Introduction
Cerebral small vessel disease (CSVD) is an important
type of cerebrovascular diseases, which is the primary cause of
aged cognitive impairment and loss of function, and is closely
associated with the pathophysiology of apoplexy, dementia and aging
(1). In western countries, CSVD is
accountable for ~25% of the causes of ischemic strokes (1), and an additional study in China
indicated that 46% of strokes are due to lacunar infarct (2). In 2012, small vessel disease was
discussed at the International Apoplexy Conference and European
Apoplexy Conference (3), and CSVD
has gained attention from clinicians in departments of neurology
and imaging (4).
CSVD is comprised of lacunar infarction,
leukodystrophy and cerebral hemorrhage, and it is recognized that
CSVD is a vascular wall lesion caused by increasing age,
hypertension and vascular amyloidosis (5). It has been identified that there are
no concomitant clinical symptoms and signs with cerebral
microbleeds (5), while tissue
damage of corresponding parts caused by the increasing of cerebral
microbleeds may induce cognitive impairment (4). It has been identified that the
majority of patients with cerebral microbleeds present with a low
score on information processing speed and executive capacity,
particularly for those patients whose cerebral microbleeds were
present in the deep brain and region below the tentorium of the
cerebellum. In addition, it is dependent on the scale of white
matter lesion and ischemic brain death, which suggests that
cerebral microbleeds caused cognitive impairment (6).
Gap expansions around the blood vessel have been
associated with lacunar infarction and leukodystrophy (7). Previous studies have indicated that
the age, hypertension, volume of leukodystrophy, lacunar infarction
and the gap expansion surrounding the blood vessel should be
regarded as the MRI indicator of advanced CSVD (7,8). A
previous study indicated that progressive CSVD is associated with
decreasing cognitive impairment, particularly with non-verbal
inference and visual-spatial ability (9). This is possibly due to the enlarged
gaps around blood vessel breaking the nerve fibers of basal ganglia
region and central semiovale center which are associated with
cognitive functions (10).
Vascular endothelial growth factor (VEGF) is present
in the central nervous system and was the first identified mature
cellular growth factor (10). In
addition to stimulating the proliferation of endothelial cells, it
is able to inhibit cell apoptosis and maintain survival of nerve
cells (11). The signal transducer
and activator of transcription (STAT) family is a group of
transcription factor proteins including STAT1, STAT2, STAT3, STAT4,
STAT5 and STAT6. During the development process of the central
nervous system, STAT proteins are present in the neurons and
neurogliocytes. As important members of the STAT family, the
expression of STAT3 is markedly increased following cerebral
ischemic injury and the activity also increases when stimulated by
cytokines and growth factors (12). Following initiation of CSVD, the
injured brain tissue releases reactive oxygen species (ROS),
cytokines and chemokines, which can activate STAT3 through common
receptor glycoprotein 130 and the STAT3 signaling pathway undergoes
phosphorylation under stimulation by ROS, cytokines and chemokines
(13).
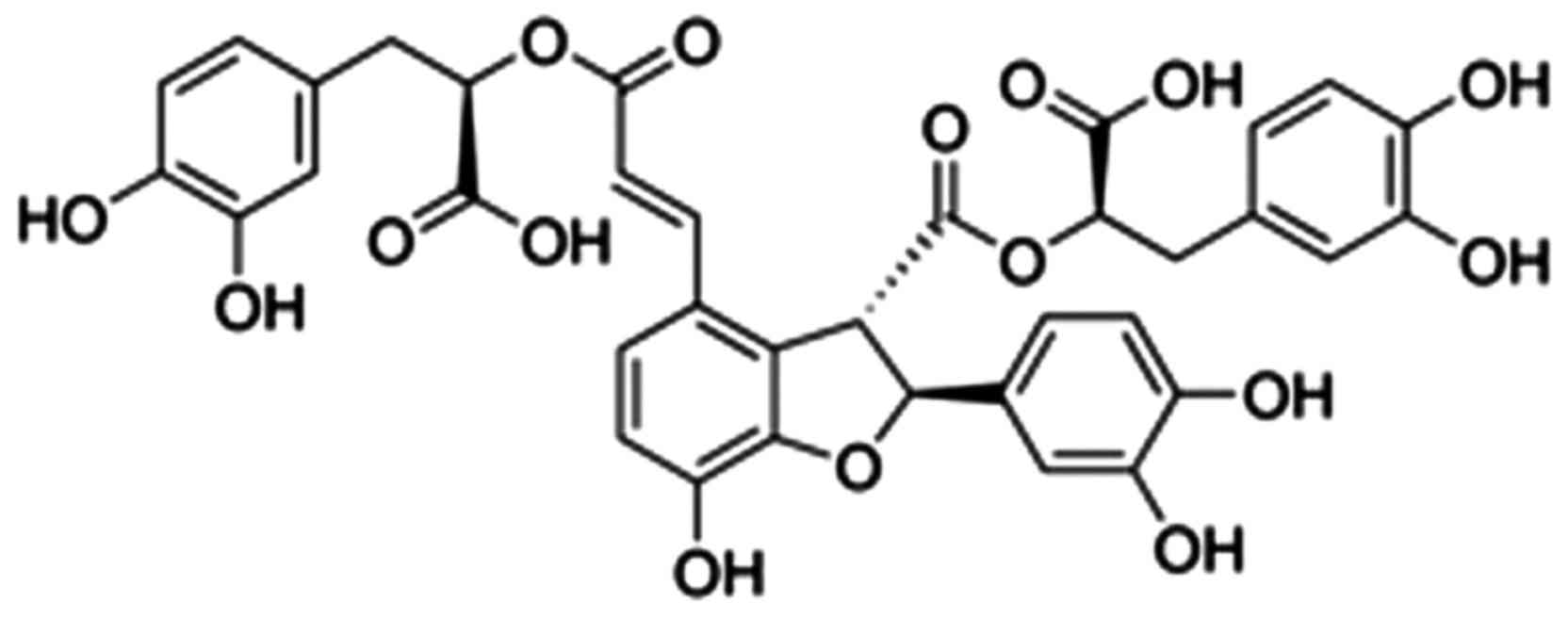
The present study indicated that salvianolic acids
were the most water-soluble in Salvia miltiorrhiza Bunge
(14). Salvianolic acid B
(Fig. 1) is the active ingredient
with the strongest biological activity in salvianolic acid
(15). A previous study has
identified that salvianolic acid can be used to protect against
myocardial ischemia/reperfusion injury, myocardial infarction and
cardiomyocyte hypertrophy (16).
It was suggested that in the early period of induction of newborn
rat myocaridial cell injury with lipopolysaccharides, the toll-like
receptor 4-nuclear factor κB-tumor necrosis factor (TNF)-α pathway
is rapidly activated and salvianolic acid B takes effect to protect
against myocardial cell injury, which is possibly mediated by
inhibition of this pathway (15,16).
Materials and methods
Animals
Male Sprague-Dawley rats (weight, 210–230 g; 8
weeks, n=6) and spontaneously hypertensive rats (SHR; n=20) were
purchased from Beijing Vital River Laboratory Animal Technology
Co., Ltd. (Beijing, China), and were maintained at a constant
temperature (23–24°C), humidity (50–55%) with a 12 h light/dark
cycle with free access to food and water. Male Sprague-Dawley rats
were assigned to sham (control) group (n=6), SHR were randomly
assigned to two groups: CSVD model group (n=10) and salvianolic
acid B treatment group (n=10). In the salvianolic acid B treatment
group, rats were treated with 80 mg/kg of salvianolic acid B
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 8 weeks. The
experiments of the current study were approved by the Medical
Ethics Committee of Beijing Chaoyang Hospital, Capital Medical
University (Beijing, China).
Behavioral tests
A circular pool (180×42 cm) was filled with water
(22±2°C) to a height of 35 cm. Briefly, rats were placed in the
arena and on the platform for 10 min on four consecutive days. The
pool was divided into quadrants and indicated by optical cues. A
transparent escape platform was placed at beneath the water surface
(2 cm) in the center of one quadrant. Rats were placed at a rotarod
drum and time (sec) of latency to fall from the rod, which was
measured three times per day was measured. Average latency was
calculated for each testing day.
Enzyme-linked immunosorbent assay
Peripheral blood was extracted from each rat and
serum was obtained subsequent to centrifugation at 2,000 × g for 10
min at 4°C. Serum was used to measure inflammation [TNF-α (H052),
interleukin (IL)-1β (H002), IL-6 (H007) and IL-18 (H015)] and
oxidative stress [superoxide dismutase (SOD, A001-1-1), catalase
(CAT, A007-1-1), glutathione (GSH, A006-2) and malondialydehyde
(MDA, A003-1)] using ELISA kits (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China).
Western blot analysis
Tissue samples were homogenized by adding lysis
buffer and a protease inhibitor and the supernatant was collected
following centrifugation at 13,800 × g for 10 min at 4°C. Total
protein concentration was determined using a bicinchoninic acid
protein assay (Beyotime Institute of Biotechnology, Nanjing,
China). The protein sample was separated by 6–10% SDS-PAGE and
transferred onto a polyvinylidenedifluoride membrane (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Membrane was blocked with
5% nonfat milk in Tris-buffered saline with 0.1% Tween 20 (TBS-T)
for 1 h at room temperature and were incubated with the following
primary antibodies: Caspase-3 (sc-98785, 1:1,000), Bcl-2-associated
X protein (Bax; sc-6236, 1:1,000), VEGF (sc-13083, 1:1,000),
VEGF-R2 (sc-504, 1:1,000), phosphorylated STAT3 (p-STAT3;
sc-8001-R, 1:1,000) and GAPDH (sc-25778, 1:1,000, Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) overnight at 4°C. The
membranes were washed three times for 10 min in TBS-T and further
incubated for 1 h at 37°C with horseradish peroxidase
(HRP)-conjugated goat anti-rabbit Immunoglobulin G (sc-2030,
1:5,000, Santa Cruz Biotechnology, Inc.) and observed using the
enhanced chemiluminescent HRP substrate (WBKLS0500; EMD Millipore,
Billerica, MA, USA).
Statistical analysis
Experiments were repeated in triplicate; data are
presented as the mean ± standard error. Comparisons between mutants
were made using one-way analysis of variance, with a Bonferonni
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Salvianolic acid B recovers cognitive
deficits in CSVD rat
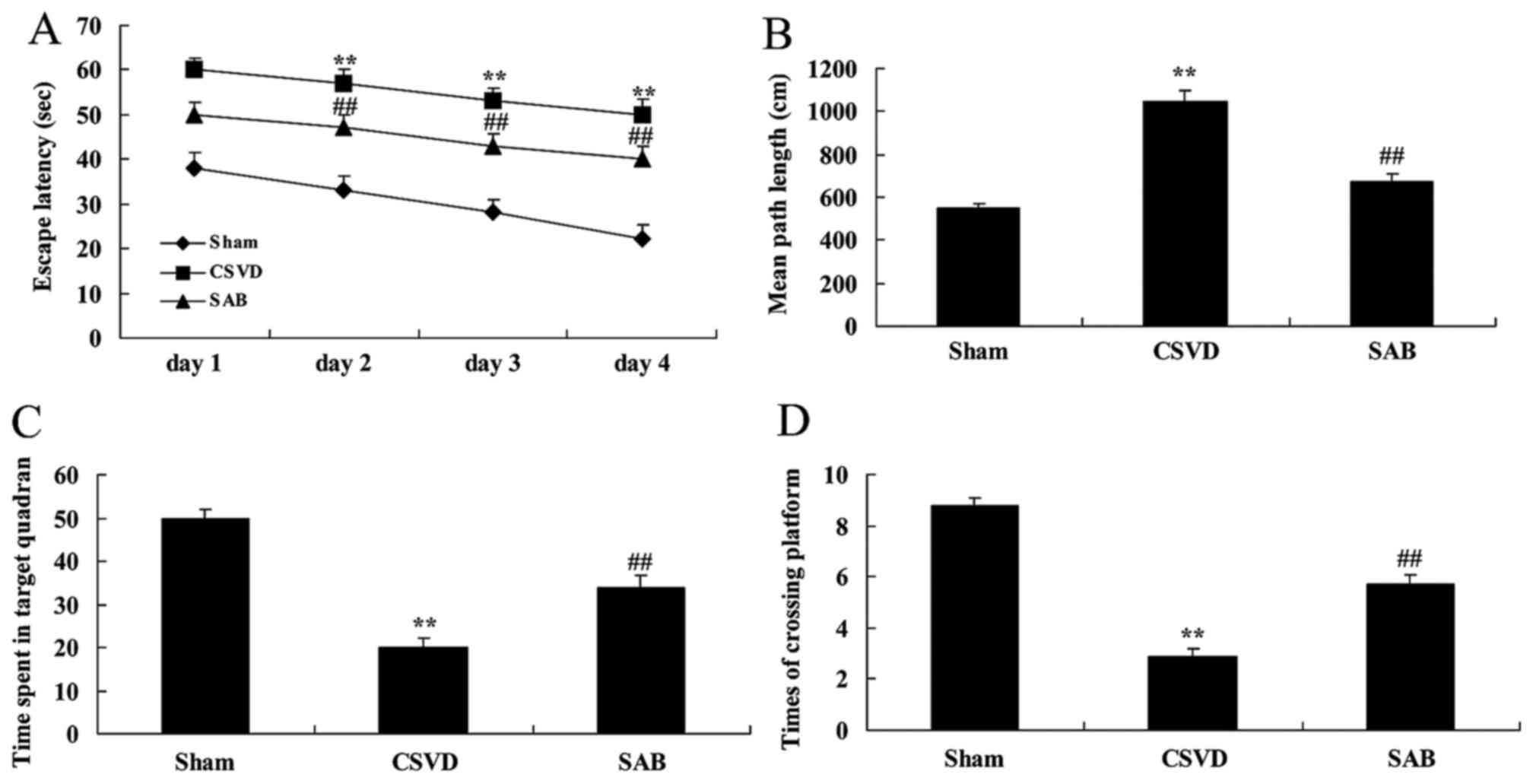
Cognitive deficits were evaluated in CSVD rats with
salvianolic acid B, which exhibited that salvianolic acid B has
nervous effects in CSVD. As presented in Fig. 2A and B, escape latency and mean
path length of CSVD rats were significantly reduced compared with
those of the sham group. However, time spent in the target quadrant
and the number of times the animals crossed the former platform
location of CSVD rats were significantly increased compared with
the sham group (Fig. 2C and D).
Following treatment with salvianolic acid B, cognitive deficits
were recovered, escape latency was reduced and mean path length was
reduced. The rats spent less time in the target quadrant and the
number of times the animals crossed the former platform location
was reduced in CSVD rats by salvianolic acid B, when compared with
CSVD model rats (Fig. 2).
Salvianolic acid B reduces
inflammation in CSVD rats
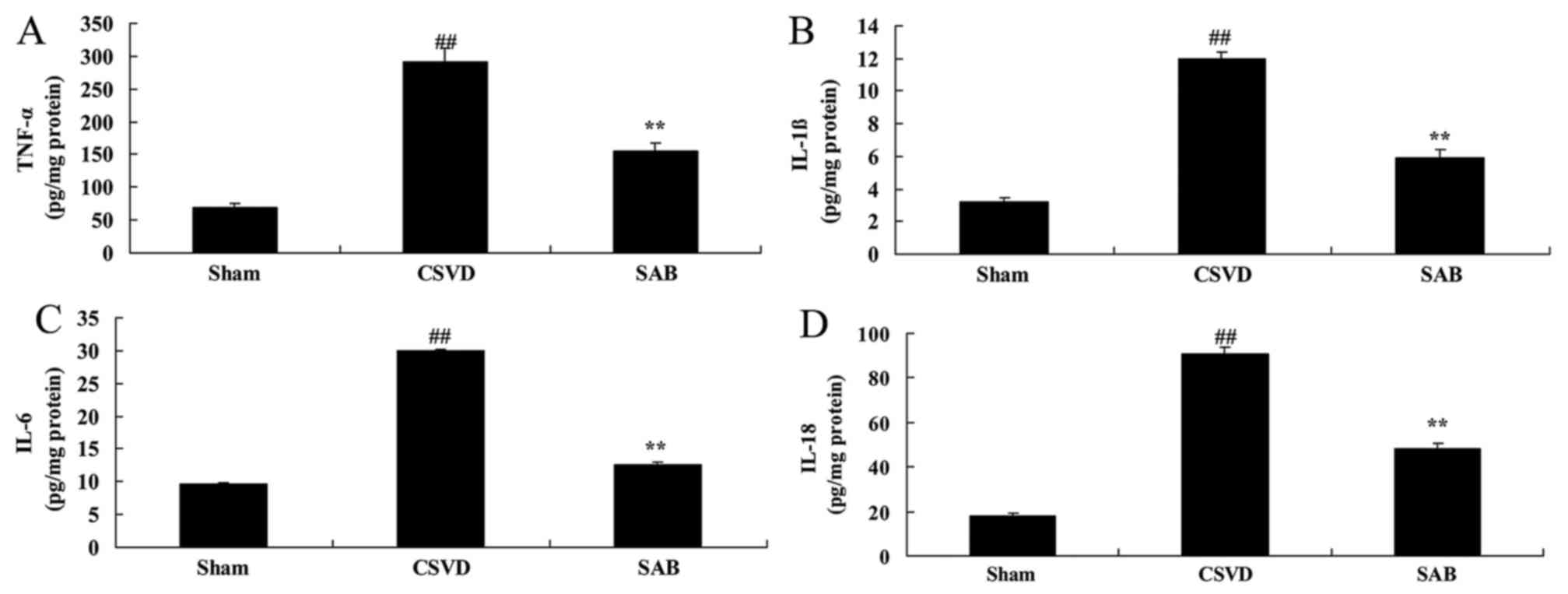
In the sham group, reduction of TNF-α, IL-1β, IL-6
and IL-18 levels was observed, compared with CSVD model group
(Fig. 3). Treatment with
salvianolic acid B significantly reduced TNF-α, IL-1β, IL-6 and
IL-18 levels compared with the CSVD model rats (Fig. 3).
Salvianolic acid B reduces oxidative
stress in CSVD rat
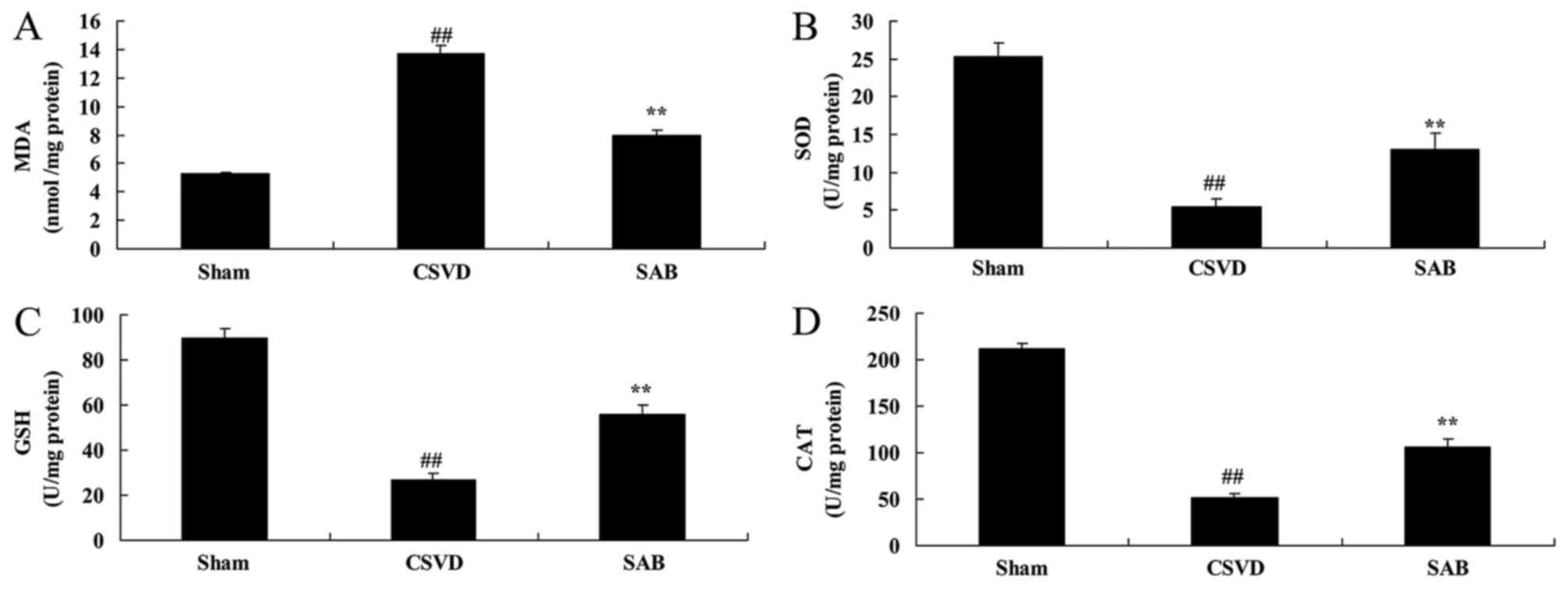
By contrast, significantly higher levels of MDA, and
reduced levels of SOD, CAT and GSH in CSVD model group, compared
with the sham rats (Fig. 4). The
administration of salvianolic acid B significantly inhibited MDA
and increased SOD, CAT and GSH levels in CSVD rats, compared with
the CSVD model rats (Fig. 4).
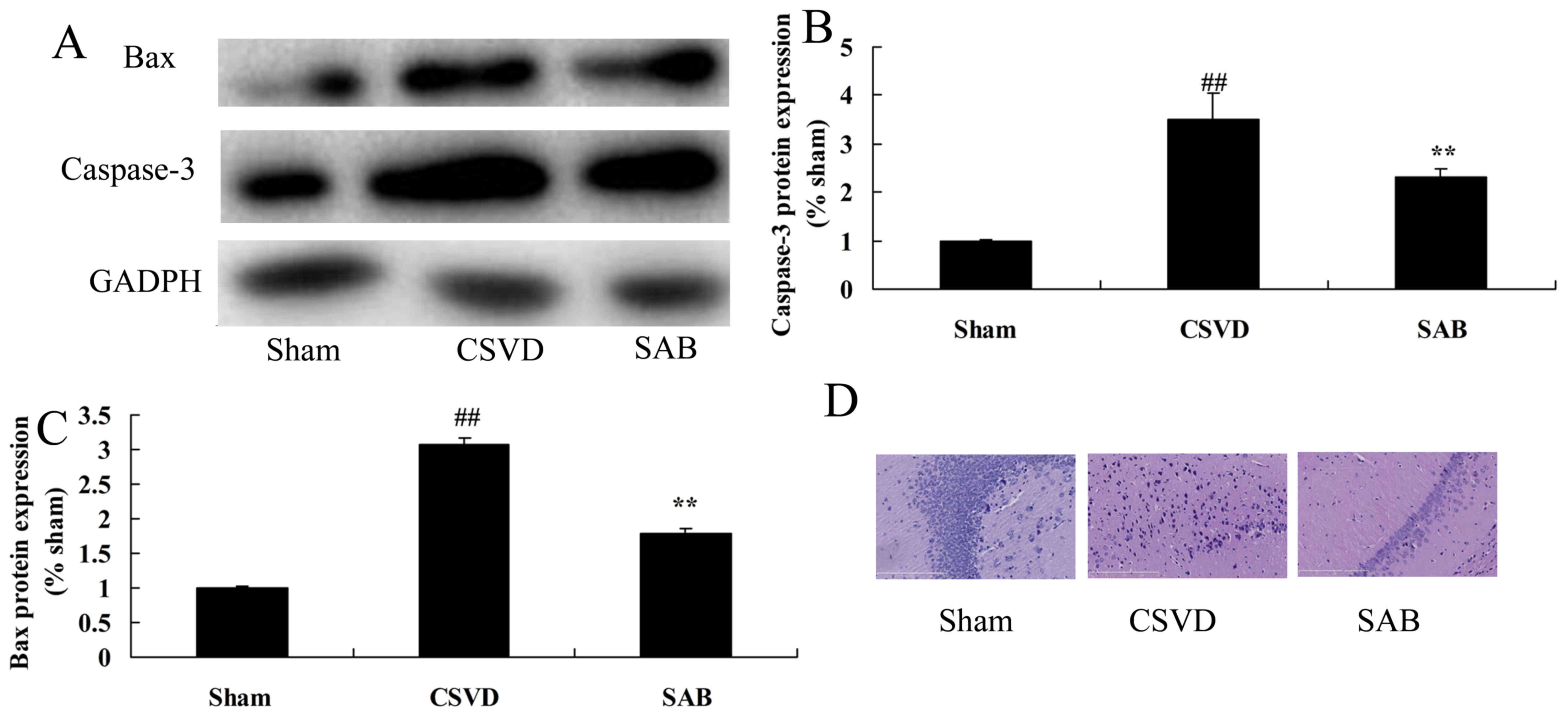
Salvianolic acid B reduces neurocyte
apoptosis (caspase-3 and Bax protein expression) in CSVD rats
To confirm the anti-apoptotic effects of salvianolic
acid B in CSVD, caspase-3 and Bax protein expression was measured
by western blot analysis. As presented in Fig. 5A-C, caspase-3 and Bax protein
expression of the CSVD model group were higher than those of the
sham group. Salvianolic acid B significantly reduced caspase-3 and
Bax protein expression of CSVD rat, compared with the CSVD model
group (Fig. 5A-C). A significantly
greater number of nerve cells was observed in the sham group
compared with the CSVD model group (Fig. 5D). Treatment with salvianolic acid
B significantly promoted the number of nerve cells in CSVD rats,
compared with the CSVD model group (Fig. 5D).
Salvianolic acid B recovers STAT3
phosphorylation, VEGF and VEGF-R2 protein expression in CSVD
rats
In order to analyze the mechanism underlying the
effects of salvianolic acid B on CSVD, STAT3 phosphorylation, VEGF
and VEGF-R2 protein expression in CSVD rats, western blot analysis
was conducted. p-STAT3, VEGF and VEGF-R2 protein expression levels
in CSVD rats were lower than those of the sham group (Fig. 6). Treatment with salvianolic acid B
significantly upregulated p-STAT3 protein expression, and induced
VEGF and VEGF-R2 protein expression in CSVD rat, compared with the
CSVD model group (Fig. 6).
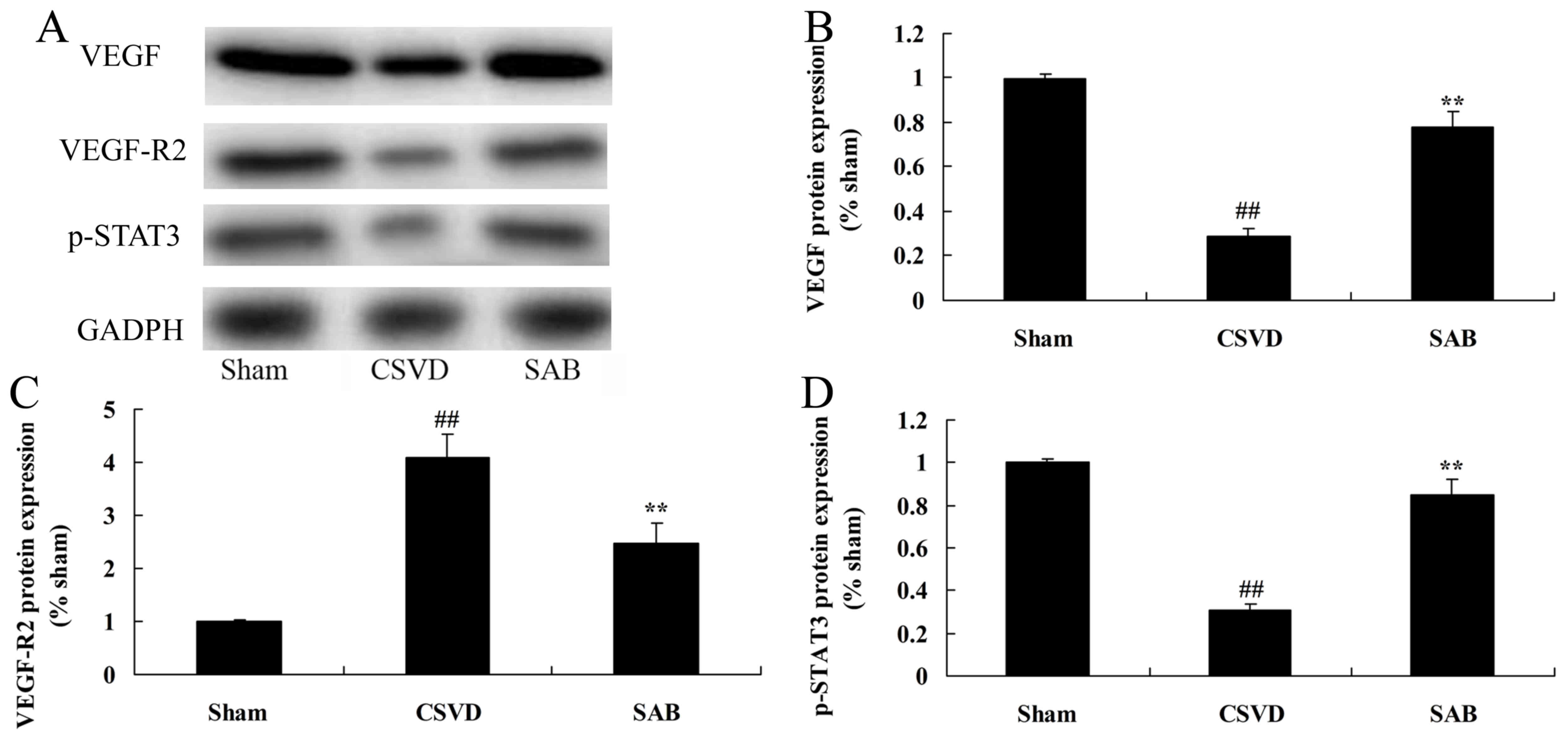 | Figure 6.SAB recovers STAT3 phosphorylation,
VEGF and VEGF-R2 protein expression in CSVD rats. SAB reduces
p-STAT3, VEGF and VEGF-R2 protein expression as identified by (A)
western blot analysis and statistical analysis of (B) VEGF, (C)
VEGF-R2 and (D) p-STAT3 protein expression in CSVD rats.
**P<0.01 vs. sham control group; ##P<0.01 vs. CSVD
model group. SAB, salvianolic acid B; STAT3, signal transducer and
activator of transcription 3; VEGF, vascular endothelial growth
factor; VEGF-R2, VEGF receptor 2; p-, phosphorylated; CSVD,
cerebral small vessel disease; sham, sham control group. |
Discussion
With the aging population in China, CSVD is a cause
of economical burden to society due to the high morbidity,
recurrence rate, disability rate and fatality rate (17), and CSVD-associated morbidity is
increasing in China (4). It is
accepted that CSVD is closely associated with numerous diseases
including apoplexy, cognitive and affective impairment and
instability of gait and function aging (18). In the present study, salvianolic
acid B was identified to recovers cognitive deficits in CSVD
rats.
The mechanism by which CSVD causes damage to the
brain is complex remains to be fully understood (19). Congenital and acquired risk factors
cause microvascular disease, and this leads to a series of
pathological changes, reduction of cerebral blood flow,
oligodendrocyte apoptosis and the destruction and inflammation of
the blood brain barrier (6). These
observations suggest that salvianolic acid B recovers neurocytes,
reduces inflammation, oxidative stress and neurocyte apoptosis in
CSVD rats. Lv et al (20)
demonstrated that salvianolic acid B attenuated apoptosis and
inflammation via sirtuin 1 activation in stroke rats.
Subsequent to CSVD in adult rats, the expression of
p-STAT3 in brain tissue increases and the location of p-STAT3 in
nerve cells varies (21). Certain
studies reported that p-STAT3 is predominantly expressed in nerve
cells and that p-STAT3 is expressed in reactive astrocyte cells and
gitter cells (21,22). In addition the excitation effect of
STAT3 for ischemic brain injury remains controversial (22). A previous study suggested that the
excitation effect of STAT3 can increase the inhibitor of apoptotic
protein Bcl-2 and Bcl-xl and decrease the Bax expression of
apoptotic protein Bax to take the excitation effect of nerves
(22). An additional study
reported that excessive activation of STAT3 can cause nerve cell
apoptosis (23). The current study
demonstrated that salvianolic acid B significantly upregulated
p-STAT3 protein expression in CSVD rat. Liu et al (14) reported that salvianolic acid B
increased proliferation rate by activating Janus kinase 2-STAT3
pathways in stem cells.
VEGFs are important downstream effector molecules
for STAT3. VEGF can promote the generation of new blood vessels and
vascular remodeling, and is also an important neurotrophic and
protective factor (24). The
protective effect on the central nervous system has been a focus of
research (11). A previous study
indicated that VEGF increased following CSVD in rats, the changing
trend of expression for VEGF was opposite to that of the trend for
apoptosis (11). This indicates
that when VEGF expression is high, the apoptosis levels are low
(25). By contrast, when VEGF
decreases, the apoptosis will increase, which may be due to the
gradual reduction of p-STAT3 protein, the regulated VEGF expression
will decrease and the protein which has protective effect to nerves
will also decrease. Therefore this will cause the exacerbation of
injury and increase of apoptosis cells. In the present study, it
was identified that salvianolic acid B significantly upregulated
VEGF and VEGF-R2 protein expression in CSVD rats.
In conclusion, the present study was, to the best of
our knowledge, the first to demonstrate that salvianolic acid B
recovers cognitive deficits and neurocytes, reduced inflammation,
oxidative stress and neurocyte apoptosis in CSVD rats through the
STAT3/VEGF signaling pathway. These results provide a potential
novel therapeutic strategy for the treatment of CSVD.
References
|
1
|
Kwon HM, Lynn MJ, Turan TN, Derdeyn CP,
Fiorella D, Lane BF, Montgomery J, Janis LS, Rumboldt Z and
Chimowitz MI; SAMMPRIS Investigators, : Frequency, risk factors,
and outcome of coexistent small vessel disease and intracranial
arterial stenosis: Results from the stenting and aggressive medical
management for preventing recurrent stroke in intracranial stenosis
(SAMMPRIS) Trial. JAMA Neurol. 73:36–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mi T, Yu F, Ji X, Sun Y and Qu D: The
interventional effect of remote ischemic preconditioning on
cerebral small vessel disease: A pilot randomized clinical trial.
Eur Neurol. 76:28–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Papma JM, de Groot M, de Koning I,
Mattace-Raso FU, van der Lugt A, Vernooij MW, Niessen WJ, van
Swieten JC, Koudstaal PJ, Prins ND and Smits M: Cerebral small
vessel disease affects white matter microstructure in mild
cognitive impairment. Hum Brain Mapp. 35:2836–2851. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavlovic AM, Pekmezovic T, Tomic G,
Trajkovic JZ and Sternic N: Baseline predictors of cognitive
decline in patients with cerebral small vessel disease. J
Alzheimers Dis. 42 Suppl 3:S37–S43. 2014.PubMed/NCBI
|
|
5
|
Benjamin P, Lawrence AJ, Lambert C, Patel
B, Chung AW, MacKinnon AD, Morris RG, Barrick TR and Markus HS:
Strategic lacunes and their relationship to cognitive impairment in
cerebral small vessel disease. Neuroimage Clin. 4:828–837. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dearborn JL, Schneider AL, Sharrett AR,
Mosley TH, Bezerra DC, Knopman DS, Selvin E, Jack CR, Coker LH,
Alonso A, et al: Obesity, insulin resistance, and incident small
vessel disease on magnetic resonance imaging: Atherosclerosis risk
in communities study. Stroke. 46:3131–3136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bridges LR, Andoh J, Lawrence AJ, Khoong
CHL, Poon W, Esiri MM, Markus HS and Hainsworth AH: Blood-brain
barrier dysfunction and cerebral small vessel disease
(arteriolosclerosis) in brains of older people. J Neuropathol Exp
Neurol. 73:1026–1033. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi Y, Thrippleton MJ, Makin SD, Marshall
I, Geerlings MI, de Craen AJ, van Buchem MA and Wardlaw JM:
Cerebral blood flow in small vessel disease: A systematic review
and meta-analysis. J Cereb Blood Flow Metab. 36:1653–1667. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Teng Z, Dong Y, Zhang D, An J and Lv P:
Cerebral small vessel disease and post-stroke cognitive impairment.
Int J Neurosci. 127:824–830. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Platt SR: Angiogenesis and cerebral
neoplasia. Vet Comp Oncol. 3:123–138. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zampetti A, Gnarra M, Borsini W,
Giurdanella F, Antuzzi D, Piras A, Smaldone C, Pieroni M, Cadeddu
C, de Waure C and Feliciani C: Vascular endothelial growth factor
(VEGF-a) in Fabry disease: Association with cutaneous and systemic
manifestations with vascular involvement. Cytokine. 61:933–939.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim HC, Kim E, Bae JI, Lee KH, Jeon YT,
Hwang JW, Lim YJ, Min SW and Park HP: Sevoflurane postconditioning
reduces apoptosis by activating the JAK-STAT pathway after
transient global cerebral ischemia in rats. J Neurosurg
Anesthesiol. 29:37–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hou YC, Liou KT, Chern CM, Wang YH, Liao
JF, Chang S, Chou YH and Shen YC: Preventive effect of silymarin in
cerebral ischemia-reperfusion-induced brain injury in rats possibly
through impairing NF-κB and STAT-1 activation. Phytomedicine.
17:963–973. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu CH, Shyu WC, Fu RH, Huang SJ, Chang
CH, Huang YC, Chen SY, Lin SZ and Liu SP: Salvianolic acid B
maintained stem cell pluripotency and increased proliferation rate
by activating Jak2-Stat3 combined with EGFR-Erk1/2 pathways. Cell
Transplant. 23:657–668. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Xu T, Du Y, Pan D, Wu W, Zhu H,
Zhang Y and Li D: Salvianolic acid a attenuates cell apoptosis,
oxidative stress, Akt and NF-κB activation in angiotensin-II
induced murine peritoneal macrophages. Curr Pharm Biotechnol.
17:283–290. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xue L, Wu Z, Ji XP, Gao XQ and Guo YH:
Effect and mechanism of salvianolic acid B on the myocardial
ischemia-reperfusion injury in rats. Asian Pac J Trop Med.
7:280–284. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Noh SM, Kim BJ and Kim JS: Cerebral small
vessel disease may be related to antiplatelet-induced
gastrointestinal bleeding. Int J Stroke. 9:E382014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arba F, Quinn T, Hankey GJ, Ali M, Lees KR
and Inzitari D; VISTA Collaboration, : Cerebral small vessel
disease, medial temporal lobe atrophy and cognitive status in
patients with ischaemic stroke and transient ischaemic attack. Eur
J Neurol. 24:276–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang S, Cai J, Lu R, Wu J, Zhang M and
Zhou X: Association between serum cystatin C level and total
magnetic resonance imaging burden of cerebral small vessel disease
in patients with acute lacunar stroke. J Stroke Cerebrovasc Dis.
26:186–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lv H, Wang L, Shen J, Hao S, Ming A, Wang
X, Su F and Zhang Z: Salvianolic acid B attenuates apoptosis and
inflammation via SIRT1 activation in experimental stroke rats.
Brain Res Bull. 115:30–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Stechishin OD, Luchman HA, Ruan Y, Blough
MD, Nguyen SA, Kelly JJ, Cairncross JG and Weiss S: On-target
JAK2/STAT3 inhibition slows disease progression in orthotopic
xenografts of human glioblastoma brain tumor stem cells. Neuro
Oncol. 15:198–207. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
D'Angelo B, Ek CJ, Sun Y, Zhu C, Sandberg
M and Mallard C: GSK3β inhibition protects the immature brain from
hypoxic-ischaemic insult via reduced STAT3 signalling.
Neuropharmacology. 101:13–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ma X, Zhou Y, Chai Y, Wang X and Huang X:
Stat3 controls maturation and terminal differentiation in mouse
hippocampal neurons. J Mol Neurosci. 61:88–95. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ahmed-Jushuf F, Jiwa NS, Arwani AS, Foot
P, Bridges LR, Kalaria RN, Esiri MM and Hainsworth AH:
Age-dependent expression of VEGFR2 in deep brain arteries in small
vessel disease, CADASIL, and healthy brains. Neurobiol Aging.
42:110–115. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SC, Lee KY, Kim YJ, Kim SH, Koh SH and
Lee YJ: Serum VEGF levels in acute ischaemic strokes are correlated
with long-term prognosis. Eur J Neurol. 17:45–51. 2010. View Article : Google Scholar : PubMed/NCBI
|