Introduction
Traumatic brain injury (TBI) is a common severe
injury of neurosurgery, which is mainly caused by mechanical forces
of tearing, shearing or stretching. TBI has been a heavy economic
and social burden and has high morbidity and mortality (1). So far plenty of researches have been
conducted to illuminate the mechanisms underlying TBI and explored
the effective neuroprotective drugs aiming to alleviate the grave
consequences of TBI. Although the precise mechanisms underlying the
development of TBI remain unclear, it is admitted that the
secondary injury is tightly linked with the prognosis of TBI.
Post-traumatic inflammation response in cerebral cortex plays an
important role in the progression of secondary injury (2).
Increasing evidences indicate that cytokines, such
as IL-1β, participate in inflammation development. IL-1β is a
potent inflammatory initiating cytokine. It can activate additional
inflammatory cells and release more inflammatory mediators and
cytokines, thus amplifying the injury signals and triggering an
inflammatory cascade (3). IL-1β is
mainly produced by NLRP3 inflammasome, which serves as a platform
to activate caspase and triggers proteolytic maturation of
pro-inflammatory cytokines, such as IL-1β and IL-18 (4). Increasing evidences indicate that
NLRP3 inflammasome participates in the development of central
nervous system disorders, such as cerebral ischemia reperfusion
injury (5), neurodegenerative
disease (6), cerebral tumor
(7) and so on. It is reported that
NLRP3 also participates in the pathogenesis of TBI (8).
Resveratrol (3,5,4′-trihydroxy-stilbene, RS) is a
stilbenoid, a natural polyphenolic agent, which has been
demonstrated to eliminate free radicals and ROS, reduce
inflammatory response and modulate programmed cell death (9). Particularly, administration of
resveratrol has potent neuroprotection effects in various animal
models including cerebral ischemia reperfusion injury (10), stroke (11), and TBI (12). However, the mechanisms through
which resveratrol exerts protective effect following TBI require
further examination. A previous study demonstrated resveratrol
exerted a protective effect in acute lung injury by suppressing the
NLRP3 inflammasome signaling (13). So it is necessary to determine
whether resveratrol is capable of modulating TBI through NLRP3
inflammasome signaling pathway.
In the present study, we sought to investigate the
neuroprotective effect of resveratrol and to explore the underlying
mechanisms of resveratrol regulating NLRP3 inflammasome in TBI.
Materials and methods
Animals and experimental groups
Healthy adult male Sprague-Dawley rats (300 g-330 g)
were used in the experiments, which were purchased from the
Experimental Animal Center of the Fourth Military Medical
University. The present study was approved by the Ethics Committee
of Animal Experiment Administration Committee of The Fourth
Military Medical University (Shaanxi, China). Rats were housed
under humidity and temperature controlled conditions as well as
under specific pathogen-free conditions. Rats were randomly divided
into the following groups: sham group (n=6), TBI group at different
time point (n=24), TBI+Resveratrol (RS) group (n=6) and
TBI+sirtinol group (n=6).
TBI model
The detailed methods to produce TBI model had been
described previously (1). Briefly,
we used a free fall device in the experiment. Rats were
anaesthetized using pentobarbital sodium (50 mg/kg) by
intraperitoneal injection and then a midline incision was created,
and the skin was retracted to expose sagittal suture and coronal
suture. When fixed to the free fall device, a ball breaker
weighting 50 g was dropped from the 90 cm height to form a
free-falling impact, resulting in closed craniocerebral trauma.
Almost all animals subjected to TBI showed brain hemorrhage and
edema, however, generally 10% mortality post injury was
observed.
Resveratrol (Sigma, St Louis, MO, USA) was dissolved
in 75% ethanol and attenuated by normal saline (2% ethanol content)
to a concentration of 100 mg/ml. 100 mg/kg resveratrol was
administered intraperitoneally in the TBI+RS group 30 min before
TBI (14), while a corresponding
volume of saline (2% ethanol content) was administered in the TBI
group and sham group 30 min before TBI model. The sham group rats
were subjected to an identical procedure with the TBI group rats,
however without ball breaker hit.
Sirtinol (Selleck Chemicals, Houston, TX, USA), a
potent SIRT1 inhibitor, was dissolved in 75% ethanol and normal
saline (2% ethanol content). Sirtinol (10 mg/kg) was administered
intraperitoneally 30 min before TBI in the TBI+sirtinol group
(15).
In the TBI group, rats were sacrificed at 6, 12, 24
and 48 h after TBI respectively, and their tissues were sampled. In
the other groups, rats were sacrificed, and their tissues and blood
were sampled 24 h after TBI. Blood was taken from aortaventralis
and centrifuged at 1,500 g for 10 min to collect serum.
Enzyme-linked immune sorbent assay
(ELISA)
The serum level of neuron-specific enolase (NSE), a
specific biomarker of brain injury, was quantified using
corresponding ELISA kit (Jiancheng Bioengineering Institute,
Nanjing, China). The level of IL-1β and IL-18 in the supernatant of
cerebral cortex homogenate was determined using specific ELISA kit
(Jiancheng Bioengineering Institute) according to the manufactures'
protocols. The optical density (OD) values were measured at a
wavelength of 450 nm using ELX808 microplate reader.
Measurement of brain water content
(BWC)
Brain water content was measured by wet/dry weight
ratio according to the protocol described previously (16). In brief, the injured cerebral
hemisphere was weighted and then transferred to desiccating oven
for dehydration at 110°C for 24 h. The dried tissue was weighted
again. BWC=[(wet brain weight-dry brain weight)/wet brain
weight]x100%.
Measurement of the level of SOD, MDA
and GSH in rats cerebral cortex
The level of SOD, MDA and GSH were
spectrophotometrically assayed using appropriate kits (Jiancheng
Bioengineering Institute) respectively. The cerebral cortex was
homogenized and centrifuged in extraction buffer to obtain a 5%
homogenate. The absorbance was measured using a DU-800
spectrophotometer (Beckman Coulter, Fullerton, CA, USA) at a
wavelength of 460 nm.
Quantitative real-time reverse
transcription PCR (qRT-PCR)
The mRNA levels of NLRP3, caspase-1, IL-1β and IL-18
were analyzed by qRT-PCR. We used TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA) to extract total RNA in injured
cerebral cortex. RNA was reverse-transcribed into cDNA by Prime
ScriptRT Reagent kit (Takara Bio, Inc., Otsu, Japan).
Using SYBR Green (Takara Bio, Inc.) and specific primers, the
levels of target gene mRNAs were measured with a real-time PCR
system (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
The qRT-PCR thermalcycle condition was employed as
follows: an initial denaturation at 95°C for 30 sec, followed by 39
cycles of denaturation at 95°C for 15 sec, annealing at 60°C for 30
sec, and elongation at 72°C for 10 sec.
The primer sequences of targeted genes used in the
present study were as follows: NLRP3: 5′-CAGCGATCAACAGGCGAGAC-3′
(F), 5′-AGAGATATCCCAGCAAACCTATCCA-3′ (R); caspase-1:
5′-ACTCGTACACGTCTTGCCCTCA-3′ (F), 5′-CTGGGCAGGCAGCAAATTC-3′ (R);
IL-1β: 5′-CCCTGAACTCAACTGTGAAATAGCA-3′ (F),
5′-CCCAAGTCAAGGGCTTGGAA-3′ (R); IL-18:
5′-GACTGGCTGTGACCCTATCTGTGA-3′ (F), 5′-TTGTGTCCTGGCACACGTTTC-3′
(R); GAPDH: 5′-GAACATCATCCCTGCATCCA-3′ (F),
5′-CCAGTGAGCTTCCCGTTCA-3′ (R).
Western blot
The injured cerebral cortex samples were homogenized
and the concentrations of protein were measured using BCA assay
method. 30 µg protein was separated by electrophoresis on 10%
sodium dodecyl sulfate-polyacrylamide gels, and transferred to
polyvinylidene difluoride membranes. The following primary
antibodies were used: NLRP3 (Biorbyt, Cambridge, UK), caspase-1
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and SIRT1 (Abcam,
Cambridge, UK). The sheared bands were observed by
chemiluminescense using the FluorChem FC system and were presented
as a densitometric ratio between the protein of target and the
loading control. Results were analyzed using the software of ImageJ
v1.46.
Statistical analysis
The experiment data were expressed as mean ± SEM.
Comparison between different groups was determined using one-way
analysis of variance (ANOVA) followed by Bonferroni t-test using
GraphPad Prism 6 software (San Diego, CA, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
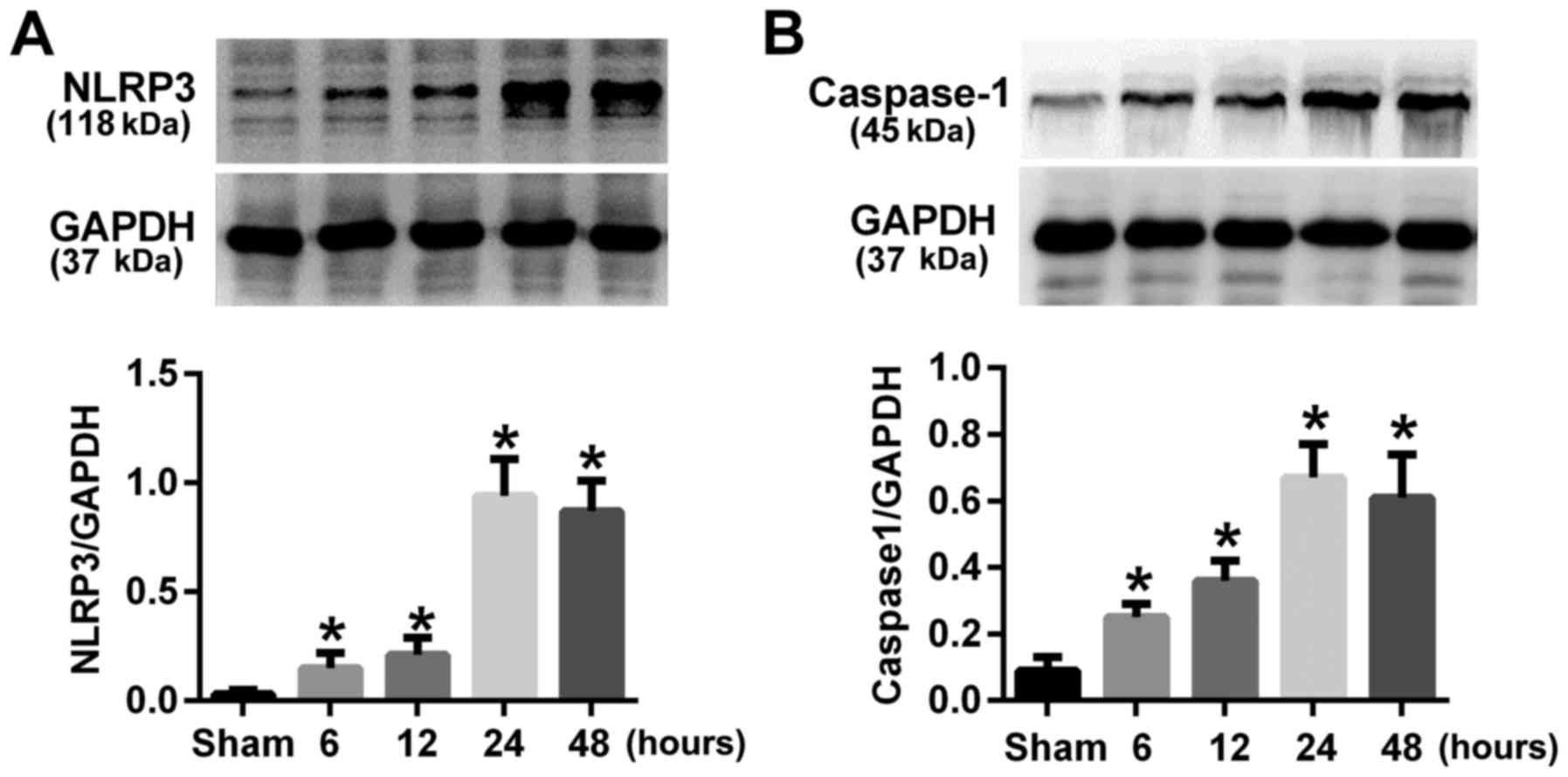
TBI resulted in elevated NLRP3 and
caspase-1 expression in rats cerebral cortex
The expression of NLRP3 and caspase-1 at different
time points after TBI were evaluated by western blot. Results
indicated that the expression of NLRP3 and caspase-1 were
up-regulated at 6 h after TBI and peaked at 24 h (P<0.05;
Fig. 1). So we chose the 24 h time
point after TBI in our following experiments.
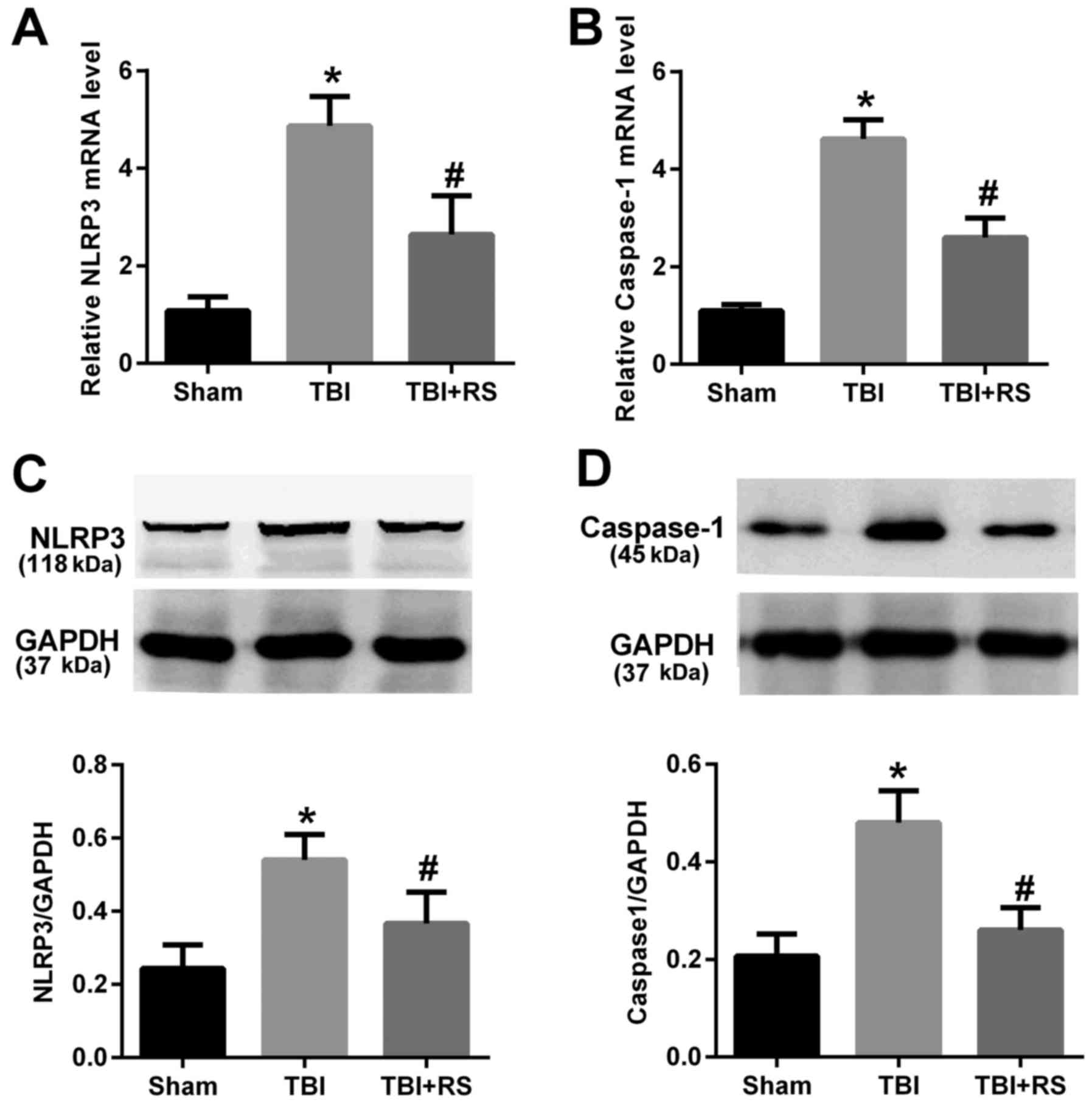
Resveratrol pretreatment reduced the
mRNA and protein expression level of NLRP3 and caspase-1 in rats
cerebral cortex after TBI
The mRNA expression of NLRP3 and caspase-1 were
detected by real time PCR. The Fig. 2A
and B showed that the mRNA expression level of NLRP3 and
caspase-1 in the TBI group was significantly elevated compared with
the sham group (P<0.05). Pretreatment with resveratrol
remarkably reduced the elevated NLRP3 and caspase-1 mRNA levels
(P<0.05). To determine whether the protein levels of NLRP3
inflammasome were corresponded to their mRNA levels, we further
conducted western blot experiment. We observed the NLRP3 and
caspase-1 protein level were increased after TBI (P<0.05), but
significantly decreased by administration of resveratrol
(P<0.05) (Fig. 2C and D).
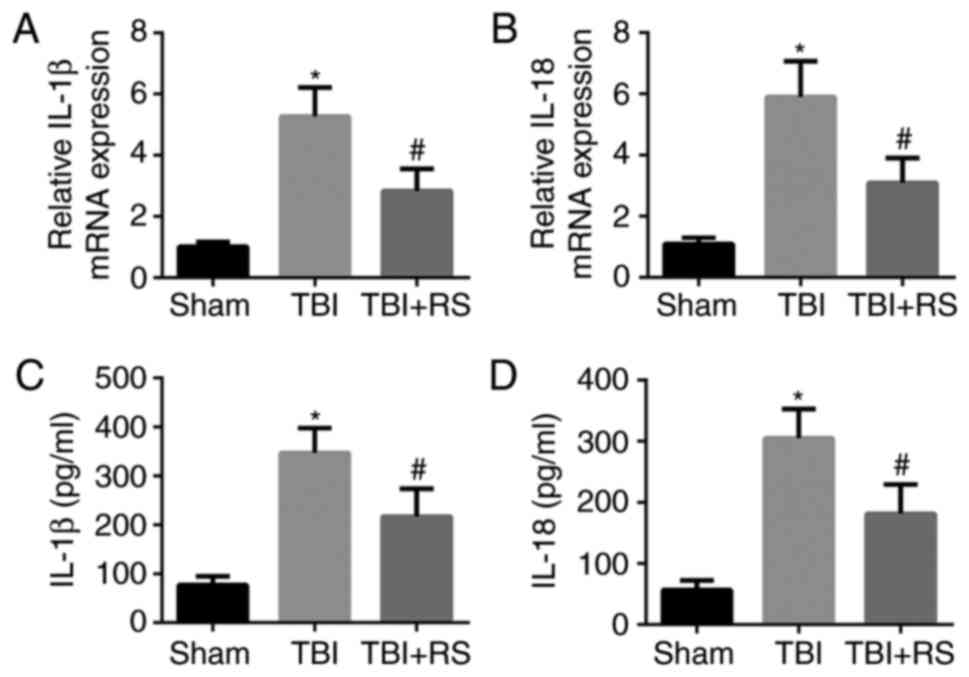
Resveratrol pretreatment decreased the
levels of IL-1β and IL-18 in rats cerebral cortex after TBI
IL-1β and IL-18 are effector molecules of NLRP3
inflammasome signals, acting as inflammatory initiating cytokines.
Therefore we analyzed the generation of IL-1β and IL-18 in rats
cerebral cortex by real time PCR and ELISA. We found that the mRNA
and protein level of IL-1β and IL-18 were relatively low in the
sham group. TBI significantly increased the mRNA and protein level
of IL-1β and IL-18 (P<0.05), while resveratrol pretreatment
markedly decreased the elevated level of IL-1β and IL-18
(P<0.05) (Fig. 3A-D).
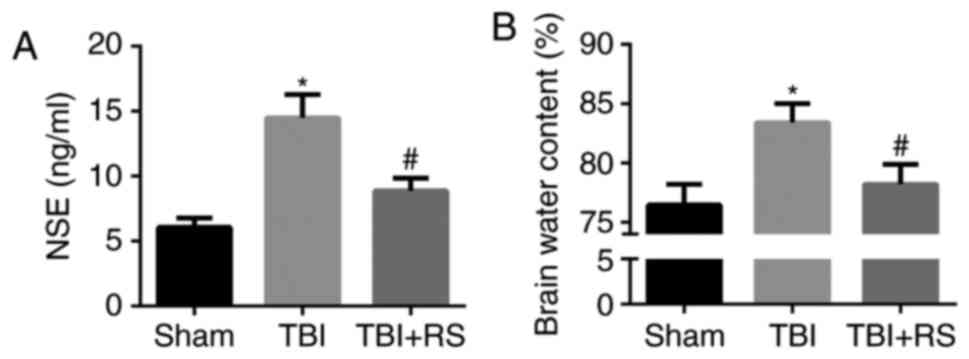
Resveratrol pretreatment decreased the
level of serum NSE and brain edema in TBI rats
It has been demonstrated that resveratrol has the
neuroprotective effect. First, we measured the serum level of NSE,
a biomarker valuing brain injury degree. We found that NSE was
significantly increased in serum of TBI rats. When pretreatment
with resveratrol, the elevated NSE was remarkably abolished
(P<0.05) (Fig. 4A). Moreover,
the BWC in the TBI group was much higher than the sham group, which
was reversed when pretreatment with resveratrol (P<0.05)
(Fig. 4B). It indicated that
pretreatment with resveratrol could alleviate the degree of
TBI.
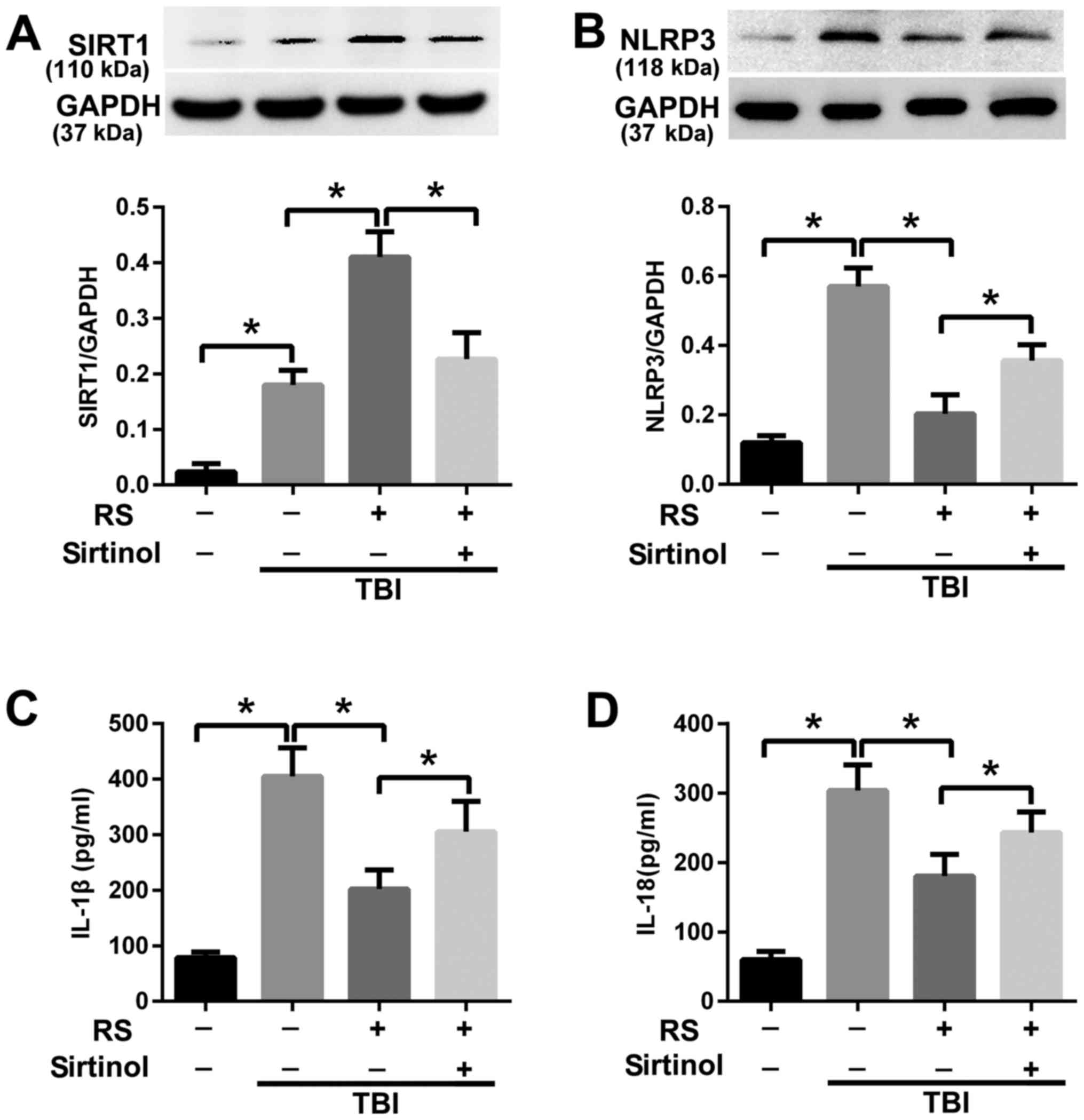
Resveratrol attenuated NLRP3
inflammasome signaling activation via enhanced expression of
SIRT1
To investigate the mechanism of resveratrol on
regulating NLRP3 inflammasome signaling, we further examined the
role of SIRT1. We introduced an inhibitor of SIRT1, sirtinol, in
conjunction with resveratrol. We found that the SIRT1 expression
was increased after TBI (P<0.05), and resveratrol pretreatment
further enhanced the expression level of SIRT1 (P<0.05). When
co-administrated with sirtinol and resveratrol, the increased level
of SIRT1 in the TBI+RS group was reduced (P<0.05) (Fig. 5A). Meanwhile, co-administrated with
sirtinol and resveratrol reversed the suppressing effect of
resveratrol on NLRP3 (P<0.05) (Fig.
5B). We further examined the level of IL-1β and IL-18. We found
co-administration with sirtinol and resveratrol could reverse the
reduced level of IL-1β and IL-18 in the TBI+RS group (P<0.05)
(Fig. 5C and D).
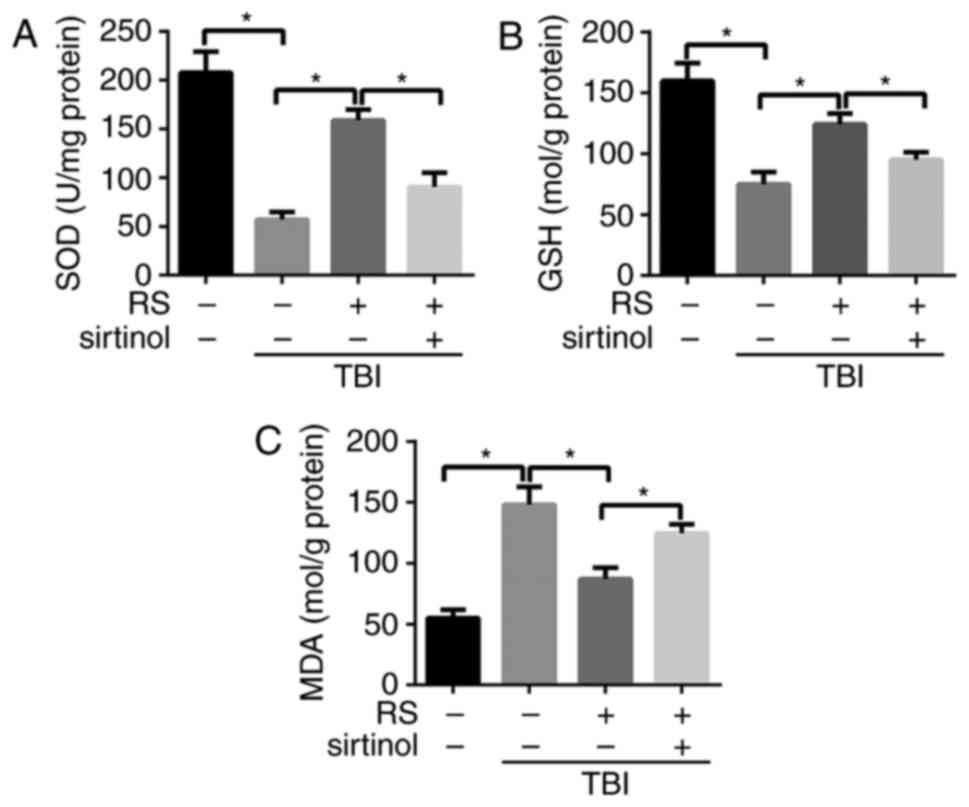
Resveratrol pretreatment inhibited the
extent of oxidative stress in rats cerebral cortex after TBI via
SIRT1
Oxidative stress plays a key role in NLRP3
inflammaoseme activation during ischemia reperfusion injury. To
determine whether resveratrol and SIRT1 had an impact on oxidative
stress in rats cerebral cortex after TBI, we assessed the changes
of endogenous antioxidants SOD, MDA and GSH. We found that the
levels of SOD and GSH were remarkably declined, while the level of
MDA was significantly elevated in the TBI group compared with the
sham group (P<0.05). When pretreatment with resveratrol, the
level of SOD and GSH were elevated while the level of MDA was
reduced (P<0.05). Moreover, sirtinol administration could
reverse the inhibiting effect of resveratrol on oxidative stress
(P<0.05) (Fig. 6A-C).
Discussion
Resveratrol has been demonstrated to exhibit
neuroprotective effects in various animal models (17). In the present study, pretreatment
with resveratrol had an obviously neuroprotective effect in rats
following TBI, as evidenced by reduced serum NSE level and BWC
level. Meanwhile, we demonstrated for the first time resveratrol
attenuated the inflammatory response and relieved TBI by reducing
ROS production and suppressing NLRP3 activation. The effect of
resveratrol on NLRP3 inflammasome and ROS production might be SIRT1
dependent.
There are two stages involved in TBI, including
immediate brain damage and secondary injury. The immediate brain
damage results in direct neuronal loss and necrotic death, which is
followed by a series of injury cascades such as oxidative stress,
mitochondrial dysfunction and inflammation (18). Of these injury cascades,
inflammatory response accounts for major reasons (19). It is reported that inflammatory
responses following various central nervous system injuries, is
associated with robust release of cytokines and activation of
inflammatory signals, which amplifies the injury (20).
The inflammasome is a multiprotein oligomer
consisting of an apoptosis associated speck like protein containing
caspase recruitment domain (ASC), a cysteine protease caspase-1 and
an NLR protein. Of all inflammasomes, the NLRP3 inflammasome is the
most important member. NLRP3 inflammasome is a component of the
innate immune system which can promote the maturation of
pro-inflammatory cytokines IL-1β and IL-18, thus initiating
inflammatory responses (21). In
fact, several studies found that the assembly of NLRP3
inflammasome, the activation of caspase-1 and the release of IL-1β
and IL-18 during TBI, indicating NLRP3 was involved in TBI
pathology (8,22,23).
In the present study, we also observed TBI induced the activation
of NLRP3 and its downstream molecule caspase-1. The expression of
pro-inflammatory cytokines pro-IL-1β and pro-IL-18 and its
maturation pattern IL-1β and IL-18 were also increased in the
cerebral cortex of TBI rats.
Literatures indicated that resveratrol had strong
neuroprotective effects on central nervous system disorders. It was
shown that pretreatment with resveratrol could reduce the
infarction volume and improve the neurological score in mouse after
middle cerebral artery occlusion (24). Resveratrol was also reported to
attenuate TBI induced brain edema and improved spatial cognitive
function and neurological impairment in rats (12,25,26).
In our study, we used NSE, a specific biomarker closely associated
with the damage of neurons, and BWC to represent the degree of
brain injury. We found that after TBI, the NSE and BWC were
significantly elevated. While pretreatment with resveratrol could
remarkably reduce the elevated NSE and brain water content, as well
as reduce the activation of NLRP3, caspase-1 and the release of
IL-1β and IL-18. The abovementioned findings indicated that
resveratrol might exert a protective effect in TBI by suppressing
the NLRP3 inflammasome signaling.
Resveratrol is also known as a potent activator of
SIRT1. SIRT1 is a class III deacetylase which involved in multiple
physiological or pathological processes such as cell longevity,
reduction of oxidative stress, inflammation and metabolism
(27–29). It is reported that enhanced
expression of SIRT1 had a neuroprotection effect in central nervous
system diseases (30).
Furthermore, studies had shown that SIRT1 was an endogenous
protective molecule in TBI (31),
and SIRT1 could negatively regulate NLRP3 in vascular endothelial
cells (32). Li et al
(32) found that lacking of CD40
could abolishe the up-regulation of NLRP3 expression in LPS-primed
ECs and SIRT1 activation could protect against inflammatory
response via CD40 both in vivo and in vitro which
demonstrated the effect of SIRT1 on NLRP3 inflammasome was partly
mediated by CD40. To explore the underlying mechanisms of
resveratrol regulating NLRP3 inflammasome in TBI, we used the SIRT1
inhibitor, sirtinol, in our experiment. The concentration of
sirtinol we used in our experiment was different from the
concentration of resveratrol. The concentration of sirtinol was
used partly according to previous article (15), and partly attributed to its high
potency. We found SIRT1 level was increased in the cerebral cortex
after TBI, while pretreatment with resveratrol resulted in a higher
level of SIRT1. When co-administrated with sirtinol, we observed
downregulated level of SIRT1. The blockade of SIRT1 activation by
sirtinol was associated with enhanced NLRP3 inflammaosme activation
in TBI. These results indicated the mechanism of resveratrol
suppressing NLRP3 inflammaosme was SIRT1 dependent.
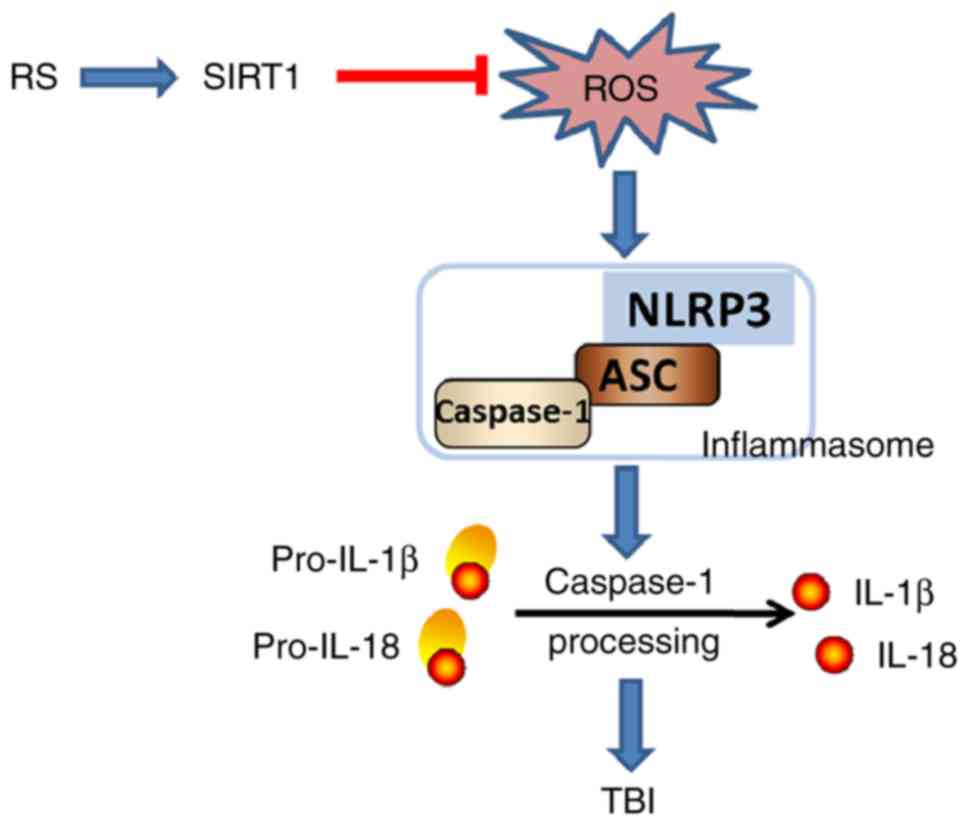
Various exogenous and endogenous molecular patterns
can activate NLRP3 inflammasome. Of them, reactive oxygen species
(ROS) have been regarded as an important NLRP3 inflammasome
activator in various diseases, such as cardiac ischemia/reperfusion
injury (33) and sepsis-induced
acute lung injury (34).
Meanwhile, SIRT1 is an important oxidative stress regulator
(29). It is reported that TBI
resulted in rapid ROS production and oxidative damage to brain
cellular components leading to neuronal dysfunction and cell death
(35). ROS is constantly generated
and eliminated in the biological homoeostasis, which is controlled
by endogenous antioxidant system, including antioxidants such as
GSH and SOD. SOD and GSH are ROS scavengers and can eliminate the
generated ROS in vivo (36). MDA is a production of oxidative
stress of lipid peroxidation, and it is generated when
polyunsaturated lipids are oxidized by ROS. ROS production can be
represented by the level of SOD, GSH and MDA (37). To determine whether resveratrol
could affect the ROS level through SIRT1 in cerebral cortex after
TBI, we conducted further experiments. In the present study, we
found that after TBI, the level of SOD and GSH were significantly
reduced while the level of MDA was remarkably increased, indicating
plenty of ROS production. When pretreatment with resveratrol, the
level of SOD and GSH were elevated while the level of MDA was
reduced, indicating resveratrol could decrease the production of
ROS. Moreover, co-administrated with sirtinol remarkably reversed
the effect of resveratrol on SOD, GSH and MDA. These results
indicated that the effect of resveratrol on suppressing NLRP3
inflammasome activation and attenuating the cerebral cortex injury
after TBI might be related to the reduced ROS production, and this
effect of resveratrol on NLRP3 inflammasome and ROS production
might be SIRT1 dependent (Fig.
7).
In conclusion, our data demonstrated that TBI could
activate NLRP3 inflammasome, thus promoting the release of
proinflammatory cytokines, IL-1β and IL-18, and amplifying brain
injury. Resveratrol might attenuate the inflammatory response and
relieve TBI by reducing ROS production and inhibiting NLRP3
activation, which prevented the over-release of pro-inflammatory
cytokines. The effect of resveratrol on NLRP3 inflammasome and ROS
production might be SIRT1 dependent. The present study enriched the
anti-inflammatory mechanisms of resveratrol and enhanced the
therapeutic potential of resveratrol for the intervention of
TBI.
References
|
1
|
Dong HJ, Shang CZ, Li G, Niu Q, Luo YC,
Yang Y, Meng HP, Yin HJ, Zhang HX, Zhao ML and Lin L: The
distribution of transplanted umbilical cord mesenchymal stem cells
in large blood vessel of rats with traumatic brain injury. J
Craniofac Surg. Mar 29–2017.(Epub ahead of print). View Article : Google Scholar
|
|
2
|
Kumar A, Stoica BA, Loane DJ, Yang M,
Abulwerdi G, Khan N, Kumar A, Thom SR and Faden AI:
Microglial-derived microparticles mediate neuroinflammation after
traumatic brain injury. J Neuroinflammation. 14:472017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jin R, Shen M, Yu L, Wang X and Lin X:
Adipose-derived stem cells suppress inflammation induced by IL-1β
through down-regulation of P2×7R mediated by miR-373 in
chondrocytes of osteoarthritis. Mol Cells. 40:222–229.
2017.PubMed/NCBI
|
|
4
|
Song L, Pei L, Yao S, Wu Y and Shang Y:
NLRP3 inflammasome in neurological diseases, from functions to
therapies. Front Cell Neurosci. 11:632017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minutoli L, Puzzolo D, Rinaldi M, Irrera
N, Marini H, Arcoraci V, Bitto A, Crea G, Pisani A, Squadrito F, et
al: ROS-mediated NLRP3 inflammasome activation in brain, heart,
kidney, and testis ischemia/reperfusion injury. Oxid Med Cell
Longev 2016. 21830262016.
|
|
6
|
Tan MS, Yu JT, Jiang T, Zhu XC and Tan L:
The NLRP3 inflammasome in Alzheimer's disease. Mol Neurobiol.
48:875–882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li L and Liu Y: Aging-related gene
signature regulated by Nlrp3 predicts glioma progression. Am J
Cancer Res. 5:442–449. 2014.PubMed/NCBI
|
|
8
|
Liu HD, Li W, Chen ZR, Hu YC, Zhang DD,
Shen W, Zhou ML, Zhu L and Hang CH: Expression of the NLRP3
inflammasome in cerebral cortex after traumatic brain injury in a
rat model. Neurochem Res. 38:2072–2083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jardim FR, de Rossi FT, Nascimento MX, da
Silva Barros RG, Borges PA, Prescilio IC and de Oliveira MR:
Resveratrol and brain mitochondria: A review. Mol Neurobiol. Mar
10–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abdel-Aleem GA, Khaleel EF, Mostafa DG and
Elberier LK: Neuroprotective effect of resveratrol against brain
ischemia reperfusion injury in rats entails reduction of DJ-1
protein expression and activation of PI3K/Akt/GSK3b survival
pathway. Arch Physiol Biochem. 122:200–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wan D, Zhou Y, Wang K, Hou Y, Hou R and Ye
X: Resveratrol provides neuroprotection by inhibiting
phosphodiesterases and regulating the cAMP/AMPK/SIRT1 pathway after
stroke in rats. Brain Res Bull. 121:255–262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng Y, Cui Y, Gao JL, Li MH, Li R, Jiang
XH, Tian YX, Wang KJ, Cui CM and Cui JZ: Resveratrol attenuates
neuronal autophagy and inflammatory injury by inhibiting the
TLR4/NF-κB signaling pathway in experimental traumatic brain
injury. Int J Mol Med. 37:921–930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang L, Zhang L, Kang K, Fei D, Gong R,
Cao Y, Pan S and Zhao M and Zhao M: Resveratrol ameliorates
LPS-induced acute lung injury via NLRP3 inflammasome modulation.
Biomed Pharmacother. 84:130–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su Q, Pu H and Hu C: Neuroprotection by
combination of resveratrol and enriched environment against
ischemic brain injury in rats. Neurol Res. 38:60–68. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang H, Gu ZT, Li L, Maegele M, Zhou BY,
Li F, Zhao M and Zhao KS: SIRT1 plays a neuroprotective role in
traumatic brain injury in rats via inhibiting the p38 MAPK pathway.
Acta Pharmacol Sin. 38:168–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang K, Zhang L, Rao W, Su N, Hui H, Wang
L, Peng C, Tu Y, Zhang S and Fei Z: Neuroprotective effects of
crocin against traumatic brain injury in mice: Involvement of notch
signaling pathway. Neurosci Lett. 591:53–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lopez MS, Dempsey RJ and Vemuganti R:
Resveratrol neuroprotection in stroke and traumatic CNS injury.
Neurochem Int. 89:75–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gardner AJ, Shih SL, Adamov EV and Zafonte
RD: Research frontiers in traumatic brain injury: Defining the
injury. Phys Med Rehabil Clin N Am. 28:413–431. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naghibi T, Mohajeri M and Dobakhti F:
Inflammation and outcome in traumatic brain injury: Does gender
effect on survival and prognosis? J Clin Diagn Res. 11:PC06–PC09.
2017.PubMed/NCBI
|
|
20
|
Russo MV and McGavern DB: Inflammatory
neuroprotection following traumatic brain injury. Science.
353:783–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pennisi M, Crupi R, Di Paola R, Ontario
ML, Bella R, Calabrese EJ, Crea R, Cuzzocrea S and Calabrese V:
Inflammasomes, hormesis, and antioxidants in neuroinflammation:
Role of NRLP3 in Alzheimer disease. J Neurosci Res. 95:1360–1372.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wallisch JS, Simon DW, Bayir H, Bell MJ,
Kochanek PM and Clark RSB: Cerebrospinal fluid NLRP3 is increased
after severe traumatic brain injury in infants and children.
Neurocrit Care. 27:44–50. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin C, Chao H, Li Z, Xu X, Liu Y, Bao Z,
Hou L, Liu Y, Wang X, You Y, et al: Omega-3 fatty acids regulate
NLRP3 inflammasome activation and prevent behavior deficits after
traumatic brain injury. Exp Neurol. 290:115–122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shah FA, Gim SA, Kim MO and Koh PO:
Proteomic identification of proteins differentially expressed in
response to resveratrol treatment in middle cerebral artery
occlusion stroke model. J Vet Med Sci. 76:1367–1374. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin CJ, Chen TH, Yang LY and Shih CM:
Resveratrol protects astrocytes against traumatic brain injury
through inhibiting apoptotic and autophagic cell death. Cell Death
Dis. 5:e11472014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gatson JW, Liu MM, Abdelfattah K,
Wigginton JG, Smith S, Wolf S and Minei JP: Resveratrol decreases
inflammation in the brain of mice with mild traumatic brain injury.
J Trauma Acute Care Surg. 74:470–475. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsushima S and Sadoshima J: The role of
sirtuins in cardiac disease. Am J Physiol Heart Circ Physiol.
309:H1375–H1389. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kleszcz R, Paluszczak J and Baer-Dubowska
W: Targeting aberrant cancer metabolism-The role of sirtuins.
Pharmacol Rep. 67:1068–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang BL: Sirt1 and the mitochondria. Mol
Cells. 39:87–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Martin A, Tegla CA, Cudrici CD, Kruszewski
AM, Azimzadeh P, Boodhoo D, Mekala AP, Rus V and Rus H: Role of
SIRT1 in autoimmune demyelination and neurodegeneration. Immunol
Res. 61:187–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao Y, Luo P, Guo Q, Li S, Zhang L, Zhao
M, Xu H, Yang Y, Poon W and Fei Z: Interactions between SIRT1 and
MAPK/ERK regulate neuronal apoptosis induced by traumatic brain
injury in vitro and in vivo. Exp Neurol. 237:489–498. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Yang X, He Y, Wang W, Zhang J, Zhang
W, Jing T, Wang B and Lin R: Negative regulation of NLRP3
inflammasome by SIRT1 in vascular endothelial cells. Immunobiology.
222:552–561. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Lian K, Zhang L, Wang R, Yi F, Gao
C, Xin C, Zhu D, Li Y, Yan W, et al: TXNIP mediates NLRP3
inflammasome activation in cardiac microvascular endothelial cells
as a novel mechanism in myocardial ischemia/reperfusion injury.
Basic Res Cardiol. 109:4152014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yin N, Peng Z, Li B, Xia J, Wang Z, Yuan
J, Fang L and Lu X: Isoflurane attenuates
lipopolysaccharide-induced acute lung injury by inhibiting
ROS-mediated NLRP3 inflammasome activation. Am J Transl Res.
8:2033–2046. 2016.PubMed/NCBI
|
|
35
|
Butterfield DA and Reed TT: Lipid
peroxidation and tyrosine nitration in traumatic brain injury:
Insights into secondary injury from redox proteomics. Proteomics
Clin Appl. 10:1191–1204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rodriguez-Rodriguez A, Egea-Guerrero JJ,
Murillo-Cabezas F and Carrillo-Vico A: Oxidative stress in
traumatic brain injury. Curr Med Chem. 21:1201–1211. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Czerska M, Mikołajewska K, Zieliński M,
Gromadzińska J and Wasowicz W: Today's oxidative stress markers.
Med Pr. 66:393–405. 2015. View Article : Google Scholar : PubMed/NCBI
|