Introduction
Intervertebral disc degeneration (IDD) is regarded
as a leading cause of lower back and leg pain (1). Due to a lack of complete
understanding of the pathogenesis of IDD, current treatments are
effective in symptomatic relief, but not biological regeneration of
degenerative disc tissue (2–4).
Further studies are required to develop effective regenerative
strategies for IDD.
The intervertebral disc (IVD) functions as a
connection structure that absorbs and transmits mechanical load
(5). Under physiological
conditions, the disc is subjected to various magnitudes of
mechanical compression (6,7). In line with previous studies, it was
demonstrated that mechanical compression significantly affected
disc biology in vitro (8,9).
Furthermore, our preliminary study identified that a high-magnitude
compression (20% deformation) promoted nucleus pulposus (NP) cell
senescence in a three-dimensional (3D) scaffold culture system
(unpublished data). As NP senescence is a classical cellular
characteristic during disc degeneration (10,11),
it is proposed that prevention of NP cell senescence may be a
potential mechanism to alleviate high-magnitude compression-induced
disc degeneration.
N-cadherin (N-CDH) is an adhesion molecule that was
initially identified in the nervous system (12,13).
Recent studies have indicated that N-CDH is a molecule that is
highly expressed in normal NP cells and is gradually downregulated
with disc degeneration (14,15).
Notably, N-CDH-mediated signaling facilitates with maintaining a
normal NP cell phenotype and NP matrix biosynthesis under the
stimulation of certain pathological factors (16,17).
However, the effects of N-CDH-mediated signaling on NP cell
senescence remain unclear.
Therefore, the aim of the present study was to
investigate the effects of N-CDH-mediated signaling on NP cell
senescence under high-magnitude compression. To achieve this
objective, a 3D scaffold culture system based upon a self-developed
perfusion bioreactor was involved (18). NP cell senescence was evaluated by
senescence-associated β-galactosidase (SA-β-Gal) activity, NP cell
proliferation, telomerase activity, senescence marker (p16 and p53)
expression levels and the matrix homeostatic phenotype.
Materials and methods
Ethical statement
All experimental animals were used in accordance
with the relevant guidelines [SYXK (YU) 2012-0012] of the Ethics
Committee at Southwest Hospital affiliated to the Third Military
Medical University (Chongqing, China).
Disc harvest and NP cell
isolation
Twenty-five healthy New Zealand rats (weight, 250 g;
age, 6–8 weeks) were obtained from the Animal Center of Third
Military Medical University (Chongqing, China) and sacrificed by
excessive carbon dioxide exposure. Briefly, after NP tissues were
separated from the harvested thoracic and lumbar discs, NP tissue
samples were sequentially digested with Gibco 0.25% trypsin (Thermo
Fisher Scientific, Inc., Waltham, MA, USA) for 3–5 min at 37°C and
Sigma-Aldrich type I collagenase (0.25%; Merck KGaA, Darmstadt,
Germany) for 10 min. Subsequently, NP cell pellets were collected
by centrifugation (500 × g at 4°C for 5 min) and cultured in
Dulbecco's modified Eagle's medium/F12 (Gibco; Thermo Fisher
Scientific, Inc. medium containing Gibco 10% fetal bovine serum
(FBS; Thermo Fisher Scientific, Inc.) and 1% (v/v)
penicillin-streptomycin (Gibco; Thermo Fisher Scientific, Inc.)
under standard conditions (37°C, 20% O2 and 5%
CO2).
NP cell transfection
NP cells were seeded in a 24-well plate and grown to
40–50% confluence. Subsequently, NP cells were incubated with 400
µl fresh culture medium containing 40 µl concentrate of recombinant
lentiviral vectors (Shanghai GenePharma Co., Ltd., Shanghai, China)
for 48 h to overexpress N-CDH in the NP cells (NP-N-CDH). NP cells
transfected with negative vectors served as controls (NP-N-CDH-NC).
Thereafter, the transfected cells were further selected via
puromycin for 4–6 days. N-CDH overexpression in NP cells was
verified by quantitative polymerase chain reaction (qPCR) and
western blotting assays.
Compression application on NP
cells
The transfected or un-transfected NP cells were
suspended in collagen solution (1 mg/ml; Shengyou Biotechnology
Co., Ltd., Hangzhou, China) and seeded into the prepared bovine
decalcified bone matrix scaffold [DBM; 10×10×5 mm (1×107
cells per DBM)], provided by Tissue Engineering Center of the Third
Military Medical University (Chongqing, China). After NP cells
seeded in the scaffold were pre-cultured under standard conditions
(37°C, 20% O2 and 5% CO2) for 2 days, NP
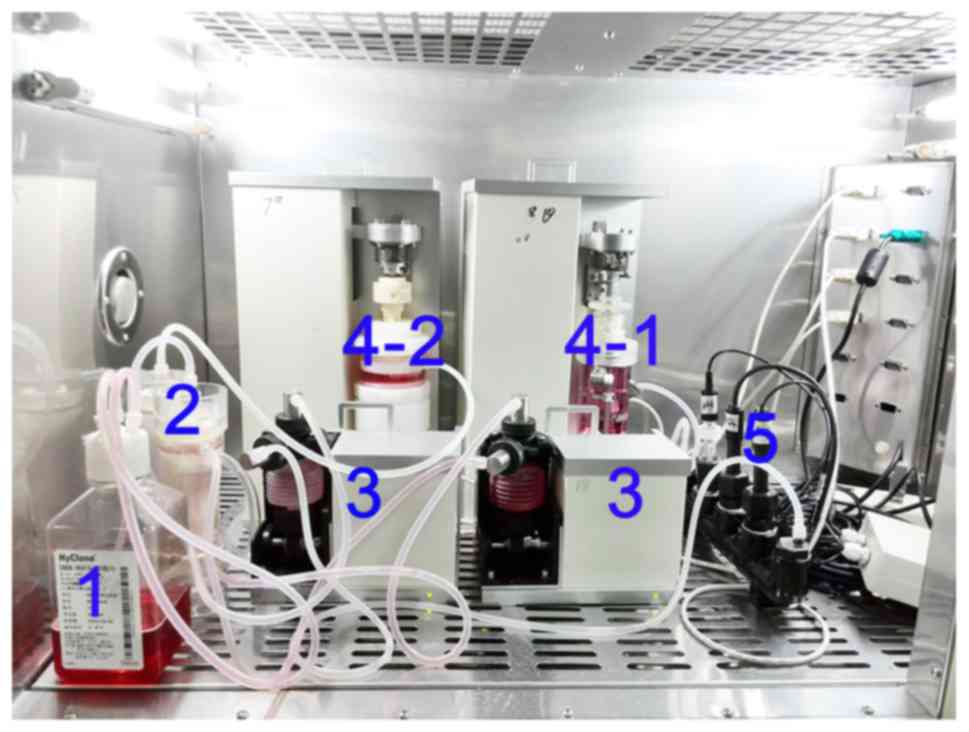
cells seeded in the DBM scaffolds were perfusion-cultured at 37°C
in the tissue culture chambers of the self-developed bioreactor
(Fig. 1) for 5 days, and
simultaneously subjected to dynamic compression (20% deformation at
a frequency of 1.0 Hz for 4 h once per day).
SA-β-Gal activity
Subsequent to compression, NP cells seeded in the
scaffold were collected by digestion with 0.05% trypsin and 0.1%
collagenase I. The NP cells (1×104 per group) were
allowed to adhere in 6-well plates within 6–8 h. Subsequently, an
SA-β-Gal staining assay was performed according to the
manufacturer's instructions (Beyotime Institute of Biotechnology,
Haimen, China). Finally, SA-β-Gal stain-positive cells were
observed under an Olympus BX51 light microscope (Olympus Corp.,
Tokyo, Japan) and SA-β-Gal activity expressed as the percentage of
SA-β-Gal stain-positive cells to the total cells was analyzed using
the Image-Pro Plus software (Version 5.1.0.20; Media Cybernetics,
Inc., Rockville, MD, USA).
Cell proliferation assay
Following compression, NP cells seeded in the
scaffold were harvested as described above. NP cells
(3×103 cells per group) were seeded in a 96-well plate
and NP cell proliferation was detected at 6, 24 and 48 h using a
Cell Counting Κit-8 (CCK-8; C0037; Beyotime Institute of
Biotechnology).
Telomerase activity detection
Subsequent to compression, NP cells seeded in the
scaffold were harvested as described above. The NP cell pellets
were incubated with RIPA lysis buffer (Beyotime Institute of
Biotechnology) and centrifuged (12,000 × g at 4°C for 5 min) to
collect the supernatant. Then, a telomerase ELISA kit (ml-003023;
Mlbio, Shanghai, China) was used to measure telomerase activity
(IU/l) according to the manufacturer's instructions.
Cell cycle analysis
Following compression, NP cells seeded in the
scaffold were harvested as described above. The NP cell pellets
were fixed with 75% ethanol overnight at 4°C and stained with
propidium iodide solution (50 µg/ml; Beyotime Institute of
Biotechnology) and RNase A (100 µg/ml; Beyotime Institute of
Biotechnology) for 30 min at 4°C. Subsequently, NP cells were
subjected to flow cytometry assay and the fraction of each cell
cycle phase (G0/G1, G2/M and S)
was calculated using the multicycle software (FlowJo 7.6.1, Engine
2.79000; Phenix Co., Ltd., Tokyo, Japan).
qPCR analysis
Gene expression of senescence markers (p16 and p53)
and matrix macromolecules (aggrecan and collagen II) was analyzed
by qPCR assay. Briefly, total RNA was extracted using TriPure
Isolation Reagent (11667157001, Roche Applied Science, Penzberg,
Germany) and synthesized into cDNA using a First Strand cDNA
Synthesis kit (04379012001, Roche Applied Science). Then, qPCR was
performed using a reaction system containing cDNA, SYBR Green Mix
(Toyobo Life Science, Osaka, Japan) and primers (Table I). The thermal cycling conditions
for all reactions were as follows: 5 min at 95°C, followed by 35
amplification cycles of 30 sec at 95°C, 20 sec at 56°C and 15 sec
at 72°C. β-actin served as an internal reference and the relative
gene expression was expressed as 2−ΔΔCq (19).
 | Table I.Primers of target genes. |
Table I.
Primers of target genes.
| Gene | Accession no. | Forward (5′-3′) | Reverse (5′-3′) |
|---|
| β-actin | NM_031144.3 |
CCGCGAGTACAACCTTCTTG |
TGACCCATACCCACCATCAC |
| Aggrecan | XM_002723376.1 |
ATGGCATTGAGGACAGCGAA |
GCTCGGTCAAAGTCCAGTGT |
| Collagen II | NM_012929.1 |
GCCAGGATGCCCGAAAATTAG |
CCAGCCTTCTCGTCAAATCCT |
| P53 | XM_008767773.1 |
CCTTAAGATCCGTGGGCGT |
GCTAGCAGTTTGGGCTTTCC |
| P16 | NM_031550.1 |
TACCCCGATACAGGTGATGA |
TACCGCAAATACCGCACGA |
Western blot analysis
Protein expression levels of senescence markers (p16
and p53) and matrix macromolecules (aggrecan and collagen II) were
analyzed by western blotting assay. Briefly, after the total
protein was extracted using RIPA lysis solution (Beyotime Institute
of Biotechnology) and the protein concentration was measured using
a BCA kit (P0009, Beyotime Institute of Biotechnology), protein
samples were subjected to an 12% SDS-PAGE system and transferred to
a polyvinylidene difluoride (PVDF) membrane (100 V for 60 min).
Then, the PVDF membrane was incubated with primary antibodies
[β-actin: ProteinTech Group, Inc., Chicago, IL, USA (cat. no.
60008–1-Ig); p16: Novus Biologicals, LLC, Littleton, CO, USA (cat.
no. NBP2-37740); p53: ProteinTech Group, Inc. (cat. no.
10442-1-AP); aggrecan: Santa Cruz Biotechnology Inc. (cat. no.
sc-16492); collagen II: Abcam, Cambridge, MA, USA. (cat. no.
ab34712); all diluted 1:1,000] at 4°C overnight and the
corresponding secondary antibodies (OriGene Technologies, Inc.,
Beijing, China; 1:2,000) at 37°C for 2 h. Protein bands were
developed using the SuperSignal West Pico Trial kit (34080, Pierce;
Thermo Fisher Scientific, Inc.) and analyzed using Image J software
(v Java 1.6.0_20 32-bit, National Institutes of Health, Bethesda,
MA, USA).
Statistical analysis
All data are expressed as means ± standard deviation
and each experiment was performed in triplicate. After the
homogeneity test for variance, comparisons between groups were
performed by one-way analysis of variance using SPSS 13.0 software,
and the post hoc test was determined by the least significant
difference test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Verification of N-CDH overexpression
in NP cells
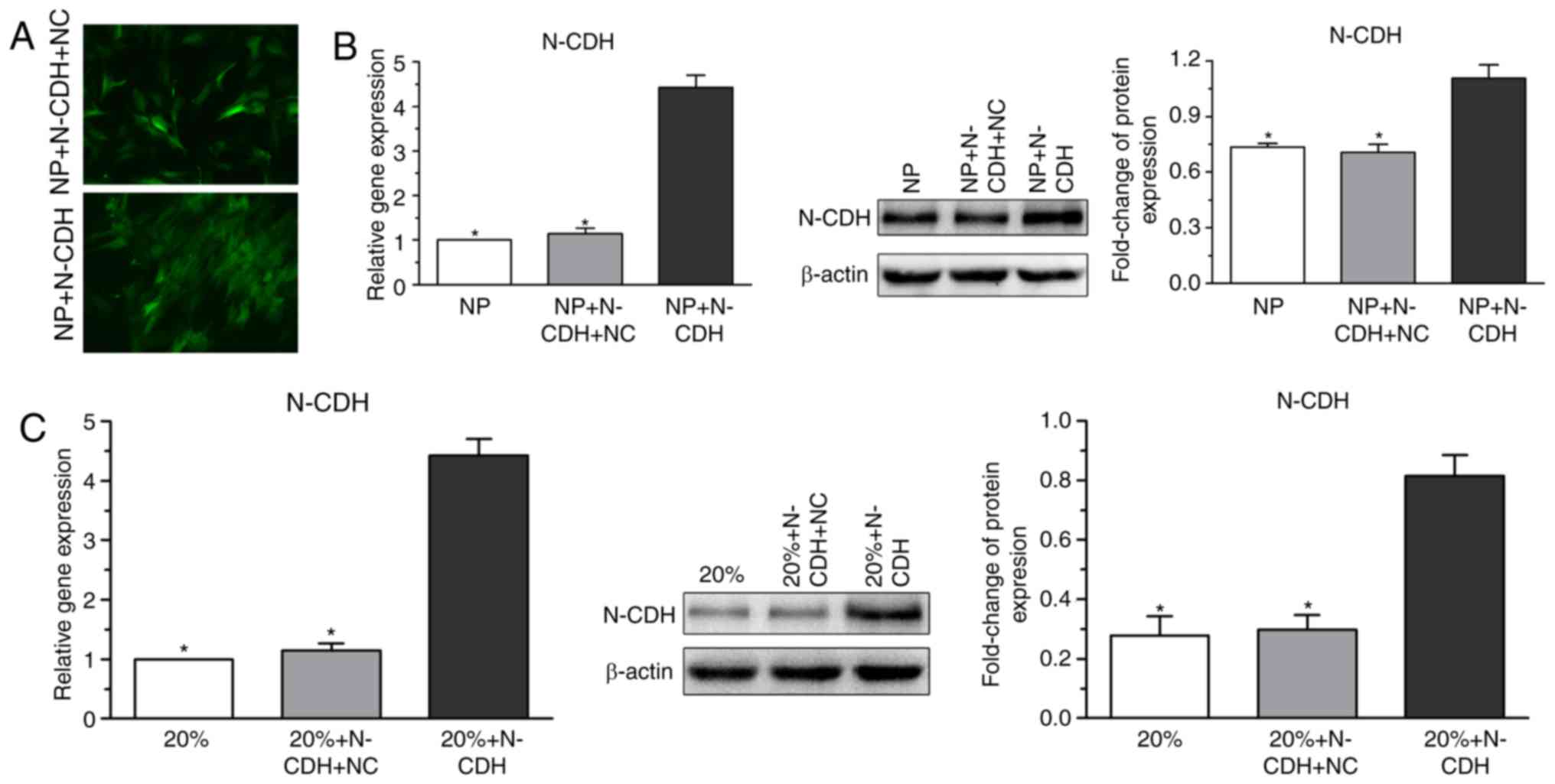
To investigate the role of N-CDH in regulating NP
cell senescence under high-magnitude compression, N-CDH expression
in NP cells was enhanced by recombinant lentiviral vectors.
Predictably, N-CDH expression in NP cells under high-magnitude
compression also increased following N-CDH overexpression (Fig. 2).
Analysis of NP cell senescence
phenotype following N-CDH overexpression under high-magnitude
compression
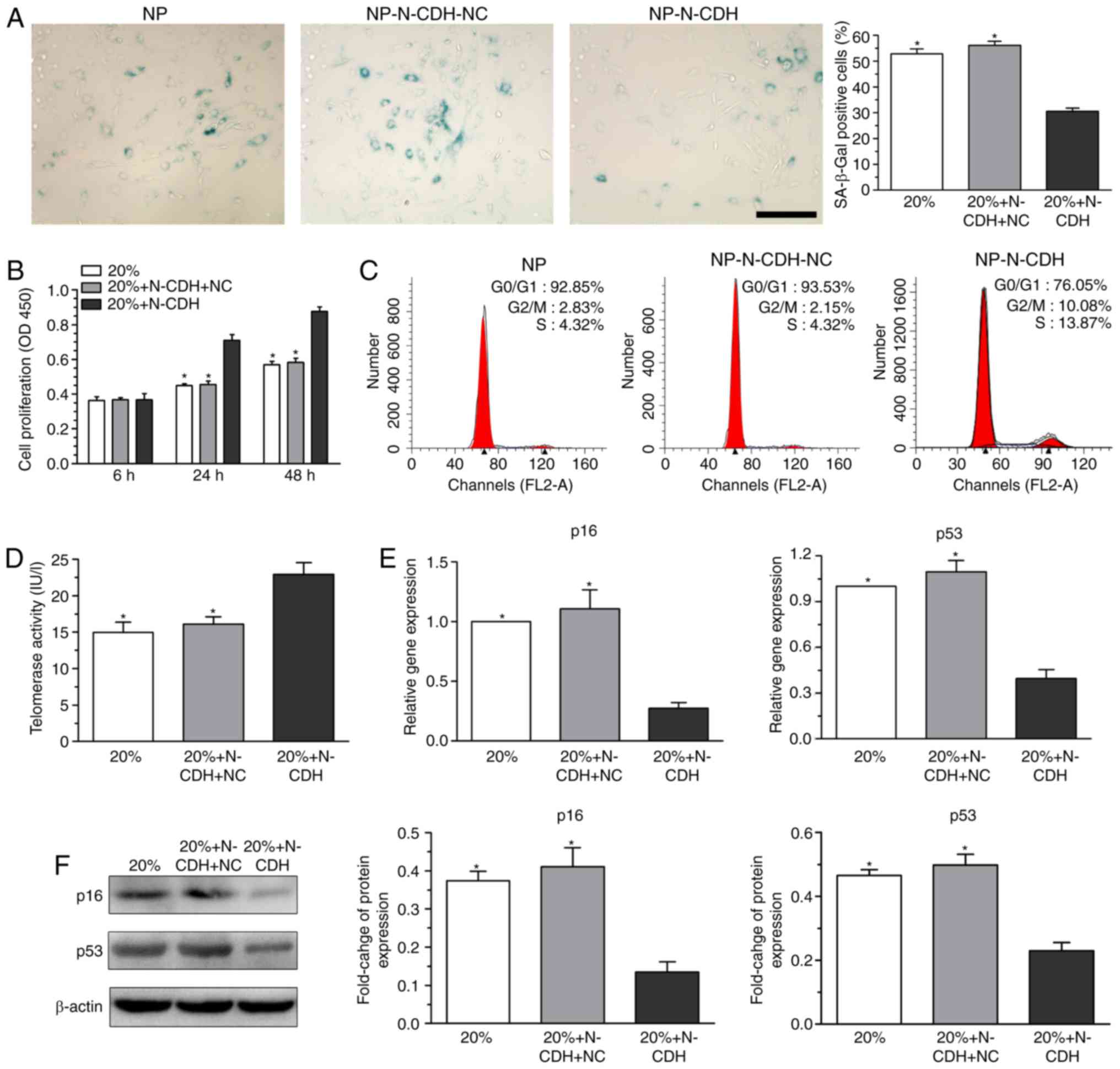
Senescent cells often exhibit increased SA-β-Gal
activity (20), decreased cell
proliferation potency (21),
aggravated G1 cell cycle arrest (22), decreased telomerase activity
(23) and upregulated expression
levels of senescence markers (p16 and p53) (24). Compared with NP cells without N-CDH
overexpression under high-magnitude compression, N-CDH
overexpressed NP cells exhibited significantly decreased SA-β-Gal
activity (Fig. 3A), increased cell
proliferation potency (Fig. 3B),
decreased percentage of cells arrested in the G1 phase
of the cell cycle (Fig. 3C),
increased telomerase activity (Fig.
3D), and downregulated gene (Fig.
3E) and protein (Fig. 3F)
expression levels of senescence markers (p16 and p53).
Analysis of the expression levels of
matrix macromolecules in NP cells following N-CDH overexpression
under high-magnitude compression
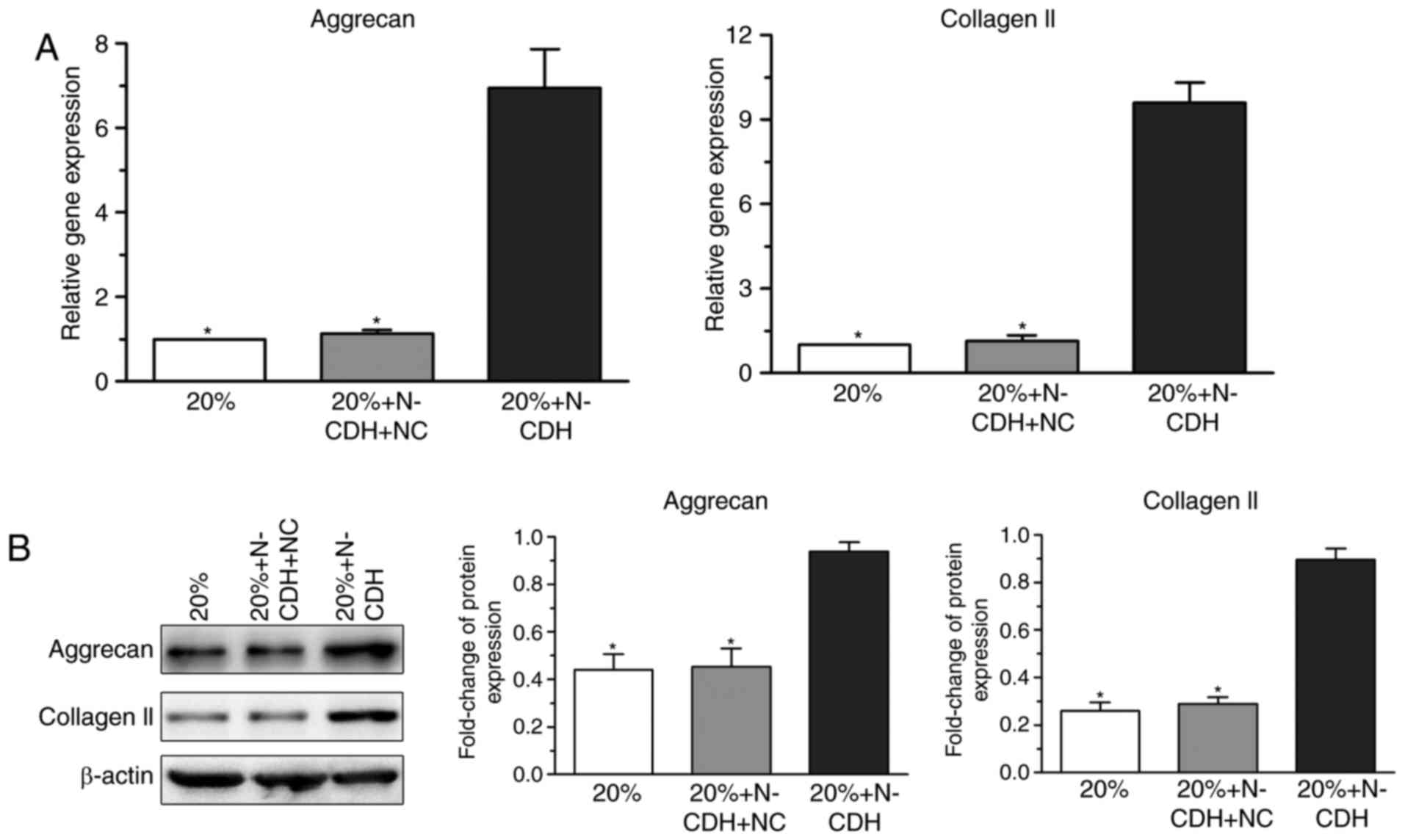
Senescent cells demonstrate altered matrix
metabolism and matrix catabolism is often promoted in senescent
cells (25,26). qPCR indicated that the gene
expression of matrix macromolecules (aggrecan and collagen II) in
N-CDH overexpressed NP cells was higher than that in NP cells
without N-CDH overexpression under high-magnitude compression
(Fig. 4A). Additionally, protein
expression levels of these matrix macromolecules presented a
similar trend (Fig. 4B).
Discussion
It is well established that mechanical load has
important effects on disc biology, and that the un-physiological
load is a validated risk factor that initiates and aggravates disc
degeneration (27–29). Disc cell senescence is a type of
typical pathology during disc degeneration (10,11).
Our preliminary study demonstrated that high-magnitude compression
(20% compressive deformation) promoted NP cell senescence in a 3D
scaffold culture system (unpublished data). The present results
demonstrated for the first time, to the best of our knowledge, that
N-CDH-mediated signaling attenuated NP cell senescence under
high-magnitude compression.
N-CDH is a molecular marker of normal juvenile disc
NP cells (14,15). Previous studies have indicated that
N-CDH-mediated signaling was helpful for promoting NP matrix
biosynthesis and maintaining a normal NP cell phenotype (16,17).
Here, to investigate whether N-CDH-mediated signaling attenuates NP
cell senescence under a high-magnitude compression, N-CDH
expression was enhanced during the current study using recombinant
lentiviral vectors (Fig. 2).
There are various parameters for evaluating cell
senescence, such as SA-β-Gal activity and telomerase activity
(20,23). The present results demonstrated
that N-CDH overexpression decreased SA-β-Gal activity, whereas it
increased telomerase activity in NP cells under high-magnitude
compression. In addition, senescent cells are often arrested in the
G1 phase of the cell cycle, which lead to a limited cell
proliferation potency (21,22).
Consistently, the present result indicated that NP cells exhibited
a decrease in the percentage of G1 phase fractions and
an increase in cell proliferation potency under the high-magnitude
compression following N-CDH overexpression. Thus, these findings
indicate that N-CDH overexpression attenuates NP cell senescence
under high-magnitude compression.
Disc cell senescence results from the natural disc
aging process, as well as possibly being induced by various
stresses, including growth factor insufficiency, oxidative damage,
inflammation reaction and mechanical injury (11). There are two approaches responsible
for the transduction senescence signal: Replicative senescence (RS)
mediated by the p53-p21-pRB signaling pathway and stress-induced
premature senescence (SIPS) mediated by the p16-pRB signaling
pathway (24). The current results
demonstrated that N-CDH overexpression downregulated expression
levels of senescence markers (p16 and p53) under high-magnitude
compression, indicating that N-CDH overexpression attenuates
mechanical overloading-induced NP cell senescence by targeting RS
and SIPS. The matrix homeostatic phenotype is an indirect indicator
for evaluating cell senescence. The current study identified that
expression levels of matrix macromolecules in N-CDH overexpressed
NP cells were significantly increased under high-magnitude
compression, further indicating that N-CDH overexpression
attenuates NP cell senescence under high-magnitude compression.
In conclusion, N-CDH overexpression attenuated NP
cell senescence under high-magnitude compression. Although this
study provides an improved understanding of NP senescence, the
potential signaling transduction behind this process requires
further investigation. However, the current study provides an
experimental basis for the protective effects of N-CDH on disc
biology under high-magnitude compression and contributes to
developing novel strategies to alleviate mechanical
overload-induced disc degeneration.
Acknowledgements
The present study received funding from the National
Natural Science Foundation of China (grant no. 81601932). We also
appreciate Dr Li P (Third Military Medical University, Chongqing,
China) for his technical help and experiment platform support.
References
|
1
|
Chen JW, Ni BB, Li B, Yang YH, Jiang SD
and Jiang LS: The responses of autophagy and apoptosis to oxidative
stress in nucleus pulposus cells: Implications for disc
degeneration. Cell Physiol Biochem. 34:1175–1189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang SD, Ma L, Gu TX, Ding WY, Zhang F,
Shen Y, Zhang YZ, Yang DL, Zhang D, Sun YP and Song YL:
17β-Estradiol protects against apoptosis induced by levofloxacin in
rat nucleus pulposus cells by upregulating integrin α2β1.
Apoptosis. 19:789–800. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang SD, Ma L, Yang DL and Ding WY:
Combined effect of 17β-estradiol and resveratrol against apoptosis
induced by interleukin-1β in rat nucleus pulposus cells via
PI3K/Akt/caspase-3 pathway. PeerJ. 4:e16402016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang SD, Yang DL, Sun YP, Wang BL, Ma L,
Feng SQ and Ding WY: 17β-estradiol protects against apoptosis
induced by interleukin-1β in rat nucleus pulposus cells by
down-regulating MMP-3 and MMP-13. Apoptosis. 20:348–357. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee CR, Iatridis JC, Poveda L and Alini M:
In vitro organ culture of the bovine intervertebral disc: Effects
of vertebral endplate and potential for mechanobiology studies.
Spine (Phila Pa 1976). 31:515–522. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Neidlinger-Wilke C, Galbusera F, Pratsinis
H, Mavrogonatou E, Mietsch A, Kletsas D and Wilke HJ: Mechanical
loading of the intervertebral disc: From the macroscopic to the
cellular level. Eur Spine J. 3 Suppl 23:S333–S343. 2014. View Article : Google Scholar
|
|
7
|
Hwang D, Gabai AS, Yu M, Yew AG and Hsieh
AH: Role of load history in intervertebral disc mechanics and
intradiscal pressure generation. Biomech Model Mechanobiol.
11:95–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li P, Gan Y, Wang H, Zhang C, Wang L, Xu
Y, Song L, Li S, Li S, Ou Y and Zhou Q: Dynamic compression effects
on immature nucleus pulposus: A study using a novel intelligent and
mechanically active bioreactor. Int J Med Sci. 13:225–234. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li P, Gan Y, Xu Y, Song L, Wang H, Zhang
C, Wang L, Zhao C, Luo L and Zhou Q: Matrix homeostasis within the
immature annulus fibrosus depends on the frequency of dynamic
compression: A study based on the self-developed mechanically
active bioreactor. Biomech Model Mechanobiol. 16:385–394. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gruber HE, Ingram JA, Norton HJ and Hanley
EN Jr: Senescence in cells of the aging and degenerating
intervertebral disc: Immunolocalization of senescence-associated
beta-galactosidase in human and sand rat discs. Spine (Phila Pa
1976). 32:321–327. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang F, Cai F, Shi R, Wang XH and Wu XT:
Aging and age related stresses: A senescence mechanism of
intervertebral disc degeneration. Osteoarthritis Cartilage.
24:398–408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brusés JL: N-cadherin signaling in synapse
formation and neuronal physiology. Mol Neurobiol. 33:237–252. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halbleib JM and Nelson WJ: Cadherins in
development: Cell adhesion, sorting, and tissue morphogenesis.
Genes Dev. 20:3199–3214. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lv F, Leung VY, Huang S, Huang Y, Sun Y
and Cheung KM: In search of nucleus pulposus-specific molecular
markers. Rheumatology (Oxford). 53:600–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Minogue BM, Richardson SM, Zeef LA,
Freemont AJ and Hoyland JA: Transcriptional profiling of bovine
intervertebral disc cells: Implications for identification of
normal and degenerate human intervertebral disc cell phenotypes.
Arthritis Res Ther. 12:R222010. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang PY, Jing L, Chen J, Lim FL, Tang R,
Choi H, Cheung KM, Risbud MV, Gersbach CA, Guilak F, et al:
N-cadherin is key to expression of the nucleus pulposus cell
phenotype under selective substrate culture conditions. Sci Rep.
6:280382016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang PY, Jing L, Michael KW, Richardson
WJ, Chen J and Setton LA: N-cadherin-mediated signaling regulates
cell phenotype for nucleus pulposus cells of the intervertebral
disc. Cell Mol Bioeng. 8:51–62. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li ST, Liu Y, Zhou Q, Lue RF, Song L, Dong
SW, Guo P and Kopjar B: A novel axial-stress bioreactor system
combined with a substance exchanger for tissue engineering of 3D
constructs. Tissue Eng Part C Methods. 20:205–214. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dimri GP, Lee X, Basile G, Acosta M, Scott
G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O,
et al: A biomarker that identifies senescent human cells in culture
and in aging skin in vivo. Proc Natl Acad Sci USA. 92:pp.
9363–9367. 1995; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gruber HE, Ingram JA, Davis DE and Hanley
EN Jr: Increased cell senescence is associated with decreased cell
proliferation in vivo in the degenerating human annulus. Spine J.
9:210–215. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Oshima J and Campisi J: Fundamentals of
cell proliferation: Control of the cell cycle. J Dairy Sci.
74:2778–2787. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chatterjee S: Telomeres in health and
disease. J Oral Maxillofac Pathol. 21:87–91. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beauséjour CM, Krtolica A, Galimi F,
Narita M, Lowe SW, Yaswen P and Campisi J: Reversal of human
cellular senescence: Roles of the p53 and p16 pathways. EMBO J.
22:4212–4222. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Deursen JM: The role of senescent
cells in ageing. Nature. 509:439–446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cristofalo VJ, Lorenzini A, Allen RG,
Torres C and Tresini M: Replicative senescence: A critical review.
Mech Ageing Dev. 125:827–848. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao X, Zhu Q and Gu W: Prediction of
glycosaminoglycan synthesis in intervertebral disc under mechanical
loading. J Biomech. 49:2655–2661. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jünger S, Gantenbein-Ritter B, Lezuo P,
Alini M, Ferguson SJ and Ito K: Effect of limited nutrition on in
situ intervertebral disc cells under simulated-physiological
loading. Spine (Phila Pa 1976). 34:1264–1271. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan SC, Ferguson SJ and Gantenbein-Ritter
B: The effects of dynamic loading on the intervertebral disc. Eur
Spine J. 20:1796–1812. 2011. View Article : Google Scholar : PubMed/NCBI
|