRP may be useful
as bio-markers in the diagnosis of uremia (Fig. 1).
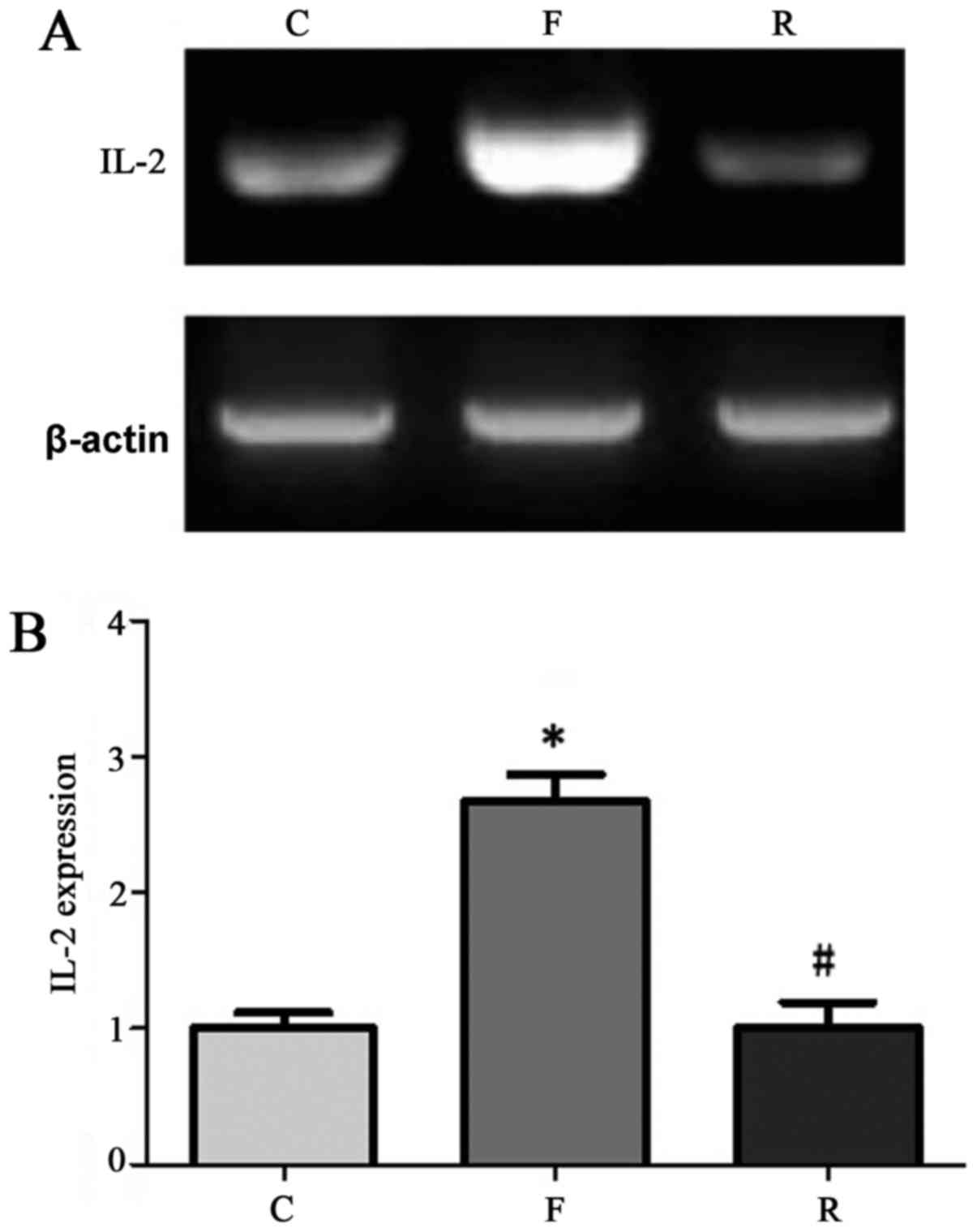
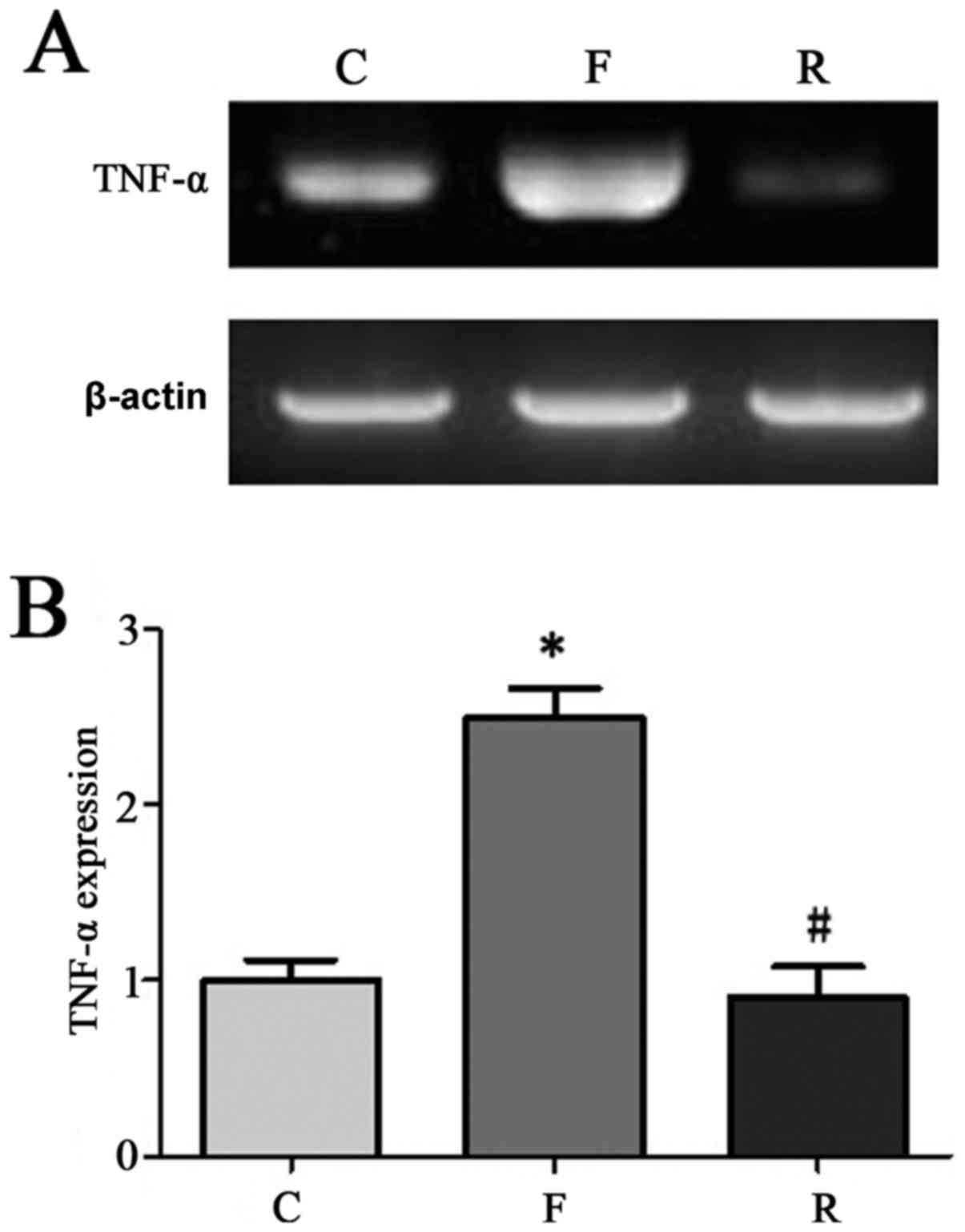
Serum IL-2, TNF-α, and CRP mRNA
expression
We randomly selected 90 samples among the 200
patients for RT-PCR detection. The results indicated that CRP, IL-2
and TNF-α levels were significantly lower at 8 months after
hemodialysis compared to before treatment (P<0.05; Figs. 2–4). CRP, IL-2, and TNF-α levels in
patients with uremia at 8 months after hemodialysis were similar
with that in normal control.
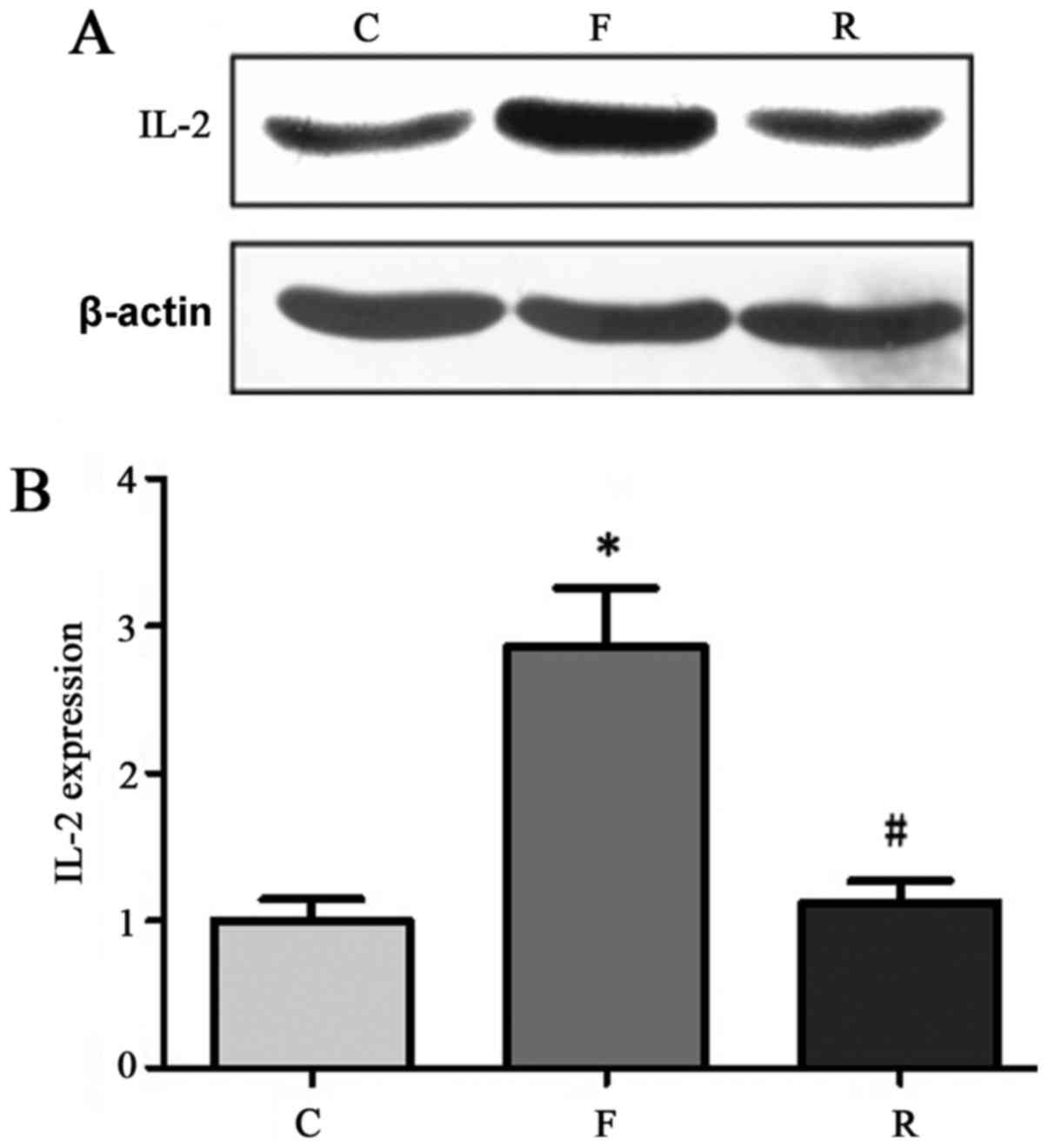
Serum IL-2, TNF-α, and CRP protein
expression
ELISA was conducted to analyze blood samples from
200 patients with uremia and controls. The results indicated that
CRP, IL-2, and TNF-α levels were reduced at 8 months after
hemodialysis compared with before treatment. CRP, IL-2, and TNF-α
levels in uremia patients at 8 months after hemodialysis were
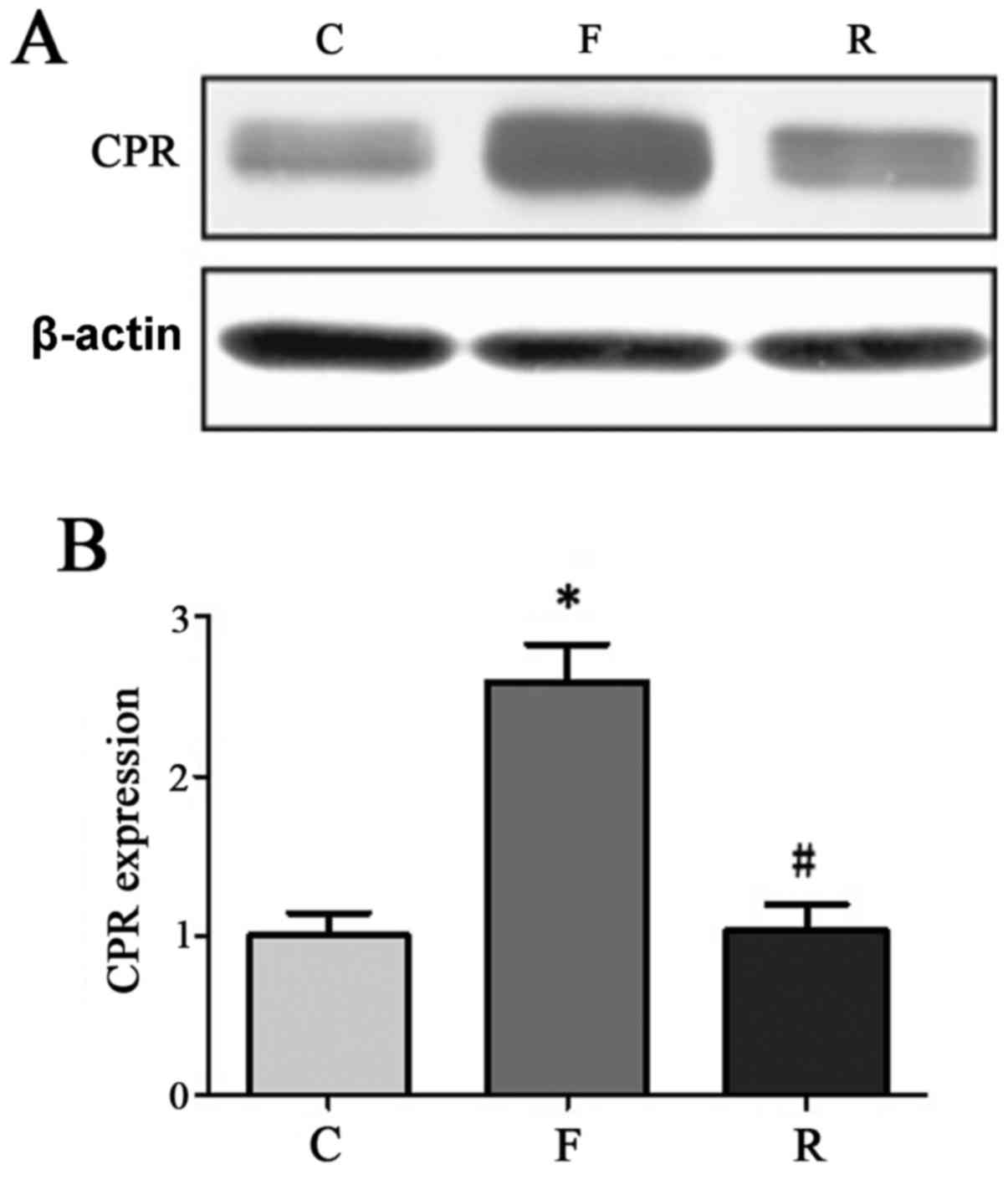
similar to that in normal control samples (Table II). Western blot analysis
validated this further. In 30 randomly-selected samples from the
200 patients CRP, IL-2 and TNF-α protein levels were significantly
decreased at 8 months after hemodialysis compared with before
treatment (P<0.05; Figs.
5–7).
 | Table II.Serum IL-2, TNF-α, and CRP protein
expression comparison. |
Table II.
Serum IL-2, TNF-α, and CRP protein
expression comparison.
| Group | Cases | IL-2 (pg/ml) | TNF-α (µg/l) | CRP (mg/l) |
|---|
| Control | 200 |
6.2±1.9 |
0.64±0.32 |
2.8±0.8 |
| Pre-dialysis | 200 |
15.7±2.8a |
1.53±0.56a |
8.4±1.6a |
| Post-dialysis (8
months) | 200 |
6.9±2.2b |
0.69±0.41b |
3.1±1.1b |
Symptoms improvement comparison after
therapy
After 8 months of hemodialysis, the patient's
symptoms of uremia, sleep and appetite obviously improved. In
addition, other symptoms, such as hypertension, peripheral
neuropathy and renal bone disease were also improved to a certain
extent with some even being recovered.
Adverse reaction
During the 8 months of hemodialysis, six cases
exhibited perspiration and precordial discomfort at 30 min after
hemodialysis treatment. For all these six cases, this may be the
first time a new dialyzer replacement was performed. After
administration with hypertonic glucose and dexamethasone, the
symptoms of these six patients were effectively relieved. No other
symptoms appeared during the following treatment period. Most
patients showed good tolerability with no other adverse reactions.
No patients opted out of the experiment over the whole treatment
process.
Discussion
Uremia is a serious threat human health (1). Infection often occurs during the
process of hemodialysis, which can aggravates patient conditions
(3). It is necessary to
investigate the levels serum inflammatory factors to improve the
effect of hemodialysis and provide valuable information for
developing anti-inflammatory treatments.
It was previous revealed that various metabolites
are abnormally accumulated in the serum of patients with uremia
(16,17). Hemodialysis and purification can
alleviate the abnormal accumulation of metabolites maintain normal
concentrations in the blood (18),
suggesting that abnormal accumulation of serum metabolites may be
important factors involved in uremia and dialysis-associated
complications. The results of the current study suggested that CRP,
IL-2 and TNF-α levels were lower at 8 months after hemodialysis
than before treatment, and the difference was statistically
significant. Additionally, CRP, IL-2 and TNF-α levels in patients
with uremia at 8 months after hemodialysis were similar to that in
normal control samples. CRP expression in patients with uremia was
positively correlated with IL-2 and TNF-α expression levels.
There were two important findings in the current
study. Firstly, patients with uremia that received MHD and
purification combined with hospital infection exhibited increased
levels of inflammatory factors in their serum compared with the
control group. Additionally, high throughput dialysis and
purification significantly reduced serum CRP, IL-2, and TNF-α
levels.
A previous study confirmed that uremia is often
accompanied by a chronic inflammatory response (12). We speculated that inflammatory
cytokines level may change. The results suggested that serum CRP,
IL-2 and TNF-α levels were elevated prior to hemodialysis compared
with the control group, which was in accordance with the previous
research (16). The potential
mechanism is that decreased immune function together with
peritoneal access or vasodilation in patients with uremia leads to
bacterial contamination and a series of complex responses.
Subsequently, the complement system is quickly activated, resulting
in an inflammatory response.
The current study has various limitations. The
number of patients enrolled in the study, was limited because of
time and other factors, and it may affect the reliability of the
results. Additionally, certain studies have reported that
inflammation in the dialysis patients is mainly induced by the type
of dialysis membrane (29,30); the association between the
different dialysis membranes and the inflammation factors was not
investigated in this study. Furthermore, the present study did not
investigate the chronic inflammatory state for enrolled patients.
These require attention in future studies.
In conclusion, uremia patients receiving MHD with
hospital-acquired infection had increased serum inflammatory
factors. Following high throughput hemodialysis, the levels of CRP,
IL-2 and TNF-α were significantly decreased in patient serum,
suggesting high throughput hemodialysis may be beneficial for
prevention of the infections in uremia patients.
References
|
1
|
Casimiro de Almeida J, Lou-Meda R, Olbert
M, Seifert M, Weiss G, Wiegerinck ET, Swinkels DW, Solomons NW and
Schümann K: The Growth Attainment, Hematological, Iron Status and
Inflammatory Profile of Guatemalan Juvenile End-Stage Renal Disease
Patients. PLoS One. 10:e01400622015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Naini AE, Asiabi RE, Keivandarian N and
Moeinzadeh F: Effect of omega-3 supplementation on inflammatory
parameters in patients on chronic ambulatory peritoneal dialysis.
Adv Biomed Res. 4:1672015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weng CH, Hu CC, Yen TH and Huang WH:
Association between ambient carbon monoxide and secondary
hyperparathyroidism in nondiabetic patients undergoing peritoneal
dialysis. Ther Clin Risk Manag. 11:1401–1408. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poesen R, Ramezani A, Claes K, Augustijns
P, Kuypers D, Barrows IR, Muralidharan J, Evenepoel P, Meijers B
and Raj DS: Associations of soluble CD14 and endotoxin with
mortality, cardiovascular disease, and progression of kidney
disease among patients with CKD. Clin J Am Soc Nephrol.
10:1525–1533. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Higuchi T, Abe M, Mizuno M, Yamazaki T,
Suzuki H, Moriuchi M, Oikawa O, Okawa E, Ando H and Okada K:
Association of restless legs syndrome with oxidative stress and
inflammation in patients undergoing hemodialysis. Sleep Med.
16:941–948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hashemian SJ, Rismanchi M, Esfahani EN,
Khoshvaghti A and Razi F: Effect of calcitriol supplementation and
tail suspension on serum biomarkers of bone formation in rats. J
Diabetes Metab Disord. 14:142015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kaysen GA, Johansen KL, Chertow GM,
Dalrymple LS, Kornak J, Grimes B, Dwyer T, Chassy AW and Fiehn O:
Associations of Trimethylamine N-Oxide with nutritional and
inflammatory biomarkers and cardiovascular outcomes in patients new
to dialysis. J Ren Nutr. 25:351–356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie LM, Ge YY, Huang X, Zhang YQ and Li
JX: Effects of fermentable dietary fiber supplementation on
oxidative and inflammatory status in hemodialysis patients. Int J
Clin Exp Med. 8:1363–1369. 2015.PubMed/NCBI
|
|
9
|
Hassan K, Hassan S, Anwar S, Zaher A,
Edgem R and Hassan F: Predictors of left ventricular hypertrophy
and their cutoffs in peritoneal dialysis patients. Int Heart J.
56:186–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mpio I, Cleaud C, Arkouche W and Laville
M: Results of therapeutics strategy of protein-energy wasting in
chronic hemodialysis: A prospective study during 12 months. Nephrol
Ther. 11:97–103. 2015.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao H, Ye H, Sun Z, Shen X, Song Z, Wu X,
He W, Dai C and Yang J: Circulatory mitochondrial DNA is a
pro-inflammatory agent in maintenance hemodialysis patients. PLoS
One. 9:e1131792014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang WH, Yen TH, Chan MJ and Su YJ:
Environmental carbon monoxide level is associated with the level of
high-sensitivity C-reactive protein in peritoneal dialysis
patients. Medicine (Baltimore). 93:e1812014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
do Sameiro-Faria M, Kohlova M, Ribeiro S,
Rocha-Pereira P, Teixeira L, Nascimento H, Reis F, Miranda V,
Bronze-da-Rocha E, Quintanilha A, et al: Potential cardiovascular
risk protection of bilirubin in end-stage renal disease patients
under hemodialysis. Biomed Res Int. 2014:1752862014.PubMed/NCBI
|
|
14
|
Hung AM, Booker C, Ellis CD, Siew ED,
Graves AJ, Shintani A, Abumrad NN, Himmelfarb J and Ikizler TA:
Omega-3 fatty acids inhibit the up-regulation of endothelial
chemokines in maintenance hemodialysis patients. Nephrol Dial
Transplant. 30:266–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mikolasevic I, Lukenda V, Racki S, Milic
S, Sladoje-Martinovic B and Orlic L: Nonalcoholic fatty liver
disease (NAFLD)-a new factor that interplays between inflammation,
malnutrition, and atherosclerosis in elderly hemodialysis patients.
Clin Interv Aging. 9:1295–1303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Janda K, Krzanowski M, Dumnicka P,
Kuśnierz-Cabala B, Kraśniak A and Sulowicz W: Transforming growth
factor beta 1 as a risk factor for cardiovascular diseases in
end-stage renal disease patients treated with peritoneal dialysis.
Clin Lab. 60:1163–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ockene IS, Matthews CE, Rifai N, Ridker
PM, Reed G and Stanek E: Variability and classification accuracy of
serial high-sensitivity C-reactive protein measurements in healthy
adults. Clin Chem. 47:444–450. 2001.PubMed/NCBI
|
|
18
|
Banerjee T, Kim SJ, Astor B, Shafi T,
Coresh J and Powe NR: Vascular access type, inflammatory markers,
and mortality in incident hemodialysis patients: The Choices for
healthy outcomes in caring for End-Stage renal disease (CHOICE)
study. Am J Kidney Dis. 64:954–961. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai MT, Hu FH, Lien TJ, Chen PJ, Huang TP
and Tarng DC: Interaction between geriatric nutritional risk index
and decoy receptor 3 predicts mortality in chronic hemodialysis
patients. Am J Nephrol. 40:191–199. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Viaene L, Meijers BK, Bammens B,
Vanrenterghem Y and Evenepoel P: Serum concentrations of p-cresyl
sulfate and indoxyl sulfate, but not inflammatory markers, increase
in incident peritoneal dialysis patients in parallel with loss of
residual renal function. Perit Dial Int. 34:71–78. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rivara MB, Ikizler TA, Ellis CD, Mehrotra
R and Himmelfarb J: Association of plasma F2-isoprostanes and
isofurans concentrations with erythropoiesis-stimulating agent
resistance in maintenance hemodialysis patients. BMC Nephrol.
16:792015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mustafar RB, Mohd R, Miswan NA, Bain A,
Cader R, Gafor AH, Mohammad M, Shah SA, Kamaruddin NA and Kong NC:
The effects of calcitriol with calcium carbonate supplementation on
inflammatory biomarkers in chronic kidney disease patients' with
low vitamin D. Cent Eur J Immunol. 39:236–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rivara MB, Mehrotra R, Linke L, Ruzinski
J, Ikizler TA and Himmelfarb J: A pilot randomized crossover trial
assessing the safety and short-term effects of pomegranate
supplementation in hemodialysis patients. J Ren Nutr. 25:40–49.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
de Vinuesa SG, Goicoechea M, Kanter J,
Puerta M, Cachofeiro V, Lahera V, Gómez-Campdera F and Luño J:
Insulin resistance, inflammatory biomarkers, and adipokines in
patients with chronic kidney disease: effects of angiotensin II
blockade. J Am Soc Nephrol. 17 12 Suppl:S206–S212. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saddadi F, Alatab S, Pasha F, Ganji MR and
Soleimanian T: The effect of treatment with N-acetylcysteine on the
serum levels of C-reactive protein and interleukin-6 in patients on
hemodialysis. Saudi J Kidney Dis Transpl. 25:66–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bossola M, Di Stasio E, Giungi S, Rosa F
and Tazza L: Fatigue is associated with serum interleukin-6 levels
and symptoms of depression in patients on chronic hemodialysis. J
Pain Symptom Manage. 49:578–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Van Buren PN, Lewis JB, Dwyer JP, Greene
T, Middleton J, Sika M, Umanath K, Abraham JD, Arfeen SS, Bowline
IG, et al: The phosphate binder ferric citrate and mineral
metabolism and inflammatory markers in maintenance dialysis
patients: Results from prespecified analyses of a randomized
clinical trial. Am J Kidney Dis. 66:479–488. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
As'habi A, Tabibi H, Hedayati M,
Mahdavi-Mazdeh M and Nozary-Heshmati B: Association of
malnutrition-inflammation score, dialysis-malnutrition score and
serum albumin with novel risk factors for cardiovascular diseases
in hemodialysis patients. Ren Fail. 37:113–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho Y, Hawley CM and Johnson DW: Clinical
causes of inflammation in peritoneal dialysis patients. Int J
Nephrol. 2014:9093732014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xing L: Effect of different dialysis
methods on cellular immunity function of maintenance haemodialysis
patients. West Indian Med J. 64:499–505. 2015.PubMed/NCBI
|