Introduction
Myocardial ischemia/reperfusion (MI/R) injury was
formerly referred to as myocardial infarction, as it is a disease
that is caused by a sudden complete interruption of part of the
myocardial blood circulation, leading to myocardial necrosis at
that site (1). MI/R has become the
primary cause of mortality in China, as the morbidity and mortality
from MI/R increases each year (2).
The major cause of mortality in acute MI/R is acute heart failure
(2). With the development of
modern medicine, measures including internal medicine thrombolysis,
surgical bypass and interventional stent surgery, have reduced the
probability of mortality caused by the development of acute MI/R
into acute heart failure (3).
However, MI/R may enter a chronic phase as the disease develops
further, which is dominated by myocardial fibrotic remodeling;
excessive remodeling with fibrosis will induce chronic heart
failure, therefore leading to the patient succumbing to heart
failure (4).
Treatment of cardiovascular disease is based on
inflammation control (5). The
following aspects are essential in order to obtain the optimal
anti-inflammatory therapeutic effects: First, excellent detection
methods should be established in order to analyze adenosine
triphosphate (ATP) ase activity in NOD-like receptor molecules and
the issue of whether NACHT, LRR, and PYD domains-containing protein
3 (NLRP3) in myocardial tissue possesses tissue specificity should
also be discussed, in order to apply the appropriate targeted
therapy (6). Secondly, the
downstream signaling mechanism following the binding of a ligand
with the receptor should be further elucidated; excluding
interleukin (IL)-1β and IL-18, NLRP3 can still activate other
cytokines and inflammatory factors. There are a number of factors
that inhibit NLRP3 in vivo, such as signaling stimulating
the downregulation of Toll-like receptors (TLRs) and the release of
the autologous IL-1 receptor antagonist (5). In addition, the IL-1 receptor
antagonist has been reported to predict disease progression more
effectively than IL-1β (6). Once
these aforementioned issues have been resolved, more effective
therapeutic strategies with higher selectivity, improved regulatory
effects and decreased side effects can be developed (7).
Peroxisome proliferator-activated receptor-γ1
(PPAR-γ1) is a type of transcription factor activated by ligand
engagement, and its activation serves an important role in
regulating multiple pathophysiological processes (8). It has been reported that the
administration of a PPAR-γ ligand, such as pioglitazone,
demonstrated protective effects on the I/R myocardium, the
mechanism of which involved the increased expression of components
of the signaling pathways associated with anti-oxidative stress, as
well as the inhibition of inflammatory factor expression; however,
each ligand was not associated with strong specificity and PPAR-γ2,
or other sub-types of PPAR, may frequently be activated during
PPAR-γ1 activation as well (8,9).
Umbelliferone (structural formula exhibited in
Fig. 1), a type of coumarin
compound with the chemical name 7-hydroxycoumarin, is the major
active ingredient of Rutaceae and Umbelliferae in Chinese herbal
medicine, and belongs to the phenol family in terms of structure
(10). Umbelliferone possesses
anti-bacterial, anti-inflammatory and anti-apoptotic effects, which
have been demonstrated in multiple Chinese herbal medicinal plants
including Chinese Stellera chamaejasme Root, Radix
Angelicae Pubescentis, Saussurea involucrata and
Aegle marmelos (11,12).
In the present study, it was hypothesized that umbelliferone may
protect cardiac tissues against MI/R via the modulation of the
inflammasome and PPAR-γ.
Materials and methods
Animals and MI/R protocol
Adult male Sprague-Dawley rats (6–7 weeks, n=24)
with a body weight of 200–230 g were provided by the Laboratory
Animal Center of Guizhou Medical University (Guizhou, China),
housed at 22–23°C, 55–60% atmosphere and maintained under a 12 h
light/dark cycle with free access to food and water. The rats were
randomly divided into the following 3 groups (n=8 rats/group):
Control, MI/R model and the umbelliferone group. The rats were
injected with sodium pentobarbital intraperitoneally (30 mg/kg) and
the pericardium was dissected open. A left thoracic incision was
conducted within rats of the MI/R and umbelliferone groups and a
6-0 silk suture slipknot was placed around the left anterior
descending coronary artery to induce MI/R injury. Following 1 h,
the slipknot was released to re-perfuse the tissue. After 1 h
following re-perfusion surgery, intraperitoneal injections of 30
mg/kg/day umbelliferone were administered for 7 days to the rats of
the umbelliferone group. The rats of the control group were only
injected with sodium pentobarbital (30 mg/kg) intraperitoneally and
the pericardium was dissected open without MI/R injury as slipknot
surgery was not performed. The protocol for the present study was
approved by the Medical Ethics Committee of the People's Hospital
of Guizhou Province (Guiyang, China).
Measurement of myocardial infarct
size
Following treatment with umbelliferone and MI/R
induction, rats were injected with 1 ml of 2% Evans Blue dye into
the aorta under sodium pentobarbital anesthesia (30 mg/kg,
intraperitoneal injection) and immediately sacrificed. Heart
tissues were then quickly frozen at −70°C for 15 min and cut
transversally into 1-mm thick slices. Samples were incubated with
1% 2,3,5-triphenyltetrazolium chloride (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at 37°C for 10 min and observed with phase
contrast microscopy (magnification, ×10, Olympus IX83; Olympus,
Tokyo, Japan).
Assessment of cardiac function
Heart tissues were homogenized and lysed with
radioimmunoprecipitation assay (RIPA) buffer (Beyotime Institute of
Biotechnology, Haimen, China) for 15 min at 4°C in the presence of
protease inhibitors to extract protein. Protein content was
isolated using a bicinchoninic acid (BCA) assay. A total of 5 µg
protein was used to measure creatine kinase (CK; A032), CK-muscle
and brain (MB; H197) and lactate dehydrogenase (LDH; A020-2)
activity using ELISA kits (Nanjing Jiangcheng Bioengineering
Institute, Nanjing, China).
Determination of myocardial apoptosis,
inflammation, oxidative stress and inducible nitric oxide synthase
(iNOS) levels
Serum was collected following centrifugation at
1,000 × g for 10 min at 4°C and was used to measure tumor necrosis
factor-α (TNF-α; PT516), IL-6 (PI328), IL-1β (PI303) (all from
Beyotime Institute of Biotechnology) and IL-18 (H015; Nanjing
Jiancheng Biology Engineering Institute, Nanjing, China) levels
using ELISA kits. Heart tissues were homogenized and lysed with
RIPA buffer (Beyotime Institute of Biotechnology) in the presence
of protease inhibitors to extract protein. Protein content was
isolated using a BCA assay. A total of 5 µg protein was used to
measure superoxide dismutase (SOD; S0101), malondialdehyde (MDA;
S0131), caspase-3 (C1116), caspase-9 (AC062) and iNOS (S0025) (all
from Beyotime Institute of Biotechnology) levels using ELISA
kits.
Western blot analysis
Heart tissues were homogenized and lysed with RIPA
assay (Beyotime Institute of Biotechnology) in the presence of
protease inhibitors (PMSF, 1:100; Beyotime Institute of
Biotechnology) to extract protein. Protein content was determined
using a BCA assay and 50 µg protein from each sample were loaded on
to 2–3% SDS-PAGE. Proteins were transferred to polyvinylidene
difluoride membranes, which were blocked with 5% non-fat milk in
Tris-buffered saline with 0.1% Tween-20 for 1 h at 37°C. Membranes
were then incubated with primary antibodies against PPAR-γ (1:500;
sc-7196), B-cell lymphoma-2-associated X protein (Bax; 1:500;
sc-6236), NLRP3 (1:500; sc-66846), caspase-1 (1:500; sc-514) and
GAPDH (1:500; sc-25778) (all from Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) at 4°C overnight. The next day, blots were washed
with TBST three times for 15 min and incubated with an anti-rabbit
immunoglobulin G (H+L) biotinylated secondary antibody (1:5,000,
14708; Cell Signaling Technology, Inc.) for 1 h at room
temperature. The results were normalized to those of GAPDH and
observed using BeyoECL Star (Beyotime Institute of Biotechnology)
and analyzed using Image Lab 3.0 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Statistical analysis
The data are presented as the mean ± standard error
of the mean (n=3) using SPSS 17.0 (SPSS Inc., Chicago, IL, USA).
For more than two groups, one-way analysis of variance with a
Bonferroni post hoc test was conducted. P<0.05 was considered to
indicate a statistically significant difference.
Results
Umbelliferone significantly prevents
myocardial injury
The protective effect of umbelliferone on myocardial
injury was evaluated, by measuring myocardial infarct size and
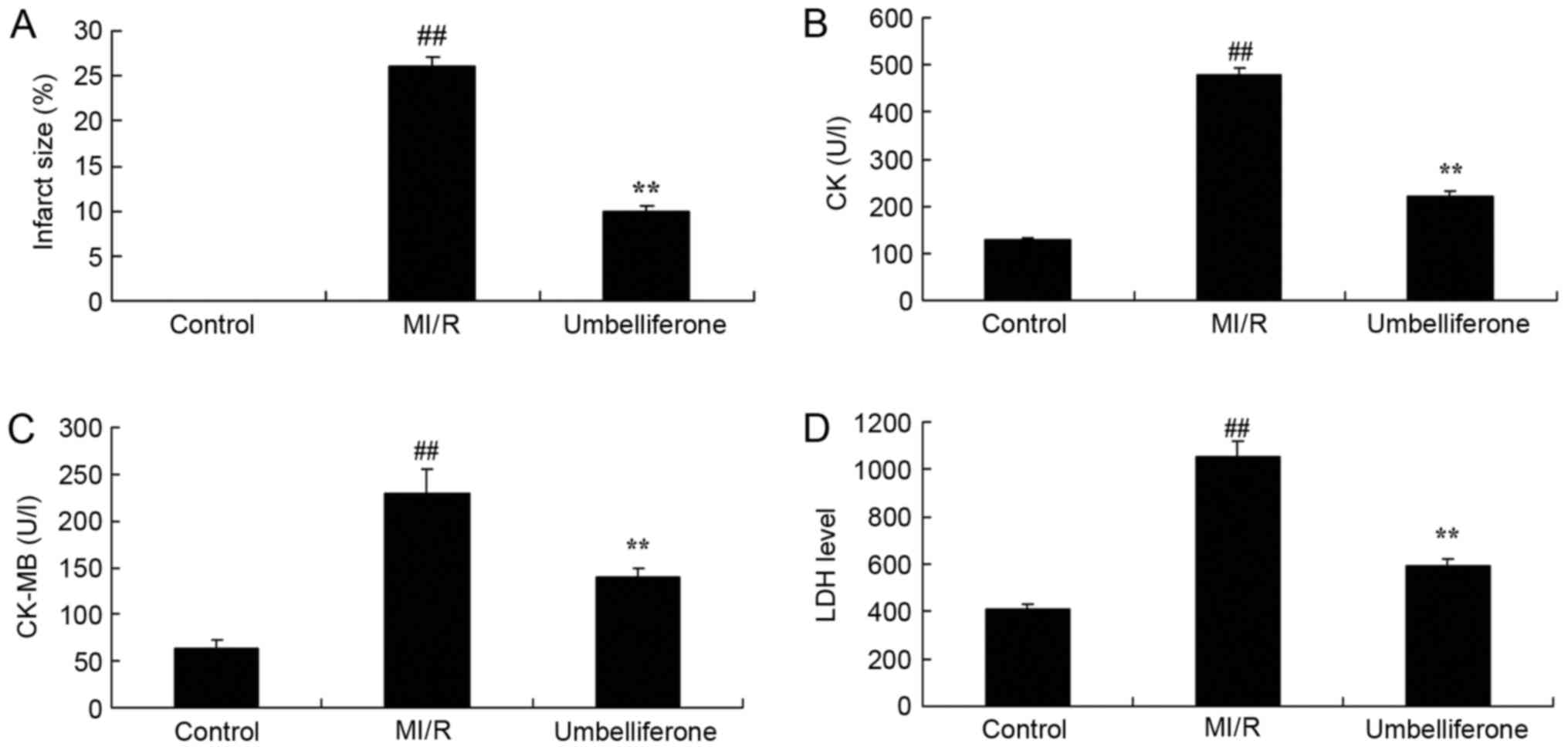
cardiac function. As demonstrated in Fig. 2, there were significant increases
in myocardial infarct size, and CK, CK-MB and LDH activity in the
MI/R model group, when compared with the control group. However,
treatment with umbelliferone significantly reduced myocardial
infarct size, and CK, CK-MB and LDH activity in MI/R mice when
compared with the MI/R group (P<0.01; Fig. 2).
Umbelliferone significantly inhibits
oxidative stress
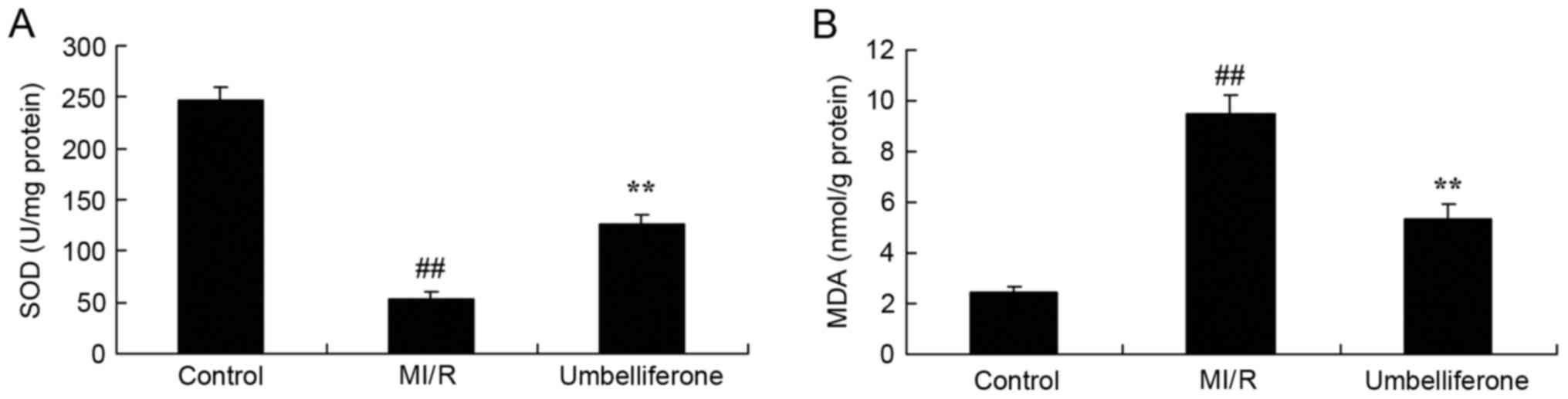
ELISA analysis demonstrated that oxidative stress
was increased, and the SOD level was lower and MDA level was higher
in the MI/R model group compared with in the control group
(P<0.01; Fig. 3). Umbelliferone
treatment significantly increased SOD level and inhibited MDA level
in MI/R mice, compared with in the MI/R model group (Fig. 3).
Umbelliferone significantly inhibits
inflammation
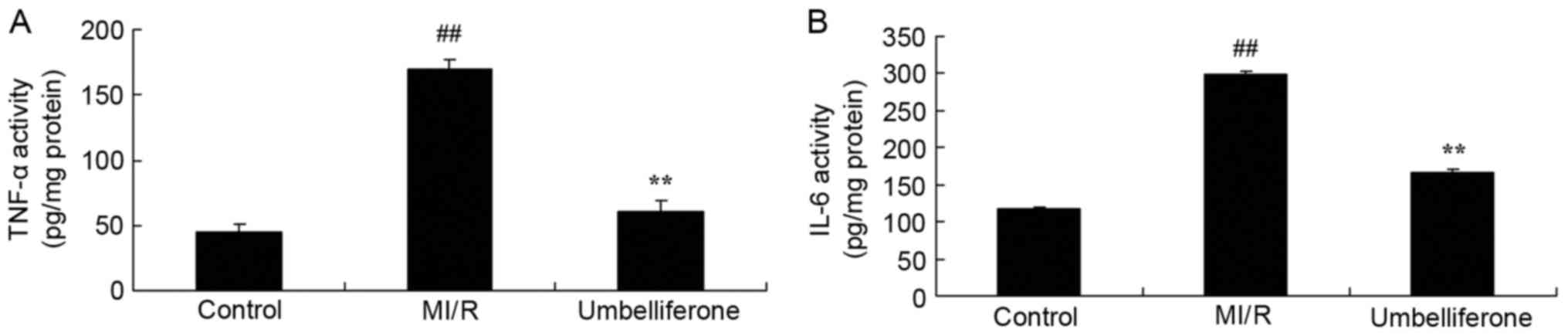
To determine the anti-inflammatory effects of
umbelliferone in MI/R, TNF-α and IL-6 levels were measured in mice
treated with umbelliferone. As shown in Fig. 4, ELISA analysis demonstrated that
there was a significant increase in TNF-α and IL-6 levels in the
MI/R model group, compared with the control group. The increase in
TNF-α and IL-6 levels in MI/R rats was significantly inhibited by
umbelliferone treatment (P<0.01; Fig. 4).
Umbelliferone significantly inhibits
myocardial caspase levels
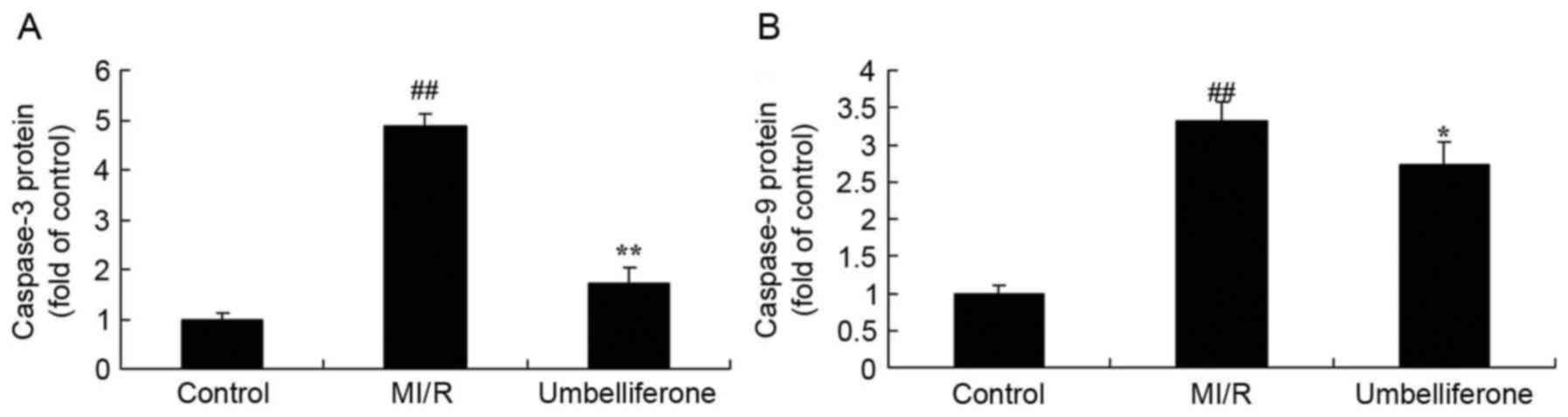
To evaluate the anti-apoptotic effects of
umbelliferone in MI/R, the activity of caspase-3 and −9 were
measured in MI/R rats treated with umbelliferone. Caspase-3 and −9
activity in the MI/R model group was significantly increased when
compared with the control group (P<0.01; Fig. 5). However, this increase in
caspase-3 and −9 activity in MI/R rats was reduced by treatment
with umbelliferone (Fig. 5).
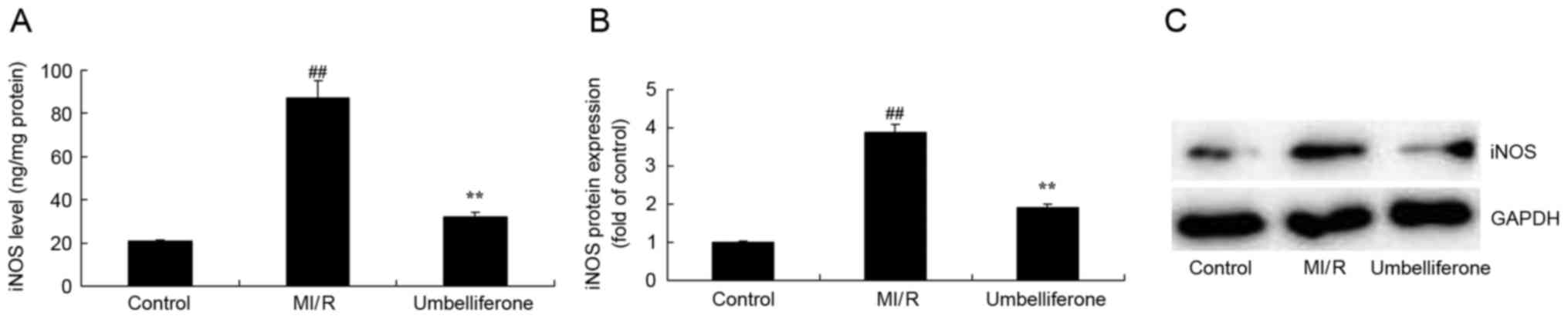
Umbelliferone significantly inhibits
iNOS activity and protein expression
To investigate the cardioprotective mechanism of
umbelliferone in MI/R, alterations in iNOS activity and protein
expression were analyzed. iNOS activity and protein expression were
significantly increased in the MI/R model group when compared with
the control group (P<0.01; Fig.
6). However, this increase in iNOS activity and protein
expression in MI/R rats was significantly inhibited by treatment
with umbelliferone (P<0.01; Fig.
6).
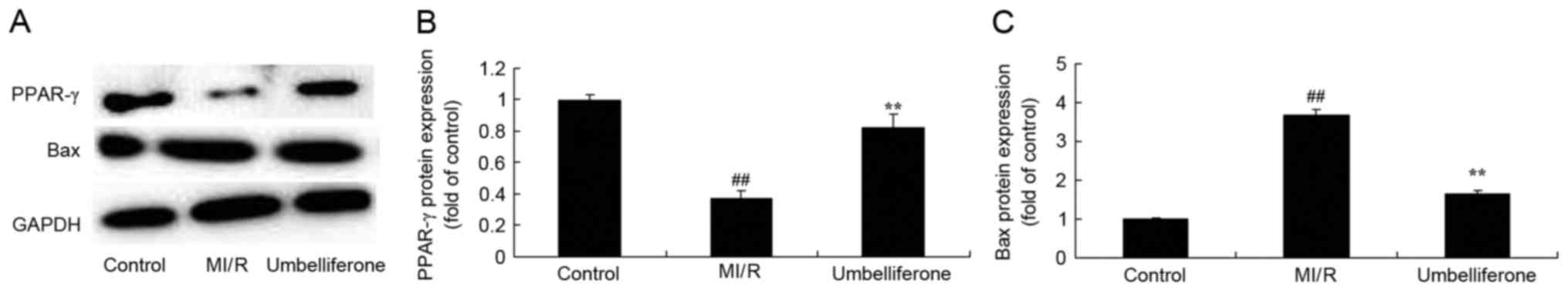
Umbelliferone significantly induces
PPAR-γ expression in rats with MI/R injury
Next, it was demonstrated that PPAR-γ protein
expression was downregulated and Bax protein expression was
upregulated in the MI/R model group when compared with the control
group (Fig. 7). Umbelliferone
significantly induced PPAR-γ protein expression and suppressed Bax
protein expression when compared with the MI/R rats (P<0.01;
Fig. 7).
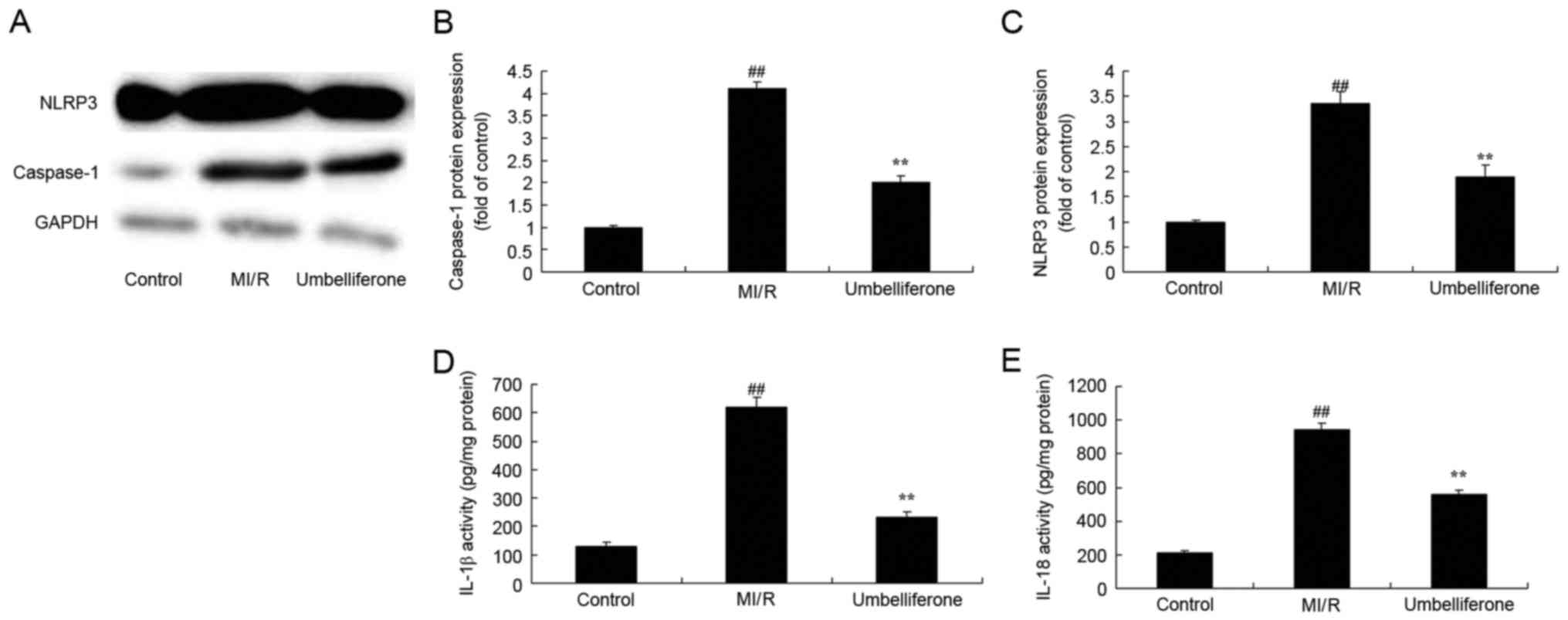
Umbelliferone significantly suppresses
NLRP3 inflammasome activation
To further investigate the anti-inflammatory effects
of umbelliferone in MI/R, the activation of the NLRP3 inflammasome
was analyzed. NLRP3 and caspase-1 protein expression were
significantly increased in the MI/R model group when compared with
the control group (P<0.01; Fig.
8A-C). IL-1β and IL-18 activity levels were also increased in
the MI/R model group when compared with the control group (Fig. 8D and E). However, umbelliferone
significantly suppressed NLRP3 and caspase-1 protein expression,
and IL-1β and IL-18 activity levels when compared with the MI/R
model group (P<0.01; Fig.
8).
Discussion
The levels of morbidity and mortality as a result of
myocardial infarction (MI) in China are increasing every year and
MI is also now affecting a younger demographic (13). At present, MI is primarily treated
by drug and interventional therapy in clinical practice, which can
partly alleviate symptoms and improve patient quality of life;
however, it cannot fundamentally repair or reconstruct the injured
or infarcted myocardium, which gives rise to the high mortality
rates associated with MI (13).
Cell therapy strategies have been previously developed, that can
repair the injured myocardium to some extent; however, they cannot
improve the hostile MI microenvironment (including ischemia,
hypoxia, apoptosis and inflammation), and therefore, produce low
retention and survival rates in the MI region, as well as
unsatisfying long-term effects (14). With the further developments made
in material science, life sciences, medical science and
engineering, the injectable myocardial tissue engineering strategy
with the basic elements of biomaterials, seeding cells and growth
factors is promising to overcome the drawbacks of cell
transplantation strategy, with an aim to realize myocardial repair
and reconstruction in a real sense; it has subsequently become a
key area of interest for research into treatments for MI (15). The results of the present study
demonstrated that treatment with umbelliferone significantly
reduced myocardial infarct size, and CK, CK-MB and LDH activity in
an MI/R rat model.
A previous study indicated that MI leads to massive
necrosis and dissolution of myocardial cells, as well as
inflammatory cell infiltration, thereby resulting in decreased
cardiac function (16). MI
produces an abnormal intracellular environment as a result of
insufficient energy supply, and the compensatory heart contraction
will give rise to an elevated level of reactive oxygen species
(ROS) induced by nicotinamide adenine dinucleotide phosphate
(17,18). An elevated ROS level will in turn
trigger a massive production of ROS by the mitochondria (16). Oxidative stress causes extensive
damage to cell membranes and organelles, and can also induce an
inflammatory response through mutual enhancement with inflammatory
factors, further aggravating MI-induced myocardial injury (19). Consequently, improving the
antioxidant enzyme level and reducing ROS content are some of the
main objectives of developing treatments for MI (19). In the present study it was
demonstrated that umbelliferone treatment significantly inhibited
SOD levels and increased MDA levels in MI/R rats. Vijayalakshmi
et al (12) suggested that
umbelliferone prevented the incidence of tumors in
7,12-dimethylbenz[a]anthracene-induced oral carcinogenesis via
antioxidant and xenobiotic metabolic effects.
The inflammatory response exhibits bidirectional
effects during the MI and post-MI repair phases; the early
inflammatory response can eliminate necrotic tissue, promote
granulation tissue formation, restrict injury of ischemia to the MI
surrounding tissues, stabilize the extracellular matrix and promote
scar formation (17). However, an
excessive inflammatory response will induce tissue injury in the MI
surrounding area, expand the MI size, enhance myocardial fibrosis,
promote ventricular remodeling, reduce cardiac function and can
lead to acute cardiac rupture (20). Furthermore, in the present study it
was demonstrated that umbelliferone significantly reduced TNF-α and
IL-6 levels in MI/R rats. Chunhua et al (11) reported that umbelliferone reverses
depression-like behavior via the nuclear receptor-interacting
protein 140/nuclear factor (NF)-κB signaling pathway in chronic
unpredictable mild stress-induced mice.
It has been demonstrated in a previous study that MI
will induce an aseptic inflammatory response (21). The following phenomena supports the
hypothesis that NLRP3 may be involved in this process (21). Expression of apoptosis-associated
speck-like protein containing a CARD (ASC) and caspase-1 is
increased in infiltrating macrophages and neutrophils in myocardial
tissue following MI in rats with ASC-deletion; in addition, NLRP3
can identify multiple damage-associated molecular patterns (DAMPs)
that are produced following myocardial injury (21). During the hepatic I/R process,
NLRP3 promotes the release of inflammatory factors and leads to I/R
injury (21). I/R injury is
reduced in NLRP3-deletion rat models (21). Sandanger et al (22) indicated that cardiac fibroblast may
serve a role in initiating inflammation during the early stage of
MI. A study proposed the following hypotheses: i) DAMPs may be
released following injury in myocardial cells as a result of
insufficient or interrupted blood supply, and TLRs on cardiac
fibroblast membranes may identify DAMPs and promote the
intracellular expression of NF-κB and NLRP3; ii) the injured
cardiac fibroblast may develop ATP-dependent K+ outflow,
and a large amount of ROS may be produced during reperfusion, which
may further activate NLRP3 in cardiac fibroblasts (5). This result demonstrated that
umbelliferone significantly suppressed NLRP3 and caspase-1 protein
expression in MI/R rats. Wang et al (23) suggested that umbelliferone may be
protective against focal cerebral ischemia partly through the NLRP3
inflammasome and activation of PPAR-γ.
PPAR-γ is a transcription factor in the nuclear
receptor superfamily that is activated by a ligand (24). The activated ligand forms a
heterodimer with the retinoic acid X receptor and then binds with
the specific PPAR response element in the target gene promoter,
thereby promoting or inhibiting the expression of its downstream
gene, and exerting regulatory effects at the transcriptional level
(24). Its activation is
associated with multiple diseases, including diabetes, lipid
metabolism, atherosclerosis and cancer (8). A previous study demonstrated that
myocardial fibers are sparsely arranged in a disorderly manner,
with a wide intercellular space and high levels of red blood cell
and inflammatory cell infiltration in the stroma; while relatively
ordered and densely arranged myocardial fibers can be seen in the
PPAR-γ group, with mild cell swelling, and little red blood cell
and inflammatory cell infiltration (25). In the present study, it was
demonstrated that umbelliferone significantly induced PPAR-γ
protein expression, and suppressed Bax and iNOS protein expression
in the MI/R rat model. Mahmoud et al (26) demonstrated that the protective
effect of umbelliferone may prevent cyclophosphamide-induced
hepatotoxicity through PPAR-γ expression.
In conclusion, the results of the present study
demonstrated that umbelliferone may ameliorate myocardial injury,
inflammation, oxidative stress and apoptosis through the
suppression of the NLRP3 inflammasome and upregulation of PPAR-γ
expression in rat. Therefore, umbelliferone demonstrates potential
as a drug for the treatment of MI/R or other cardiovascular
diseases.
Acknowledgements
The present study was supported by Qiankehe Lh Zi
(grant no. 2016, 7181).
References
|
1
|
Prasad A, Gersh BJ, Mehran R, Brodie BR,
Brener SJ, Dizon JM, Lansky AJ, Witzenbichler B, Kornowski R,
Guagliumi G, et al: Effect of ischemia duration and door-to-balloon
time on myocardial perfusion in ST-segment elevation myocardial
infarction: An analysis from HORIZONS-AMI trial (harmonizing
outcomes with revascularization and stents in acute myocardial
infarction). JACC Cardiovasc Interv. 8:1966–1974. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grygier M, Araszkiewicz A, Lesiak M, Janus
M, Kowal J, Skorupski W, Pyda M, Mitkowski P and Grajek S: New
method of intracoronary adenosine injection to prevent
microvascular reperfusion injury in patients with acute myocardial
infarction undergoing percutaneous coronary intervention. Am J
Cardiol. 107:1131–1135. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Woo JS, Kim W, Ha SJ, Kim JB, Kim SJ, Kim
WS, Seon HJ and Kim KS: Cardioprotective effects of exenatide in
patients with ST-segment-elevation myocardial infarction undergoing
primary percutaneous coronary interventio: Results of exenatide
myocardial protection in revascularization study. Arterioscler
Thromb Vasc Biol. 33:2252–2260. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamarneh A, Sivaraman V, Bulluck H,
Shanahan H, Kyle B, Ramlall M, Chung R, Jarvis C, Xenou M, Ariti C,
et al: The effect of remote ischemic conditioning and glyceryl
trinitrate on perioperative myocardial injury in cardiac bypass
surgery patients: Rationale and design of the ERIC-GTN study. Clin
Cardiol. 38:641–646. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toldo S, Marchetti C, Mauro AG, Chojnacki
J, Mezzaroma E, Carbone S, Zhang S, Van Tassell B, Salloum FN and
Abbate A: Inhibition of the NLRP3 inflammasome limits the
inflammatory injury following myocardial ischemia-reperfusion in
the mouse. Int J Cardiol. 209:215–220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu SY, Tang L, Zhao GJ and Zhou SH: Statin
protects the heart against ischemia-reperfusion injury via
inhibition of the NLRP3 inflammasome. Int J Cardiol. 229:23–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Valle Raleigh J, Mauro AG, Devarakonda T,
Marchetti C, He J, Kim E, Filippone S, Das A, Toldo S, Abbate A and
Salloum FN: Reperfusion therapy with recombinant human relaxin-2
(Serelaxin) attenuates myocardial infarct size and NLRP3
inflammasome following ischemia/reperfusion injury via
eNOS-dependent mechanism. Cardiovasc Res. 113:609–619.
2017.PubMed/NCBI
|
|
8
|
Wu Y, Tan X, Tian J, Liu X, Wang Y, Zhao
H, Yan Z, Liu H and Ma X: PPARγ agonist ameliorates the impaired
fluidity of the myocardial cell membrane and cardiac injury in
hypercholesterolemic rats. Cardiovasc Toxicol. 17:25–34. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ravingerova T, Adameova A, Carnicka S,
Nemcekova M, Kelly T, Matejikova J, Galatou E, Barlaka E and Lazou
A: The role of PPAR in myocardial response to ischemia in normal
and diseased heart. Gen Physiol Biophys. 30:329–341. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rezaee R, Behravan E, Behravan J, Soltani
F, Naderi Y, Emami B and Iranshahi M: Antigenotoxic activities of
the natural dietary coumarins umbelliferone, herniarin and
7-isopentenyloxy coumarin on human lymphocytes exposed to oxidative
stress. Drug Chem Toxicol. 37:144–148. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chunhua M, Lingdong K, Hongyan L and
Zhangqiang M: Retracted: Umbelliferone reverses depression-like
behavior in chronic unpredictable mild stress-induced mice via
RIP140/NF-κB pathway. IUBMB Life. 69:7672017. View Article : Google Scholar
|
|
12
|
Vijayalakshmi A and Sindhu G: Dose
responsive efficacy of umbelliferone on lipid peroxidation,
anti-oxidant, and xenobiotic metabolism in DMBA-induced oral
carcinogenesis. Biomed Pharmacother. 88:852–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hofsten DE, Kelbaek H, Helqvist S,
Kløvgaard L, Holmvang L, Clemmensen P, Torp-Pedersen C, Tilsted HH,
Bøtker HE, Jensen LO, et al: The third DANish study of optimal
acute treatment of patients with ST-segment elevation myocardial
infarction: Ischemic postconditioning or deferred stent
implantation versus conventional primary angioplasty and complete
revascularization versus treatment of culprit lesion only:
Rationale and design of the DANAMI 3 trial program. Am Heart J.
169:613–621. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hausenloy DJ, Candilio L, Laing C, Kunst
G, Pepper J, Kolvekar S, Evans R, Robertson S, Knight R, Ariti C,
et al: Effect of remote ischemic preconditioning on clinical
outcomes in patients undergoing coronary artery bypass graft
surgery (ERICCA): Rationale and study design of a multi-centre
randomized double-blinded controlled clinical trial. Clin Res
Cardiol. 101:339–348. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu YL, Kampinga MA, Wieringa WG, Fokkema
ML, Nijsten MW, Hillege HL, Van den Heuvel AF, Tan ES, Pundziute G,
van der Werf R, et al: Intracoronary versus intravenous
administration of abciximab in patients with ST-segment elevation
myocardial infarction undergoing primary percutaneous coronary
intervention with thrombus aspiration: The comparison of
intracoronary versus intravenous abciximab administration during
emergency reperfusion of ST-segment elevation myocardial infarction
(CICERO) trial. Circulation. 122:2709–2717. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pei H, Song X, Peng C, Tan Y, Li Y, Li X,
Ma S, Wang Q, Huang R, Yang D, et al: TNF-α inhibitor protects
against myocardial ischemia/reperfusion injury via Notch1-mediated
suppression of oxidative/nitrative stress. Free Radic Biol Med.
82:114–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yue R, Xia X, Jiang J, Yang D, Han Y, Chen
X, Cai Y, Li L, Wang WE and Zeng C: Mitochondrial DNA oxidative
damage contributes to cardiomyocyte ischemia/reperfusion-injury in
rats: Cardioprotective role of lycopene. J Cell Physiol.
230:2128–2141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Z, Wang S, Zhang X, Li Y, Zhao Q and
Liu T: Pterostilbene protects against myocardial
ischemia/reperfusion injury via suppressing oxidative/nitrative
stress and inflammatory response. Int Immunopharmacol. 43:7–15.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zheng P, Xie Z, Yuan Y, Sui W, Wang C, Gao
X, Zhao Y, Zhang F, Gu Y, Hu P, et al: Plin5 alleviates myocardial
ischaemia/reperfusion injury by reducing oxidative stress through
inhibiting the lipolysis of lipid droplets. Sci Rep. 7:425742017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Zhang J, Yu P, Chen M, Peng Q,
Wang Z and Dong N: Remote ischaemic preconditioning and sevoflurane
postconditioning synergistically protect rats from myocardial
injury induced by ischemia and reperfusion partly via Inhibition
TLR4/MyD88/NF-κB signaling pathway. Cell Physiol Biochem. 41:22–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sandanger Ø, Ranheim T, Vinge LE, Bliksøen
M, Valen G, Aukrust P and Yndestad A: A role for NLRP3 inflammasome
in acute myocardial ischaemia-reperfusion injury? Reply. Cardiovasc
Res. 99:226–227. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandanger Ø, Ranheim T, Vinge LE, Bliksøen
M, Alfsnes K, Finsen AV, Dahl CP, Askevold ET, Florholmen G,
Christensen G, et al: The NLRP3 inflammasome is up-regulated in
cardiac fibroblasts and mediates myocardial ischaemia-reperfusion
injury. Cardiovasc Res. 99:164–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Li R, Wang X, Fu Q and Ma S:
Umbelliferone ameliorates cerebral ischemia-reperfusion injury via
upregulating the PPAR gamma expression and suppressing TXNIP/NLRP3
inflammasome. Neurosci Lett. 600:182–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Yu Z, Huang X, Gao Y, Wang X, Gu J
and Xue S: Peroxisome proliferator-activated receptor γ (PPARγ)
mediates the protective effect of quercetin against myocardial
ischemia-reperfusion injury via suppressing the NF-κB pathway. Am J
Transl Res. 8:5169–5186. 2016.PubMed/NCBI
|
|
25
|
Qian J, Chen H, Birnbaum Y, Nanhwan MK,
Bajaj M and Ye Y: Aleglitazar, a balanced dual PPARα and -γ
agonist, protects the heart against ischemia-reperfusion injury.
Cardiovasc Drugs Ther. 30:129–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mahmoud AM, Germoush MO, Alotaibi MF and
Hussein OE: Possible involvement of Nrf2 and PPARγ up-regulation in
the protective effect of umbelliferone against
cyclophosphamide-induced hepatotoxicity. Biomed Pharmacother.
86:297–306. 2017. View Article : Google Scholar : PubMed/NCBI
|