Introduction
Ischemic stroke caused by blood vessel occlusion is
a major cause of morbidity and mortality in humans. Numerous
pathological processes are associated with ischemic neuronal
damage, including inflammation, energy metabolism disturbance,
necrotic and apoptotic cell death, and excitotoxicity (1). Previous studies have aimed to improve
the understanding of the underlying mechanisms of ischemic stroke;
however, further investigation is required to identify a potential
target for the effective prevention and treatment of stroke. Recent
clinical and experimental studies have demonstrated that
alternative complementary medicine may exhibit a unique efficacy in
the treatment of stroke (2,3).
MicroRNA (miRNA/miR), which are non-coding RNAs, are
frequently employed in research and are characterized by their
regulatory effects on gene expression by inhibiting mRNA
translation and/or mediating mRNA degradation (4,5). As
numerous pathways are implicated in cerebral ischemic injury,
including oxidative stress, excitotoxicity, mitochondrial
dysfunction and inflammation, miRNAs have been associated with
cerebral ischemic injury due to their roles in ischemic processes,
including neuronal cell differentiation (6,7),
dendritic plasticity and neuronal outgrowth modulation (8). As a multifunctional miRNA, miR-155
expression is affected by cerebral ischemia (9–11)
and has been reported to be involved in the regulation of a variety
of physiological and pathological processes, including inflammation
and immunity (12,13). Several inflammatory and cytotoxic
factors, including tumor necrosis factor-α, interleukin (IL)-1β and
IL-6, secreted by activated microglia may exert an effect on
neuronal cell survival (14).
miR-155 may regulate the immune response mediated by microglia in
various pathological states (15–17).
Gardenia is the fruit of Gardenia jasminoides
Ellis (Rubiaceae), which is a commonly used Chinese herb. Gardenia
has been reported to exhibit therapeutic effects on hepatic
disorders, brain diseases, inflammatory diseases and contusions
(18–20). A previous study reported that
geniposide, an active component of Gardenia, reduces neuronal cell
death and increases cell viability following oxygen and glucose
deprivation (OGD) (21). Our
previous study also revealed that geniposide exhibits protective
effects against infarction volume, microglial activation and
inflammatory cytokine release in middle cerebral artery occlusion
(MCAO) model rats (22). In
clinical and experimental research, Gardenia has previously been
used in combination with Panax notoginseng to treat brain
diseases, including cerebrovascular disease and Alzheimer's disease
(23,24). For rats injured by local ischemia,
ginsenoside Rg1, a component of Panax notoginseng, was
reported to alleviate neurological function deficits, as determined
by improvements in the learning and memory abilities, in addition
to a reduction in the apoptosis of neurons in the hippocampus CA1
zone (25,26). The present study aimed to
investigate whether tail vein-administered geniposide in
combination with ginsenoside Rg1 may protect against ischemic
stroke via miR-155 inhibition. An in vivo investigation into
miR-155-5p expression, and the effects of geniposide + ginsenoside
Rg1 on miR-155-5p expression within the brain and activation of
microglia, as determined by immunohistochemistry for CD11b, was
performed. In addition, an in vitro study was performed to
analyze the effects of geniposide + ginsenoside Rg1 on miR-155-5p
expression in BV2 microglial cells.
Materials and methods
Animals and grouping
Specific pathogen and virus antibody-free grade male
Sprague-Dawley rats (n=56; 253.4±20.3 g; 7–8 weeks-old) were
obtained from Beijing Vital River Laboratory Animal Technology Co.,
Ltd. (Beijing, China). In accordance with the UK Animals
(Scientific Procedures) Act of 1986 (27), the animal experiments were
performed with the approval of the ethics committee of the
Institute of Basic Theory, China Academy of Chinese Medical
Sciences (Beijing, China). The rats were housed in rooms maintained
at 23±2°C in a 12 h light/dark cycle and fed a rodent standard diet
with free access to water. The animals were randomly divided into
three groups (n=8 per group): Sham, model and geniposide (30 mg/kg)
+ ginsenoside Rg1 (6 mg/kg) groups. For the sham group, the rats
underwent similar surgery to the model group, without nylon
filament insertion; rats in the model group were administrated
saline via the tail vein immediately following MCAO. Rats in the
geniposide + ginsenoside Rg1 group were administered geniposide (30
mg/kg) + ginsenoside Rg1 (6 mg/kg) via the tail vein immediately
following MCAO. Geniposide (purity, >95%; cat. no.
110749-201718) and ginsenoside Rg1 (purity, >95%; cat. no.
110703-201731) were purchased from the National Institutes for Food
and Drug Control (Beijing, China).
MCAO model establishment
The MCAO model was established as described by Longa
et al (28). Rats were
anesthetized with 10% chloral hydrate (350 mg/kg body weight,
intraperitoneal injection) (29,30).
Subsequently, a midline incision was made to expose the left common
carotid artery and the external carotid artery (ECA), including the
occipital artery branches. Following this, blood from the occipital
artery and superior thyroid artery branches of the ECA was
coagulated using a bipolar electrocoagulator. The internal carotid
artery (ICA) was isolated and carefully separated from the adjacent
vagus nerve. Following ICA exposure, the origin of the left middle
cerebral artery was occluded using a 0.25 mm diameter nylon
filament coated with poly-L-lysine, which was inserted into the ICA
via the ECA. Rectal temperature was maintained at 37°C during and
following surgery with a temperature-controlled heating pad (CMA
150 Carnegie Medicin AB, Solna, Sweden). After 2, 6 and 24 h, the
ischemic procedure was complete.
Infarct size and brain edema
detection
Rats of the 24 h ischemia group were selected for
in vivo analysis; statistical calculations demonstrated no
significant difference when comparing the 2 and 6 h ischemia group
rats with the control group. Following the performance of ischemic
injury for different time periods, all animals were sacrificed.
Brain tissues were removed and prepared into 2 mm coronal sections.
The sections were stained with 1.5% 2,3,5-triphenyltetrazolium
chloride at 37°C for 10 min and fixed in 4% paraformaldehyde for 24
h at room temperature. The images of the stained slices were
analyzed using Image-Pro Plus 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA) to determine the infarct size, which was
expressed as the percentage of the whole brain tissue. Brain edema
was calculated according to the following equation: [(volume of
ipsilateral hemisphere-volume of contralateral hemisphere)/volume
of contralateral hemisphere] ×100. The experiment was independently
repeated three times.
Cell culture and in vitro model
Mouse BV2 microglial cells were purchased from the
Institute of Basic Medical Sciences of the China Science Academy
(Beijing, China) and cultured in Dulbecco's modified Eagle
medium/Ham's F12 (DMEM/F12; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) culture medium containing 10% fetal bovine serum
(Thermo Fisher Scientific, Inc.) and 1% penicillin and streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in a humidified 5%
CO2/95% air atmosphere at 37°C. The microglial cells
were passaged at 90% confluence, with two to three passages per
week. To mimic the OGD pathological process, glucose-free DMEM
(Gibco; Thermo Fisher Scientific, Inc.) was used instead of the
culture medium and oxygen was restricted in the flasks by keeping
the culture flasks in a sealed tank with a persistent low-flow (1.5
l/min) of 95% N2 and 5% CO2 mixture. The
inlet and outlet ends of the tubes were then clipped and the sealed
tank was returned to the incubator. DMEM was used to dissolve
geniposide and ginsenoside Rg1 and prepare the storage solution. A
total of 40 µg/ml geniposide + 8 µg/ml ginsenoside Rg1 was
dissolved within the culture media. After 4, 8 and 16 h of OGD
exposure at 37°C, with or without geniposide (40 µg/ml) +
ginsenoside Rg1 (8 µg/ml), BV2 cells were collected for the
analysis of miR-155-5p expression. The experiment was independently
repeated three times.
miR-155-5p, pri-miR-155 and
pre-miR-155 quantification by reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA of brain tissue and cultured BV2
microglial cells was isolated and analyzed using a Reverse
Transcriptase kit (Suzhou GenePharma, Co., Ltd., Suzhou, China).
The temperature and duration of reverse transcription was performed
at 30°C for 10 min, 42°C for 30 min and 99°C for 5 min. A stem-loop
real-time PCR system was used to determine the level of miRNAs
using the Maxima SYBR Green qPCR Master Mix (Fermentas; Thermo
Fisher Scientific, Inc., Waltham MA, USA) and a StepOne Sequence
Detector (Applied Biosystems; Thermo Fisher Scientific, Inc.). qPCR
was performed at 95°C for 10 min followed by 40 cycles at 95°C for
15 sec and 60°C for 60 sec. The following primer sequences were
used: miR-155 forward, 5′-GCTTCGGTTAATGCTAATCGTG-3′; miR-155
reverse, 5′-AGAGCAGGGTCCGAGGTA-3′; U6 forward,
5′-ATTGGAACGATACAGAGAAGATT-3′; U6 reverse,
5′-GGAACGCTTCACGAATTTG-3′; pre-miR-155 forward,
5′-TGCTAATTGTGATAGGGGTTTT-3′; pre-miR-155 reverse,
5′-TATGGTTGTTCACGACTCCTTCAC-3′; pri-miR-155 forward,
5′-AGGCTTTTCCTGGGCACC-3′; and pri-miR-155 reverse,
5′-CATGAACAAACCACAACGAGC-3′. RT-qPCR was performed in duplicate and
relative expression of miR-155-5p, pri-miR-155 and pre-miR-155 were
normalized to U6 expression and determined by the 2−ΔΔCq
method (31). The experiment was
independently repeated three times.
Immunohistochemistry
Brain tissues from rats were removed for
immunohistochemistry analysis at the termination of ischemia. Brain
tissues were fixed in 4% paraformaldehyde overnight at room
temperature. Sections (5 µm) embedded in paraffin were
deparaffinized in Histo-Clear™ (HS-200; National
Diagnostics, Atlanta, GA, USA) rehydrated and washed with 0.01 M
PBS; 3% hydrogen peroxide in methanol was used at room temperature
for 30 min to inhibit the endogenous peroxidase. Sections were
incubated with rabbit anti-CD11b (1:500) antibody (cat. no.
ab133357; Abcam, Cambridge, MA) at 4°C overnight following blocking
of nonspecific antigens with goat serum (Gibco; Thermo Fisher
Scientific, Inc.) at 37°C for 20 min. Subsequently, sections were
incubated with horseradish peroxidase-conjugated secondary
antibodies at 37°C for 2 h. Peroxidase detection was performed
using a mixture containing 0.05% 3,3′-diaminobenzidine. Following
staining, all sections were coverslipped and analyzed. The
immunohistochemistry images were acquired using an Olympus Fluoview
FV1000 microscope (LSI3-FV1000-Inverted; Olympus Corporation,
Tokyo, Japan) and Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.). The experiment was independently repeated three times.
Statistical analysis
Data are presented as the mean + standard deviation.
The statistical analysis was conducted with SPSS software (SPSS,
Inc. Chicago, IL, USA). Significant differences among the groups
were determined by one-way analysis of variance, followed by a
Student-Newman-Keuls post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
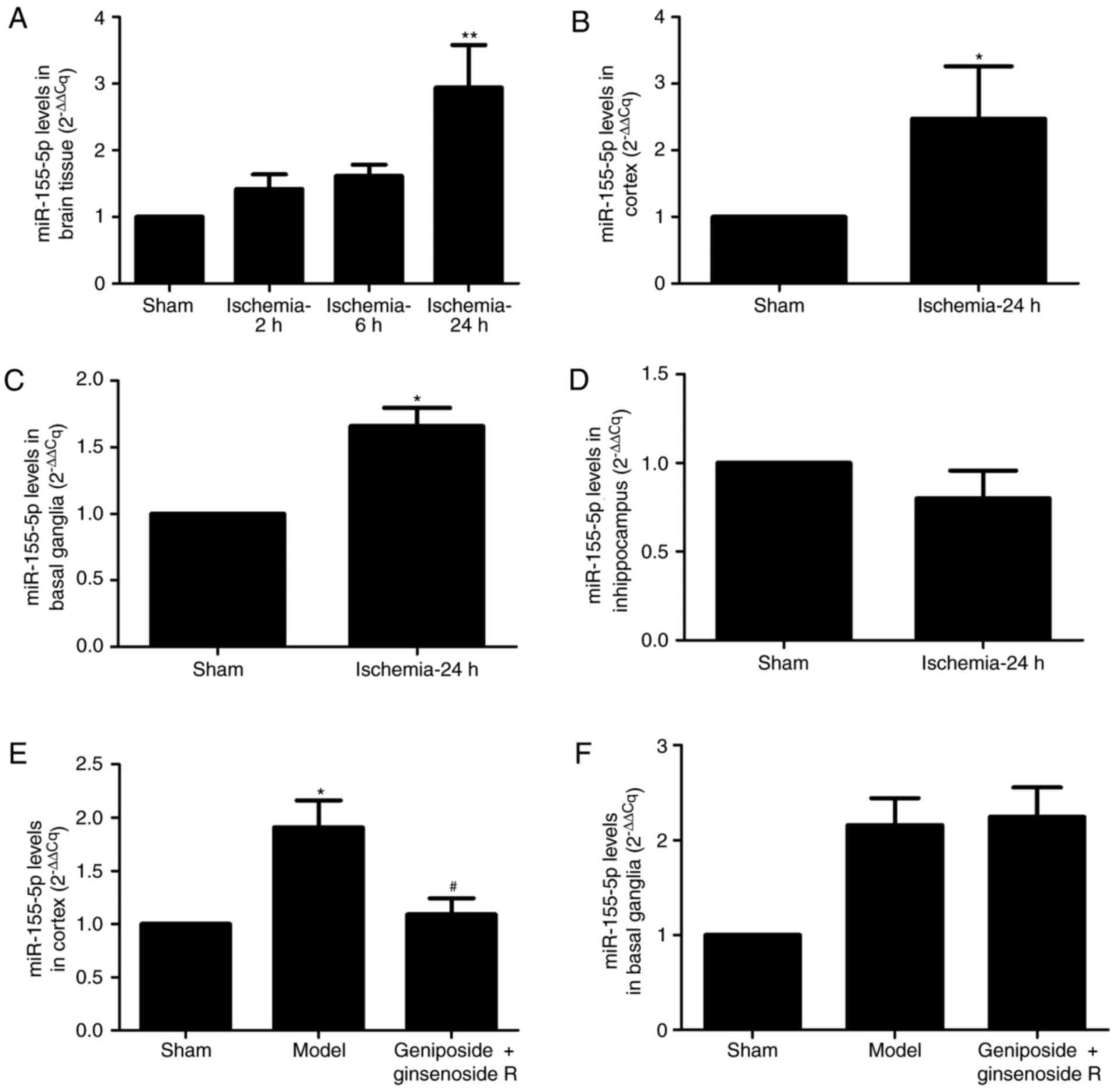
miR-155-5p expression in rats
In the present study, a rat MCAO model was
established. miR-155-5p expression levels increased in a
time-dependent manner; miR-155-5p expression levels were
significantly increased in the ipsilateral brain tissue of rats in
the 24 h ischemia group compared with the sham group (P<0.01;
Fig. 1A). The Cq values of 2, 6
and 24 h ischemia groups increased by 1.42-, 1.62- and 2.94-fold,
respectively, compared with the sham group (Fig. 1A). To investigate whether the
increase of miR-155-5p in the brain was region specific, miR-155-5p
expression levels were observed within various brain regions at 24
h post-ischemia. The results demonstrated that miR-155-5p
expression was significantly increased in the cortex and basal
ganglia of the ipsilateral brain in MCAO models, compared with in
the sham group (P<0.05; Fig. 1B and
C). However, a significant increase was not observed in the
expression levels of miR-155-5p within the hippocampus of MCAO
model rats, compared with in the sham group (Fig. 1D). Treatment with geniposide +
ginsenoside Rg1 attenuated the miR-155-5p Cq value markedly
compared with the model group (1.67±0.52 and 2.93±0.87,
respectively; P<0.05; Fig. 1E).
However, geniposide + ginsenoside Rg1 had no effect on the
miR-155-5p expression levels in the basal ganglia of MCAO model
rats (Fig. 1F).
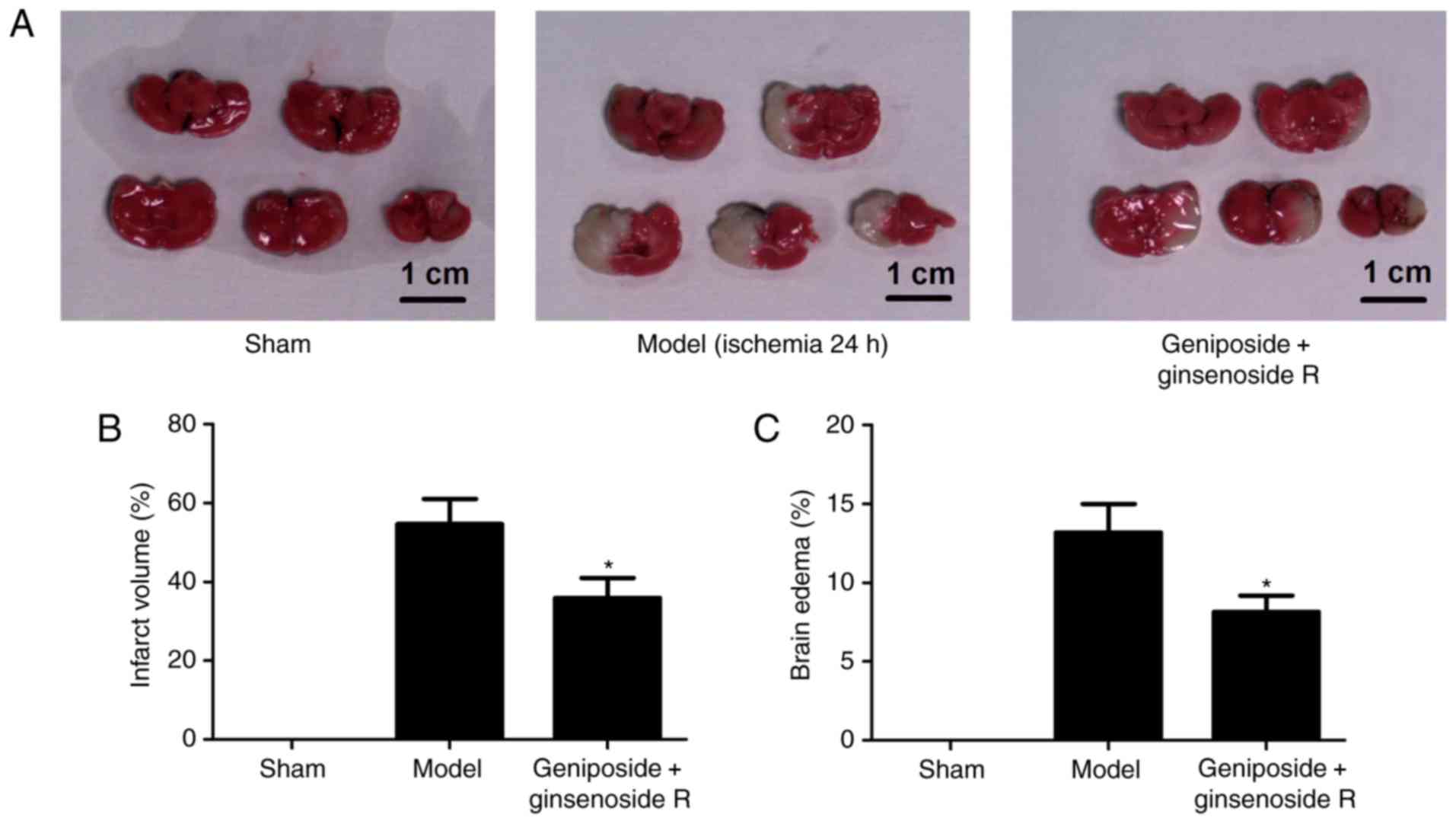
Geniposide + ginsenoside Rg1 protects
brain tissue against ischemic injury
To assess the in vivo protective effect of
geniposide + ginsenoside Rg1 against ischemic injury, the
percentage of cerebral infarction and edema was investigated within
an MCAO rat model. Infarction was not observed in the sham group.
Cerebral infarction within the model group was observed primarily
in the frontal and parietal lobe of the cortex, the caudate nucleus
and posterior limbs of the internal capsule (Fig. 2A). The results revealed that
geniposide + ginsenoside Rg1 treatment significantly reduced brain
infarction size and edema volume compared with the model group
(P<0.05). In particular, the percentage of cerebral infarction
and brain edema of rats subjected to geniposide + ginsenoside Rg1
was decreased by 34.2 and 42.6%, respectively, compared with the
MCAO model group (Fig. 2B and
C).
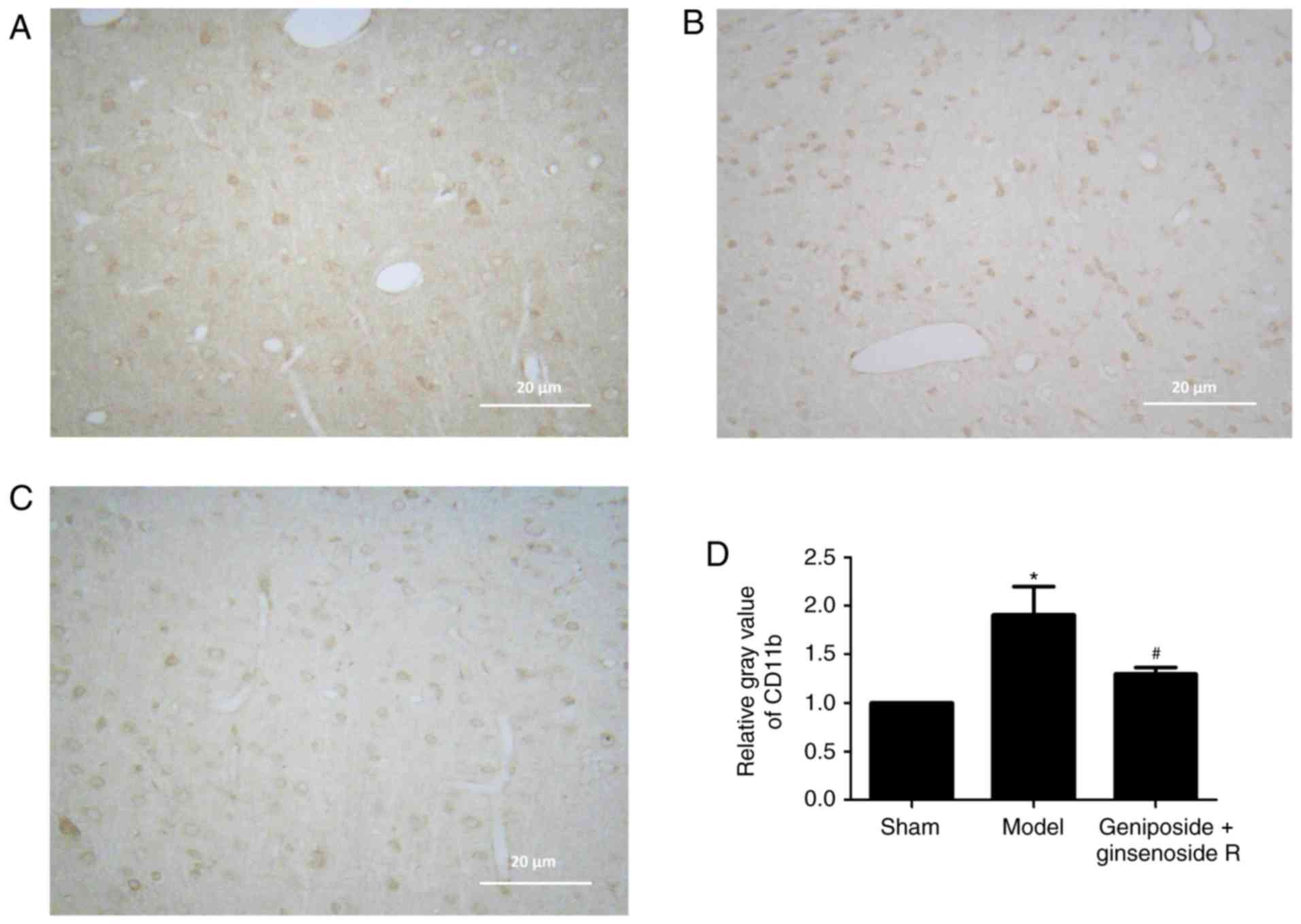
Geniposide + ginsenoside Rg1 inhibits
microglia stimulation
Immunohistochemistry demonstrated low levels of
CD11b expression within the sham group (Fig. 3A). However, after 24 h of ischemia,
CD11b immunoreactivity was visibly increased in the model group
(Fig. 3B), which was primarily
present in the cortex region, indicating a marked activation of
microglial cells within ischemic penumbra. The positive staining of
CD11b was primarily located within the cell membranes, while the
microglial morphology altered from branch- to amoeba-like,
indicating a marked stimulation. Following treatment with
geniposide + ginsenoside Rg1, the expression of CD11b was
suppressed in the brain parenchyma, compared with the model group
(Fig. 3C), as indicated by a
reduced number of CD11b-positive cells in the ischemic penumbra
region. Semi-quantitative analysis revealed the relative gray
values of immunohistochemical staining (Fig. 3D), which indicated that the number
of CD11b-positive cells was markedly reduced by geniposide +
ginsenoside Rg1 treatment in MCAO model rats (P<0.05).
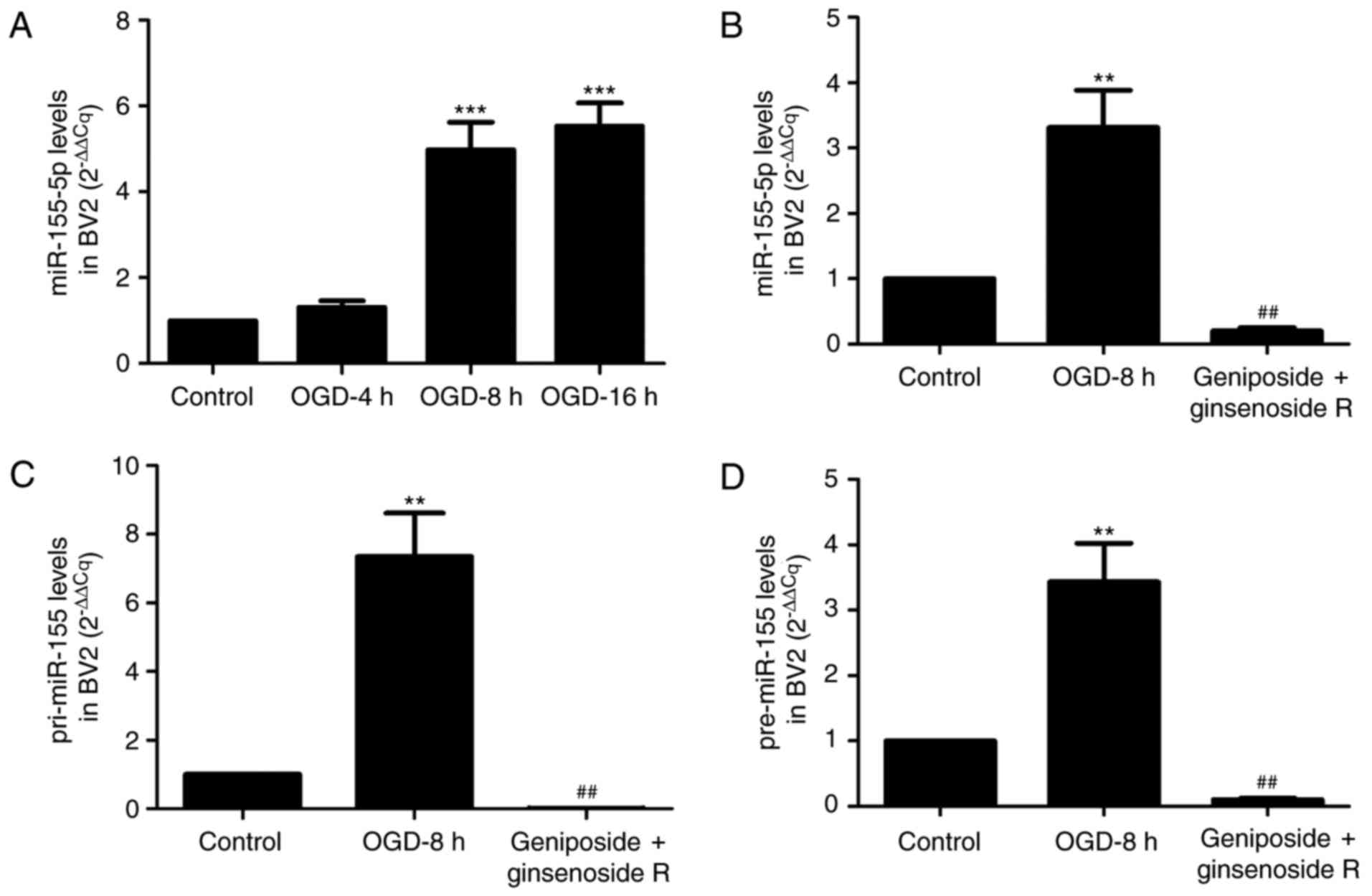
miR-155-5p expression within BV2
microglial cells
To further investigate the protective effects of
geniposide + ginsenoside Rg1 against ischemic injury in
vitro, BV2 microglial cells were cultured and the levels of
immature forms of miR-155 (pri-miR-155 and pre-miR-155) and mature
miR-155-5p were observed by RT-qPCR. As presented in Fig. 4A, miR-155-5p expression levels
within the OGD 4 h group were not markedly altered compared with in
control group. However, when the duration of ischemic was extended
to 8 or 16 h, miR-155-5p expression levels within BV2 microglial
cells were significantly increased compared with the control group
(P<0.001). Consequently, OGD 8 h was selected as the duration
for stimulation for subsequent analysis of BV2 cells. Treatment of
cells with geniposide + ginsenoside Rg1 for 8 h significantly
decreased the expression levels of the immature and mature forms of
miR-155. In particular, miR-155-5p expression within BV2 microglial
cells incubated with geniposide + ginsenoside Rg1 was reduced by
93.9% compared with the model group (Fig. 4B; P<0.01). In addition,
following treatment with geniposide + ginsenoside Rg1, the
expression levels of pri-miR-155 and pre-miR-155, the immature
forms of miR-155, were significantly inhibited by 97.9 and 97%,
respectively, compared with the model group (P<0.01; Fig. 4C and D).
Discussion
Ischemic stroke leads to a series of complex,
multipart-cascade events that contribute to irreversible brain
injury and neurovascular network dysfunction within the ischemic
core (32). Activation of
microglia and astrocytes following stroke leads to enhanced release
of proinflammatory factors, which may persist for several weeks
(33). Although the immune
responses mediated by microglia serve an important role in pathogen
clearance and tissue regeneration, without treatment, the
inflammatory state may worsen neuronal injury (16). miRNAs are a type of small
non-coding RNA that regulate target gene expression and are highly
conserved in various eukaryotes (34). Numerous miRNAs have been reported
to be involved in the regulation of several inflammation phases and
responses to cerebral ischemia (35–37).
Of these miRNAs, miR-155 has been demonstrated to be associated
with the regulation of various physiological and pathological
processes, including inflammation, immunity, cancer and
cardiovascular diseases (12,13).
As a critical inflammation-associated miRNA, miR-155 regulates the
host immune response by repressing the expression of target genes
(38). The majority of target
genes of miR-155 are reported to be associated with the
inflammatory regulation process. For example, it has been
demonstrated that inflammatory responses mediated by miR-155 in
ischemic cerebral tissues were modulated by Toll-like receptor
(TLR)4/myeloid differentiation primary response gene 88 and
suppressor of cytokine signaling 1 expression, while
acetylbritannilactone may suppress miR-155 expression and inhibit
inflammatory actions (39). In
addition, an association was reported between the expression of
TLRs 3, 7 and 9, and miR-155 transcription, in the olfactory bulbs
(40). Based on the reported
associations between miR-155 and post-ischemic brain inflammation,
therapeutic approaches that target miR-155 may be promising for the
treatment of cerebral ischemic damage.
Gardenia, the fruit of Gardenia jasminoides
Ellis, exhibits varying concentrations of iridoid glycosides. The
root of Panax notoginseng (Burk.) F. H. Chen is a widely
used traditional Chinese medicine that belongs to the genus
Panax and family Araliaceae. Panax notoginseng is
also termed Radix notoginseng or Sanchi. In clinical and
experimental research, Gardenia is usually used in combination with
Panax notoginseng to treat central nervous system diseases,
including cerebrovascular disease and Alzheimer's disease.
Geniposide and ginsenoside Rg1 are the active components of
Gardenia and Panax notoginseng, respectively. In a
carrageenan-induced rat paw edema model, Gardenia and Panax
notoginseng have been reported to exhibit anti-inflammatory
effects via the inhibition of nitric oxide production (41). These compounds were also
demonstrated to ameliorate neurological injury, brain infarct
volume and the permeability of the blood-brain barrier in MCAO rat
models by reducing the expression of proteinase-activated receptor
1 (42). However, studies
concerning the role of geniposide + ginsenoside Rg1 have
predominantly been performed in neurons; to the best of our
knowledge, investigation regarding their combined effects on
microglial cells is limited.
The results of the present study indicated that
ischemia caused severe damage in cerebral tissue with a large area
of cerebral infarction and brain edema. Geniposide combined with
ginsenoside Rg1 reduced the percentage of cerebral infarction size
and brain edema in rats subjected to MCAO, which demonstrated a
protective effect on ischemia-induced cerebral injury in
vivo. RT-qPCR revealed that miR-155 expression levels were
unaltered in the 2 and 6 h ischemia groups; however, when the
duration of ischemia was prolonged to 24 h, miR-155 expression
levels increased within the brain tissue, compared with the sham
group. Notably, miR-155 expression levels were increased in the
cortex and basal ganglia in MCAO rats, compared with the sham
group, indicating the potential of miR-155 overexpression as a
target for the treatment of cerebral ischemic injury. Rats treated
with geniposide combined with ginsenoside Rg1 demonstrated
significantly attenuated expression levels of miR-155 expression
levels. Based on the results for infarction size and brain edema,
the neuroprotective effects of geniposide + ginsenoside Rg1 may be
associated with the downregulation of miR-155. In the present
study, 10% chloral hydrate was employed for anesthesia during the
course of MCAO surgery, according to the previously described
method (29,30) in which all mice or rats were
treated with 10% chloral hydrate for anesthesia; however, no
symptoms of peritonitis were determined in the present study. In
the present study, only the brain tissue was dissected for
observation and the abdominal cavity was not observed as there were
no obvious symptoms of peritonitis in rats. However, a previous
study reported that intraperitoneal administration of 10% chloral
hydrate resulted in adynamic ileus and peritonitis in rats, gastric
ulcers in rats and peritonitis in swine (43). Therefore, in future experiments, we
plan to change to gas anesthesia with isoflurane.
To the best of our knowledge, activated microglia
become hypertrophic, rapidly proliferate and migrate to the
inflammatory area where they generate excessive amounts of
proinflammatory and neurotoxic cytokines that cause neuronal damage
(44,45). In the current study, the results of
immunohistochemistry demonstrated that microglial cells increased
in number and appeared to possess an activated morphology within
ischemic-injured regions. Geniposide + ginsenoside Rg1 markedly
reversed the CD11b expression, indicating an inhibitory effect of
geniposide + ginsenoside Rg1 on microglial cells. As microglial
miR-155 expression serves an important role in the pathological
process following ischemic stroke, an in vitro experiment
was performed to observe the effects of geniposide + ginsenoside
Rg1 on microglial cells. The results demonstrated that miR-155
expression levels were elevated and microglial cells were activated
following OGD injury for 8 h, compared with the control group,
indicating microglial activation and high inflammatory potential
following OGD. However, geniposide + ginsenoside Rg1 reduced the
expression of mature miR-155 and the immature precursors,
pri-miR-155 and pre-miR-155, indicating a potential protective role
of geniposide + ginsenoside Rg1 in microglial activation.
Geniposide + ginsensoside Rg1 treatment may activate microglial
cells to release inflammatory factors. miR-155 is an important
miRNA that regulates the mRNA expression of inflammatory factors;
based on the inhibitory effect on miR-155 in the current study, the
anti-inflammatory effect of geniposide + ginsenoside Rg1 may be
achieved by inhibiting miR-155 expression.
In conclusion, the in vivo and in
vitro experiments of the present study demonstrated that the
administration of geniposide in combination with ginsenoside Rg1
via tail vein injection inhibited ischemia-induced microglial cell
activation, which may be associated with the inhibition of
microglial miR-155-5p expression. The results of the present study
indicated that geniposide in combination with ginsenoside Rg1 may
serve as a potential alternative for the prevention of microglial
cell injury in ischemic diseases.
Acknowledgements
The present study was supported by the Natural
Science Foundation in China (grant no. 81473449).
References
|
1
|
Xu F, Li J, Ni W, Shen YW and Zhang XP:
Peroxisome proliferator-activated receptor-gamma agonist
15d-prostaglandin J2 mediates neuronal autophagy after cerebral
ischemia-reperfusion injury. PLoS One. 8:e550802013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng CY and Lee YC: Anti-Inflammatory
effects of traditional chinese medicines against ischemic injury in
in vivo models of cerebral ischemia. Evidence Based Complement
Alternat Med. 2016:57394342016. View Article : Google Scholar
|
|
3
|
Liu X, Tao Y, Wang F, Yao T, Fu C, Zheng
H, Yan Y, Liang X, Jiang X and Zhang Y: Kudiezi injection mitigates
myocardial injury induced by acute cerebral ischemia in rats. BMC
Complement Alternat Med. 17:82017. View Article : Google Scholar
|
|
4
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lau P and Hudson LD: MicroRNAs in neural
cell differentiation. Brain Res. 1338:14–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rybak A, Fuchs H, Smirnova L, Brandt C,
Pohl EE, Nitsch R and Wulczyn FG: A feedback loop comprising lin-28
and let-7 controls pre-let-7 maturation during neural stem-cell
commitment. Nat Cell Biol. 10:987–993. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
White RE and Giffard RG: MicroRNA-320
induces neurite outgrowth by targeting ARPP-19. Neuroreport.
23:590–595. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim KY, Chua JH, Tan JR, Swaminathan P,
Sepramaniam S, Armugam A, Wong PT and Jeyaseelan K: MicroRNAs in
Cerebral Ischemia. Transl Stroke Res. 1:287–303. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu DZ, Tian Y, Ander BP, Xu H, Stamova
BS, Zhan X, Turner RJ, Jickling G and Sharp FR: Brain and blood
microRNA expression profiling of ischemic stroke, intracerebral
hemorrhage, and kainate seizures. J Cereb Blood Flow Metab.
30:92–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu Y, Zhang J, Han R, Liu H, Sun D and
Liu X: Downregulation of serum brain specific microRNA is
associated with inflammation and infarct volume in acute ischemic
stroke. J Clin Neurosci. 22:291–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Faraoni I, Antonetti FR, Cardone J and
Bonmassar E: miR-155 gene: A typical multifunctional microRNA.
Biochim Biophys Acta. 1792:497–505. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O'Connell RM, Kahn D, Gibson WS, Round JL,
Scholz RL, Chaudhuri AA, Kahn ME, Rao DS and Baltimore D:
MicroRNA-155 promotes autoimmune inflammation by enhancing
inflammatory T cell development. Immunity. 33:607–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dheen ST, Kaur C and Ling EA: Microglial
activation and its implications in the brain diseases. Curr Med
Chem. 14:1189–1197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butovsky O, Jedrychowski MP, Cialic R,
Krasemann S, Murugaiyan G, Fanek Z, Greco DJ, Wu PM, Doykan CE,
Kiner O, et al: Targeting miR-155 restores abnormal microglia and
attenuates disease in SOD1 mice. Ann Neurol. 77:75–99. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cardoso AL, Guedes JR, Pereira de Almeida
L and Pedroso de Lima MC: miR-155 modulates microglia-mediated
immune response by down-regulating SOCS-1 and promoting cytokine
and nitric oxide production. Immunology. 135:73–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pareek S, Roy S, Kumari B, Jain P,
Banerjee A and Vrati S: MiR-155 induction in microglial cells
suppresses Japanese encephalitis virus replication and negatively
modulates innate immune responses. J Neuroinflammation. 11:972014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen QC, Zhang WY, Kim H, Lee IS, Ding Y,
Youn UJ, Lee SM, Na M, Min BS and Bae K: Effects of Gardeniae
Fructus extract and geniposide on promoting ligament cell
proliferation and collagen synthesis. Phytother Res. 24 Suppl
1:S1–S5. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang J, Li PT, Du H, Hou JC, Li WH, Pan YS
and Chen HC: Tong Luo Jiu Nao injection, a traditional Chinese
medicinal preparation, inhibits MIP-1β expression in brain
microvascular endothelial cells injured by oxygen-glucose
deprivation. J Ethnopharmacol. 141:151–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang SW, Lai CY and Wang CJ: Inhibitory
effect of geniposide on aflatoxin B1-induced DNA repair synthesis
in primary cultured rat hepatocytes. Cancer Lett. 65:133–137. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee P, Lee J, Choi SY, Lee SE, Lee S and
Son D: Geniposide from Gardenia jasminoides attenuates neuronal
cell death in oxygen and glucose deprivation-exposed rat
hippocampal slice culture. Biol Pharm Bull. 29:174–176. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Hou J, Zhang P, Li D, Zhang C and
Liu J: Geniposide reduces inflammatory responses of oxygen-glucose
deprived rat microglial cells via inhibition of the TLR4 signaling
pathway. Neurochem Res. 37:2235–2248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Lin SX, Hua Q, Yang KY, Yao N, Yi
LS, Wang AM, Chen WJ and Chen JY: Research on the mechanism of the
active ingredients of Sanchi and Gardenia removing the early
amyloid in the AD transgenic mouse brain. Chin Pharmacol Bull.
28:179–84. 2012.(In Chinese).
|
|
24
|
Zhai YS, Du SY, Lu Y, Wang Y, Xu B and Gao
Y: Impact of notoginseng total saponin on intestinal absorption
kinetics of jasminoidin. China J Trad Chin Med Pharm. 25:459–462.
2010.
|
|
25
|
Lanou W, Heqin Z, Wenjun H and Wenfeng C:
Analysis of behavioral index of ginsenoside_RG 1 intervention on
brain ischemia. Chin J Lab Ani Sci. 14:283–285. 2004.
|
|
26
|
Xia L, Cuifen B, Jia W, Jia L and Shujian
Q: The protective role and mechanism of Ginsenoside Rg1 on the
ischemic-reperfusion injuried neurons in hippocampus CA1 of rat.
Progress Anat Sci. 16:177–180. 2010.
|
|
27
|
Hollands C: The animals (scientific
procedures) act 1986. Lancet. 2:32–33. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu JH, Feng D, Zhang YF, Shang Y, Wu Y,
Li XF and Pei L: Chloral hydrate preconditioning protects against
ischemic stroke via upregulating annexin A1. CNS Neuroscience Ther.
21:718–726. 2015. View Article : Google Scholar
|
|
30
|
Chang CF, Lin SZ, Chiang YH, Morales M,
Chou J, Lein P, Chen HL, Hoffer BJ and Wang Y: Intravenous
administration of bone morphogenetic protein-7 after ischemia
improves motor function in stroke rats. Stroke. 3:558–564. 2003.
View Article : Google Scholar
|
|
31
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Brouns R and De Deyn PP: The complexity of
neurobiological processes in acute ischemic stroke. Clin Neurol
Neurosurg. 111:483–495. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liguz-Lecznar M and Kossut M: Influence of
inflammation on poststroke plasticity. Neural Plast.
2013:2585822013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Taganov KD, Boldin MP and Baltimore D:
MicroRNAs and immunity: Tiny players in a big field. Immunity.
26:133–137. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
O'Connell RM, Rao DS, Chaudhuri AA and
Baltimore D: Physiological and pathological roles for microRNAs in
the immune system. Nat Rev Immunol. 10:111–122. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xiao C and Rajewsky K: MicroRNA control in
the immune system: Basic principles. Cell. 136:26–36. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhu J, Yue H, Qiao C and Li Y: Association
between single-nucleotide polymorphism (SNP) in miR-146a,
miR-196a2, and miR-499 and risk of ischemic stroke: A
meta-analysis. Med Sci Monit. 21:3658–3663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang J, Yang K, Zhou L, Minhaowu, Wu Y,
Zhu M, Lai X, Chen T, Feng L, Li M, et al: MicroRNA-155 promotes
autophagy to eliminate intracellular mycobacteria by targeting
Rheb. PLoS Pathog. 9:e10036972013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wen Y, Zhang X, Dong L, Zhao J, Zhang C
and Zhu C: Acetylbritannilactone modulates MicroRNA-155-mediated
inflammatory response in ischemic cerebral tissues. Mol Med.
21:197–209. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oliveira BRSM, Vieira FV, De S Vieira D,
da Silva SEL, Gameiro R, Flores EF and Cardoso TC: Expression of
miR-155 associated with Toll-like receptors 3, 7, and 9
transcription in the olfactory bulbs of cattle naturally infected
with BHV5. J Neurovirol. 23:772–778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zheng X, Yang D, Liu X, Wang N, Li B, Cao
H, Lu Y, Wei G, Zhou H and Zheng J: Identification of a new
anti-LPS agent, geniposide, from Gardenia jasminoides Ellis, and
its ability of direct binding and neutralization of
lipopolysaccharide in vitro and in vivo. Int Immunopharmacol.
10:1209–1219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xie CL, Li JH, Wang WW, Zheng GQ and Wang
LX: Neuroprotective effect of ginsenoside-Rg1 on cerebral
ischemia/reperfusion injury in rats by downregulating
protease-activated receptor-1 expression. Life Sci. 121:145–151.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Silverman J and Muir WW III: A review of
laboratory animal anesthesia with chloral hydrate and chloralose.
Lab Anim Sci. 43:210–216. 1993.PubMed/NCBI
|
|
44
|
Smith JA, Das A, Ray SK and Banik NL: Role
of pro-inflammatory cytokines released from microglia in
neurodegenerative diseases. Brain Res Bull. 87:10–20. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vilhardt F: Microglia: Phagocyte and glia
cell. Int J Biochem Cell Biol. 37:17–21. 2005. View Article : Google Scholar : PubMed/NCBI
|