Introduction
Rheumatoid arthritis (RA) is a heterogenic and
systemic autoimmune disease that is characterized by inflammation
of the joint lining and subsequent joint destruction (1). Fibroblast-like synoviocytes (FLS) are
the primary effectors of cartilage destruction in RA and have been
reported to serve a crucial role in initiating and maintaining the
inflammatory and destructive processes that occur in the rheumatoid
joint (2,3). Therefore, a better understanding of
the molecular mechanisms involved in the progression of RA,
particularly in FLS, may facilitate the development of novel
biomarkers and therapies for RA.
The roles of microRNAs (miRNAs) have been examined
in a number of diseases (4).
miRNAs are a class of small, noncoding RNAs that regulate gene
expression by binding to the 3′-untranslated region (UTR), leading
mRNA degradation or translation inhibition (5,6).
Aberrant miRNA expression has been reported to regulate diverse
biological processes, such as cell proliferation, differentiation
and apoptosis, be have been associated with various diseases and
cancers (7). In addition, an
increasing number of reports have suggested that miRNAs serve
crucial roles in the pathogenesis of RA, and may serve as
diagnostic biomarkers and therapeutic agents (8–10).
The miRNA miR-137 is located on chromosome 1p22 and
has been revealed to serve as a regulator of susceptibility genes
in certain diseases, such as non-small cell lung cancer (10), gastric cancer (11), renal cancer (12), breast cancer (13), Alzheimer's disease (14) and schizophrenia (15). However, the biological functions
and underlying molecular mechanisms ofmiR-137 in RA remain unclear.
Therefore, the present study aimed to analyze miR-137 expression in
RA-FLS, to examine the effects of miR-137 on proliferation,
migration, invasion and the expression of inflammatory cytokines,
and to investigate its role in RA-FLS.
Materials and methods
Animals and RA model preparation
A total of 10 male Wistar rats (weight, 160-180 g;
age, 5–6 weeks) were obtained from the Animal Center of Jilin
University (Changchun, China), and were maintained under specific
pathogen-free conditions (SPF), fed standard chow and provided with
tap water ad libitum at room temperature (20–25°C) and 12-h
light dark cycle. Rats were randomly divided into 2 groups
(n=5/group): The RA group and the normal control group. The RA
mouse model was established as described previously (16). Briefly, rats were treated with
Complete Freund's Adjuvant (0.1 ml/100 g bodyweight, administered
via left paw injection; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 21 days. Rats in the normal control group received
injections of normal PBS (0.1 ml/100 g body weight) in the left
paw. Animal experimental protocols were approved by The Animal Care
and Use Committee of Jilin University (Changchun, China).
Cell culture and transfection
FLSs were obtained from the synovial tissues of RA
and normal control rats as described previously (17). Briefly, synovial tissues were
obtained and were cut into blocks of 1×1×1 mm in Dulbecco's
modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) under sterile conditions. The tissues were
aspirated using a sterile pipette and sprayed evenly onto the
bottom of the 25 cm2 flasks, then were cultured in DMEM
supplemented with supplemented with 20% (v/v) heat-inactivated
fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at
37°C in 5% CO2. The medium was replaced every two day.
The tissue blocks were washed with PBS one week later and after the
primary culture reached 70% confluence, the cells were passaged.
Cells from passages 3–6 were used in the present study. FLSs were
grown in cell culture flasks in 10% high-glucose DMEM supplemented
with 10% (v/v) FBS, 100 U/ml penicillin and 100 mg/ml streptomycin
(both from the Beyotime Institute of Biotechnology, Haimen, China)
and maintained at 37°C in humidified air with 5%
CO2.
For transfections, FLS were seeded (1×104
cells/well) in 6 well plates and cultured for 24 h. RA-FLS were
transfected with miR-137 mimic (5′-GAUGCGCAUAAGAAUUCGUUAUU-3′) or
the corresponding negative control (miR-NC;
5′-UCGCUUGGUGCAGGUCGGGAA-3′; both from Shanghai GenePharma Co.,
Ltd., Shanghai, China) at final concentration 100 nM using the
Lipofectamine 2000 Reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Then transfected
cells were cultured at 37°C in humidified air with 5%
CO2 for 24–72 h to test miR-137 role in RA-FLS.
Transfection efficiency was determined by RT-qPCR at 24 h
post-transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA including miRNAs was isolated from
2×106 cultured cells and 100 mg frozen fresh tissues
using TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according
to the manufacturer's protocol. The purity and concentration of
total RNA were determined with a dual-beam ultraviolet
spectrophotometer (Eppendorf, Hamburg, Germany). To quantify
miR-137, cDNA was synthesized using the TaqMan miRNA Reverse
Transcription kit (Thermo Fisher Scientific, Inc.), then was
quantified using TaqMan Human MicroRNA Assay kit (Thermo Fisher
Scientific, Inc.) on an ABI7900 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The primers of miR-137
and U6 used in the present study were as described previously
(18) (miRNA-137, forward
5′-GCGCTTATTGCTTAAGAATAC-3′ and reverse, 5′-CAGTGCAGGGTCCGAGGT-3′;
U6, forward 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse
5′-CGCTTCACGAATTTGCGTGTCAT-3′). For the detection of C-X-C motif
chemokine ligand 12 (CXCL12) and GAPDH, cDNA was synthesized from
total RNA using the PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). RT-qPCR was performed
using the QuantiFast SYBR-Green RT-PCR kit (Qiagen GmbH, Hilden,
Germany), according to the manufacturer's protocol, and an ABI7900
Real-Time PCR System. The CXCL12 and GAPDH primers used in the
present study were described previously (19). The primer of CXCL12 was as follows:
Forward 5′-CTCTGCATCAGTGACGGTAAGC-3′; and reverse,
5′-AATCTGAAGGGCACAGTTTGG-3′; the primer for GAPDH was as follows:
Forward 5′-TGGAATCCACTGGCGTCTTC-3′; and reverse,
5′-GGTTCACGCCCATCACAAAC-3′. The PCR amplification conditions were
as follows: 95°C for 40 sec and 40 cycles of 95°C for 5 sec, 60°C
for 40 sec, finally extend for 72°C 5 min. U6 small nuclear RNA was
used to normalize miR-137 expression levels, and GAPDH was used to
normalize CXCL12 mRNA levels. The relative expression levels were
evaluated using the 2−∆∆Cq method (20).
Cell proliferation
RA-FLS were seeded (2×104 cells/well) in
96-well plates and cultured in DMEM medium containing 10% FBS at
37°C in humidified air with 5% CO2 for 24 h prior to
transfection with miR-137 mimic or miR-NC for and additional 48 h
at 37°C. Following the addition of MTT solution (20 µl, 5 g/l;
Sigma-Aldrich; Merck KGaA) to each well, plates were cultured for 4
h at 37°C. The medium was removed, 150 µl dimethylsulfoxide
(Sigma-Aldrich; Merck KGaA) was added and the plates were
oscillated for 10 min. The absorbance of each well was measured at
490 nm using a Microplate Spectrophotometer (BioTek Instruments,
Inc., Winooski, VT, USA).
Cell migration and invasion
The migratory ability of miR-137 mimic- or
miR-NC-transfected RA-FLS was examined by wound-healing assay.
Briefly, RA-FLS (2×104 cells/well) were seeded into
24-well tissue culture plates for 24 h at 37°C in humidified air
with 5% CO2, and grown to ~80% confluence, a linear
wound was made in the cellular monolayer with a 200 µl pipette tip.
Following wounding, the debris was removed, DMEM was added and the
cells were incubated for 24 h. Wound closure was observed and
imaged at 0 and 24 h using an IX71 inverted light microscope
(Olympus Corporation, Tokyo, Japan). The migration index was
calculated as follows: Experimental group scratch distance (0–24
h)/control group (miR-NC) scratch distance (0 h-24 h).
For the Transwell invasion assay, RA-FLS
(2×104) were suspended in serum-Free DMEM medium and
seeded in the upper Transwell chamber that was pre-coated with
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). DMEM containing
20% FBS was added to the lower chamber as the chemoattractant and
the cells were incubated for 48 h. Non-invading RA-FLS were removed
from the upper membrane surface with cotton swabs, and the RA-FLS
that migrated to the lower membrane were fixed with 100% methanol
at room temperature for 30 min and stained with 0.1% crystal violet
(Sigma-Aldrich; Merck KGaA) at room temperature for 5 min. Cells
were imaged with an IX71 inverted light microscope (Olympus
Corporation) and counted in five randomly selected fields.
Measurement of tumor necrosis factor-α
(TNF-α), interleukin (IL)-6, IL-8 and IL-1β protein expression
Following transfection, protein expression levels of
TNF-α, IL-6, IL-8 and IL-1β were measured in each FLSs using ELISA.
Briefly, transfected RA-FLS were seeded into a 24-well plate at a
density of 2×105 cells/well and grown in DMEM medium
with 10% FBS for 48 h at 37°C. The supernatants were collected by
centrifugation at 1,000 × g for 5 min at 4°C TNF-α, IL-6, IL-8 and
IL-1β production in supernatants were determined using human
Cytokine 25-Plex Panel (Invitrogen; Thermo Fisher Scientific, Inc.;
cat no. LHC0009) according to the manufacturer's protocol. The
concentrations of each protein were determined using ELISA
multi-well spectrophotometer (Molecular Devices, LLC, Sunnyvale,
CA, USA).
Luciferase reporter assay
Prediction of miR-137 targets was performed using
two publicly available algorithms: TargetScan (www.targetscan.org) and miRanda (www.microrna.org). Human CXCL12 3′UTR oligonucleotides
containing the wild-type (Wt) or mutant (Mut) binding site of
miR-137 were synthesized by Shanghai GenePharma Co., Ltd.,
(Shanghai, China) and inserted into the pGL3-control vector
(Ambion; Thermo Fisher Scientific, Inc.) at the NheI
(Sigma-Aldrich; Merck KGaA) and XhoI (Sigma-Aldrich; Merck
KGaA) sites. For luciferase reporter assay, RA-FLS were seeded into
24-well plates for 24 h, and transiently co-transfected with 100 ng
of either Wt-CXCL12 or Mut-CXCL12 plasmid, and 100 nM of either
miR-137 mimic or miR-NC using Lipofectamine 2,000. The cells were
incubated for 48 h, harvested and lysed. Luciferase activities were
determined using the Dual-Luciferase Reporter 1000 Assay System
(Promega Corporation, Madison, WI, USA). Renilla-luciferase
was used for normalization.
Western blotting
Cultured FLS (2×106 cells) were harvested
and lysed with radioimmunoprecipitation assay buffer (Beyotime
Institute of Biotechnology). The supernatants were collected by
centrifugation at 1,000 × g for 5 min at 4°C, and protein
concentrations were determined using the Bicinchoninic Acid Protein
Assay kit (Beyotime Institute of Biotechnology). A total of 30 µg
protein were separated by 10% SDS-PAGE and transferred onto a
nitrocellulose membrane (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Membranes were blocked with 5% non-fat milk in Tris-buffered
saline (TBS; Sigma-Aldrich; Merck KGaA) for 1 h at room
temperature, and incubated overnight at 4°C with the following
primary antibodies: Mouse monoclonal anti-human CXCL12 (1:1,000;
cat no. sc-74271) and mouse monoclonal anti-human GAPDH (1:5,000;
cat no. sc-293335); both were purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Following primary antibody
incubation, the membranes were washed three times in TBS/0.1%
Tween-20 (TBST; Sigma-Aldrich; Merck KGaA) and incubated for 1 h
with goat anti-mouse horseradish peroxidase-conjugated secondary
antibody (1:10,000; cat no. 516102; Santa Cruz Biotechnology, Inc.)
in TBS/Tween-20 at room temperature. Protein bands were detected
using the Enhanced Chemiluminescence-Plus kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. GAPDH
was used as an internal control.
Statistical analysis
Data are presented as the mean ± standard deviation
of at least three separate experiments, and were analyzed using
SPSS software version 19.0 (IBM Corp., Armonk, NY, USA).
Statistical differences were determined by using two-tailed
Student's t-test for two comparisons or one-way analysis of
variance followed by a post hoc Tukey's test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
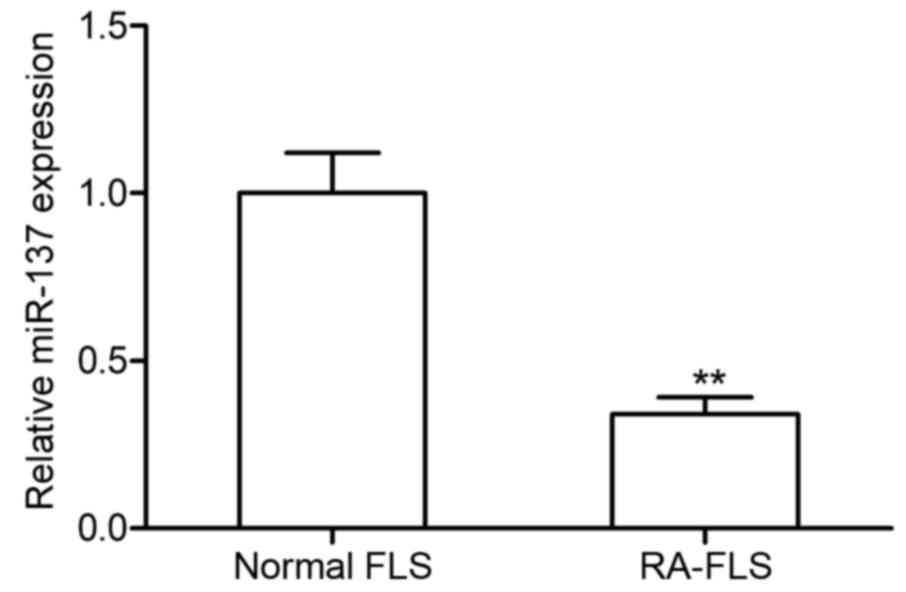
miR-137 expression levels are
upregulated in RA-FLS
The levels of miR-137 expression in RA-FLS and
normal FLS were examined using RT-qPCR (Fig. 1). The miR-137 expression level was
significantly higher in RA-FLS compared with the level of miR-137
expression in normal FLS. This result indicated that miR-137 maybe
involved in RA pathogenesis.
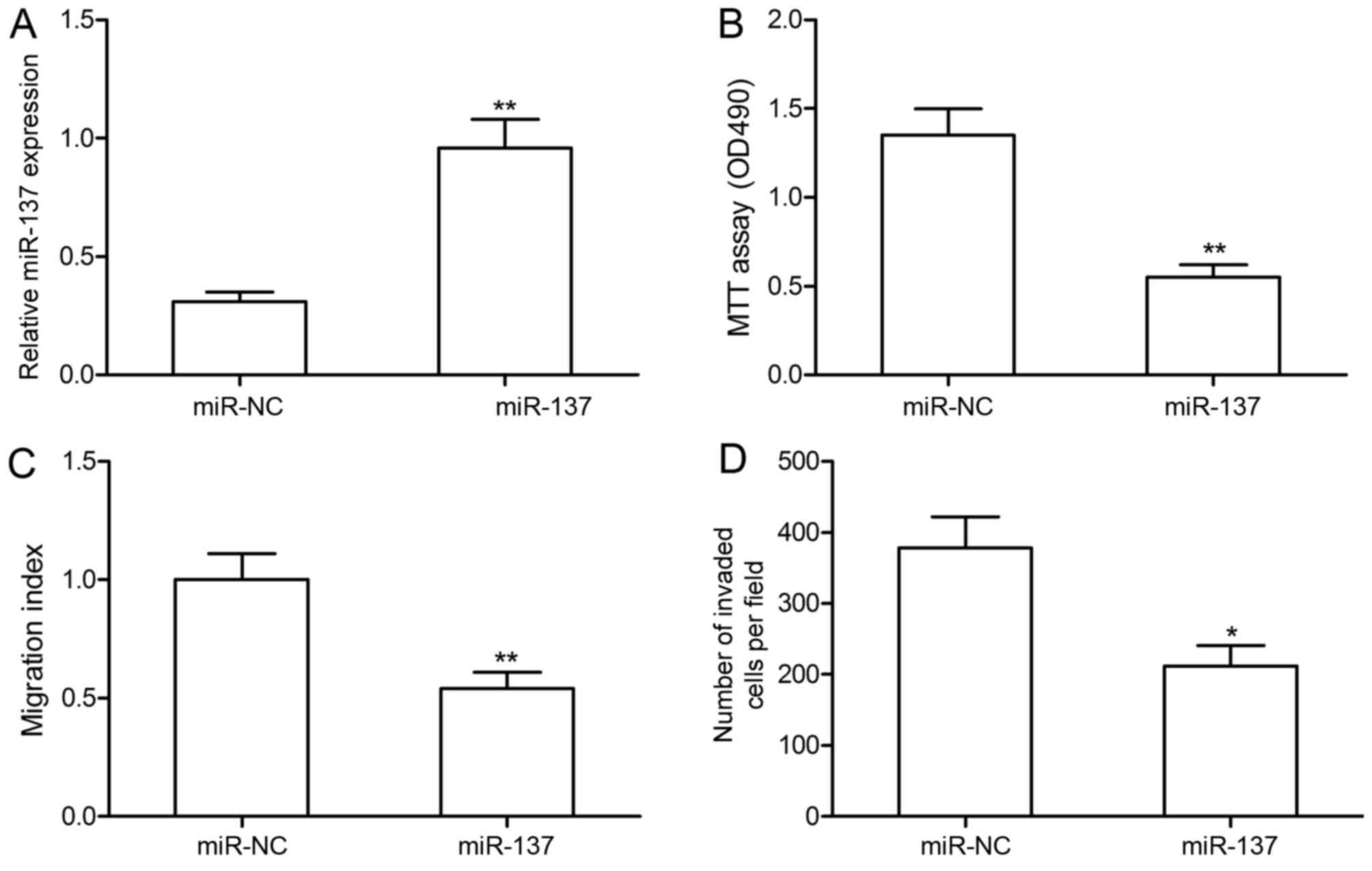
miR-137 overexpression inhibits RA-FLS
proliferation, migration and invasion
To investigate the biological functions of miR-137,
RA-FLS were transfected with miR-137 mimic or miR-NC. RA-FLS
transfected with miR-137 mimics exhibited a significant increase in
miR-137 expression compared with RA-FLS transfected with miR-NC
(Fig. 2A). Cell proliferation,
migration and invasion were determined in by MTT, wound healing and
Transwell invasion assays, respectively. miR-137 overexpression
significantly inhibited RA-FLS proliferation, migration and
invasion compared with RA-FLS transfected with miR-NC (Fig. 2B-D). These results suggested that
miR-137 may serve an inhibitory role in RA-FLS.
miR-137 overexpression inhibits TNF-α,
IL-6, IL-8 and IL-1β protein expression in RA-FLS
To investigate the effects ofmiR-137 on inflammatory
cytokines, TNF-α, IL-6, IL-8 and IL-1β protein expression levels
were measured in RA-FLS by ELISA (Fig.
3). The results revealed that RA-FLS transfected with miR-137
mimic exhibited significantly decreased expression levels of TNF-α,
IL-6, IL-8 and IL-1β compared with cytokine expression levels in
RA-FLS transfected with miR-NC.
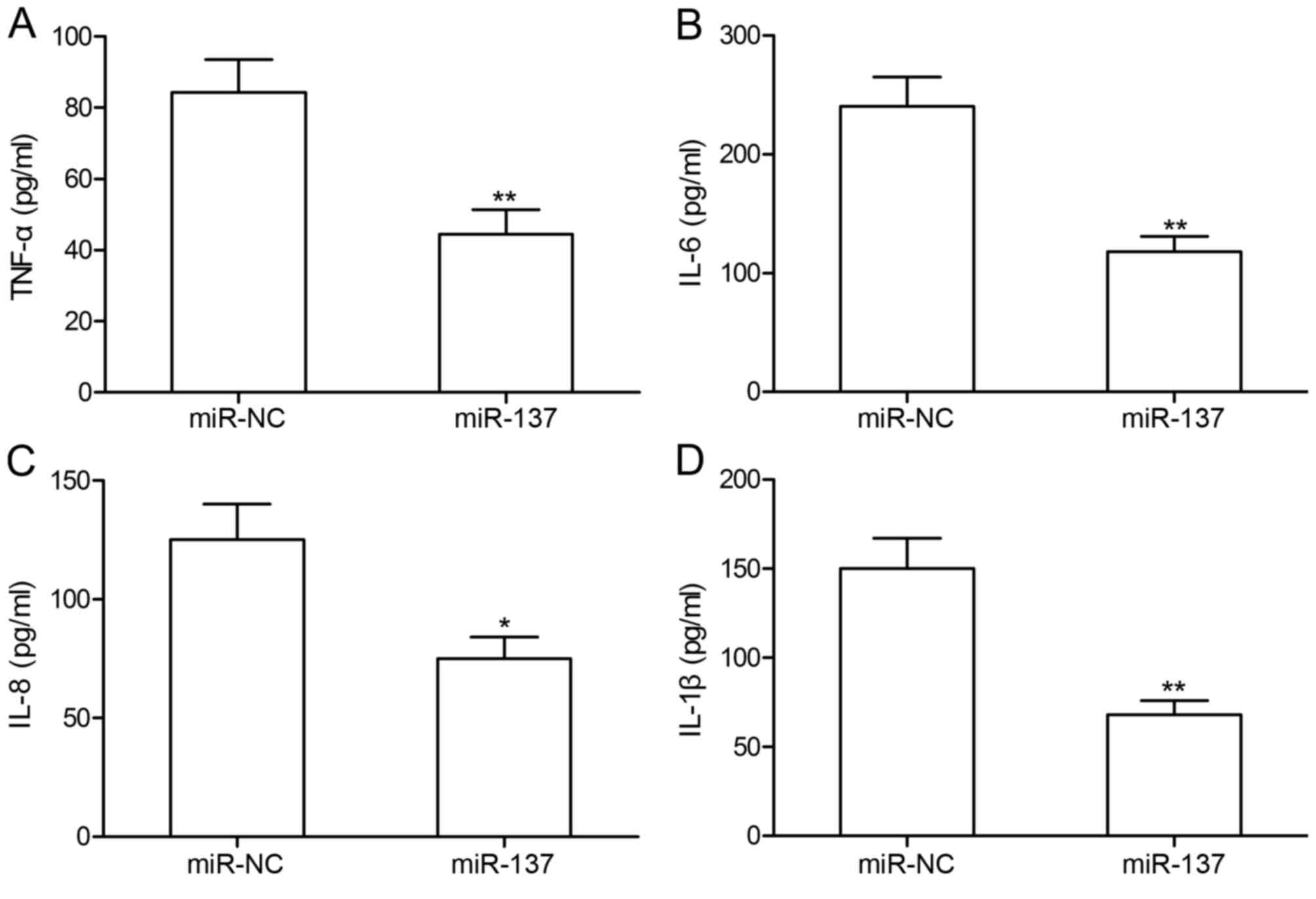 | Figure 3.miR-137 overexpression inhibits TNF-α,
IL-6, IL-8 and IL-1β protein expression in RA-FLS. ELISA was
performed to measure the protein expression levels of the
inflammatory cytokines (A) TNF-α, (B) IL-6, (C) IL-8 and (D) IL-1β
in RA-FLS transfected with miR-137 mimic or miR-NC by ELISA.
*P<0.05 and **P<0.01 vs. miR-NC. FLS, fibroblast-like
synoviocytes; IL, interleukin; miR, microRNA; NC, negative control;
RA, rheumatoid arthritis; TNF, tumor necrosis factor. |
CXCL12 mRNA is a direct target of
miR-137 in RA-FLS
Potential miR-137 targets were predicted with two
bioinformatics databases (TargetScan and miRanda), CXCL12 was
selected for further analysis as it has been previously reported to
serve crucial roles in RA development (21). To verify whether CXCL12 is a direct
target of miR-137 in RA-FLS, human Wt-CXCL12 and Mut-CXCL12 3′UTR
reporter plasmids were constructed (Fig. 4A). The Wt or Mut reporter plasmid
was co-transfected with either miR-137 mimic or miR-NC into RA-FLS
and luciferase activities were determined. miR-137 overexpression
in RA-FLS co-transfected with miR-137 mimics and Wt-CXCL12 resulted
in a significant decrease in luciferase activity compared with the
luciferase activity in RA-FLS co-transfected with miR-NC and
Wt-CXCL12 (Fig. 4B). No
significant differences were identified between RA-FLS
co-transfected with Mut-CXCL12 and either miR-137 mimic or miR-NC
(Fig. 4B). These results suggested
that CXCL12 maybe a direct target of miR-137.
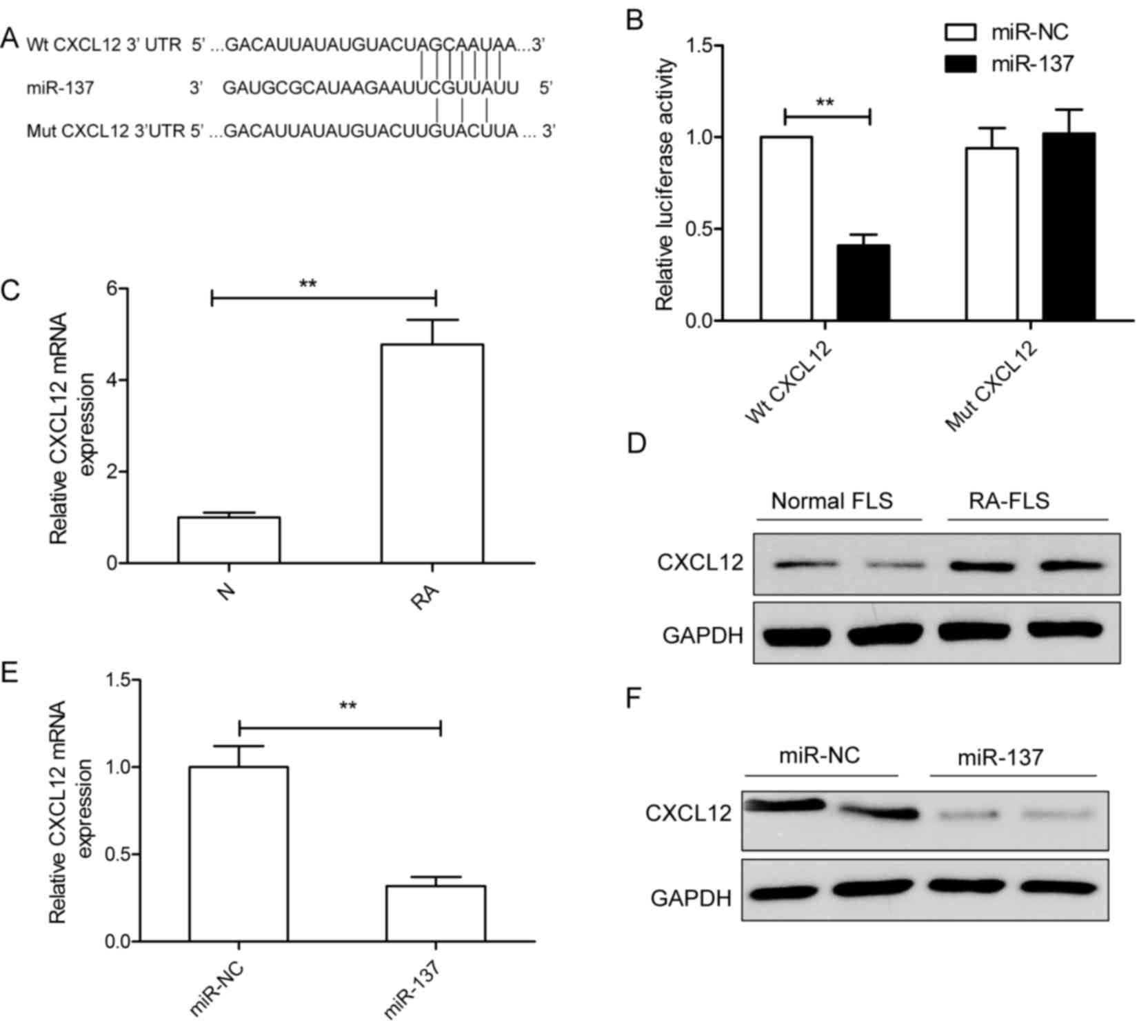 | Figure 4.CXCL12 is a direct target of miR-137
in RA-FLS. (A) Predicted binding site for miR-137 in the 3′UTR of
Wt-CXCL12; mutations in the binding sites in the Mut-CXCL12
sequence are also indicated. (B) Relative luciferase activity of in
RA-FLS co-transfected with either Wt-CXCL12 or Mut-CXCL12 3′UTR
reporter plasmid and either miR-137 mimic or miR-NC. **P<0.01
vs. miR-NC. The expression levels of CXCL12 (C) mRNA and (D)
protein were detected in FLS isolated the RA model rat group and
the normal control group by RT-PCR and western blotting,
respectively. GAPDH was used as an internal control. **P<0.01
vs. normal FLS. (E and F) RA-FLS were transfected with either
miR-137 mimic or miR-NC, and CXCL12 (E) mRNA and (F) protein
expression levels were determined. GAPDH was used as an internal
control. **P<0.01 vs. miR-NC. CXCL12, C-X-C motif chemokine
ligand 12; FLS, fibroblast-like synoviocytes; miR, microRNA; Mut,
mutant; NC, negative control; RA, rheumatoid arthritis; UTR,
untranslated region; Wt, wild-type. |
The levels of CXCL12 mRNA and protein expression in
FLS were analyzed by RT-qPCR and western blotting, respectively.
CXCL12 mRNA expression was significantly increased in RA-FLS
compared with normal FLS (Fig.
4C); similarly, CXCL12 protein expression was notably higher in
RA-FLA compared with normal FLS (Fig.
4D). To determine whether miR-137 regulated CXCL12 expression
in FLS, RA-FLS were transfected with miR-137 mimics or miR-NC, and
CXCL12 mRNA and protein expression levels were determined in by
RT-qPCR and western blotting, respectively. As expected,
overexpression of miR-137 in RA-FLS resulted in a significant
decrease in CXCL12 mRNA expression (Fig. 4E), and a notable decrease in CXCL12
protein expression (Fig. 4F).
These results indicated that CXCL12 was a direct target of miR-137
in RA-FLS.
Discussion
An increasing number of studies have indicated that
the aberrant expression of miRNAs was involved in RA progression
through regulating cell proliferation, migration and invasion
(8,9). For example, a recent study reported
that increased miR-21 expression in FLS in RA model rats promoted
cell proliferation by facilitating the nuclear translocation of
NF-κB (17). Another recent study
demonstrated that decreased miR-221 expression led to a significant
reduction in the expression of pro-inflammatory cytokines, and
inhibited FLS migration and invasion through the inhibition of
vascular endothelial growth factor, matrix metalloproteinase
(MMP)-3 and MMP-9 expression (22). miR-20a has been revealed to
regulate the formation of the NACHT, LRR and PYD domains-containing
protein 3 inflammasome and the release of cytokines in RA-FLS by
targeting thioredoxin-interacting protein expression (23). miR-137 has previously been reported
to be involved in the development of certain human diseases and
cancers (10–15). However, whether miR-137 serves a
role in RA development remains unclear. The present study
demonstrated that the expression levels of miR-137 were
significantly lower in FLS from RA model rats compared with normal
FLS, and that overexpression of miR-137 (via miR-137 mimics
transfection) significantly inhibited proliferation, migration and
invasion, and reduced the expression of inflammatory cytokines.
These results indicated that miR-137 may serve an inhibitory role
in RA pathogenesis.
A number of in vitro and in vivo
studies have reported that certain inflammatory cytokines,
including TNF-α, IL-1β, IL-18 and IL-6, were closely related to RA
development (24–26). These cytokines stimulate synovial
fibroblast hyperplasia, leading to the secretion of chemokines and
cytokines and subsequent joint damage (24,27–29).
Therefore, the present study aimed to determine whether miR-137
effected inflammation in RA-FLS, and the results revealed that the
overexpression of miR-137 substantially decreased the expression of
TNF-α, IL-6, IL-8 and IL-1β in RA-FLS, suggesting that miR-137 may
have potential as an anticytokine therapy for RA.
CXCL12 is a potent chemoattractant that has been
identified for band-T-lymphocytes, monocytes, and progenitor cells
(30). It has been reported that
CXCL12 was involved in physiological processes, such as
angiogenesis and tissue repair, and pathological processes, such as
chronic inflammation and neoplasia (21,30).
CXCL12 expression was revealed to be increased in a variety of
inflammatory conditions, including RA, and may aid in the
recruitment of leukocytes and endothelial progenitors (31,32).
Although a previous study demonstrated that CXCL12 was a target of
miR-137 in thyroid cancer (33),
an interaction between miR-137 and CXCL12 has not been demonstrated
experimentally in RA-FLS. The present study used a luciferase
reporter assay to confirm CXCL12 as a direct target of miR-137. In
addition, CXCL12 mRNA and protein expression levels were revealed
to be upregulated in RA-FLS, and these high expression levels were
reduced when miR-137 was overexpressed. These results indicated
that miR-137 may exert its inhibitory role in RA, at least partly,
through targeting CXCL12.
In conclusion, the present study demonstrated that
miR-137 expression was downregulated in RA-FLSs, and that the
overexpression of miR-137 in RA-FLS significantly inhibited
proliferation, migration and invasion, and reduced the expression
of inflammatory cytokines. In addition, CXCL12 was confirmed as a
direct target of miR-137 in RA-FLSs. These results provided further
insight into the molecular mechanisms of RA progression, and
suggested that miR-137 may be a target for the treatment RA.
References
|
1
|
Firestein GS: Evolving concepts of
rheumatoid arthritis. Nature. 423:356–361. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bottini N and Firestein GS: Duality of
fibroblast-like synoviocytes in RA: Passive responders and
imprinted aggressors. Nat Rev Rheumatol. 9:24–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miossec P: Rheumatoid arthritis: Still a
chronic disease. Lancet. 381:884–886. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Esteller M: Non-coding RNAs in human
disease. Nat Rev Genet. 12:861–874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McManus MT: MicroRNAs and cancer. Semin
Cancer Biol. 13:253–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
O'Connell RM, Rao DS and Baltimore D:
microRNA regulation of inflammatory responses. Annu Rev Immunol.
30:295–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stanczyk J, Pedrioli DM, Brentano F,
Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S and Kyburz
D: Altered expression of MicroRNA in synovial fibroblasts and
synovial tissue in rheumatoid arthritis. Arthritis Rheum.
58:1001–1009. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu X, Li Y, Shen H, Li H, Long L, Hui L
and Xu W: miR-137 inhibits the proliferation of lung cancer cells
by targeting Cdc42 and Cdk6. FEBS Lett. 587:73–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cheng Y, Li Y, Liu D, Zhang R and Zhang J:
miR-137 effects on gastric carcinogenesis are mediated by targeting
Cox-2-activated PI3K/AKT signaling pathway. FEBS Lett.
588:3274–3281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H and Li H: miR-137 inhibits renal
cell carcinoma growth in vitro and in vivo. Oncol Lett. 12:715–720.
2016.PubMed/NCBI
|
|
13
|
Zhao Y, Li Y, Lou G, Zhao L, Xu Z, Zhang Y
and He F: MiR-137 targets estrogen-related receptor alpha and
impairs the proliferative and migratory capacity of breast cancer
cells. PLoS One. 7:e391022012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Geekiyanage H and Chan C:
MicroRNA-137/181c regulates serine palmitoyltransferase and in turn
amyloid β, novel targets in sporadic Alzheimer's disease. J
Neurosci. 31:14820–14830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Siegert S, Seo J, Kwon EJ, Rudenko A, Cho
S, Wang W, Flood Z, Martorell AJ, Ericsson M, Mungenast AE and Tsai
LH: Addendum: The schizophrenia risk gene product miR-137 alters
presynaptic plasticity. Nat Neurosci. 19:11152016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Brand DD, Latham KA and Rosloniec EF:
Collagen-induced arthritis. Nat Protoc. 2:1269–1275. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Y, Xian PF, Yang L and Wang SX:
MicroRNA-21 promotes proliferation of fibroblast-like synoviocytes
through mediation of NF-κB nuclear translocation in a rat model of
collagen-induced rheumatoid arthritis. Biomed Res Int.
2016:92790782016.PubMed/NCBI
|
|
18
|
Zhang H and Li H: miR-137 inhibits renal
cell carcinoma growth in vitro and in vivo. Oncol Lett. 12:715–720.
2016.PubMed/NCBI
|
|
19
|
Li Z, Wang W, Xu H, Ning Y, Fang W, Liao
W, Zou J, Yang Y and Shao N: Effects of altered CXCL12/CXCR4 axis
on BMP2/Smad/Runx2/Osterix axis and osteogenic gene expressions
during osteogenic differentiation of MSCs. Am J Transl Res.
9:1680–1693. 2017.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pablos JL, Santiago B, Galindo M, Torres
C, Brehmer MT, Blanco FJ and García-Lázaro FJ: Synoviocyte-derived
CXCL12 is displayed on endothelium and induces angiogenesis in
rheumatoid arthritis. J Immunol. 170:2147–2152. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang S and Yang Y: Downregulation of
microRNA-221 decreases migration and invasion in fibroblast-like
synoviocytes in rheumatoid arthritis. Mol Med Rep. 12:2395–2401.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li XF, Shen WW, Sun YY, Li WX, Sun ZH, Liu
YH, Zhang L, Huang C, Meng XM and Li J: MicroRNA-20a negatively
regulates expression of NLRP3-inflammasome by targeting TXNIP in
adjuvant-induced arthritis fibroblast-like synoviocytes. Joint Bone
Spine. 83:695–700. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Choy EH and Panayi GS: Cytokine pathways
and joint inflammation in rheumatoid arthritis. N Engl J Med.
344:907–916. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stokes MB, Foster K, Markowitz GS,
Ebrahimi F, Hines W, Kaufman D, Moore B, Wolde D and D'Agati VD:
Development of glomerulonephritis during anti-TNF-α therapy for
rheumatoid arthritis. Nephrol Dial Transplant. 20:1400–1406. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hashizume M and Mihara M: The roles of
interleukin-6 in the pathogenesis of rheumatoid arthritis.
Arthritis. 2011:7656242011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Croft M: The TNF family in T cell
differentiation and function-unanswered questions and future
directions. Semin Immunol. 26:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schenten D, Nish SA, Yu S, Yan X, Lee HK,
Brodsky I, Pasman L, Yordy B, Wunderlich FT, Brüning JC, et al:
Signaling through the adaptor molecule MyD88 in CD4+ T cells is
required to overcome suppression by regulatory T cells. Immunity.
40:78–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nish SA, Schenten D, Wunderlich FT, Pope
SD, Gao Y, Hoshi N, Yu S, Yan X, Lee HK, Pasman L, et al: T
cell-intrinsic role of IL-6 signaling in primary and memory
responses. Elife. 3:e019492014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vicente-Manzanares M, Montoya MC, Mellado
M, Frade JM, del Pozo MA, Nieto M, de Landazuri MO, Martínez-A C
and Sánchez-Madrid F: The chemokine SDF-1α triggers a chemotactic
response and induces cell polarization in human B lymphocytes. Eur
J Immunol. 28:2197–2207. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nagasawa T, Tachibana K and Kishimoto T: A
novel CXC chemokine PBSF/SDF-1 and its receptor CXCR4: Their
functions in development, hematopoiesis and HIV infection. Semin
Immunol. 10:179–185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Santiago B, Calonge E, Del Rey MJ,
Gutierrez-Cañas I, Izquierdo E, Usategui A, Galindo M, Alcamí J and
Pablos JL: CXCL12 gene expression is upregulated by hypoxia and
growth arrest but not by inflammatory cytokines in rheumatoid
synovial fibroblasts. Cytokine. 53:184–190. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dong S, Jin M, Li Y, Ren P and Liu J:
miR-137 acts as a tumor suppressor in papillary thyroid carcinoma
by targeting CXCL12. Oncol Rep. 35:2151–2158. 2016. View Article : Google Scholar : PubMed/NCBI
|