Introduction
Diseases associated with cerebral ischemia are a
major cause of mortality in developing countries. Ischemic stroke
is associated with the acute loss of neurons, astroglia and
oligodendroglia, in addition to disruption to synaptic
architecture, as a result of cerebral artery occlusion (1). Certain studies have focused on the
potential use of mesenchymal stem cell (MSC) transplantation in the
treatment of central nervous system (CNS) diseases and injures,
such as cerebral ischemia (2,3). MSC
therapy is considered a novel and promising strategy for the
treatment of ischemic stroke, and may exert neuroprotective effects
and promote the repair of neurons by secreting various neural
trophic factors and replacing damaged neurons (4). However, the ischemic microenvironment
negatively influences the survival rate of transplanted MSCs in
injured CNS conditions due to oxidative stress (5,6).
Thus, improving the survival of MSCs during oxidative stress may
improve the efficacy of MSC-based therapies.
Gigantol is a biphenolic compound that is primarily
extracted from the stem of Dendrobium aurantiacum (7). Phenols derived from natural plants
contain numerous antioxidants and therefore are typically used to
study antioxidative activities (8–11).
Furthermore, gigantol is reported to exhibit numerous biological
functions, including anti-osmosis effects (12), antitumor effects in human liver
(13) and lung (14) cancer, antimutagenic effects
(15) and immunomodulatory
activities (16). Additionally,
gigantol was reported to be a potent compound for restoring sight
in diabetics with cataracts (17).
However, to the best of our knowledge, no previous studies have
investigated the protective effect of gigantol on hydrogen peroxide
(H2O2)-induced oxidative stress in rat bone
marrow MSCs (rBMSCs). Therefore, the present study investigated
whether gigantol protects against
H2O2-induced oxidative stress in rBMSCs and
whether the antioxidant mechanism of gigantol involves the
phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt)
pathway.
Materials and methods
Chemicals and materials
Male 4-week-old Sprague-Dawley rats (n=10) weighing
80–100 g were used in the present study and were obtained from
Guangzhou Laboratory Animal Center, Guangzhou University of Chinese
Medicine (Guangzhou, China). Low glucose Dulbecco's modified
Eagle's medium (DMEM) and PBS were acquired from Gibco (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
H2O2 was purchased from Guangzhou Chemical
Reagent Factory (Guangzhou, China). Basal medium of Sprague-Dawley
rat MSCs, fetal bovine serum (FBS), glutamine,
penicillin-streptomycin and trypsin were purchased from Cyagen
Biosciences, Inc. (Guangzhou, China). MTT and dimethyl sulfoxide
were acquired from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Gigantol was purchased from the National Institute for Food and
Drug Control (cat. no. 111875; Beijing, China). The chemical
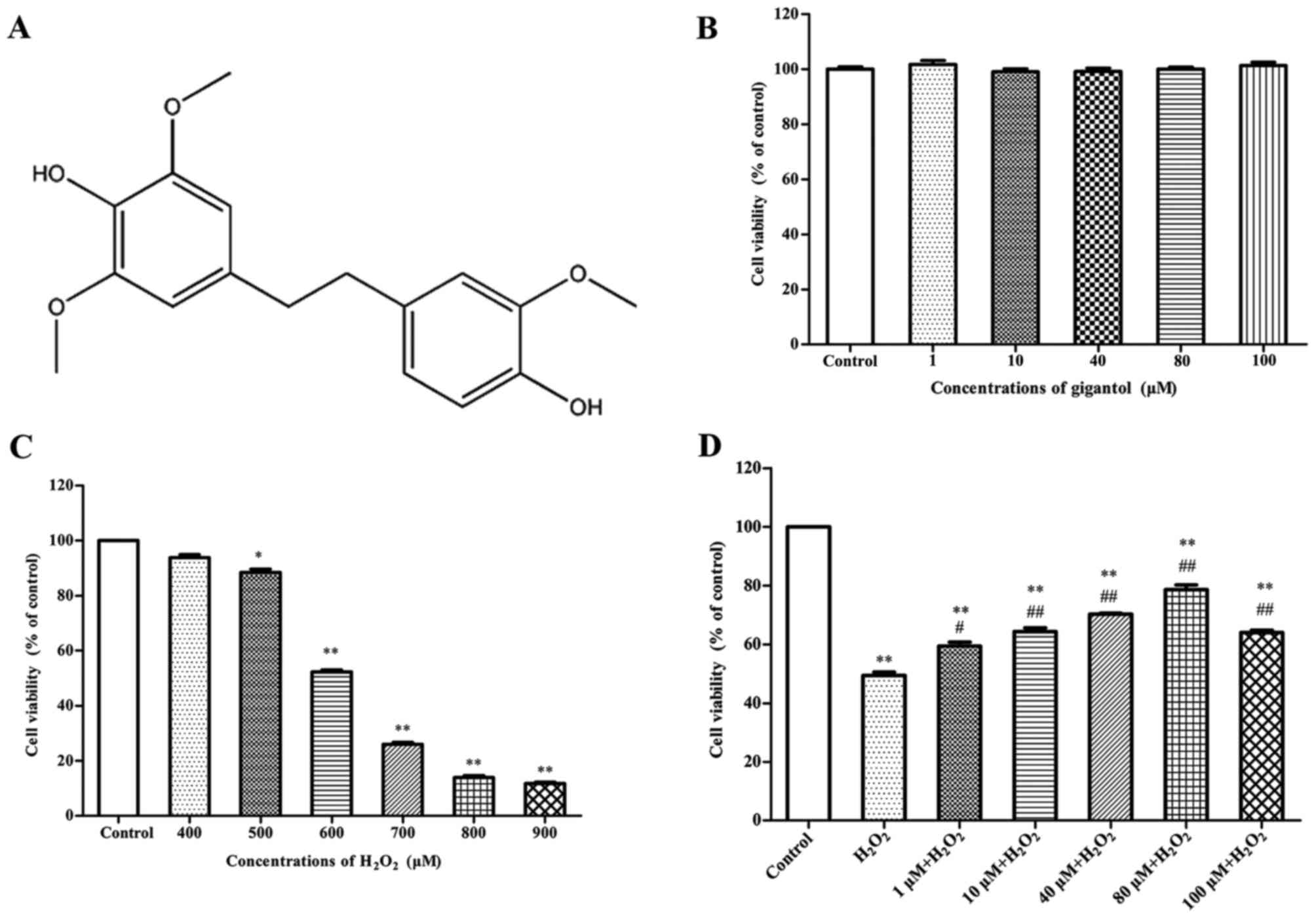
structure of gigantol is presented in Fig. 1A. Annexin V-fluorescein
isothiocyanate (FITC) apoptosis, Hoechst 33258 and reactive oxygen
species (ROS) assay kits were provided by Nanjing KeyGen Biotech
Co., Ltd. (Nanjing, China). The PI3K/Akt inhibitor LY294002 was
purchased from Selleck Chemicals (Houston, TX, USA). All other
chemicals were of analytical grade.
Isolation and culture of rBMSCs
MSCs were immediately isolated from the
Sprague-Dawley rats as previously described, with minor
modifications (18). Briefly,
Sprague-Dawley rats were sacrificed by cervical dislocation. The
experimental procedures were approved by the Laboratory Animal
Committee of Guangdong Province (Guangzhou, China). All treatments
on animals were performed in accordance with the Guide for the Care
and Use of Laboratory Animals (19). The femurs and tibias of rats were
carefully cleaned of adherent soft tissue, the marrow was harvested
and flushed with serum-free DMEM with 1% penicillin-streptomycin
until the bone washed pale. Cells were resuspended in DMEM medium
with 10% FBS and 1% penicillin -streptomycin of Sprague-Dawley
rBMSCs at 37°C with 5% CO2 After being allowed to attach
for 24 h, hematopoietic and non-adherent cells were removed by
changing the medium. Subsequently, rBMSCs were harvested for the
experiments described below between the second and third passage.
Cells were pretreated with gigantol for 12 h followed by treatment
with H2O2 for 2 h, both at room temperature.
To determine the effect of LY294002, cells were pretreated with
LY294002 (25 µmol/l) for 1 h at room temperature, followed by the
treatments with gigantol and H2O2.
Cell viability assay
Cells were seeded in 96-well plates
(1×105 cells/ml) for 24 h at room temperature. To
determine the effects of gigantol and H2O2 on
rBMSC viability, cells were treated with 1, 10, 40, 80 and 100 µM
gigantol for 12 h, or 400, 500, 600, 700, 800 and 900 µM
H2O2 for 2 h, respectively. As a control,
cells were treated with DMEM medium only. Furthermore, in another
cell viability assay, cells were pretreated with different
concentrations of gigantol (1, 10, 40, 80 and 100 µM) for 12 h
followed by treatment with 600 µM H2O2 for 2
h, both at room temperature. Subsequently, 20 µl MTT was added to
each well and incubated at 37°C for 4 h prior to removal and
addition of 100 µl dimethyl sulfoxide. The absorbance value was
measured in a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at 490 nm. Statistical analysis was performed on
absorbance value readings.
Assessment of morphological
changes
Cells were cultured in 24-well plates
(5×105 cells/well) and treated with 80 µM gigantol for
12 h followed by the addition of 600 µM H2O2
for 2 h. Cells in the H2O2 group were treated
with 600 µM H2O2 only. Cells were fixed with
4% paraformaldehyde for 10 min and washed with PBS twice prior to
staining with Hoechst 33258 for 5 min at 4°C in the dark. Condensed
nuclei and cell shrinkage were observed using an inverted and
fluorescence microscope (Leica Microsystems GmbH, Wetzlar,
Germany). A bright blue stain indicated apoptotic cell nuclei.
Measurement of ROS
Cells were cultured in 6-well plates
(1×106 cells/well) and treated with 80 µM gigantol for
12 h followed by the addition of 600 µM H2O2
for 2 h, both at room temperature. Cells in the
H2O2 group were treated with 600 µM
H2O2 only. Cells were stained with 10 µM
2′7′-dichlorofluorescin diacetate (DCFH-DA) diluted with serum-free
medium at 37°C for 20 min and later washed with serum-free medium
three times. Fluorescence intensity was analyzed using a microplate
reader (Bio-Rad Laboratories, Inc.) at excitation and emission
wavelengths of 488 and 525 nm, respectively. Images were captured
using a fluorescence microscope (Leica Microsystems GmbH). The
absorbance values were obtained for statistical analysis.
Flow cytometric analysis of cell
apoptosis
Cells were seeded in 6-well plates (1×106
cells/well) for 24 h and and treated with 80 µM gigantol for 12 h
followed by the addition of 600 µM H2O2 for 2
h. Cells in the H2O2 group were treated with
600 µM H2O2 only. Subsequently, cells were
harvested and washed twice using PBS, and were resuspended in 500
µl binding buffer. Annexin V-FITC stock (5 µl) and propidium iodide
solution (5 µl) was added to the cells and incubated for 10 min at
room temperature in the dark, and immediately analyzed using flow
cytometer (BD FACSCanto II). The percentage of apoptotic cells was
obtained for statistical analysis.
Protein extraction and western blot
analysis
Cells were seeded in 6-well plates (1×106
cells/well) for 24 h and treated with 80 µM gigantol for 12 h
followed by the addition of 600 µM H2O2 for 2
h, both at room temperature. Cells in the
H2O2 group were treated with 600 µM
H2O2 only. Cells in the gigantol +
H2O2 + LY294002 group were pretreated with
LY294002 (25 µmol/l) for 1 h prior to gigantol with
H2O2 treatment. Subsequently, cells were
washed with PBS and lysed in cold radioimmunoprecipitation assay
lysis buffer (Beyotime Institute of Biotechnology, Haimen, China)
with protein inhibitor. Cellular proteins were collected and their
concentrations were determined using a Bradford assay. Equal
amounts of protein (40 µg/lane) were separated on 15%
SDS-polyacrylamide gels and transferred onto polyvinylidene
difluoride membranes via electrophoresis. After blocking with
tris-buffered saline (TBS) containing 5% skimmed milk and 0.05%
Tween-20 for 1 h at room temperature, the membranes were incubated
with the following primary antibodies: p-Akt (ser 473; cat. no.
Sc7985r; 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
Akt (ser 473; cat. no. Sc8312; 1:200; Santa Cruz Biotechnology,
Inc.), B-cell lymphoma-2 (Bcl-2)-associated X (Bax; cat. no. 2772;
1:1,000; CST Biological Reagents Co., Ltd., Shanghai, China), Bcl-2
(cat. no. 2872; 1:1,000; CST Biological Reagents Co., Ltd.),
Caspase-3 (cat. no. 9662; 1:1,000; CST Biological Reagents Co.,
Ltd.), Caspase-9 (cat. no. 9504; 1;1,000; CST Biological Reagents
Co., Ltd.) and β-actin (cat. no. sc58673; Santa Cruz Biotechnology,
Inc.) at 4°C overnight. After washing with TBS three times, the
membranes were incubated with goat anti-rabbit immunoglobulin G
antibodies conjugated with horseradish peroxidase (cat. no.
111-035-003; 1:1,000; Jackson Immuno Research Laboratories, Inc.,
West Grove, PA, USA) for 1 h at room temperature. Following three
washes with TBS-Tween-20, the intensity of bands was visualized
using an enhanced chemiluminescence western blotting kit (Merck
KGaA) and quantified by densitometric analysis with ImageJ software
(version 3.0; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All experiments were conducted at least three times.
Data are presented as the mean + standard error of the mean.
Differences among groups were analyzed by one-way analysis of
variance, followed by Dunnett's post-hoc test, using SPSS version
20 (IBM Corp., Armonk, NY, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Gigantol inhibits
H2O2-induced inhibition of cell viability in
rBMSCs
To determine an appropriate concentration of
gigantol, cells were treated with gigantol (1, 10, 40, 80 and 100
µM), and the results indicated that none of these concentrations
exhibited a damaging effect on cell viability (Fig. 1B). Cell viability was reduced in a
dose-dependent manner when treated with 400, 500, 600, 700 and 800
µM H2O2 for 2 h, compared with the control
group. H2O2 at the concentration of 600 µM
significantly reduced cell viability compared with the control by
51.6±3.2% (Fig. 1C). In addition,
results in Fig. 1D demonstrated
that gigantol significantly increased the cell viability of rBMSCs
in a dose-dependent manner compared with cells treated with
H2O2 only. Furthermore, pretreatment with 80
µM gigantol significantly enhanced cell viability compared with the
H2O2 only group (Fig. 1D). Concentrations of gigantol
>80 µM reduced the stimulatory effect. Therefore, 600 µM
H2O2 and 80 µM gigantol were selected for the
following experiments.
Assessment of morphological
changes
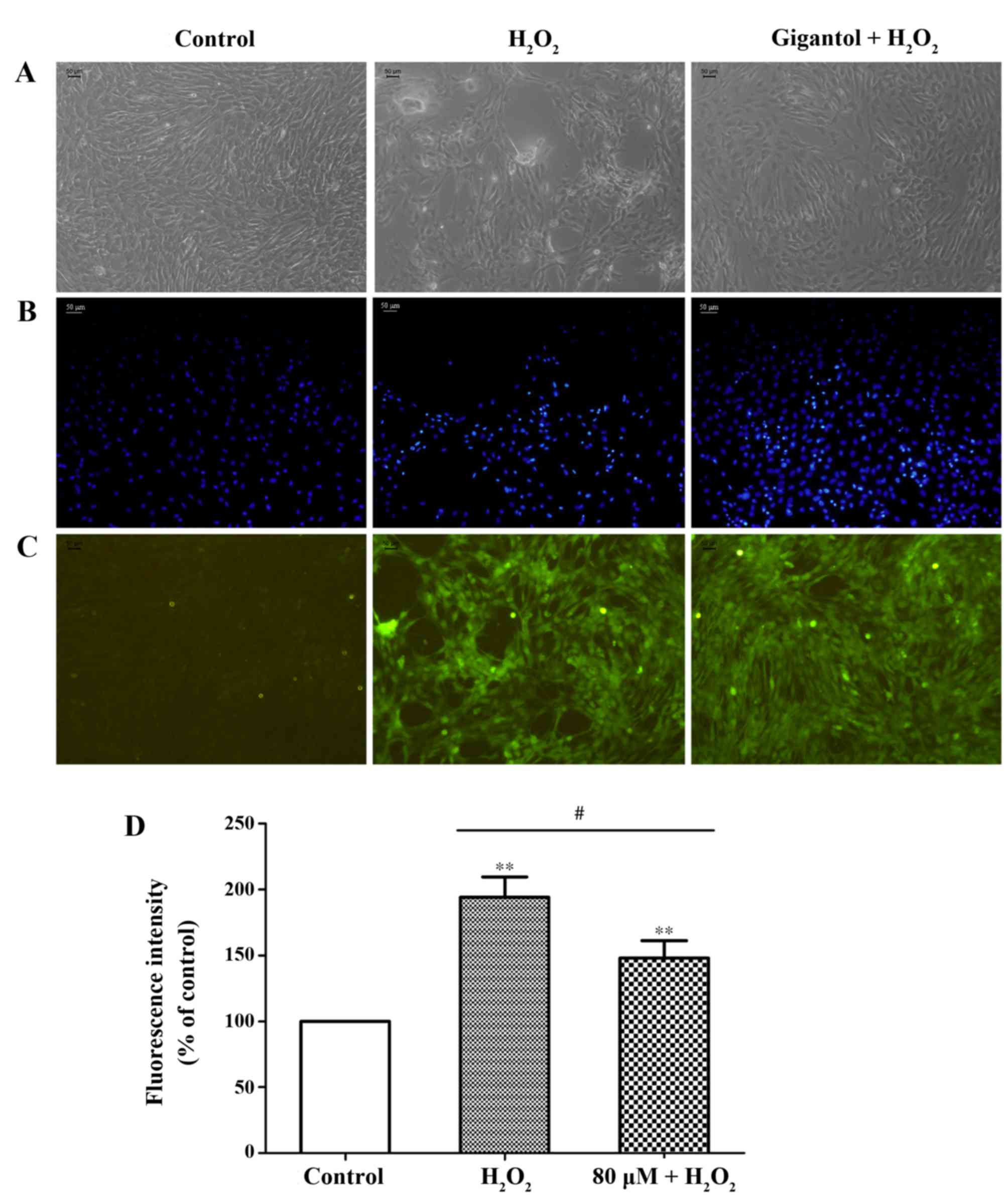
Following treatment with H2O2,
apoptosis-associated morphology was observed in rBMSCs, including
detachment, irregular shape and nuclear shrinkage. However, the
number of apoptosis-like cells decreased in the group pretreated
with gigantol, which indicated a potential protective effect of
gigantol from apoptosis induction (Fig. 2A and B).
Detection of ROS
Cellular oxidative stress was examined by a DCFH-DA
assay. The results demonstrated that, in the
H2O2-treated group, a significant increase in
2′,7′-dichlorofluorescein fluorescence was observed (Fig. 2C and D). However, pretreatment with
gigantol significantly reduced the intracellular production of ROS
compared with the H2O2-treated group
(Fig. 2C and D).
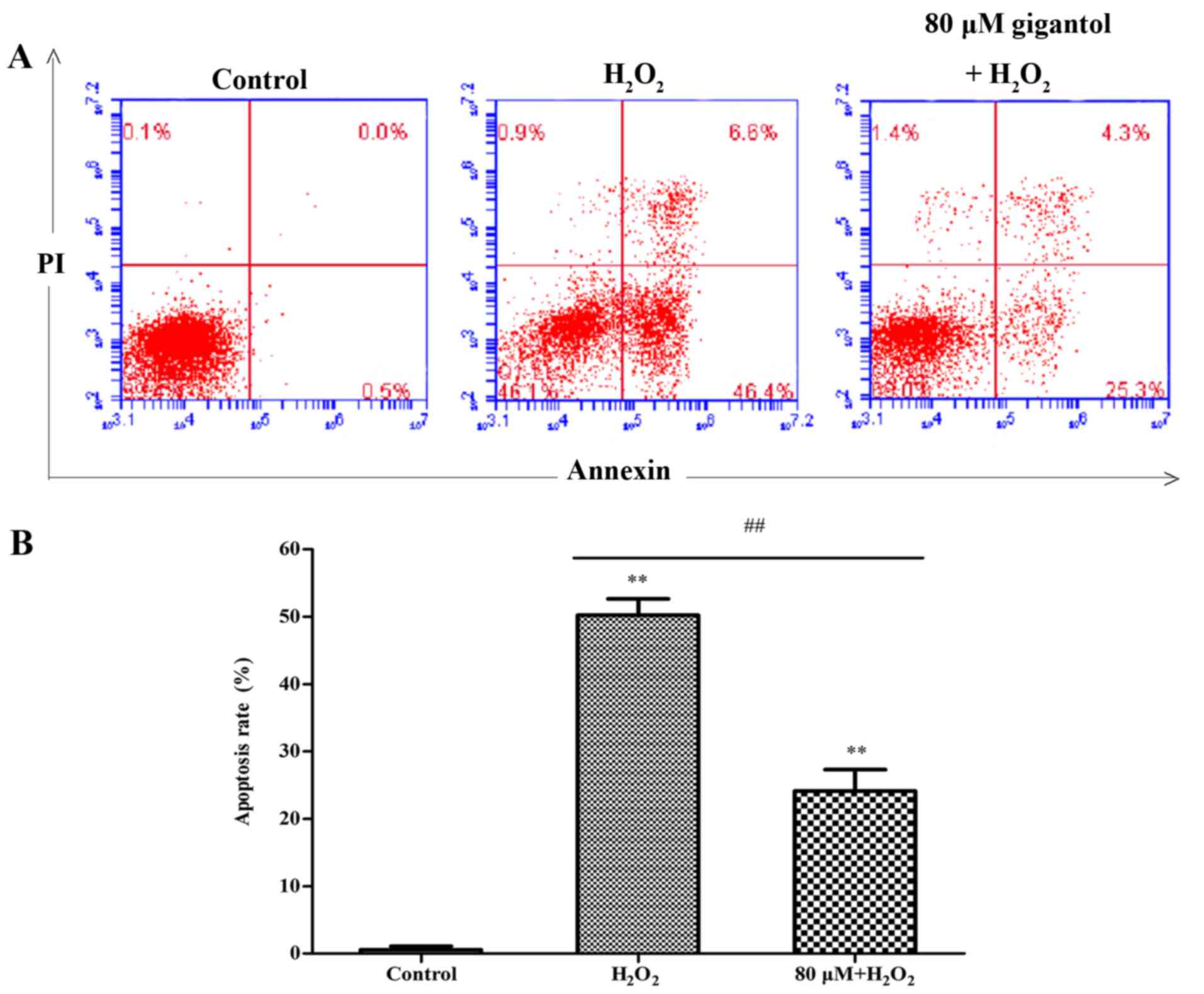
Analysis of cell apoptosis
Cell apoptosis was analyzed using an Annexin V and
propidium iodide double-staining assay by flow cytometry. The
percentage of apoptotic cells in Q2 and Q4 increased from 0.5±0.45%
in the control group to 49.5±3.30% in the
H2O2 group, while apoptosis was significantly
reduced to 23.4±2.06% in the gigantol + H2O2
group, compared with the H2O2 only group
(Fig. 3).
Gigantol activates the PI3K/Akt
pathway
The results of western blot analysis demonstrated
that H2O2 treatment reduced the protein
levels of phosphorylated (p)-Akt and the antiapoptotic protein
Bcl-2 (Fig. 4A and B), and
increased the levels of the proapoptotic proteins Bax, caspase-3
and caspase-9 (Fig. 4B-D).
However, gigantol pretreatment lowered the caspase-3, caspase-9 and
Bax levels, and increased the levels of p-Akt and Bcl-2, compared
with the H2O2 only group (Fig. 4). Furthermore, LY294002 (a PI3K
inhibitor) significantly inhibited the protective effect of
gigantol against H2O2-induced apoptosis by
increasing the levels of caspase-3, caspase-9 and the ratio of
Bax/Bcl-2, and decreasing the ratio of p-Akt/Akt (Fig. 4).
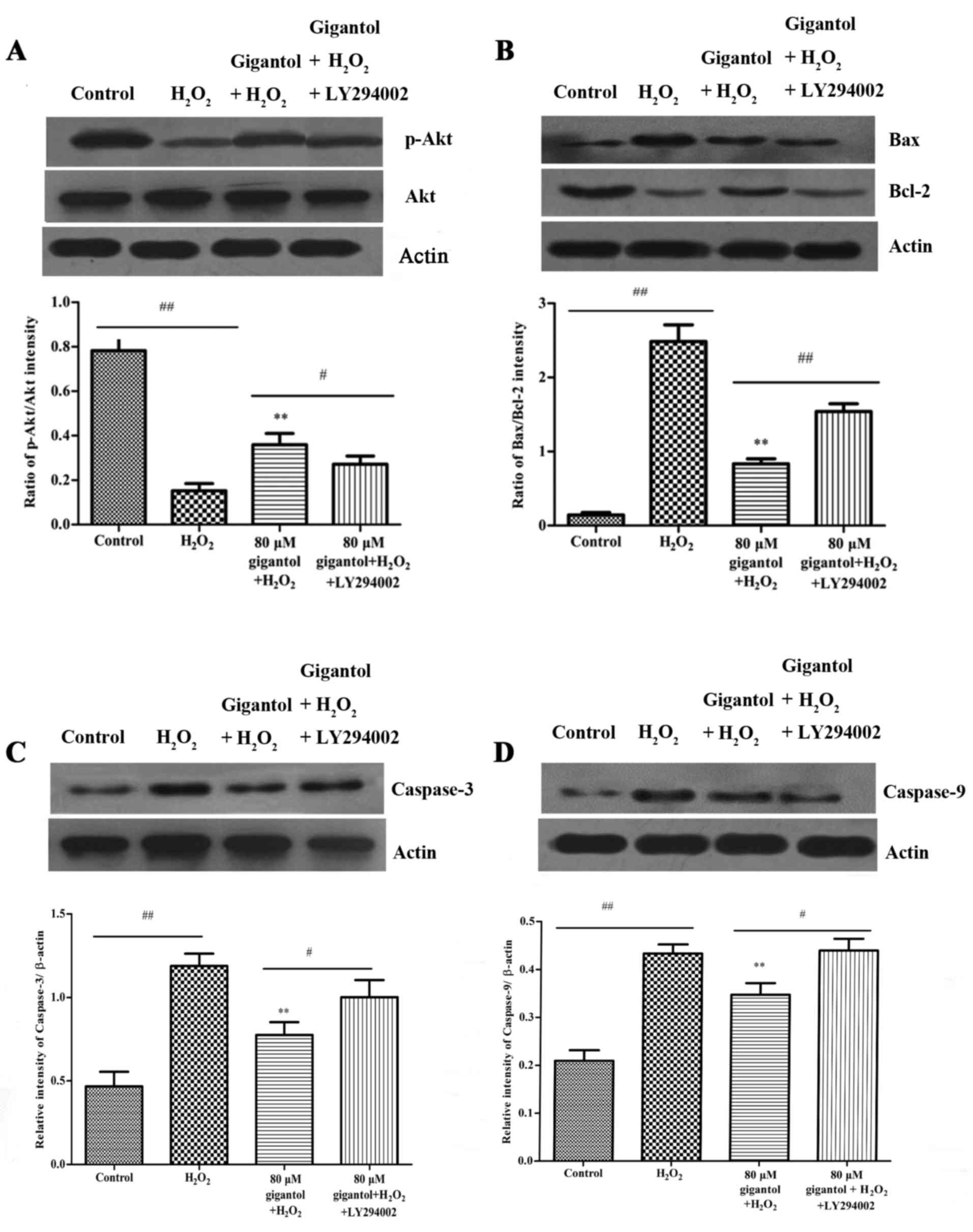 | Figure 4.The effect of gigantol and LY294002
on the expression of apoptosis-associated proteins was investigated
by western blot analysis. (A) Exposure of rBMSCs to 80 µM gigantol
significantly increased the expression of p-Akt compared with the
H2O2 only group, while LY294002 reduced the
increase in p-Akt. Akt was used as an internal control and
densitometric analysis indicates the p-Akt/Akt ratio. (B) Gigantol
pretreatment attenuated H2O2-induced
upregulation of Bax and downregulation of Bcl-2, and these effects
were reversed by the application of LY294002. β-actin was used as
an internal control and densitometric analysis indicates the
Bax/Bcl-2 ratio. (C) Gigantol inhibited the protein expression of
caspase-3 in H2O2-induced rBMSCs and LY294002
reversed this inhibition partially. β-actin was used as an internal
control. (D) Gigantol suppressed the expression of caspase-9 in
H2O2-induced rBMSCs, while LY294002 reversed
the inhibition partially. β-actin was used as an internal control.
**P<0.01 vs. H2O2 group;
#P<0.05 and ##P<0.01, as indicated.
rBMSCs, rat bone marrow mesenchymal stem cells; p-,
phosphorylated-; Akt, protein kinase B; H2O2,
hydrogen peroxide; Bcl-2, B-cell lymphoma-2; Bax, Bcl-2-associated
X; actin, β-actin. |
Discussion
Previous studies have reported that the
transplantation of human or rat MSCs led to a substantial
functional improvement in stroke treatment (20–22).
However, the low survival rate of MSCs that are transplanted for
the treatment of an ischemic myocardium indicates that the hypoxic
microenvironment may impair the survival of MSCs.
H2O2 has successfully been used to induce
oxidative stress, which led to cell apoptosis and mimicked the
hypoxic microenvironment of the ischemic brain (23–25).
In addition, Sun et al (26) employed a
H2O2-induced cytotoxicity model of BMSCs to
investigate damage induced by oxidative stress.
We previously reported that gigantol is abundant in
Dendrobium aurantiacum among the herbal medicines grouped as
Huangcao Shihu, which includes Dendrobium nobile,
Dendrobium fimbriatum and Dendrobium aurantiacum
(27). It is uncommon for such
high concentrations of active compounds to occur naturally within
plants; therefore, gigantol may be of clinical value if beneficial
effects are observed. The present study, to the best of our
knowledge, is the first to indicate that gigantol may have
protective activities against ischemic diseases, as MTT and flow
cytometry results demonstrated that gigantol inhibited
H2O2-induced cell apoptosis in rBMSCs.
Furthermore, gigantol reduced the generation of ROS in
H2O2-treated rBMSCs, which indicates that
gigantol may exhibit beneficial antiapoptotic activities through
inhibition of ROS generation.
A previous report demonstrated that extracellular
H2O2 enhanced intracellular concentrations of
ROS, which subsequently inactivated p-Akt (28). In the present study, treatment of
MSCs with H2O2 led to decreased levels of
p-Akt, indicating that the PI3K/Akt signaling pathway may be
inhibited in MSCs following exposure to H2O2.
In the present study, treatment with gigantol activated the
expression of p-Akt in H2O2-induced rBMSCs.
Previous studies have demonstrated that the PI3K/Akt pathway is
involved in various biological processes, including cell growth,
survival and apoptosis, and also has roles in cell metabolism,
proliferation and migration (29–31).
PI3K/Akt is reported to prevent cell apoptosis by
reducing the expression of various proapoptotic proteins, including
caspase-3, caspase-9 and Bax, and by elevating the levels of the
antiapoptotic protein Bcl-2 (32).
These results are consistent with those of an earlier report, which
indicated that PI3K-Akt signaling increased the intracellular
levels of ROS and activated the proapoptotic proteins caspase-3,
caspase-9 and Bax, and inhibited the expression of Bcl-2 (33). The results of the current study
demonstrated that H2O2 treatment increased
the Bax/Bcl-2 ratio, and caspase-3 and caspase-9 protein
expression, in rBMSCs. However, pretreatment with gigantol
suppressed the Bax/Bcl-2 ratio, and caspase-3 and caspase-9 levels,
which indicates that gigantol may protect against rBMSC apoptosis
via the PI3K/Akt signaling pathway. Furthermore, LY294002, a
specific PI3K/Akt inhibitor, blocked the protective effects of
gigantol. These results confirmed that PI3K/Akt may be activated by
gigantol to protect rBMSCs from H2O2-induced
apoptosis.
In conclusion, the present study demonstrated that
gigantol significantly inhibited H2O2-induced
apoptosis in rBMSCs. The protective effect of gigantol was
accompanied by reductions in intracellular ROS generation, the
expression ratio of Bax/Bcl-2, and caspase-3 and caspase-9 protein
expression, in addition to increases in the ratio of p-Akt/Akt and
Bcl-2 expression. Therefore, gigantol may have the potential to be
developed as a protective agent for the clinical treatment of
patients with ischemic diseases. Regarding the utilization of
gigantol in ischemic stroke, however, further in vitro and
in vivo experiments are required to investigate the effect
of gigantol on transport and differentiation in rBMSCs.
Acknowledgements
The present study was financially supported by the
Special Foundation of 2015 High Level University Construction
(grant no. 2050205), the Special Foundation of High Level
University Construction of Guang Zhou University of Chinese
Medicine (grant no. A1-AFD018171Z11024) and the Science and
Technology Planning Project of Guangdong Province (grant no.
2013B060400022).
Glossary
Abbreviations
Abbreviations:
|
rBMSCs
|
rat bone marrow mesenchymal stem
cells
|
|
Bcl-2
|
B-cell lymphoma-2
|
|
Bax
|
Bcl-2-associated X
|
|
H2O2
|
hydrogen peroxide
|
|
PI3K
|
phosphatidylinositol 3-kinase
|
|
Akt
|
protein kinase B
|
|
ROS
|
reactive oxygen species
|
References
|
1
|
Tuan RS, Boland G and Tuli R: Adult
mesenchymal stem cells and cell-based tissue engineering. Arthritis
Res Ther. 5:32–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Keyser J: Autologous mesenchymal stem
cell transplantation in stroke patients. Ann Neurol. 58:653–654.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Monsel A, Zhu YG, Gennai S, Hao Q, Liu J
and Lee JW: Cell-based therapy for acute organ injury: Preclinical
evidence and ongoing clinical trials using mesenchymal stem cells.
Anesthesiology. 121:1099–1121. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hao L, Zou Z, Tian H, Zhang Y, Zhou H and
Liu L: Stem cell-based therapies for ischemic stroke. Biomed Res
Int. 2014:4687482014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee JH, Jung HK, Han YS, Yoon YM, Yun CW,
Sun HY, Cho HW and Lee SH: Antioxidant effects of Cirsium setidens
extract on oxidative stress in human mesenchymal stem cells. Mol
Med Rep. 14:3777–3784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei H, Li Z, Hu S, Chen X and Cong X:
Apoptosis of mesenchymal stem cells induced by hydrogen peroxide
concerns both endoplasmic reticulum stress and mitochondrial death
pathway through regulation of caspases, p38 and JNK. J Cell
Biochem. 111:967–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Y, Wang ZT and Xu LS: Phenols and a
triterpene from Dendrobium aurantiacum var. Denneanum
(Orchidaceae). Biochem Syst Ecol. 34:658–660. 2006. View Article : Google Scholar
|
|
8
|
Kuppusamy S, Thavamani P, Megharaj M,
Nirola R, Lee YB and Naidu R: Assessment of antioxidant activity,
minerals, phenols and flavonoid contents of common plant/tree waste
extracts. Ind Crop Prod. 83:630–634. 2016. View Article : Google Scholar
|
|
9
|
Wang ST, Gao W, Fan YX, Liu XG, Liu K, Du
Y, Wang LL, Li HJ, Li P and Yang H: Phenol profiles and antioxidant
capacities of bistort rhizoma (Polygonum bistorta L.) extracts. Rsc
Adv. 6:27320–27328. 2016. View Article : Google Scholar
|
|
10
|
Temel E, Alasalvar C, Gokce H, Güder A,
Albayrak Ç, Alpaslan YB, Alpaslan G and Dilek N: DFT calculations,
spectroscopy and antioxidant activity studies on
(E)-2-nitro-4-((phenylimino)methyl)phenol. Spectrochim Acta A.
136:534–546. 2015. View Article : Google Scholar
|
|
11
|
Menshchikova EB, Wisman NY, Zenkov NK,
Tkachev VO and Kandalintseva NV: ARE-inducing phenol antioxidant
TC-13 improves survival of drosophila melanogaster in oxidative
stress. B Exp Med. 154:260–264. 2012. View Article : Google Scholar
|
|
12
|
Fang H, Hu X, Wang M, Wan W, Yang Q, Sun
X, Gu Q, Gao X, Wang Z, Gu L, et al: Anti-osmotic and antioxidant
activities of gigantol from Dendrobium aurantiacum var. Denneanum
against cataractogenesis in galactosemic rats. J Ethnopharmacol.
172:238–246. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen H, Huang Y, Huang J, Lin LZ and Wei
G: Gigantol attenuates the proliferation of human liver cancer
through the PI3K/Akt/NF-κB signaling pathway. Oncol Rep.
37:865–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bhummaphan N and Chanvorachote P: Gigantol
suppresses cancer stem cell-like phenotypes in lung cancer cells.
Evid Based Complement Alternal Med. 2015:1–10. 2015. View Article : Google Scholar
|
|
15
|
Miyazawa M, Shimamura H, Nakamura S and
Kameoka H: Antimutagenic activity of gigantol from Dendrobium
nobile. J Agrc Food Chem. 45:2849–2853. 1997. View Article : Google Scholar
|
|
16
|
Won JH, Kim JY, Yun KJ, Lee JH, Back NI,
Chung HG, Chung SA, Jeong TS, Choi MS and Lee KT: Gigantol isolated
from the whole plants of cymbidium goeringii inhibits the
LPS-induced iNOS and COX-2 expression via NF-κB inactivation in RAW
264.7 macrophages cells. Planta Med. 72:1181–1187. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu J, Lu C, Li X, Fang H, Wan W, Yang Q,
Sun X, Wang M, Hu X, Chen CY and Wei X: Synthesis and biological
evaluation of novel gigantol derivatives as potential agents in
prevention of diabetic cataract. PLoS One. 10:e01410922015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tropel P, Noël D, Platet N, Legrand P,
Benabid AL and Berger F: Isolation and characterisation of
mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res.
295:395–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals: Guide for the Care and Use of Laboratory Animals. 8th.
National Academies Press (US); Washington, DC: 2011
|
|
20
|
Gutiérrez-Fernández M, Rodríguez-Frutos B,
Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdán S and
Díez-Tejedor E: Effects of intravenous administration of allogenic
bone marrow- and adipose tissue-derived mesenchymal stem cells on
functional recovery and brain repair markers in experimental
ischemic stroke. Stem Cell Res. 4:112013. View Article : Google Scholar
|
|
21
|
Wei L, Fraser JL, Lu ZY, Hu X and Yu SP:
Transplantation of hypoxia preconditioned bone marrow mesenchymal
stem cells enhances angiogenesis and neurogenesis after cerebral
ischemia in rats. Neurobiol Dis. 46:635–645. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsai LK, Wang Z, Munasinghe J, Leng Y,
Leeds P and Chuang DM: Mesenchymal stem cells primed with valproate
and lithium robustly migrate to infarcted regions and facilitate
recovery in a stroke model. Stroke. 42:2932–2939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin YC, Huang YC, Chen SC, Liaw CC, Kuo
SC, Huang LJ and Gean PW: Neuroprotective effects of ugonin K on
hydrogen peroxide-induced cell death in human neuroblastoma SH-SY5Y
cells. Neurochem Res. 34:923–930. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Q, Huang WD, Lv XY and Yang YM:
Puerarin protects differentiated PC12 cells from H2O2-induced
apoptosis through the PI3K/Akt signalling pathway. Cell Biol Int.
36:419–426. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee AY, Wu TT, Hwang BR, Lee J, Lee MH,
Lee S and Cho EJ: The neuro-protective effect of the methanolic
extract of Perilla frutescens var. Japonica and rosmarinic acid
against H2O2-induced oxidative stress in C6 glial cells. Biomol
Ther (Seoul). 24:338–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun B, Feng M, Tian X, Lu X, Zhang Y, Ke
X, Huang S, Cao J and Ding X: Dl-3-n-Butylphthalide protects rat
bone marrow stem cells against hydrogen peroxide-induced cell death
through antioxidation and activation of PI3K-Akt pathway. Neurosci
Lett. 516:247–252. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tao SC, Chen ZH, Huang KW, Yan MX and Wei
G; Guangzhou University of Chinese Medicine, ; Foshan Hospital of
Traditional Chinese Medicine Affiliated to Guangzhou University of
Chinese Medicine, : Comparative study of HPLC characteristic
spectrum of Dendrobium fimbriatum hook and other huangcao
dendrobium species. Chin Med Phar. 27:238–241. 2016.
|
|
28
|
Li Y, Xue F, Xu SZ, Wang XW, Tong X and
Lin XJ: Lycopene protects bone marrow mesenchymal stem cells
against ischemia-induced apoptosis in vitro. Eur Rev Med Pharmaco.
18:1625–1631. 2014.
|
|
29
|
Stambolic V and Woodgett JR: Functional
distinctions of protein kinase B/Akt isoforms defined by their
influence on cell migration. Trends Cell Bio. 16:461–466. 2006.
View Article : Google Scholar
|
|
30
|
Osaki M, Oshimura M and Ito H: PI3KAkt
pathway: Its functions and alterations in human cancer. Apoptosis.
9:667–676. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song G, Ouyang G and Bao S: The activation
of Akt/PIK13 signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Downward J: PI 3-kinase, Akt and cell
survival. Semin Cell Dev Biol. 15:177–182. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim JY, Lee JS, Han YS, Lee JH, Bae I,
Yoon YM, Kwon SM and Lee SH: Pretreatment with lycopene attenuates
oxidative stress-induced apoptosis in human mesenchymal stem cells.
Biomol Ther. 23:517–524. 2015. View Article : Google Scholar
|