Introduction
Gastric cancer (GC) is the most common
gastrointestinal malignancy in Asia (1). With the advancement of diagnostic and
therapeutic techniques, certain preventative measures and treatment
strategies have been developed, thus resulting in a decline in GC
incidence and mortality rate. However, GC remains a serious threat
to human health (2–5). During the complex development and
progression of GC, various molecules have been confirmed to be
involved in tumor cell proliferation, apoptosis, invasion,
metastasis and drug resistance (6,7).
Although numerous studies have been conducted regarding GC, the
exact molecular mechanisms underlying GC remain unclear (8,9). In
recent studies, apoptotic resistance has been suggested as an
important feature of GC cells (10). Due to their marked anti-apoptotic
activity, GC cells exhibit strong survivability, which may lead to
the progression and metastasis of GC. It has previously been
confirmed that some molecules and signaling pathways are involved
in the development of apoptotic resistance in GC (11–13);
however, the key molecules and signaling pathways remain to be
elucidated.
The complex molecular mechanisms underlying
apoptotic resistance of GC cells have been explored in numerous
studies (14–16); however, these studies have been
unsuccessful in applying apoptosis-promoting strategies in the
treatment of GC. Previous studies demonstrated that microRNAs
(miRNAs/miR) serve a regulatory role in the apoptosis of GC cells
(17,18). Since miRNAs exert a potent
inhibitory effect on numerous target genes, they are considered to
possess a stronger biological function compared with single genes.
Numerous studies have reported that miR-185 is involved in tumor
proliferation, apoptosis, invasion and multidrug resistance
(19–21). miR-185 may also be involved in the
apoptosis of GC cells; however, the association between miR-185 and
GC cell apoptosis, as well as the underlying molecular mechanisms,
remain unclear. The present study analyzed the association between
miR-185 and apoptosis of GC cells, and demonstrated that miR-185
expression was significantly decreased in GC tissues and cell
lines; in particular, miR-185 expression was lower in poorly
differentiated cell lines. Following transfection with miR-185
mimics, in order to upregulate miR-185 expression in GC cells, the
viability of GC cells was significantly decreased; apoptotic rate
was increased; and the expression levels of apoptosis-associated
genes [B-cell lymphoma 2 (Bcl-2), survivin, X-linked inhibitor of
apoptosis protein (XIAP)], and the expression and activity of
caspase-3 and caspase-8 were altered. These results suggested that
miR-185 was associated with apoptosis of GC cells by downregulating
the expression of anti-apoptotic genes. miR-185 may promote
apoptosis of GC cells; therefore, miR-185 may serve as a tumor
marker and as a potential target for the regulation of GC cell
apoptosis.
Materials and methods
Materials
Human GC cell lines MKN74, SGC7901, BGC823 and
MGC803 were obtained from the Research Center of the Fourth
Affiliated Hospital of Hebei Medical University (Shijiazhuang,
China); and the GES-1 gastric epithelial cell line was purchased
from the Institute of Biochemistry and Cell Biology (Shanghai,
China). RPMI-1640 culture medium and trypsin were purchased from
Gibco (Thermo Fisher Scientific, Inc., Waltham, MA, USA); TRIzol
reagent and Lipofectamine™ 2000 transfection reagents
were purchased from Invitrogen (Thermo Fisher Scientific, Inc.).
Reverse transcription (RT) kit and fluorescence RT-quantitative
polymerase chain reaction (qPCR) reagents were purchased from
Promega Corporation (Madison, WI, USA); PCR primers and miR-185
mimics were synthesized by Sangon Biotech Co., Ltd. (Shanghai,
China). Protein extraction kit was purchased from Beyotime
Institute of Biotechnology (Haimen, China); Bcl-2 (cat. no.
sc-23960), Bcl-2-associated X protein (Bax) (cat. no. sc-7480),
survivin (cat. no. sc-17779), XIAP (cat. no. sc-55551), livin (cat.
no. sc-393237), caspase-3 (cat. no. sc-7272), caspase-8 (cat. no.
sc-5263) and β-actin (cat. no. sc-8432) antibodies were all
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA).
MTT was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany). Caspase-3 (cat. no. G015) and caspase-8 (cat. no. G017)
Activity Colorimetric Assay kits were purchased from Nanjing KeyGen
Biotech Co., Ltd. (Nanjing, China).
Clinical sample preparations
The present study was approved by the Ethics
Committee of the Fourth Affiliated Hospital, Hebei Medical
University, and all patients provided written informed consent. A
total of 30 patients with GC (21 males and 9 females; average age,
58.45±14.1 years), which had been surgically removed and
pathologically confirmed, were recruited from the Fourth Affiliated
Hospital of Hebei Medical University. None of the patients received
preoperative radiotherapy or chemotherapy. Specimens (~1.0×0.5×0.5
cm), including cancerous and adjacent tissues (>3 cm from the
edge of cancerous tissue, no cancer cells present), were harvested
and stored at −80°C.
Cell culture
MKN74, SGC7901, BGC823, MGC803 and GES-1 cells were
cultured in RPMI-1640 containing 10% fetal calf serum (both from
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100
mg/ml streptomycin. The cells were incubated at 37°C in an
atmosphere containing 5% CO2. Cells were trypsinized in
a 0.25% trypsin solution containing 0.02% of EDTA.
Synthesis and transfection of miR-185
mimics
Prior to transfection, miR-185 synthetic mimics
(has-miR-185-5p-mimic, 5′-UGGAGAGAAAGGCAGUUCCUGA-3′; has-miR-185-5p
inhibitor, 5′-ACCUCUCUUUCCGUCAAGGACU-3′; Guangzhou RiboBio Co.,
Ltd., Guangzhou, China) were dissolved at a concentration of 20
µmol/l. MGC803 cells were seeded in 6-well plates at a density of
4×105 cells/ml, incubating at 37°C for 24 h. Prior to
transfection, cells were rinsed with RPMI-1640 medium free of serum
and antibiotics. According to the manufacturer's instructions,
untransfected control group and miR-185 mimics diluted with
RPMI-1640 medium were mixed with Lipofectamine® 2000.
miR-185 mimics were then transfected into MGC803 cells. After 24 h,
he transfection efficiency was determined, and subsequent
experiments were conducted after another 24 h.
MTT assay to determine cell
viability
MGC803 cells were seeded in 96-well plates at a
density of 5×104 cells/ml. Cells at 60–70% confluence
were transfected with miR-185 mimics, or with only the negative
transfection reagent Lipofectamine™ 2000. A total of six
replicates per group were analyzed. A total of 4 h prior to the end
of the experiment, 20 µl MTT (5 mg/ml) was added to each group. The
cells were cultured at 37°C for 4 h, and the culture medium was
then discarded. Subsequently, 150 µl dimethyl sulfoxide was added
to each well and the plates were agitated at room temperature for
15 min. Absorbance (A value) was then measured at a wavelength of
490 nm; A value represented cell viability. This experiment was
repeated three times at 24, 48 and 72 h.
Flow cytometry to detect apoptotic
rate of GC cells
Apoptosis was quantified using an Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) detection
kit (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. Briefly, cells were harvested and
resuspended in binding buffer (106 cells/ml). Following
the addition of 5 µl Annexin V-FITC and 10 µl PI, the cells were
mixed and were incubated for 15 min at room temperature in the
dark. Annexin V-FITC binding was detected using a FACSCalibur flow
cytometer (BD Biosciences, Franklin Lakes, NJ, USA). Data were
analyzed using Cell Quest software 5.1 (BD Biosciences). The
experiment was repeated three times.
Caspase-3 and caspase-8 activity
assay
According to the protocol of the spectrophotometric
detection kit, cells were collected, and lysed in 50 µl cold lysis
buffer for 20 min. The cells were were centrifugated at 12,500 g
for 10 min, after which the supernatant was transferred to new
tubes and the protein concentration of the cell lysates was
measured by Bio-Rad DC™ Protein Assay (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Protein (100 µg) was taken
from each group and was adjusted to 50 µl with lysis buffer. A
total of 50 µl 2X Reaction Buffer and 5 µl appropriate substrate
was added to each sample and was incubated at 37°C in the dark for
4 h. Subsequently, A value was measured at a wavelength of 405 nm
using a microplate reader. Caspase enzyme activity within a unit
volume of protein represented caspase-3 and caspase-8
activation.
RNA isolation and RT-qPCR to detect
target mRNA expression
Total RNA was extracted from cells and tissues,
using the TRIzol one-step method and 2 µg RNA was reverse
transcribed to cDNA. cDNA (2 µl) underwnt PCR to detect the mRNA
expression of target molecules. GAPDH served as an internal
reference gene. According to the manufacturer's instructions, PCR
was conducted in a final volume of 20 µl, as follows: 2 µl cDNA, 10
µl SYBR Green Mix (Promega Corporation, Madison, WI, USA), and 0.5
µl downstream and upstream primers (10 µmol/l), 7 µl deionized
water. The following cycling conditions were conducted: 1 cycle at
95°C for 5 min, followed by 45 cycles of 94°C for 30 sec, 60°C for
30 sec, and 72°C for 30 sec, and a final extension at 72°C for 10
min). Primers were designed using Primer 5.0 software (Premier
Biosoft International, Palo Alto, CA, USA) and were detected for
specificity using primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/).
Primer sequences for each gene were as follows: miR-185-5p forward,
5′-TCCGCTGGAGAGAAAGGC-3′ and reverse, 5′-ATGGAGGCTGAGGAGCACTG-3′;
Bcl-2 (98 bp) forward, 5′-TGTGTGGAGAGCGTCAACC-3′ and reverse,
5′-TGGATCCAGGTGTGCAGGT-3′; Bax (129 bp) forward,
5′-TTTCTGACGGCAACTTCAA-3′ and reverse, 5′-AGTCCAATGTCCAGCCCAT-3′;
survivin (185 bp) forward, 5′-GCCAGATTTGAATCGCGGGA-3′ and reverse,
5′-GCAGTGGATGAAGCCAGCCT-3′; XIAP (292 bp) forward,
5′-CCGTGCGGTGCTTTAGTTGT-3′ and reverse,
5′-TTCCTCGGGTATATGGTGTCTGAT-3′; livin (312 bp) forward,
5′-TCCACAGTGTGCAGGAGACT-3′ and reverse, 5′-ACGGCACAAAGACGATGGAC-3′;
caspase-3 (148 bp) forward, 5′-AGAGCTGGACTGCGGTATTGAG-3′ and
reverse, 5′-GAACCATGACCCGTCCCTTG-3′; caspase-8 (163 bp) forward,
5′-GATGAGGCAGACTTTCTGCT-3′ and reverse,
5′-CATAGTTCACGCCAGTCAGGAT-3′; and GAPDH (138 bp) forward,
5′-GACCCCTTCATTGACCTCAAC-3′ and reverse,
5′-CGCTCCTGGAAGATGGTGAT-3′. qPCR results were analyzed using the
2−ΔΔCq method (22).
GAPDH was used as an internal reference.
Western blot anlaysis of target
proteins
Cell samples were lysed with lysis buffer: 1% Triton
X-100, 150 mM NaCl, 10 mM Tris-HCl (pH 7.4), 1 mM EDTA, 1 mM EGTA
(pH 8.0), 0.2 mM Na3VO4, 0.2 mM
phenylmethylsulfonyl fluoride and 0.5% NP-40. Following protein
quantification detected by Bicinchoninic acid Protein Quantitation
kit (MultiSciences Biotech Co., Ltd., Hangzhou, China), 40 µg
protein from each sample were separated by 10% SDS-PAGE and were
electrotransferred onto polyvinylidene fluoride membranes (GE
Healthcare Life Sciences, Little Chalfont, UK). Membranes were
blocked with 5% bovine serum albumin (Sigma-Aldrich, Merck KGaA) at
room temperature for 2 h, and were incubated with primary
antibodies (all 1:1,000) overnight at 4°C. Membranes were then
incubated at room temperature for 2 h with anti-mouse horseradish
peroxidase-conjugated secondary antibody (1:105) (cat.
no. 610-103-121-0100; Rockland Immunochemicals Inc., Pottstown, PA,
USA) and target bands were detected using an enhanced
chemiluminescence detection kit (Santa Cruz Biotechnology, Inc.).
β-actin was used as the internal control. The experiment was
repeated three times.
Statistical analysis
SPSS software version 16.0 (SPSS, Inc., Chicago, IL,
USA) was used to analyze data. Experimental data were expressed as
the mean ± standard deviation (n≥3). Data were analyzed by one-way
analysis of variance sand Dunnett post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
miR-185 expression in GC tissues and
cell lines
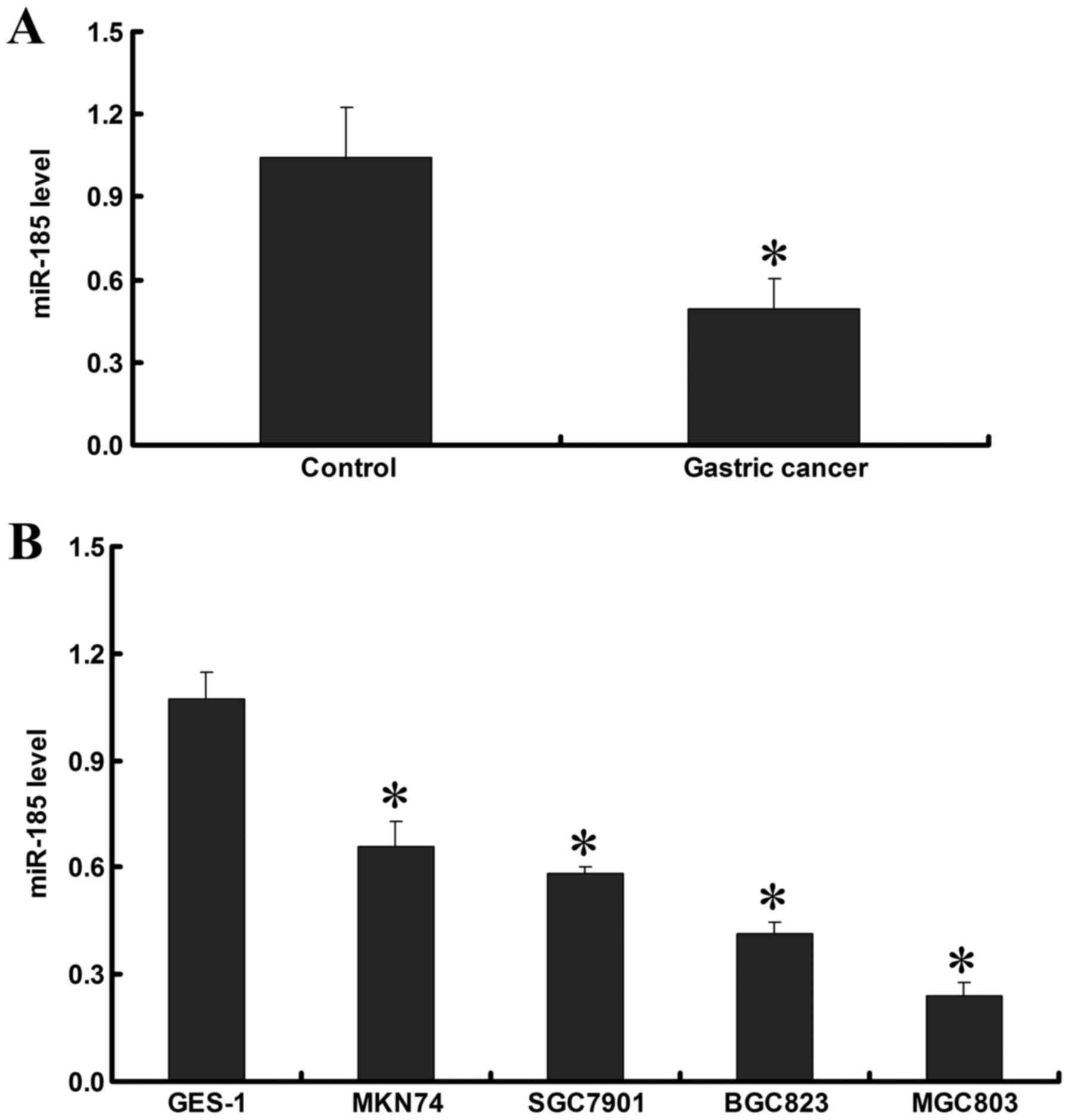
RT-qPCR demonstrated that miR-185 expression in GC
tissues was lower than in adjacent noncancerous tissues (P<0.05;
Fig. 1A). In addition, the
expression of miR-185 was detected in the cell lines, and the
results demonstrated that miR-185 expression in GC cell lines was
lower than in the GES-1 normal gastric epithelial cell line
(P<0.05). In the GC cell lines, miR-185 expression was the
highest in MKN74, followed by SGC7901 and BGC823, and the lowest
miR-185 expression was detected in the MGC803 cell line (P<0.05;
Fig. 1B). These data indicated
that miR-185 expression in GC tissues and cell lines is decreased,
which may be associated with the development and progression of
GC.
Association between miR-185 expression
and clinicopathological characteristics
The present study demonstrated that miR-185
expression was not significantly correlated with gender (t=−0.123,
P=0.903), age (t=0.170, P=0.866) or tumor-node-metastasis stage
(t=−0.270, P=0.789). However, miR-185 expression was correlated
with tumor size, differentiation and lymphatic metastasis. miR-185
expression was reduced in tumors ≥5 cm compared with in tumors
<5 cm (t=−2.318, P=0.028). In addition, miR-185 expression was
decreased in poorly differentiated/undifferentiated tumors
(t=5.958, P<0.001), whereas miR-185 expression was significantly
higher in GC patients without lymph node metastasis compared with
in patients with lymph node metastasis (t=−4.032, P<0.001)
(Table I).
 | Table I.Association of miR-185 expression
with clinical characteristics of patients with GC. |
Table I.
Association of miR-185 expression
with clinical characteristics of patients with GC.
| Characteristic | n | miR-185 expression
in GC tissues | t-value | P-value |
|---|
| Sex |
|
| −0.123 | 0.903 |
|
Male | 21 |
0.490±0.166 |
|
|
|
Female | 9 |
0.498±0.155 |
|
|
| Age (years) |
|
| 0.170 | 0.866 |
|
≥60 | 9 |
0.500±0.177 |
|
|
|
<60 | 21 |
0.489±0.156 |
|
|
| Tumor size
(cm) |
|
| −2.318 | 0.028 |
| ≥5 | 22 |
0.453±0.159 |
|
|
|
<5 | 8 |
0.596±0.116 |
|
|
|
Tumor-node-metastasis stage |
|
| −0.270 | 0.789 |
|
I–II | 7 |
0.478±0.054 |
|
|
|
III–IV | 23 |
0.497±0.182 |
|
|
|
Differentiation |
|
| 5.958 | <0.001 |
|
High/moderate | 21 |
0.572±0.126 |
|
|
|
Poor/undifferentiated | 9 |
0.316±0.032 |
|
|
| Lymphatic
metastasis |
|
| −4.032 | <0.001 |
|
Positive | 24 |
0.443±0.142 |
|
|
|
Negative | 6 |
0.681±0.030 |
|
|
Effects of miR-185 mimics on miR-185
expression in MGC803 cells
Post-transfection of MGC803 cells with 80 nM miR-185
mimics for 24 h, level of miR-185 in transfected cells was
107.85±19.58, which in untransfected cells was 2.48±0.41, and
miR-185 expression was significantly increased (P<0.001), which
provided a promising basis for further study of miR-185 function in
GC.
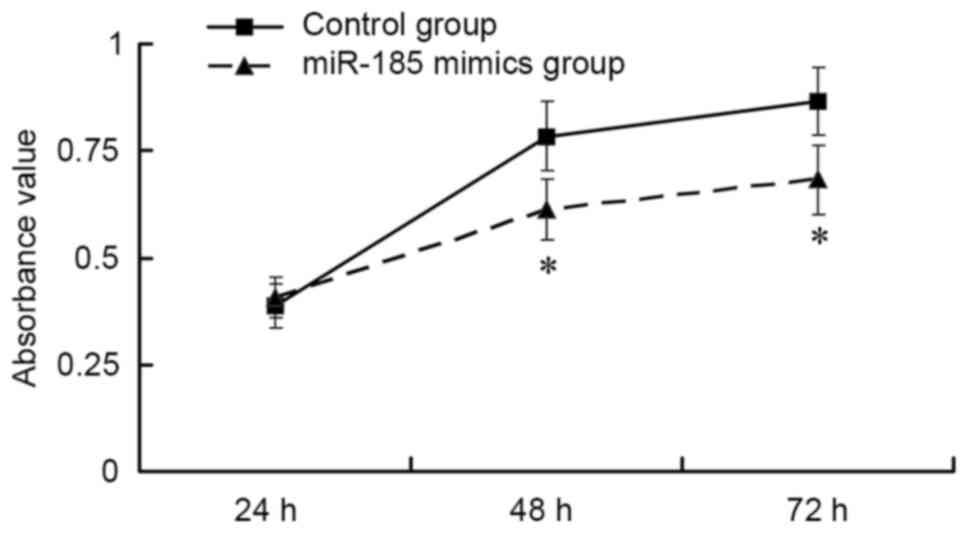
Effects of miR-185 mimics on MGC803
cell viability
The results of an MTT assay indicated that,
post-transfection of MGC803 cells with 80 nM miR-185 mimics, GC
cell viability was significantly suppressed compared with in the
control group in a time-dependent manner (P<0.01; Fig. 2). These findings indicated that
upregulation of miR-185 may inhibit the viability of GC cells.
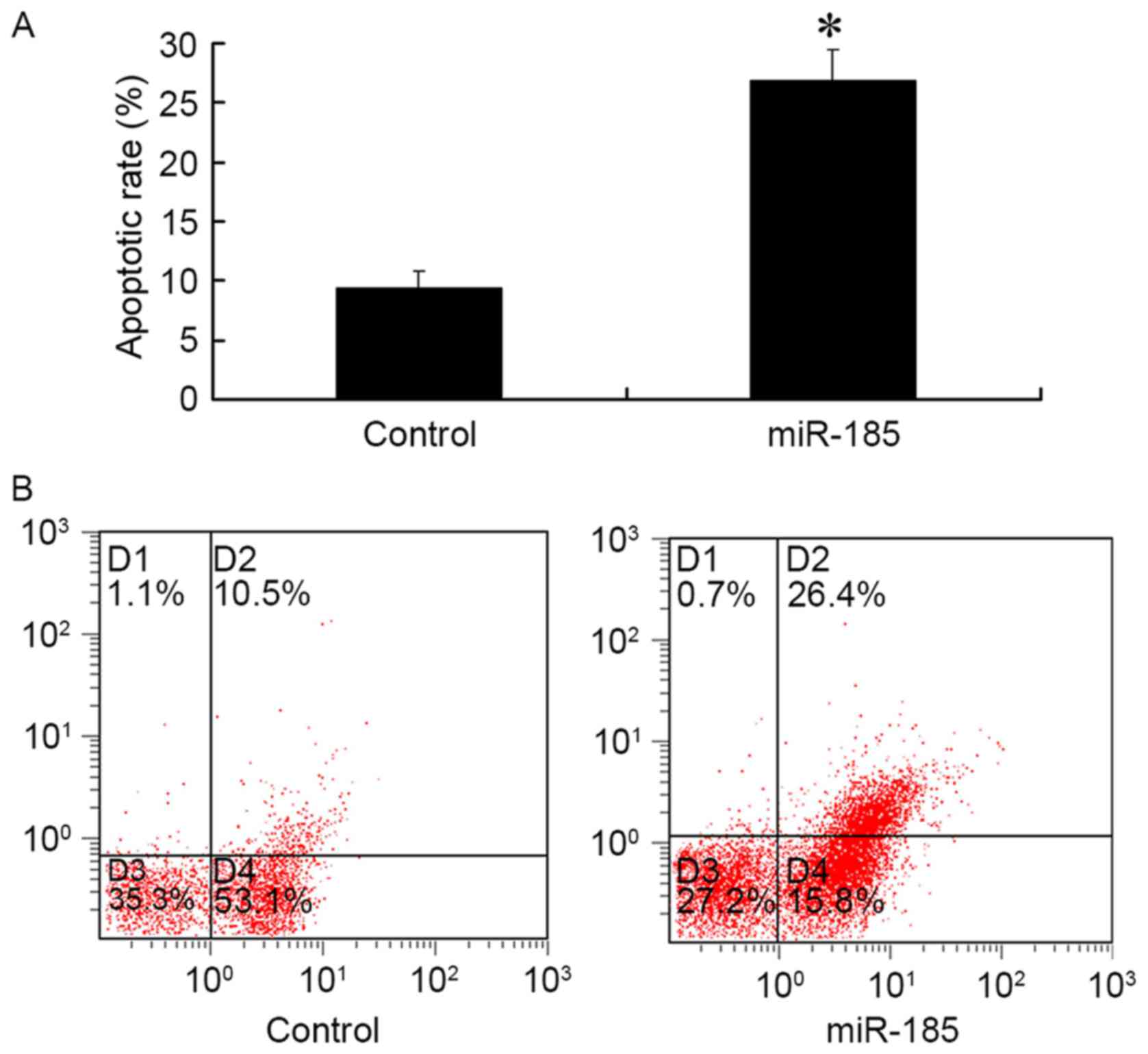
Effects of miR-185 mimics on MGC803
apoptosis
The results of FCM demonstrated that 48 h
post-transfection of MGC803 cells with 80 nM miR-185 mimics, the
apoptotic rate of GC cells was significantly increased compared
with in the control group (P<0.01; Fig. 3). These results suggested that
upregulated miR-185 expression in GC cells may promote
apoptosis.
Effects of miR-185 mimics on the
expression of apoptotic factors in MGC803 cells
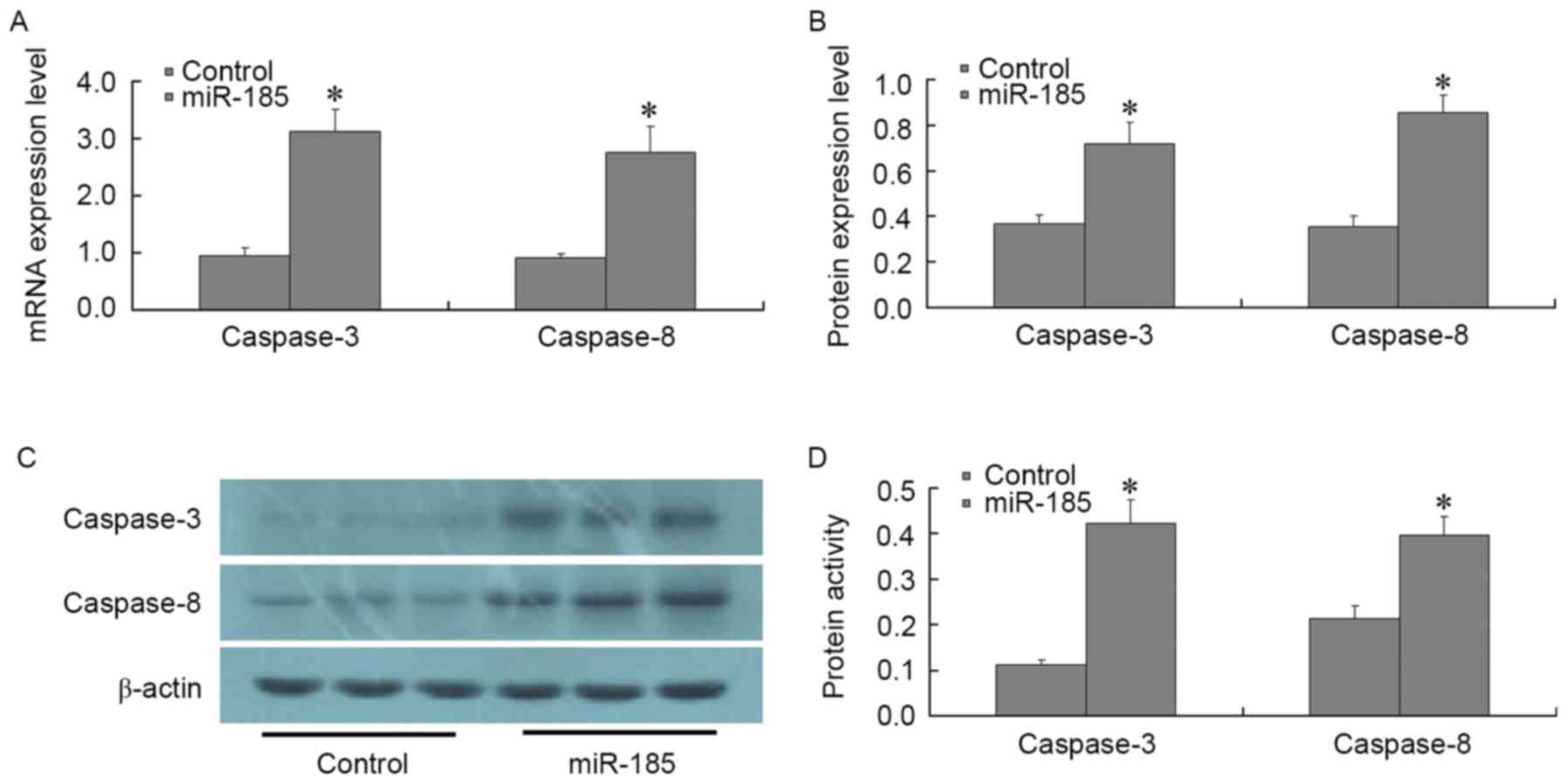
A total of 48 h post-transfection of MGC803 cells
with 80 nM miR-185 mimics, the expression levels of the following
apoptosis-associated factors were detected: Bcl-2, Bax, survivin,
XIAP, livin, caspase-3 and caspase-8. RT-qPCR and western blotting
results demonstrated that post-transfection of MGC803 cells with
miR-185 mimics, the expression levels of Bcl-2, survivin and XIAP
were significantly decreased (P<0.01), whereas the expression
levels of Bax and livin were not significantly altered (P>0.01;
Fig. 4). In addition, the
expression levels of caspase-3 and caspase-8 were significantly
increased, as determined by RT-qPCR and western blotting
(P<0.01; Fig. 5A-C). The
results of a spectrophotometric analysis indicated that caspase-3
and caspase-8 activity was significantly increased in MGC803 cells
post-transfection with miR-185 mimics (P<0.01; Fig. 5D). These results suggested that, in
GC cells, miR-185 may exert an inhibitory effect on anti-apoptotic
factors, such as Bcl-2, survivin and XIAP. In addition, miR-185 may
increase the expression and activity of core apoptotic genes
caspase-3 and caspase-8, thus promoting apoptosis of GC cells.
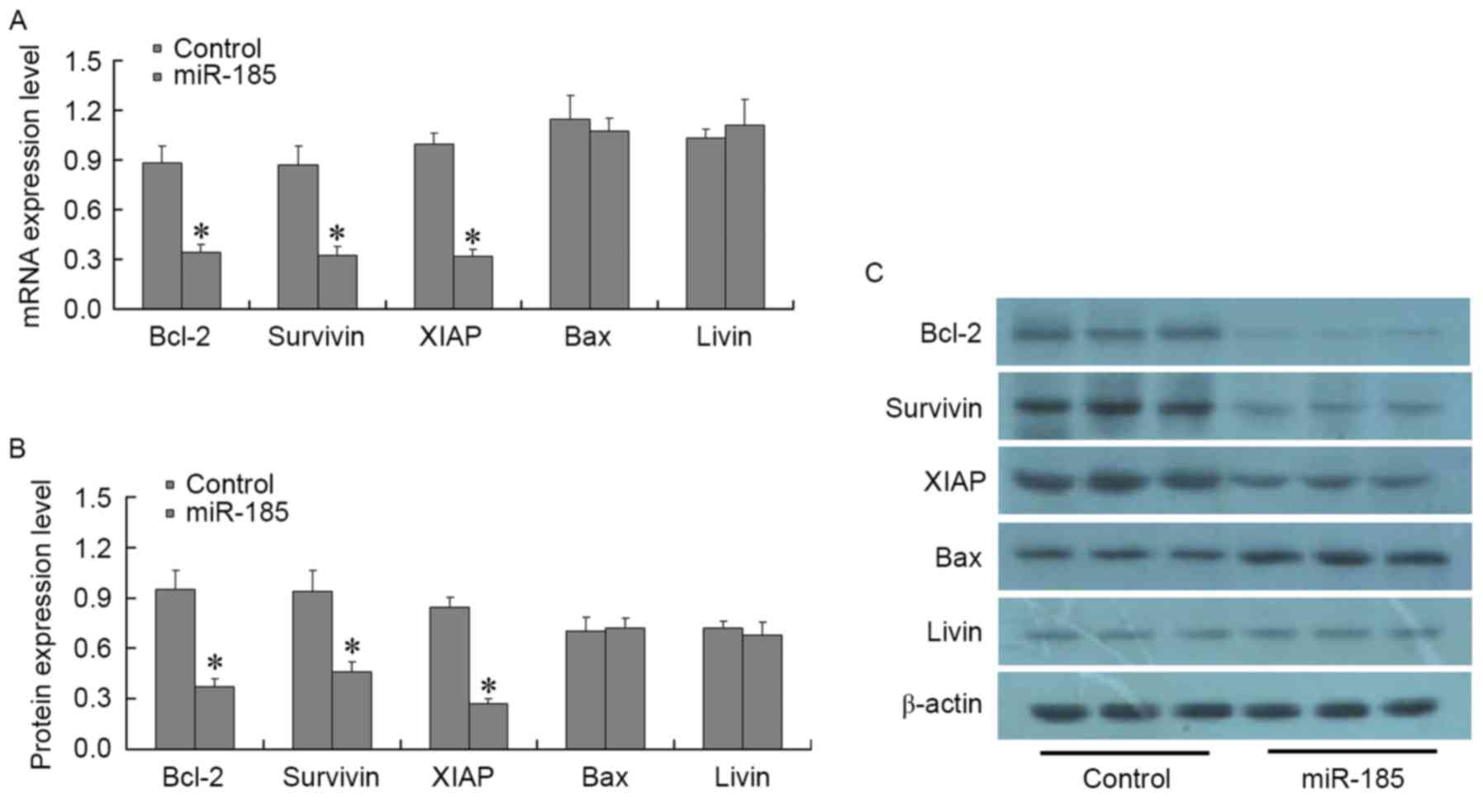 | Figure 4.Effects of miR-185 mimics on the
expression of apoptotic factors, Bcl-2, Bax, survivin, XIAP and
livin, in MGC803 cells. A total of 48 h post-transfection of MCG803
cells with 80 nM miR-185 mimics, (A) mRNA and (B and C) protein
expression levels of Bcl-2, Bax, survivin, XIAP and livin were
detected using quantitative polymerase chain reaction and western
blotting, respectively. Bcl-2, survivin and XIAP were significantly
decreased in miR-185 mimics-transfected MGC803 cells, whereas Bax
and livin expressions was not significantly altered. *P<0.01 vs.
the control group. miR-185, microRNA-185; Bcl-2, B-cell lymphoma 2;
Bax, Bcl-2-associated X protein; XIAP, X-linked inhibitor of
apoptosis protein. |
Discussion
Although the worldwide incidence of GC has decreased
in recent years, there remains a high incidence in Asia (23,24),
which seriously endangers the health of the population. Compared
with normal cells, GC cells exhibit increased proliferation,
angiogenesis, invasion and metastasis, reduced apoptosis, and
immune evasion. Various molecules at various stages contribute to
the development and progression of GC. Previous studies have
investigated the associated genes and signaling pathways; however,
the regulatory mechanism underlying GC remains unclear (25,26).
miRNAs are a class of widely distributed,
single-stranded, non-protein coding RNAs, ~22 nucleotides in
length. The main function of miRNAs is to inhibit the expression of
downstream genes (27–31). It has previously been confirmed
that miRNAs are closely associated with GC, and various miRNAs,
such as miR-21, let-7 and miR-27, are invovled in invasion and
metastasis of GC (32–34). miR-185 is a newly discovered miRNA,
which is closely associated with numerous malignancies. Zhi et
al reported that miR-185 exhibits tumor-suppressing activity;
patients with hepatocellular carcinoma with low miR-185 expression
had a low survival rate and short survival period (35). In addition, miR-185 has been
revealed to be abnormally expressed in various malignancies, and is
involved in the proliferation, invasion and metastasis of tumors,
as well as the resistance of endometrial cancer cells to cisplatin
(19–21). The present study indicated that
miR-185 expression was significantly reduced in GC tissues and cell
lines, thus suggesting that miR-185 is associated with GC. miR-185
was upregulated in GC cells post-transfection with miR-185 mimics,
after which the viability of tumor cells was significantly reduced,
suggesting that miR-185 may serve a role in GC cells by inhibiting
proto-oncogenes, including tripartite motif-containing protein 29,
and zinc finger protein SNAI1 and 2 (36,37).
Therefore, miR-185 may be considered a potential novel target for
GC biotherapy.
The detailed mechanism of action of miR-185 in GC
remains unclear. Li et al demonstrated that miR-185 was able
to induce apoptosis of prostate cancer cells (33); however, the association between
miR-185 and GC cell apoptosis remains unclear. The present study
confirmed that following upregulation of miR-185 expression in GC
cells, the apoptotic rate was significantly increased;
consequently, it was hypothesized that miR-185 may promote
apoptosis by regulating the expression of apoptosis-associated
factors. Therefore, the expression of apoptosis-associated factors
were detected.
GC cells have a marked resistance to apoptosis, and
disturbance of apoptotic regulation is an important mechanism
underlying the progression of GC. Therefore, it is of great
significance to explore the apoptotic mechanism of GC cells, so as
to research the pathogenesis and potential treatment strategies of
GC. The present study analyzed the alterations in the expression of
apoptosis-associated factors post-transfection with miR-185 mimics,
thus providing a basis for analyzing the mechanism of miR-185 in GC
cells. Bcl-2/Bax, which are important members of the mitochondrial
pathway, can be combined into dimers. Alterations in the proportion
of these dimers may lead to changes in the apoptotic ability of GC
cells (38,39). Survivin is an important member in
the inhibitor of apoptosis protein family, which directly inhibits
caspase family members, enhancing tumor cell resistance to
apoptosis (11). XIAP may inhibit
caspase-3 and −7 by inhibiting the death receptor and mitochondrial
pathways (40,41), thus serving a role in apoptotic
suppression. Livin has an anti-apoptotic role via activation of the
c-Jun N-terminal kinase 1 signal transduction pathway, and can also
directly inhibit caspase-3 or inhibit apoptosis by inhibiting
caspase-9 (42,43). Caspase-3 and −8, as core apoptotic
genes, can directly promote apoptosis (12,44).
The present study demonstrated that when miR-185 expression is
upregulated in GC cells, the expression levels of Bcl-2, survivin
and XIAP were significantly decreased, whereas caspase-3 and
caspase-8 expression and activity were significantly increased,
thus suggesting that miR-185 may activate caspase-3 and caspase-8
by inhibiting the expression of Bcl-2, survivin and XIAP, thus
serving a role in promoting apoptosis. However, the underlying
molecular mechanisms require further investigation.
In conclusion, the present study indicated that
miR-185 expression in GC tissues and cell lines was significantly
decreased. Upregulation of miR-185 was able to suppress the
viability of GC cells and to increase their apoptotic rate by
regulating apoptosis-associated genes. These results suggested that
miR-185 may be instrumental in GC cell apoptosis, and may be
considered a potential novel target in human GC biotherapy.
References
|
1
|
Sugano K: Screening of gastric cancer in
Asia. Best Pract Res Clin Gastroenterol. 29:895–905. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nishizawa M, Seshimo A, Miyake K, Amano K
and Kameoka S: Usefulness of the TRC method in the peritoneal
washing cytology for gastric cancer. Hepatogastroenterology.
61:240–244. 2014.PubMed/NCBI
|
|
3
|
Li Y, Tan BB, Zhao Q, Fan LQ, Wang D and
Liu Y: ZNF139 promotes tumor metastasis by increasing migration and
invasion in human gastric cancer cells. Neoplasma. 61:291–298.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katanoda K, Matsuda T, Matsuda A, Shibata
A, Nishino Y, Fujita M, Soda M, Ioka A, Sobue T and Nishimoto H: An
updated report of the trends in cancer incidence and mortality in
Japan. Jpn J Clin Oncol. 43:492–507. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naito Y, Uchiyama K, Kinoshita Y, Fukudo
S, Joh T, Suzuki H, Takahashi S, Ueno F, Fujiwara Y, Arakawa T, et
al: A questionnaire-based survey on screening for gastric and
colorectal cancer by physicians in East Asian countries in 2010.
Digestion. 86:94–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang YJ, Liu JZ, Lv P, Dang Y, Gao JY and
Wang Y: Long non-coding RNA CCAT2 promotes gastric cancer
proliferation and invasion by regulating the E-cadherin and LATS2.
Am J Cancer Res. 6:2651–2660. 2016.PubMed/NCBI
|
|
7
|
Sun Y, Zhang D, Mao M, Lu Y and Jiao N:
Roles of p38 and JNK protein kinase pathways activated by compound
cantharidin capsules containing serum on proliferation inhibition
and apoptosis of human gastric cancer cell line. Exp Ther Med.
14:1809–1817. 2017.PubMed/NCBI
|
|
8
|
Jia S, Qu T, Feng M, Ji K, Li Z, Jiang W
and Ji J: Association of Wnt1-inducible signaling pathway protein-1
with the proliferation, migration and invasion in gastric cancer
cells. Tumour Biol. 39:10104283176997552017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu YY, Chen ZH, Peng JJ, Wu JL, Yuan YJ,
Zhai ET, Cai SR, He YL and Song W: Up-regulation of long non-coding
RNA XLOC_010235 regulates epithelial-to-mesenchymal transition to
promote metastasis by associating with Snail1 in gastric cancer.
Sci Rep. 7:24612017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu M, Zhou X, Du Y, Huang Z, Zhu J, Xu J,
Cheng G, Shu Y, Liu P, Zhu W and Wang T: miR-20a induces cisplatin
resistance of a human gastric cancer cell line via targeting CYLD.
Mol Med Rep. 14:1742–1750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang QP, Wang Y, Wang XD, Mo XM, Gu J, Lu
ZY, Pan ZL and Zhu YX: Survivin up-regulates the expression of
breast cancer resistance protein (BCRP) through attenuating the
suppression of p53 on NF-κB expression in MCF-7/5-FU cells. Int J
Biochem Cell Biol. 45:2036–2044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wittkopf N, Günther C, Martini E, He G,
Amann K, He YW, Schuchmann M, Neurath MF and Becker C: Cellular
FLICE-like inhibitory protein secures intestinal epithelial cell
survival and immune homeostasis by regulating caspase-8.
Gastroenterology. 145:1369–1379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sikdar S, Mukherjee A, Ghosh S and
Khuda-Bukhsh AR: Condurango glycoside-rich components stimulate DNA
damage-induced cell cycle arrest and ROS-mediated caspase-3
dependent apoptosis through inhibition of cell-proliferation in
lung cancer, in vitro and in vivo. Environ Toxicol Pharmacol.
37:300–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hayakawa Y, Hirata Y, Sakitani K, Nakagawa
H, Nakata W, Kinoshita H, Takahashi R, Takeda K, Ichijo H, Maeda S
and Koike K: Apoptosis signal-regulating kinase-1 inhibitor as a
potent therapeutic drug for the treatment of gastric cancer. Cancer
Sci. 103:2181–2185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Korbakis D and Scorilas A: Treatment of
gastric cancer cells with 5-fluorouracil/leucovorin and irinotecan
induces distinct alterations in the mRNA expression of the
apoptosis-related genes, including the novel gene BCL2L12. Tumour
Biol. 30:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhuo Z, Zhang L, Mu Q, Lou Y, Gong Z, Shi
Y, Ouyang G and Zhang Y: The effect of combination treatment with
docosahexaenoic acid and 5-fluorouracil on the mRNA expression of
apoptosis-related genes, including the novel gene BCL2L12, in
gastric cancer cells. In Vitro Cell Dev Biol Anim. 45:69–74. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Wang JX, He YQ, Feng C, Zhang XJ,
Sheng JQ and Li PF: MicroRNA-185 regulates chemotherapeutic
sensitivity in gastric cancer by targeting apoptosis repressor with
caspase recruitment domain. Cell Death Dis. 24:e11972014.
View Article : Google Scholar
|
|
18
|
Wu XL, Cheng B, Li PY, Huang HJ, Zhao Q,
Dan ZL, Tian DA and Zhang P: MicroRNA-143 suppresses gastric cancer
cell growth and induces apoptosis by targeting COX-2. World J
Gastroenterol. 19:7758–7765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li X, Chen YT, Josson S, Mukhopadhyay NK,
Kim J, Freeman MR and Huang WC: MicroRNA-185 and 342 inhibit
tumorigenicity and induce apoptosis through blockade of the SREBP
metabolic pathway in prostate cancer cells. PLoS One. 8:e709872013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qu F, Cui X, Hong Y, Wang J, Li Y, Chen L,
Liu Y, Gao Y, Xu D and Wang Q: MicroRNA-185 suppresses
proliferation, invasion, migration, and tumorigenicity of human
prostate cancer cells through targeting androgen receptor. Mol Cell
Biochem. 377:121–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xiang Y, Ma N, Wang D, Zhang Y, Zhou J, Wu
G, Zhao R, Huang H, Wang X, Qiao Y, et al: miR-152 and miR-185
co-contribute to ovarian cancer cells cisplatin sensitivity by
targeting DNMT1 directly: A novel epigenetic therapy independent of
decitabine. Oncogene. 33:378–386. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Tan BB, Zhao Q, Fan LQ, Liu Y and
Wang D: Regulatory mechanism of ZNF139 in multi-drug resistance of
gastric cancer cells. Mol Biol Rep. 41:3603–3610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao LY, Tong DD, Xue M, Ma HL, Liu SY,
Yang J, Liu YX, Guo B, Ni L, Liu LY, et al: MeCP2, a target of
miR-638, facilitates gastric cancer cell proliferation through
activation of the MEK1/2-ERK1/2 signaling pathway by upregulating
GIT1. Oncogenesis. 6:e3682017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee H, Saini N, Parris AB, Zhao M and Yang
X: Ganetespib induces G2/M cell cycle arrest and apoptosis in
gastric cancer cells through targeting of receptor tyrosine kinase
signaling. Int J Oncol. 51:967–974. 2017.PubMed/NCBI
|
|
27
|
Xu X, Wu J, Li S, Hu Z, Xu X, Zhu Y, Liang
Z, Wang X, Lin Y, Mao Y, et al: Downregulation of microRNA-182-5p
contributes to renal cell carcinoma proliferation via activating
the AKT/FOXO3a signaling pathway. Mol Cancer. 13:1092014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang HY, Shen J, Jiang CP and Liu BR: How
to explain the contradiction of microRNA 200c expression and
survival in solid tumors? A meta-analysis. Asian Pac J Cancer Prev.
15:3687–3690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Jin X, Deng X, Zhang G, Zhang B and
Ma L: The putative tumor suppressor microRNA-497 modulates gastric
cancer cell proliferation and invasion by repressing eIF4E. Biochem
Biophys Res Commun. 449:235–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan W, Xiaoyun H, Haifeng Q, Jing L,
Weixu H, Ruofan D, Jinjin Y and Zongji S: MicroRNA-218 enhances the
radiosensitivity of human cervical cancer via promoting radiation
induced apoptosis. Int J Med Sci. 11:691–696. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Z, Zhang Z, Liu Z, Qiu B, Liu K and
Dong G: MicroRNA-335 inhibits invasion and metastasis of colorectal
cancer by targeting ZEB2. Med Oncol. 31:9822014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang SM, Huang C, Li XF, Yu MZ, He Y and
Li J: miR-21 confers cisplatin resistance in gastric cancer cells
by regulating PTEN. Toxicology. 306:162–168. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li ZH, Pan XM, Han BW, Guo XM, Zhang Z,
Jia J and Gao LB: A let-7 binding site polymorphism rs712 in the
KRAS 3′ UTR is associated with an increased risk of gastric cancer.
Tumour Biol. 34:3159–3163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao X, Yang L and Hu J: Down-regulation
of miR-27a might inhibit proliferation and drug resistance of
gastric cancer cells. J Exp Clin Cancer Res. 30:552011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhi Q, Zhu J, Guo X, He S, Xue X, Zhou J,
Hu B, Li H, Chen S, Zhao H and Kuang Y: Metastasis-related miR-185
is a potential prognostic biomarker for hepatocellular carcinoma in
early stage. Biomed Pharmacother. 67:393–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Qiu F, Xiong JP, Deng J and Xiang XJ:
TRIM29 functions as an oncogene in gastric cancer and is regulated
by miR-185. Int J Clin Exp Pathol. 8:5053–5061. 2015.PubMed/NCBI
|
|
37
|
Yoon JH, Choi WS, Kim O, Choi BJ, Nam SW,
Lee JY and Park WS: Gastrokine 1 inhibits gastric cancer cell
migration and invasion by downregulating RhoA expression. Gastric
Cancer. 20:274–285. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Golestani Eimani B, Sanati MH, Houshmand
M, Ataei M, Akbarian F and Shakhssalim N: Expression and prognostic
significance of bcl-2 and bax in the progression and clinical
outcome of transitional bladder cell carcinoma. Cell J. 15:356–363.
2014.PubMed/NCBI
|
|
39
|
Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang
C, Li M, Bao S and Zhu R: Dihydromyricetin reduced Bcl-2 expression
via p53 in human hepatoma HepG2 cells. PLoS One. 8:e768862013.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li S, Sun J, Yang J, Zhang L, Wang L, Wang
X and Guo Z: XIAP expression is associated with pancreatic
carcinoma outcome. Mol Clin Oncol. 1:305–308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chui YL, Ma CH, Li W, Xu Z, Yao Y, Lin FK,
Chan JY and Lee KK: Anti-apoptotic protein BRE/BRCC45 attenuates
apoptosis through maintaining the expression of caspase inhibitor
XIAP in mouse Lewis lung carcinoma D122 cells. Apoptosis.
19:829–840. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu M, Xia LP, Fan LJ, Xue JL, Shao WW and
Xu D: Livin and caspase-3 expression are negatively correlated in
cervical squamous cell cancer. Eur J Gynaecol Oncol. 34:152–155.
2013.PubMed/NCBI
|
|
43
|
Yang D, Song X, Zhang J, Ye L, Wang S, Che
X, Wang J, Zhang Z and Wang L: Suppression of livin gene expression
by siRNA leads to growth inhibition and apoptosis induction in
human bladder cancer T24 cells. Biosci Biotechnol Biochem.
74:1039–1044. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sikdar S, Mukherjee A, Ghosh S and
Khuda-Bukhsh AR: Condurango glycoside-rich components stimulate DNA
damage-induced cell cycle arrest and ROS-mediated caspase-3
dependent apoptosis through inhibition of cell-proliferation in
lung cancer, in vitro and in vivo. Environ Toxicol Pharmacol.
37:300–314. 2014. View Article : Google Scholar : PubMed/NCBI
|