Introduction
Oral health status has become one of the most
important determinants of quality of life in elderly people for a
variety of physical, social and psychological reasons (1). Elderly individuals are now
increasingly more likely to seek dental treatment, including
implant restoration, periodontal and orthodontic treatment
(2). All of these treatments
involve the alveolar bone, which is the major tissue supporting the
teeth. Osteoporosis is a common systemic skeletal disease
associated with aging (3). The
effect of osteoporosis on the alveolar bone and on oral health in
general has been the focus of clinical dentistry and of basic
research for a number of years. A number of studies have provided
evidence that osteoporosis can affect human alveolar bones
(4–6). For example, osteoporotic women had
less mandibular bone mass and density and a thinner cortex at the
gonion compared with non-osteoporotic women (5).
The most destructive effects of alveolar bone
resorption result from postmenopausal osteoporosis induced by
excessive osteoclast activation (7). Therefore, it is plausible that
anti-resorptive agents could be employed in patients with
postmenopausal osteoporosis to prevent alveolar bone resorption
(8). Pharmacological agents for
the treatment of osteoporosis may be classified as either
antiresorptive or anabolic, and mainly include estrogen, selective
estrogen-receptor modulators (SERMs), bisphosphonates, denosumab
and teriparatide (9). Concerns
regarding the nonskeletal risks associated with estrogen use,
including breast cancer and coronary, cerebrovascular and
thrombotic events, have led to recommendations against using
estrogen as a first-line therapy for osteoporosis (10). Raloxifene, a SERM that has been
approved by the Food and Drug Administration (FDA) to treat
osteoporosis, increases the risk of thromboembolic events (11,12).
The long-term use of bisphosphonates presents a risk for the
development of oral osteonecrosis and atypical femoral fracture
(13,14). Denosumab inhibits bone resorption
by binding to the receptor activator of nuclear factor-κβ ligand,
thereby decreasing the differentiation of osteoclasts. The most
common side effects of denosumab include urinary and respiratory
tract infections, cataracts, constipation, rashes and joint pain
(15). Teriparatide is an anabolic
agent that functions primarily by increasing bone formation rather
than by decreasing resorption (16). Ongoing toxicology studies in rats
have demonstrated that a longer duration and larger dose of therapy
is a risk factor for osteosarcoma (9,16).
For this reason, the FDA has limited the use of teriparatide to 2
years and use is not permitted in patients with a history of
Paget's disease or any type of cancer. Therefore, the authors
hypothesized that a novel class of anti-resorptive agents that
inhibit osteoclast activation via a mechanism different from that
of the currently used bisphosphonates may be beneficial in reducing
the occurrence of oral osteonecrosis.
Enoxacin, a fluoroquinolone antibiotic, is widely
used to treat patients with bacterial infections. A recent study
has indicated that enoxacin can inhibit osteoclast formation and
bone resorption by blocking the interactions between the V-ATPase
B2-subunit or V-ATPase a3-subunit and microfilaments in osteoclasts
(17). Enoxacin has a stronger
affinity for bone than bisphosphonates, and inhibits bone
resorption via a mechanism different from that of bisphosphonates.
Therefore, it is possible that a combination of bisphosphonates and
enoxacin in the form of bis-enoxacin (BE) could be used to treat
alveolar bone resorption in postmenopausal osteoporosis. The use of
BE is advantageous as it reduces the risk of oral osteonecrosis
with bisphosphonates and dysbacteriosis with enoxacin.
BE is a bisphosphonate derivative of enoxacin.
Enoxacin is widely used to treat patients with chronic infections
associated with osteomyelitis. However, enoxacin has a weaker
ability to bind to, concentrate in, and/or be retained by infected
bone, a site often difficult to treat clinically. Therefore, in
2002, Herczegh et al (18)
synthesized a novel antibacterial drug, BE, by conjugating
bone-binding bisphosphonate groups to enoxacin. However, whether BE
is able to prevent osteoporosis in vivo has yet to be
elucidated. Based on this research, our group proposed the
hypothesis that BE is able to prevent osteoporosis in vivo
via the anti-bone resorptive property of bisphosphonates and
enoxacin. Hence, the aim of the present study was to investigate
the effect of BE on the maxillary alveolar bone in a rat model of
osteoporosis.
Materials and methods
Animals and study design
All animal care and experimental procedures were
carried out in strict accordance with the recommendations provided
in the Guide for Ethical Conduct in the Care and Use of Nonhuman
Animals in Research produced by the American Psychological
Association (http://www.apa.org/science/leadership/care/guidelines.aspx)
and were approved by the Animal Care Committee of Shanghai Ninth
People's Hospital, Shanghai Jiao Tong University School of Medicine
[Shanghai, China; HKDL (2014)125]. A total of 30 6-month-old female
Sprague–Dawley rats (approximately 300–330 g) were obtained from
the Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China).
All rats were housed in filter-top cages in a temperature and
humidity controlled room (23±1°C and 60±5%, respectively) with a
12-h light/dark cycle and free access to food and water.
The rats were randomly divided into 5 groups of 6
rats each: Group 1, Sham saline control (Sham); group 2,
ovariectomized (OVX) group (Vehicle); group 3, OVX rats treated
with 50 µg/kg/day zoledronic acid (ZOL); group 4, OVX rats treated
with 50 µg/kg/day BE (BE-L; low concentration group); group 5, OVX
rats treated with 100 µg/kg/day BE (BE-H; high concentration
group).
The rats were either sham operated or bilaterally
OVX through a vertical dorsal incision. In the OVX groups (groups
2–5), the bilateral ovaries were ligated and ablated under aseptic
conditions. The remaining rats in the Sham group were subjected to
sham operations in which the ovaries were not removed. At 8 weeks
following the ovariectomy or Sham surgery, rats in the Sham and
Vehicle groups were injected intraperitoneally with vehicle (0.9%
saline) and used as controls. Rats in the ZOL group were treated
with 50 µg/kg/day ZOL (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) and those in BE-L and BE-H groups received 50 and 100
µg/kg/day BE (SynQuest Laboratories, Inc., Alachua, FL, USA),
respectively. All treatments were performed on alternate days over
a period of 8 weeks (Table I). At
16 weeks post-surgery, all rats were sacrificed by exsanguination
under 10% chloral hydrate anesthesia and both sides of the maxillae
were removed from each rat. The surgical procedures were performed
according to FDA guidelines (19).
All rats were housed under the conditions stated above for 16 weeks
and body weight was measured once a month during the course of the
study.
 | Table I.Experimental design and the associated
treatments in each group of rats. |
Table I.
Experimental design and the associated
treatments in each group of rats.
| Group no. | Surgery type | Treatment type | Dose applied | Duration | Group
abbreviation |
|---|
| 1 | Sham | Vehicle | Saline | 8 weeks | Sham |
| 2 | OVX | Vehicle | Saline |
| Vehicle |
| 3 | OVX | Zoledronic acid | 50 µg/kg/day ZOL |
| ZOL |
| 4 | OVX | Low concentration of
BE | 50 µg/kg/day BE |
| BE-L |
| 5 | OVX | High concentration of
BE | 100 µg/kg/day BE |
| BE-H |
A polychrome sequential fluorescent bone labeling
method was performed to label the mineralized tissue and to assess
the time course of new bone mineral apposition rate (MAR) and
mineralizing surface/bone surface (MS/BS) of the maxilla alveolar
bone. Following the initiation of BE, ZOL or vehicle treatment,
rats were injected intraperitoneally with 10 µg/kg calcein
(Sigma-Aldrich; Merck KGaA) on day 20 and 20 µg/kg alizarin red
(Sigma-Aldrich; Merck KGaA) on day 40.
Micro-computed tomography (CT)
scanning and assessment of the alveolar bone
The two sides of the maxillae were scanned using a
high-resolution micro-CT scanner (µCT80; Scanco Medical,
Brüttisellen, Switzerland). The scanning protocol was set using an
isometric resolution of 20 µm, and X-ray energy settings of 70 kV
and 1,170 mA. Scans were reconstructed to generate
three-dimensional digitized models, and microstructure parameters
of bone volume/tissue volume (BV/TV), trabecular number (Tb.N),
trabecular thickness (Tb.Th) and trabecular separation (Tb.Sp) were
calculated in a three-dimensional region of interest (ROI) using a
calculated Hounsfield Unit grayscale threshold value. The ROI was a
cuboidal bone body that encompassed the roots of M1 and M2. The
length of the ROI extended from the most mesial aspect of the M1
root to the most distal aspect of the M2 root. The width of the ROI
extended from the most buccal aspect of either root of the two
molars to the most palatal aspect of either root. The height of the
ROI extended from the most apical aspect of either root to the most
coronal part of the alveolar bone crest. The ROI was generated as
an indicator of the border of the volumetric analysis. The 3D
reconstructed images were processed to 2D parasagittal images, with
a single investigator drawing the outline of the desired alveolar
bone region in order to maximize the quantification of bone and
minimize the inclusion of roots.
Bone histology
Following the completion of micro-CT scanning,
decalcified sections were prepared from the left maxilla specimens
and undecalcified sections were prepared from the right maxilla
specimens.
The left maxillae specimens were decalcified with
10% ethylenediaminetetraacetic acid disodium salt for ~2 months and
then embedded in paraffin. Sections were prepared from the occlusal
surface of the tooth crown to the alveolar bone mesiodistally along
a plane parallel to the long axis of the tooth, and then cut into
5-µm thick serial sagittal sections. Half of these sections were
stained with hematoxylin (200 µg/l) and eosin (500 µg/l; H&E;
Sigma-Aldrich; Merck KGaA) at 37°C for 2 h for descriptive
analysis. The remaining sections were stained with
tartrate-resistant acid phosphatase (7%; TRAP; Sigma-Aldrich; Merck
KGaA) at 37°C for 2 h to identify osteoclasts on the bone surface.
Trabecular bone volume fractions (expressed as the percentage of
BV/TV) in H&E-stained sections were analyzed by Image-Pro Plus
software (version 6.0; Media Cybernetics, Inc., Rockville, MD, USA)
using an IX71 inverted microscope (Olympus Corporation, Tokyo,
Japan). The average number of TRAP-positive multinucleated
osteoclasts per mm2 was counted by randomly selecting 5
regions of each section.
The right maxilla specimens were processed and
embedded without decalcification in methyl methacrylate (35g/l;
MMA; M55909; Sigma-Aldrich; Merck KGaA) at 37°C for 1 month. A
confocal laser-scanning microscope (Leica TCS SP5; Leica
Microsystems GmbH, Wetzlar, Germany) was used to examine the
fluorescence labeling of the undecalcified sections (~20 µm. The
excitation/emission wavelengths used for fluorescence were 488/517
nm (calzein) and 543/617 nm (alizarin red). Three section slides
from each specimen were selected for histomorphometric analysis.
Trabecular bone dynamic parameters were measured in a
1-mm2 square area positioned at the inter-radicular
region of M1 at magnification, ×200, using calcein and alizarin red
as labels. The dynamic parameters included single-label perimeter
(sL.Pm), double-label perimeter (dL.Pm), trabecular bone perimeter
(Tb.Pm) and interlabel width (Ir.L.Wi). The mean Ir.L.Wi, was
obtained dynamically by averaging the distances between points
randomly selected from 5 intervals. MAR (The mean Ir.L.Wi/interval
period, µm/day) was measured using BioQuant OSTEO II software
(version 8.00.20; BioQuant Image Analysis Corporation, Nashville,
TN, USA). The mineralizing surface [MS/BS=(dL.Pm + sL.Pm/2)/Tb.Pm ×
100%] was analyzed using Image-Pro Plus software (version 6.0;
Media Cybernetics, Inc.). The sections were then stained with van
Gieson's picro fuchsin (Sigma-Aldrich; Merck KGaA) at 37°C for 4 h
for histological observation.
Statistical analysis
Results are expressed as the mean ± standard
deviation. One-way analysis of variance followed by the Bonferroni
post hoc test was used to measure statistically significant
differences among groups. SPSS version 17.0 software (SPSS Inc.,
Chicago, IL, USA) was used for all analyses. P<0.05 was
considered to indicate a statistically significant difference.
Results
Body weight
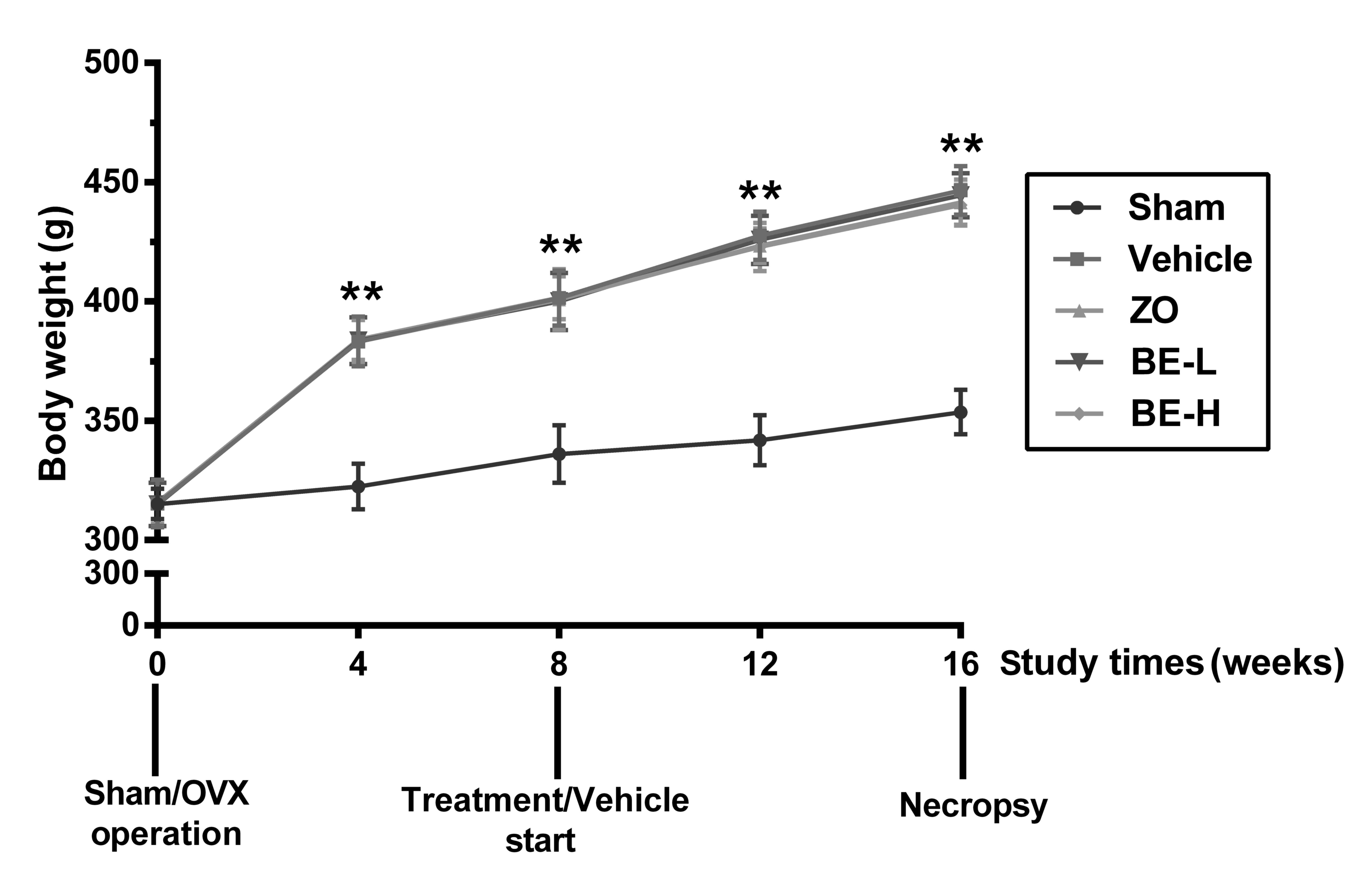
Monthly records of body weight are shown in Fig. 1. The rats in each group had a
similar initial mean body weight (315±10 g) and the difference
between the groups was insignificant. Ovariectomy generally
resulted in weight gain. At 4, 8, 12 and 16 weeks post-ovariectomy,
the body weights of the Vehicle group were 383.25±10.3,
401.42±11.53, 427.58±10.1 and 446.5±10.16 g. respectively. When
compared with the Sham group at the same time points, these
differences were all significant (all P<0.01). The trend in body
weight among the ZOL or BE administration groups (groups 3–5) and
the Vehicle group was similar throughout the course of the
experiment and the differences between them were not statistically
significant.
Micro-CT analysis of alveolar bone
microarchitecture
The three-dimensional images provided a clear view
of the microarchitecture of the inter-radicular alveolar bone in
rats. Statistical analysis of the microarchitectural parameters
demonstrated that the BV/TV and Tb.Th were significantly reduced,
whereas Tb.Sp was significantly increased, in the Vehicle group
when compared with the Sham group (P<0.01; Fig. 2B, C and E). Notably, no significant
difference in Tb.N was identified between the groups (P>0.05;
Fig. 2D). The trabecular and
cortical thicknesses were greater in the Sham group than in the
Vehicle group. Taken together, these results indicate that there
was a loss of bone and deterioration of the trabeculae of the
alveolar bone in the OVX rats. The ZOL, BE-L and BE-H groups had an
increased BV/TV and Tb.Th, and a decreased Tb.Sp, when compared
with the Vehicle group (P<0.05; Fig. 2B, C and E). There was a
statistically significant difference between the BE-L and BE-H
groups in BV/TV (P<0.01; Fig.
2B) and Tb.Sp (P<0.05; Fig.
2E). The BE-H group had a significantly lower Tb.Sp value
compared with the ZOL group (P<0.05; Fig. 2E).
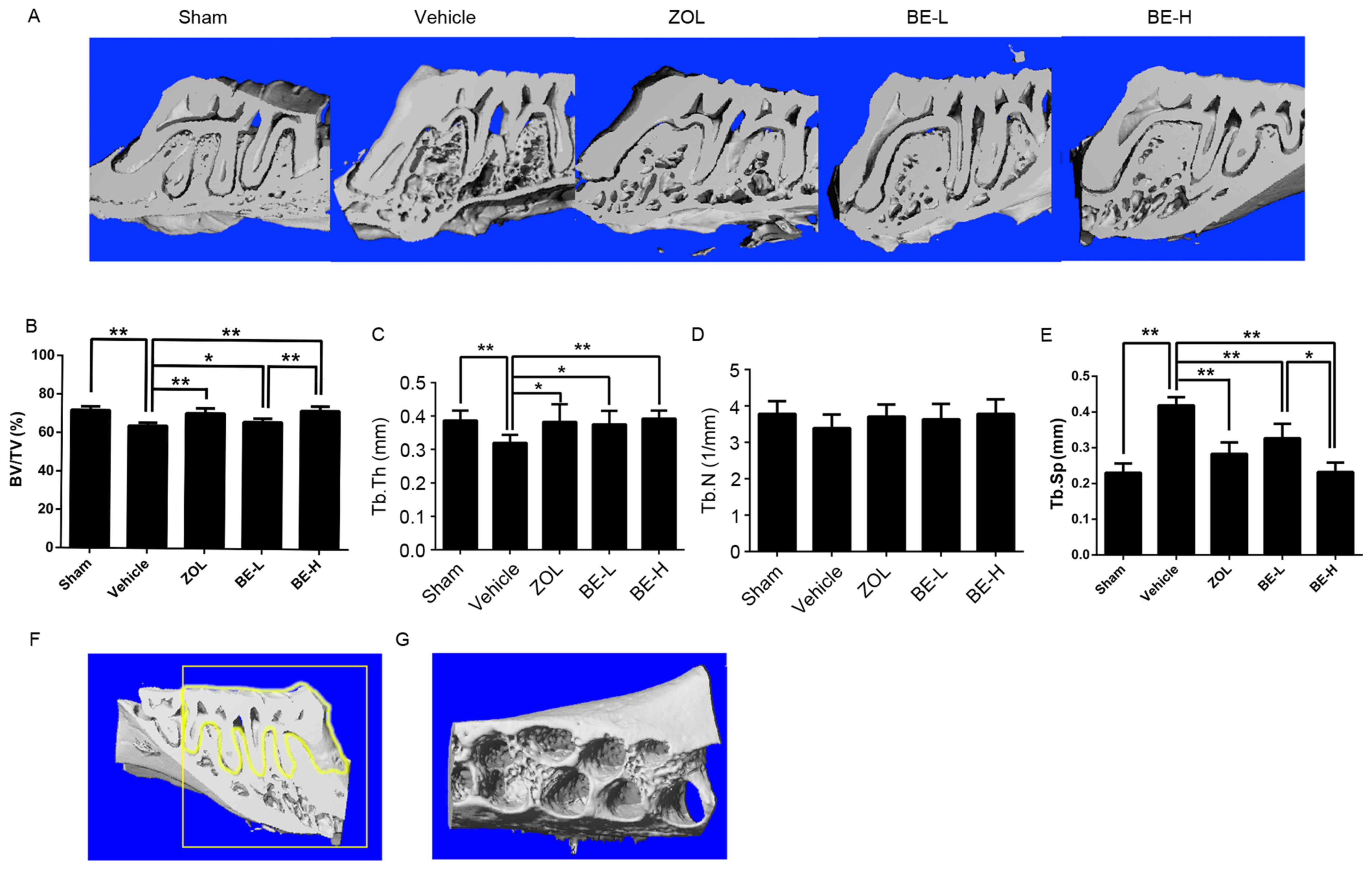 | Figure 2.The effect of BE on the maxilla
assessed by micro-CT analysis of bone volumetric parameters. (A)
Longitudinal-sectional micro-CT images of the first molar (M1) and
the second molar (M2) in the maxilla. Analysis of micro-CT
volumetric parameters: (B) BV/TV, (C) Tb.Th, (D) Th.N and (E)
Tb.Sp. (F) The contour of the desired alveolar bone region was
transposed on to two-dimensional parasagittal images. (G) The
region of interest was a cuboidal bone body that encompassed the
roots of the M1 and M2 molars. Values are expressed as the mean ±
standard deviation (n=6). *P<0.05 and **P<0.01 cf. Vehicle.
BE, bis-enoxacin; CT, computed tomography; BV/TV, bone
volume/tissue volume; Tb.Th, trabecular thickness; Th.N, trabecular
number; Tb.Sp, trabecular separation; ZOL, zoledronic acid; BE-L,
low concentration of bis-enoxacin (50 µg/kg/day); BE-H, high
concentration of bis-enoxacin (100 µg/kg/day). |
Histomorphometric and histological
analysis of the alveolar bone
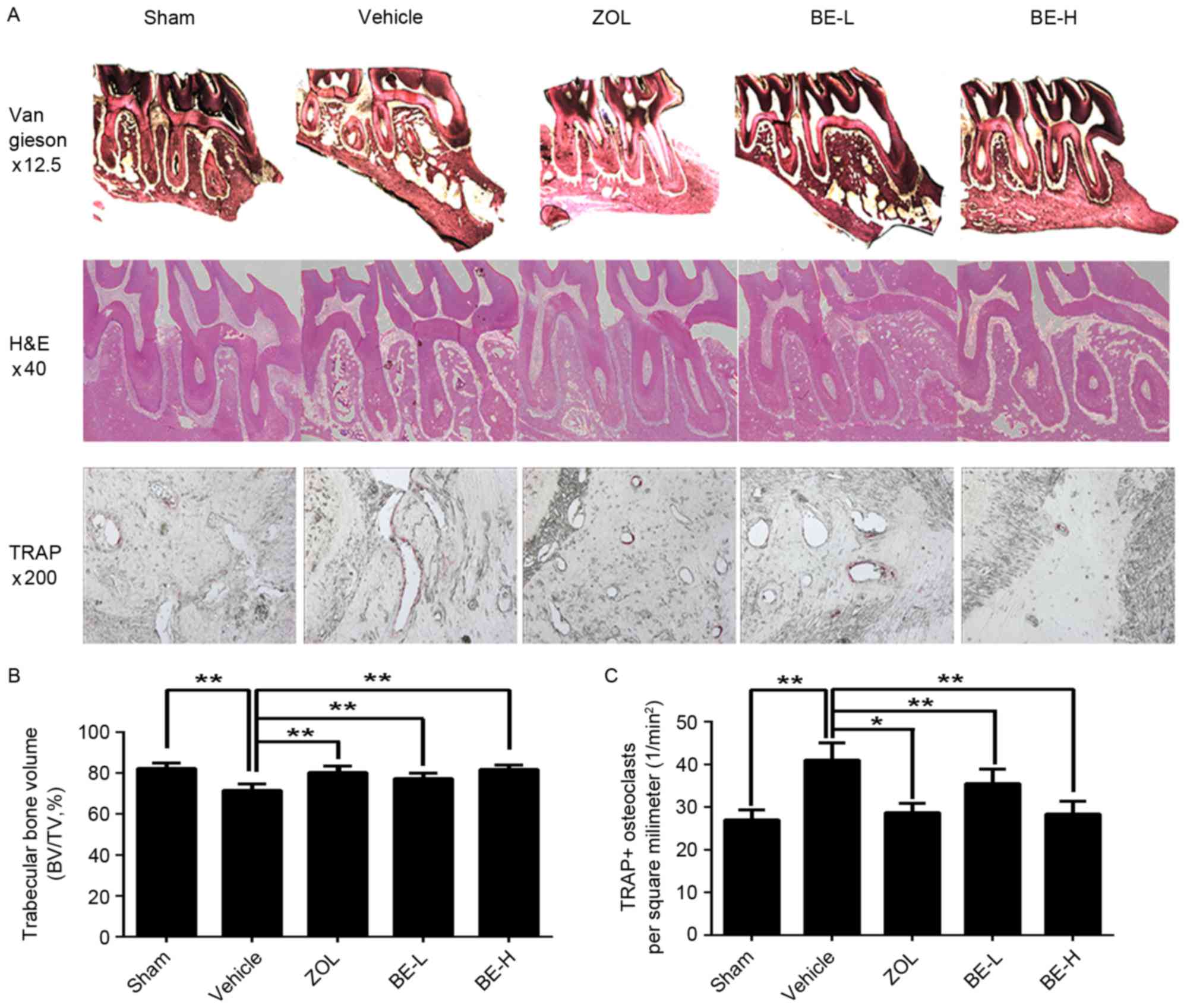
Analysis of the van Gieson-stained sections and
H&E-stained sections from the maxilla revealed that the
trabecular bone was affected by the ovariectomy operation, with the
Sham group having an increased bone marrow volume when compared
with the Vehicle group (Fig. 3A).
The alveolar bone from the Sham group had a thicker interconnected
trabecula and in the Vehicle group the alveolar bone exhibited
lower connectivity and a thinner trabecular; however, it did
exhibit an abundance of bone marrow (Fig. 3A). The amount of bone tissue was
higher in all of the ZOL and BE administration groups when compared
with the Vehicle group, indicating a potential protective effect of
BE administration in the preservation of alveolar bone (Fig. 3A). In the H&E-stained sections,
analysis of the trabecular bone fraction (percentage of BV/TV)
revealed a higher value for the Sham group when compared with the
Vehicle group (~15%; Fig. 3B). The
trabecular bone area increased in the ZOL, BE-L and BE-H groups
when compared with the Vehicle group (~12, 8 and 14%, respectively;
Fig. 3B). The microscopic
evaluation of the histological sections clearly supported the
results of the micro-CT.
Osteoclasts were defined as multinuclear cells that
were stained wine-red by TRAP staining (Fig. 3A). In alveolar bone, osteoclasts
were observed lining the bone surface of the marrow space or in the
periodontal ligament. While the osteoclasts were densely clustered
in the Vehicle group, a significant decrease was observed in the
ZOL and BE administration groups when compared with the Sham group,
where only sporadic osteoclasts were visible (Fig. 3A). The number of TRAP-positive
osteoclasts were significantly decreased in the ZOL, BE-L and BE-H
groups when compared with the Vehicle group (~30, 13 and 31%,
respectively), suggesting that BE may inhibit osteoclast formation
(P<0.05 and P<0.01; Fig.
3C).
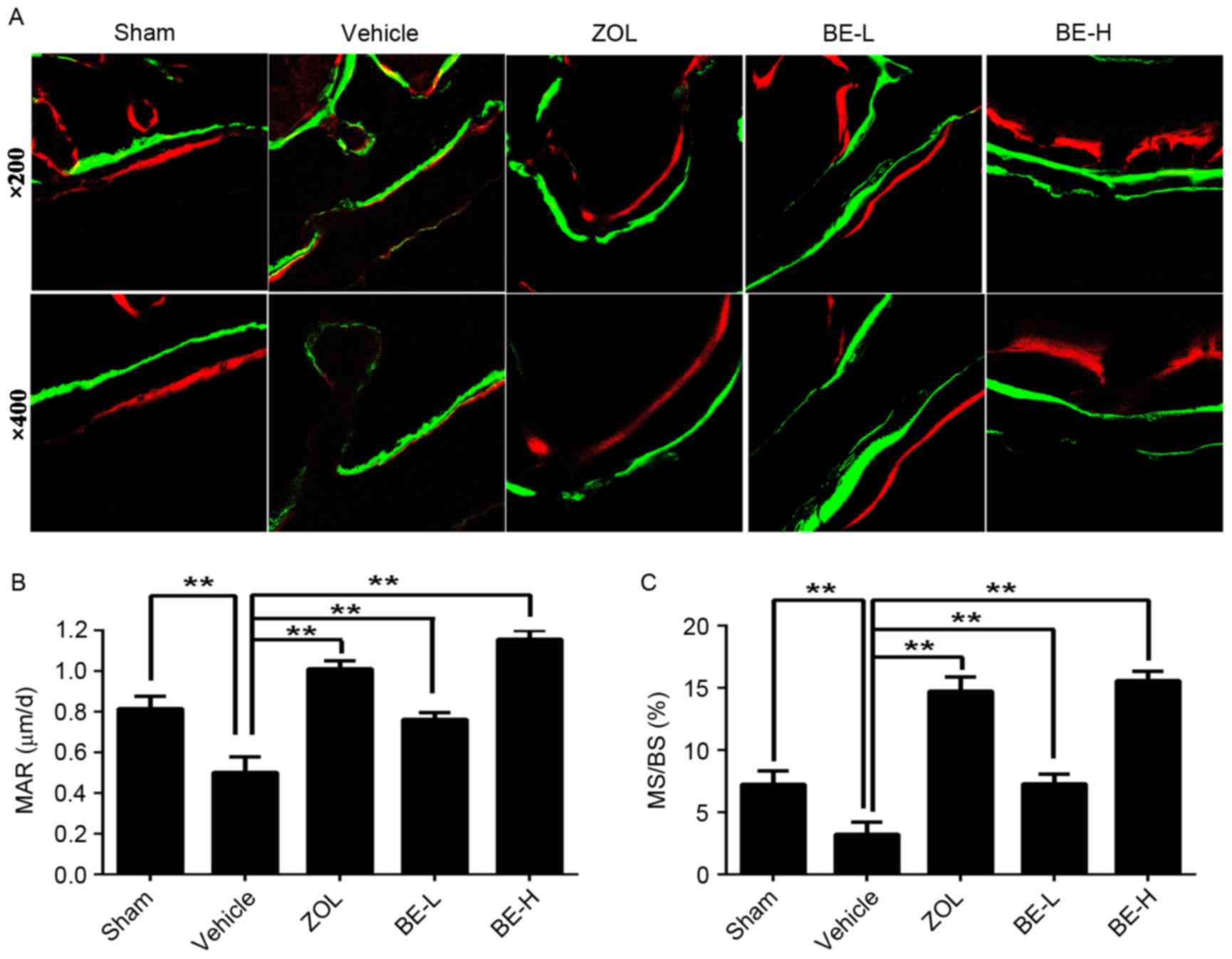
Fluorochrome microscopy
The deposition of mineralized bone matrix was
observed, which was demonstrated by calcein (green) and alizarin
red S (red) staining. The distance between the labels decreased
notably in the Vehicle group compared with the other four groups
(Fig. 4A). The Vehicle group
exhibited a significantly lower MAR and MS/BS when compared with
the Sham group (~38 and 55%, respectively; Fig. 4B and C). A significantly increased
MAR and MS/BS were observed in the ZOL, BE-L and BE-H groups when
compared with the Vehicle group (~101, 52 and 131%, and 355, 124
and 382%, increases, respectively; Fig. 4B and C).
Discussion
Ovarian hormone deficiency leads to postmenopausal
osteoporosis as a result of increased bone turnover and a rapid
loss of cancellous bone (20,21).
However, the rate and magnitude of such changes vary markedly
between different skeletal sites (22,23).
Alveolar bone is an irregular protuberance of the jawbone that
encompasses the roots of the teeth. Although alveolar bone forms a
relatively small part of the bone system, it serves the most
important role in supporting the teeth, and its anatomic study is
therefore important for therapies including implant restoration,
periodontal, endodontic, orthodontic and maxillofacial treatments
(24). As a consequence, research
into the connection between postmenopausal osteoporosis and
alveolar bone is receiving an increasing amount of attention. A
previous study examined the effects of osteoporosis on periodontal
disease, subsequent tooth loss and on the capacity of the maxilla
and mandible to integrate endosseous dental implants (25). In orthodontics, consolidation
therapy in estrogen-deficient patients usually takes longer, and
relapse and therapeutic failures are more common (26). Osteoporosis in the jaw may present
a risk for accentuation of alveolar bone loss in patients with full
dentures (27). Taken together,
these findings suggest that postmenopausal osteoporosis may have a
substantial effect on alveolar bone. Thus, identifying therapeutic
agents to alleviate osteoporosis in alveolar bone would have
significant clinical value. To investigate this, the present study
established an OVX rat model; such models have been widely used to
study postmenopausal osteoporosis in humans and have helped to
reveal the underlying mechanisms.
The primary purpose of the present study was to
characterize the alveolar bone changes in the maxilla of OVX rats
by simultaneously assessing micro-CT and histomorphometry. The
micro-CT results revealed a significant decrease in the BV/TV, and
a significant increase in the Tb.Th and Tb.Sp, parameters in the
Vehicle group when compared with the Sham group. No significant
differences between the groups were observed in the
microarchitecture parameter of Tb.N (P>0.05). This suggests that
ovariectomy may not affect the density of the trabecular bone. The
microscopic evaluation of histological sections using H&E and
van Gieson's staining clearly supported the findings obtained by
micro-CT. In the present study, animal body weights in OVX rats
(including the Vehicle, ZOL, BE-L and BE-H groups) were notably
different at the first observation time point of 4 weeks. A rapid
increase in body weight was observed in OVX rats when compared with
those in the Sham group at every point following surgery
(**P<0.01). Ovariectomy is associated with a disorder in fat
metabolism that is induced by estrogen deficiency, resulting in an
increase in body fat rather than lean body mass, which is in line
with previous reports (19,28).
In 2002, Herczegh et al (18) synthesized a novel antibacterial
drug, BE, by conjugating bone-binding bisphosphonate to enoxacin.
BE has been demonstrated to have the ability to bind to bone and to
inhibit the growth of bacteria, and may offer additional treatment
options for highly concentrated targeting of an antibacterial to
the site of a pathogen (18). A
recent study (29) has identified
other unexpected properties of enoxacin. Enoxacin has been shown to
inhibit osteoclast formation and function by interfering with the
interactions between the V-ATPase B2 subunit or V-ATPase a3 subunit
and microfilaments. Based on previous studies, it was hypothesized
that BE may prevent osteoporosis disease in vivo via the
combined anti-bone resorptive properties of bisphosphonates and
enoxacin (18,29). As the long-term use of
bisphosphonates may present a certain degree of risk for the
development of oral osteonecrosis, the authors of the present study
speculated that BE may be beneficial in reducing the occurrence of
oral osteonecrosis through enoxacin replacement therapy by allowing
for a reduction in the dose of bisphosphonates.
To the best of our knowledge, the present study has
confirmed for the first time that BE has the potential to restore
maxilla alveolar bone mass induced by OVX, and has further
demonstrated its role in inhibiting osteoclasts in vivo,
suggesting that its use may have potential therapeutic benefits for
the treatment of patients with alveolar bone osteoporosis. This
conclusion is in agreement with the results of two previous
studies, which demonstrated that BE administration had a protective
effect on alveolar bone for orthodontics and the treatment of
periodontitis (30,31). In the present study, micro-CT
measurement demonstrated that the ZOL and BE administration groups
exhibited an increased BV/TV, an increased Tb.Th and a decreased
Tb.Sp when compared with the Vehicle group. Consistent with these
results, histological observation of the region between M1 and M2
in the maxilla alveolar bone demonstrated that ovariectomy induced
trabecular bone destruction; however, ZOL and BE administration
alleviated this process. In addition, analysis of the TRAP-stained
paraffin sections and the line distance data from fluorochrome
labeling demonstrated that ZOL and BE administration was protective
against the deterioration of trabecular bone induced by excessive
osteoclast production, and also accelerated the rate of alveolar
bone formation. BE exhibited dose-dependent bone-resorption
inhibiting effects. Specifically, the anti-resorptive effect in the
BE-H group was close to, or better than, that of the ZOL group,
while the effect was poorer in the BE-L group. It is possible that
the unique anti-resorptive mechanism of BE made it particularly
useful in blocking the alveolar bone resorption triggered by
osteoporosis in the present study (30).
In conclusion, BE, a bisphosphonate derivative of
enoxacin, inhibits osteoclast formation and bone resorption in a
manner that resembles the novel mechanism by which enoxacin
functions (30). Notably, the
present study demonstrated that BE inhibits maxilla osteoporosis, a
process that is dependent on osteoclast activity in vivo.
However, the conclusions of the present study on BE are confined to
oral medicine, and so whether it will improve local or systemic
bone osteoporotic changes has yet to be elucidated. The present
study proposes that BE has the ability to reduce alveolar bone
resorption following periodontal infection and osteoporosis induced
by ovariectomy, as well as reducing orthodontic tooth movement,
which makes it a promising candidate for clinical use (30,31).
Acknowledgements
The present was supported by grants from the Science
and Technology Commission of Shanghai Municipality (grant no.
17140903400), the Shanghai Municipal Commission of Health and
Family Planning (grant no. M20170365), the Opening Project of
Shanghai Key Laboratory of Orthopaedic Implant (grant no.
KFKT2015002) and the National Natural Science Foundation of China
(grant no. 81570948).
References
|
1
|
McGrath C and Bedi R: The importance of
oral health to older people's quality of life. Gerodontology.
16:59–63. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Howson CP: Perspectives and needs for
health in the 21st century: 20th-century paradigms in 21st-century
science. J Hum Virol. 3:94–103. 2000.PubMed/NCBI
|
|
3
|
Dempster DW, Birchman R, Xu R, Lindsay R
and Shen V: Temporal changes in cancellous bone structure of rats
immediately after ovariectomy. Bone. 16:157–161. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kribbs PJ, Chesnut CH III, Ott SM and
Kilcoyne RF: Relationships between mandibular and skeletal bone in
an osteoporotic population. J Prosthet Dent. 62:703–707. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kribbs PJ: Comparison of mandibular bone
in normal and osteoporotic women. J Prosthet Dent. 63:218–222.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeffcoat MK: Osteoporosis: A possible
modifying factor in oral bone loss. Ann Periodontol. 3:312–321.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Purdue PE, Koulouvaris P, Potter HG,
Nestor BJ and Sculco TP: The cellular and molecular biology of
periprosthetic osteolysis. Clin Orthop Relat Res. 454:251–261.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun W, Wang YQ, Yan Q, Lu R and Shi B:
Effects of Er-Zhi-Wan on microarchitecture and regulation of
Wnt/β-catenin signaling pathway in alveolar bone of ovariectomized
rats. J Huazhong Univ Sci Technolog Med Sci. 34:114–119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Black DM and Rosen CJ: Postmenopausal
osteoporosis. N Engl J Med. 374:254–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cauley JA, Robbins J, Chen Z, Cummings SR,
Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, et
al: Effects of estrogen plus progestin on risk of fracture and bone
mineral density: The Women's Health Initiative randomized trial.
JAMA. 290:1729–1738. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cummings SR, Tice JA, Bauer S, Browner WS,
Cuzick J, Ziv E, Vogel V, Shepherd J, Vachon C, Smith-Bindman R and
Kerlikowske K: Prevention of breast cancer in postmenopausal women:
Approaches to estimating and reducing risk. J Natl Cancer Inst.
101:384–398. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Crandall CJ, Newberry SJ, Diamant A, Lim
YW, Gellad WF, Booth MJ, Motala A and Shekelle PG: Comparative
effectiveness of pharmacologic treatments to prevent fractures: An
updated systematic review. Ann Intern Med. 161:711–723. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khosla S, Bilezikian JP, Dempster DW,
Lewiecki EM, Miller PD, Neer RM, Recker RR, Shane E, Shoback D and
Potts JT: Benefits and risks of bisphosphonate therapy for
osteoporosis. J Clin Endocrinol Metab. 97:2272–2282. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shane E, Burr D, Abrahamsen B, Adler RA,
Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, et
al: Atypical subtrochanteric and diaphyseal femoral fractures:
Second report of a task force of the American Society for Bone and
Mineral Research. J Bone Miner Res. 29:1–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tella SH and Gallagher JC: Prevention and
treatment of postmenopausal osteoporosis. J Steroid Biochem Mol
Biol. 142:155–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Black DM, Bilezikian JP, Ensrud KE,
Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA and Rosen CJ:
PaTH Study Investigators: One year of alendronate after one year of
parathyroid hormone (1–84) for osteoporosis. N Engl J Med.
353:555–565. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ostrov DA, Magis AT, Wronski TJ, Chan EK,
Toro EJ, Donatelli RE, Sajek K, Haroun IN, Nagib MI, Piedrahita A,
et al: Identification of enoxacin as an inhibitor of osteoclast
formation and bone resorption by structure-based virtual screening.
J Med Chem. 52:5144–5151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herczegh P, Buxton TB, McPherson JC III,
Kovács-Kulyassa A, Brewer PD, Sztaricskai F, Stroebel GG, Plowman
KM, Farcasiu D and Hartmann JF: Osteoadsorptive bisphosphonate
derivatives of fluoroquinolone antibacterials. J Med Chem.
45:2338–2341. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Thompson DD, Simmons HA, Pirie CM and Ke
HZ: FDA Guidelines and animal models for osteoporosis. Bone. 17 4
Suppl:125S–133S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Assessment of fracture risk and its
application to screening for postmenopausal osteoporosis. Report of
a WHO Study Group. World Health Organ Tech Rep Ser. 843:1–129.
1994.PubMed/NCBI
|
|
21
|
Tuck SP and Francis RM: Osteoporosis.
Postgrad Med J. 78:526–532. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang J, Pham SM and Crabbe DL: Effects of
oestrogen deficiency on rat mandibular and tibial
microarchitecture. Dentomaxillofac Radiol. 32:247–251. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mavropoulos A, Rizzoli R and Ammann P:
Different responsiveness of alveolar and tibial bone to bone loss
stimuli. J Bone Miner Res. 22:403–410. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai QG, Zhang P, Wu YQ, Ma XH, Pang J,
Jiang LY and Fang B: Ovariectomy induces osteoporosis in the
maxillary alveolar bone: An in vivo micro-CT and histomorphometric
analysis in rats. Oral Dis. 20:514–520. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Al Habashneh R, Azar W, Shaweesh A and
Khader Y: The relationship between body mass index and
periodontitis among postmenopausal women. Obes Res Clin Pract.
10:15–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Salazar M, Hernandes L, Ramos AL, Salazar
Bde O, Micheletti KR, Paranhos LR, de Mendonça MR and Cuoghi OA:
Effect of alendronate sodium on tooth movement in ovariectomized
rats. Arch Oral Biol. 60:776–781. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
von Wowern N: General and oral aspects of
osteoporosis: A review. Clin Oral Investig. 5:71–82. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chen H, Xu X, Liu M, Zhang W, Ke HZ, Qin
A, Tang T and Lu E: Sclerostin antibody treatment causes greater
alveolar crest height and bone mass in an ovariectomized rat model
of localized periodontitis. Bone. 76:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu X, Qu X, Wu C, Zhai Z, Tian B, Li H,
Ouyang Z, Xu X, Wang W, Fan Q, et al: The effect of enoxacin on
osteoclastogenesis and reduction of titanium particle-induced
osteolysis via suppression of JNK signaling pathway. Biomaterials.
35:5721–5730. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toro EJ, Zuo J, Gutierrez A, La Rosa RL,
Gawron AJ, Bradaschia-Correa V, Arana-Chavez V, Dolce C, Rivera MF,
Kesavalu L, et al: Bis-enoxacin inhibits bone resorption and
orthodontic tooth movement. J Dent Res. 92:925–931. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rivera MF, Chukkapalli SS, Velsko IM, Lee
JY, Bhattacharyya I, Dolce C, Toro EJ, Holliday LS and Kesavalu L:
Bis-enoxacin blocks rat alveolar bone resorption from experimental
periodontitis. PLoS One. 9:e921192014. View Article : Google Scholar : PubMed/NCBI
|