Introduction
Femoral head disease (FH) is one of the refractory
diseases in the field of orthopedics, which may be clinically
divided into invasive and non-invasive categories; the former is
primarily due to skeletal trauma, including femoral neck fracture
or hip dislocation, and the latter is principally caused by the
application of corticosteroids and long-term heavy alcohol
consumption (1,2). Steroid-induced FH is the most common
type of non-invasive FH, accounting for 50% of mortalities
(3). Hormones are widely-used in
the anti-inflammatory treatment of severe acute infections. Hormone
treatment may prevent certain sequelae of inflammation, autoimmune
diseases, including severe rheumatic fever, rheumatic myocarditis,
rheumatic and rheumatoid arthritis, systemic lupus erythematosus,
autoimmune anemia and nephrotic syndrome, organ transplant
rejection, and allergic diseases, including urticaria, angioedema,
bronchial asthma and anaphylactic shock; therefore, high-dose or
long-term use of hormones may lead to FH (2,3). It
is of particular importance that effective marrow protection
treatment methods be identified, and early diagnosis and
intervention holds the greatest promise for the treatment of
steroid-induced FH.
FH is one of the most serious complications of
glucocorticoid therapy, and, in recent years, research has focused
on the etiology, pathology and pathogenesis of the disease,
particularly the theories of fat metabolism, intravascular
coagulation, bone high pressure and osteoporosis, to provide novel
approaches towards pharmaceutical prevention and early intervention
for steroid-induced FH (4).
However, precise mechanisms underlying steroid-induced FH remain
unclear; although different theories have emerged from clinical and
experimental research, they cannot explain all the clinical
phenomena and experimental results, and it is therefore important
to investigate the exact pathogenesis of steroid-induced FH, and
develop sensitive early diagnostic methods, effective early
treatment or preventative approaches (4).
Peroxisome proliferator-activated receptor (PPAR)γ
is a ligand-activated nuclear transcription factor, which belongs
to the PPAR superfamily. The aldehyde reductase (ARY) gene, located
on chromosome 3p25, may be divided into three subtypes: ARYl,
present in numerous tissues; PPARY3, highly expressed in
macrophages, adipocytes and colonic epithelial cells; and PPARγ
(5), primarily expressed in fat
cells, with relative specificity (6). Previous experimental results have
demonstrated that ARY serves an important role in the
differentiation of bone marrow stromal cells, and that PPARY3 is
considered to regulate the differentiation of bone marrow stromal
cells into adipocytes, and is the necessary
differentiation-specific transcription factor for the generation of
fat cells (7). PPARγ serves a
positive role in the regulation of the differentiation of bone
marrow stromal cells into adipocytes, and an increased expression
level in bone marrow stromal cells may promote the formation of
adipocytes, subsequently leading to FH (8).
The neurogenic locus notch homolog protein 1
(Notch1) signaling pathway serves an important role in the
differentiation of human bone marrow mesenchymal stem cells into
neuronal cells. It has been reported that the Notch1-mediated
signaling pathway is the essential factor in determining the
differentiation potential of bone marrow mesenchymal stem cells
(9). The weakening of Notch
signaling pathway is associated with the proliferation and
differentiation of bone marrow mesenchymal stem cells in
osteoporosis patients following the menopause, which may be one of
the causes for the decrease in postmenopausal bone mass of patients
with osteoporosis (10). Estrogen
may reverse the decreased expression of important molecules in the
Notch signaling pathway of bone marrow mesenchymal stem cells in
postmenopausal osteoporotic patients; following treatment with
estrogen, the expression of receptor Notch1 and its ligand protein
jagged-1 is increased, and the expression of transcription factor
HES1 is increased markedly. It is therefore indicated that an
association may exist between estrogen and the Notch signaling
pathway (10). Following estrogen
stimulation in human bone marrow mesenchymal stem cells during the
osteogenic and adipogenic differentiation processes, it was
previously observed that the expression of key molecules increases
compared with a group without estrogen stimulation in the early
stage of osteogenic differentiation and adipogenic differentiation
(11).
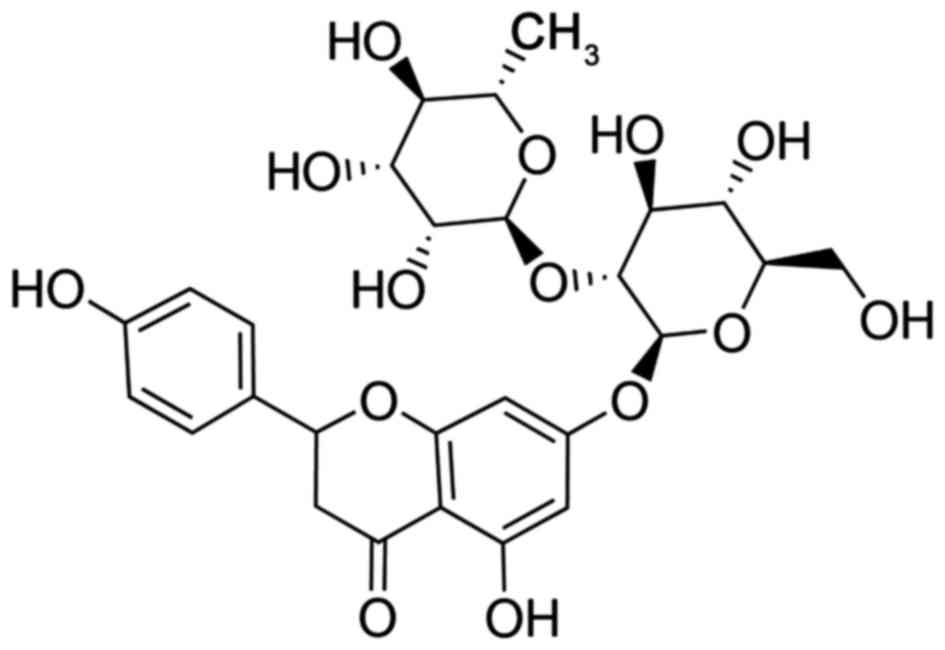
Drynaria (Fig. 1)
is the dried rhizome of Drynaria fortunei (Kunze) J. Sm.,
which was described in ‘Herbal Supplements’ of the Tang Dynasty as
bitter and warm, with the effects of strengthening bones and
reinforcing kidney and liver function, alleviating injury and
providing analgesia (12).
Drynaria is a type of Chinese herbal medicine used to promote
fracture healing, and is commonly prescribed for the treatment of
osteoporosis (13). Drynaria
contains sterols, triterpenes, phenolic acids and a variety of
flavonoids, and a previous study demonstrated that drynaria water
extract and flavonoids may effectively inhibit bone loss and
prevent osteoporosis (14).
Naringin is the most abundant flavonoid in drynaria, with a range
of biological activities (14). A
previous study indicated that naringin may promote proliferation
and osteogenic differentiation in human bone marrow mesenchymal
stem cells, which serves a role in the proliferation of osteoblasts
(15). The present study examined
the role of naringin in steroid-induced avascular necrosis of the
femoral head (SANFH) with the aim of elucidating the potential
modulatory mechanisms.
Materials and methods
Experimental animal and
instruments
The Ethics Committee of Zhejiang Provincial Hospital
of TCM (Hangzhou, China) approved the present study. A total of 30
male healthy adult Japanese white rabbits (5 months old; 2.5±0.5
kg) were provided by the Animal Breeding Center of Shantou
University Medical College (Shantou, China) and were kept in a
temperature-(21±2°C) and humidity-(55±10%) controlled room on a
12-h light/dark cycle. The rabbits were fed with a standard diet
with free access to food and water, and housed individually in
cages.
Establishment of the experimental
animal model and grouping
All the rabbits were randomly divided into 5 groups:
Control group (n=6); model group (n=6); 5 mg/kg naringin group
(n=6); 10 mg/kg naringin group (n=6) and 20 mg/kg naringin group
(n=6). A total of three injections of methylprednisolone (MPS; 20
mg/kg body weight; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
were administered intramuscularly at 24 h intervals into rabbits to
establish the SANFH model. Doses of 5, 10 and 20 mg/kg naringin
(Sigma-Aldrich; Merck KGaA) were administered intramuscularly into
SANFH model rabbits every week for 8 weeks. In the control group,
rats were administered intramuscularly with equal volume of
vehicle.
Determination of biological
indicator
Femoral head tissue was fixed using
5%-paraformaldehyde for 24 h at room temperature and embedded into
paraffin. Then, samples were cut into 0.5 µM sections, stained with
hematoxylin and eosin at 37°C for 15 min and observed using a light
microscope. Osteonecrosis was identified by the necrosis of the
medullary hematopoietic cells in osteocytes. Osteonecrosis
incidence rate was calculated using the amount of
osteonecrosis/total amount. SANFH tissue was washed and homogenized
using a radioimmunoprecipitation assay (RIPA) buffer (Beyotime
Institute of Biotechnology, Haimen, China) on ice for 1 h.
Following centrifugation at 12,000 × g for 10 min at 4°C, the
supernatant was collected to analyze the protein content using a
bicinchoninic acid (BCA) assay. The caspase-3 activity was detected
with a Caspase-3 Colorimetric Assay kit (cat. no. C1115; Beyotime
Institute of Biotechnology), and osteocalcin (cat. no. H152), total
cholesterol (cat. no. F002-1), the ratio of low-density lipoprotein
to high-density lipoprotein (LDL/HDL ratio; cat. no. A113-1/A112-1)
and alkaline phosphatase (ALP; cat. no. A059-2) activity was
detected using Colorimetric Assay kits (Nanjing Jiancheng
Bioengineering Institute, Nanjing, China). Caspase-3 activity was
expressed in terms of absorbance units [optical density (OD) 405
nm], and osteocalcin, total cholesterol, LDL/HDL ratio and ALP
activity was expressed in terms of absorbance units (OD 450 nm)
using an ELISA reader (Beckman Coulter, Inc., Brea, CA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA from the SANFH tissue was isolated using
TRIzol® Reagent (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
Total RNA (500 ng) was synthesized into cDNA using an RT kit,
according to the manufacturer's protocol (Fermentas; Thermo Fisher
Scientific, Inc.). The 7500 Real-Time PCR system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) was used to perform the
qPCR, using Power SYBR-Green PCR Master mix (Bio-Rad Laboratories,
Inc.). The PCR program involved 40 cycles of 94°C for 15 sec, an
annealing step at 60°C for 30 sec, and 72°C for 30 sec. Primers
used were: RUNX2, 5′-AAGTGCGGTGCAAACTTTCT-3′ and
5′-TCTCGGTGGCTGCTAGTGA-3′, OSX, 5′-GCCAGAAGCTGTGAAACCTC-3′ and
5′-GCTGCAAGCTCTCCATAACC-30), and β-actin, 5′-TCCTAGCACCATGAAGATC-3′
and 5′AAA CGC AGC TCA GTA ACA G-3′ as control. miRNA expression was
quantified using the 2−ΔΔCq method (16).
Western blot analysis
SANFH tissue was washed and homogenized using RIPA
buffer on ice for 1 h. Following centrifugation at 12,000 × g for
10 min at 4°C, the supernatant was obtained to analyze the protein
content using a BCA assay. Proteins in the lysates (50 µg) were
subsequently separated via 8–12% SDS-PAGE l and electrophoretically
transferred onto polyvinylidene difluoride membranes (Bio-Rad
Laboratories, Inc.). The membranes were blocked with 5% w/v non-fat
dried milk in TBS/Tween-20 for 1 h at 37°C and incubated with
anti-collagen I (cat. no. sc-25974; 1:500), anti-PPARγ (cat. no.
sc-9000; 1:500), anti-Notch (cat. no. sc-9170; 1:500),
anti-β-catenin (cat. no. sc-16743-R; 1:500), anti-phosphorylated
(p-)Rac-α serine/threonine protein kinase (p-AKT; cat. no.
sc-16646-R; 1:500) and anti-GAPDH (cat. no. sc-25778; 1:2,000; all
from Santa Cruz Biotechnology, Inc., Dallas, TX, USA), overnight at
4°C. Membranes were washed with TBS/Tween-20 three times and
incubated with anti-rabbit horseradish peroxidase-conjugated
secondary polyclonal antibody (cat. no. sc-2004; 1:5,000; Santa
Cruz Biotechnology, Inc.) at room temperature for 1 h. Positive
antibody interactions were visualized using an enhanced
chemiluminescence-plus kit (Thermo Fisher Scientific Inc.) and
quantified using densitometry and Quantity One software version 3.0
(Bio-Rad Laboratories, Inc.).
Statistical analysis
Quantitative data are expressed as the mean ±
standard deviation of 3 independent experiments. Statistical
analysis was performed using SPSS software version 17.0 (SPSS,
Inc., Chicago, IL, USA). Data were analyzed using one-way analysis
of variance followed by Tukey's post test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Protective effects of naringin on
steroid-induced serum osteocalcin level and osteonecrosis incidence
rate
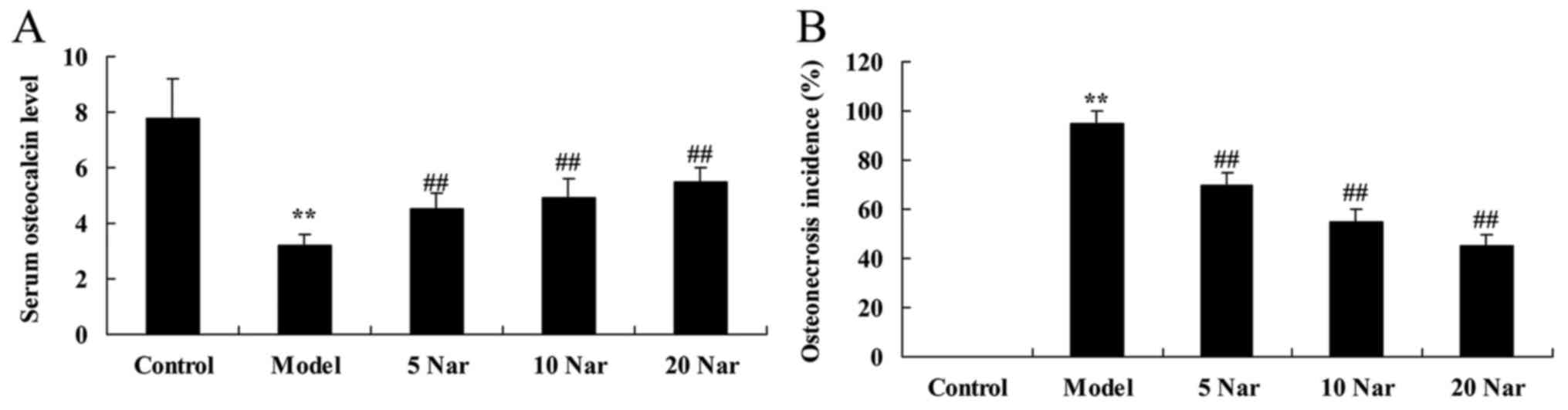
In the SANFH model group, a significant inhibition
of serum osteocalcin was observed, compared with the control group
(Fig. 2A). There was a significant
increase in osteonecrosis incidence in the SANFH model group,
compared with the control group (Fig.
2B). Treatment with naringin increased the serum osteocalcin
levels and inhibited osteonecrosis in SANFH rabbits, compared with
the SANFH model group (Fig.
2).
Protective effects of naringin on
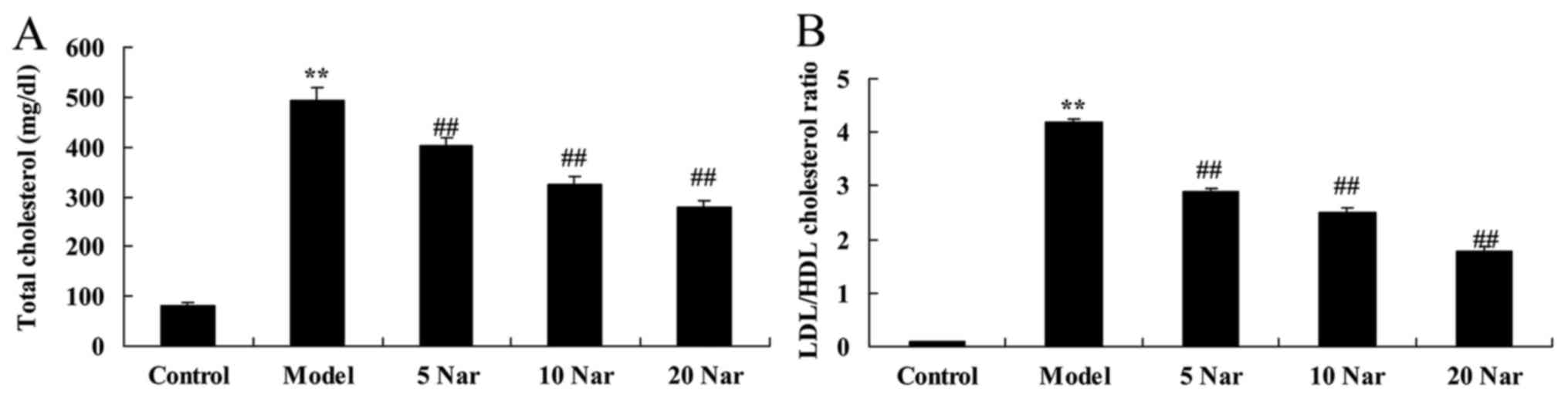
total cholesterol and LDL/HDL cholesterol ratio
The total cholesterol and LDL/HDL cholesterol ratio
of the SANFH model group were increased compared with the control
group (Fig. 3). Treatment with
naringin reversed this increase in total cholesterol and the
LDL/HDL cholesterol ratio in SANFH rabbits, compared with the SANFH
model group (Fig. 3).
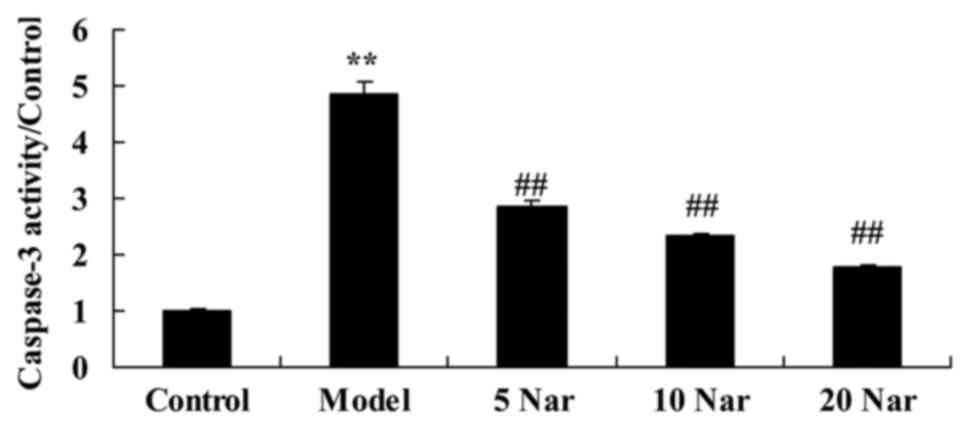
Protective effects of naringin on
caspase-3 activity
Compared with the control group, there was a
significant increase in caspase-3 activity in the SANFH rabbit
model group (Fig. 4). Treatment
with naringin significantly suppressed caspase-3 activity in SANFH
rabbits, compared with the SANFH model group (Fig. 4).
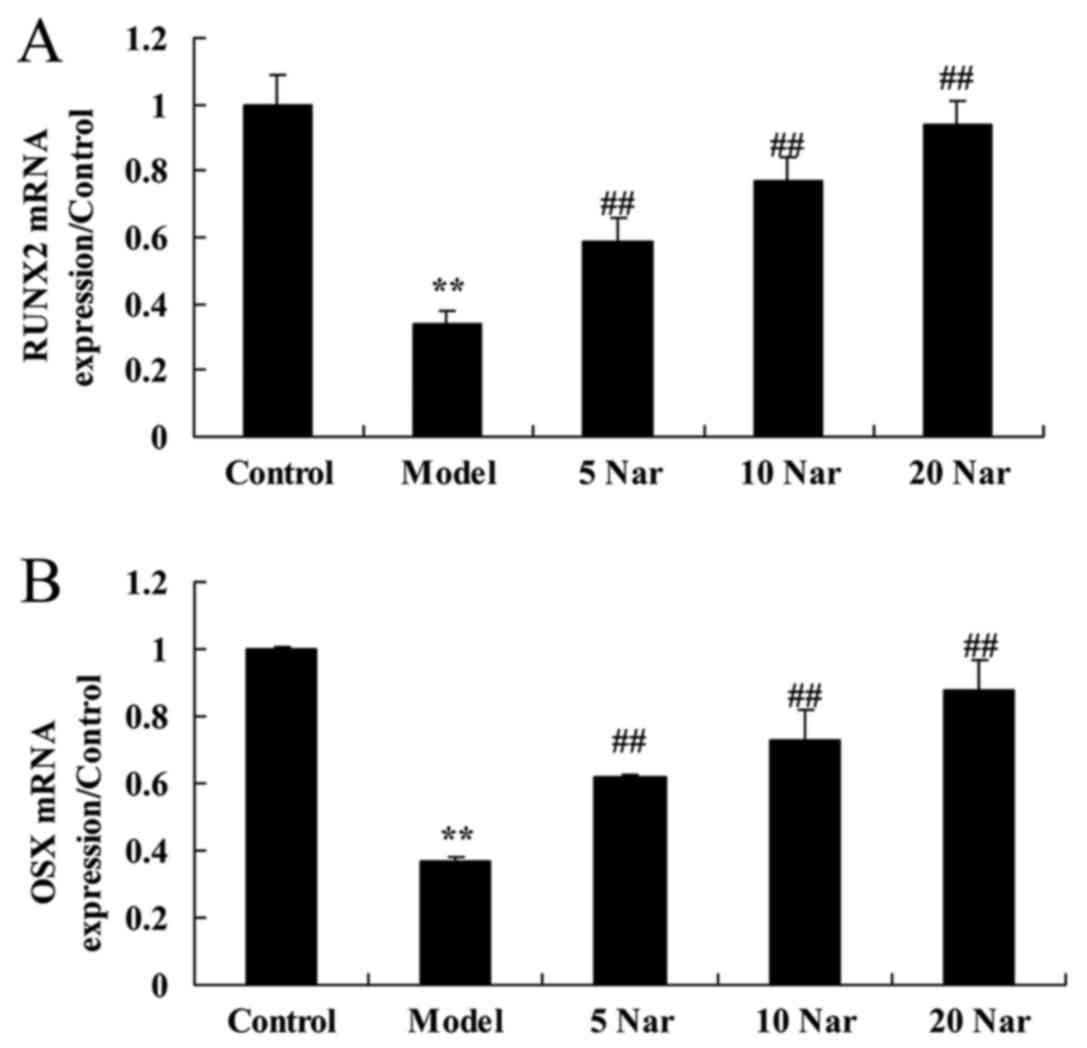
Protective effects of naringin on
runt-related transcription factor 2 (RUNX2) and transcription
factor sp7 (OSX) mRNA expression
RUNX2 and OSX mRNA expression in the SANFH rabbit
model group were significantly decreased compared with the control
group (Fig. 5). The decrease of
RUNX2 and OSX mRNA expression in the SANFH rabbit was significantly
reversed by treatment with naringin, compared with the SANFH model
group (Fig. 5).
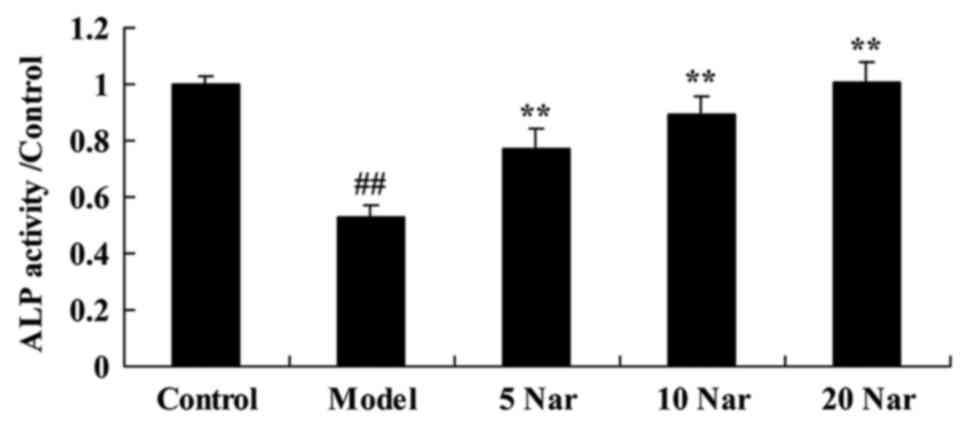
Protective effects of naringin on ALP
activity
It was observed that ALP activity was significantly
decreased in the SANFH rabbit model group, compared with the
control group (Fig. 6). Treatment
with naringin significantly improved ALP activity in the SANFH
rabbit, compared with the SANFH model group (Fig. 6).
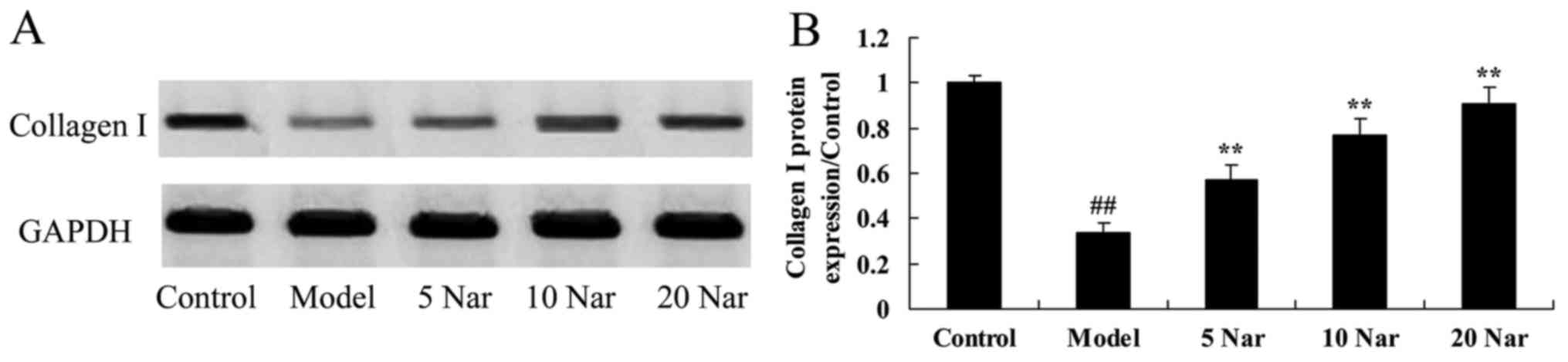
Protective effects of naringin on
collagen I protein expression
In addition, it was observed that collagen I protein
expression in the SANFH rabbit model was decreased compared with
the control group (Fig. 7).
Naringin treatment significantly enhanced collagen I protein
expression in the SANFH rabbit, compared with the SANFH model group
(Fig. 7).
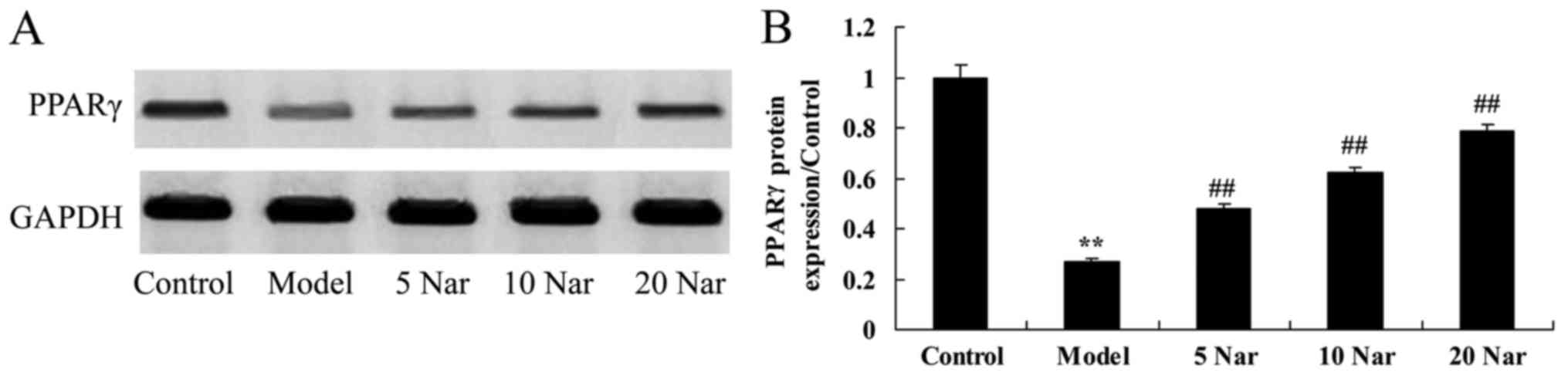
Protective effects of naringin on
PPARγ protein expression
As presented in Fig.
8, PPARγ protein expression in the SANFH rabbit model was
significantly suppressed, compared with the control group. Naringin
significantly promoted PPARγ protein expression in the SANFH
rabbit, compared with the SANFH model group (Fig. 8).
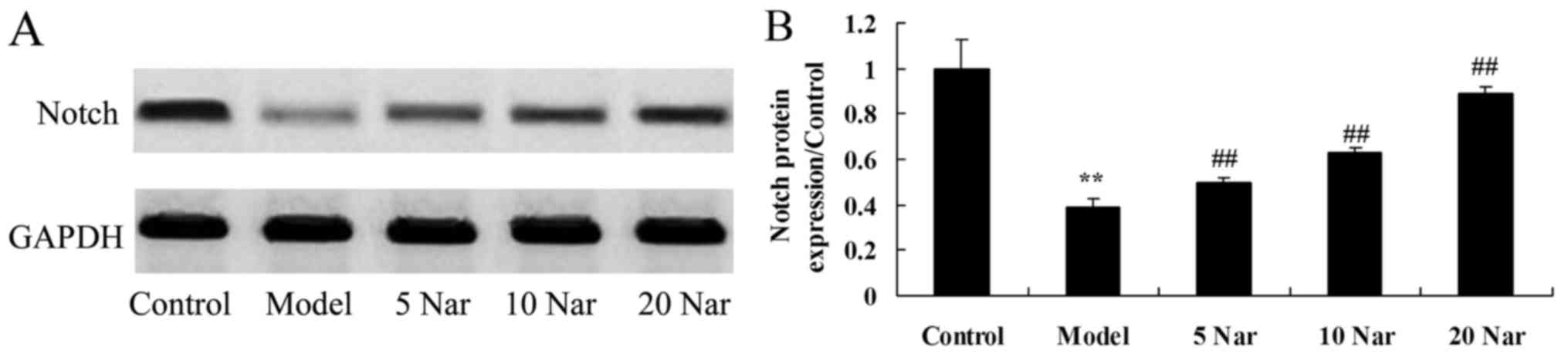
Protective effects of naringin on
Notch protein expression
In the present study, it was demonstrated that the
protein expression of Notch protein in the SANFH rabbit model group
was decreased compared with the control group (Fig. 9). Treatment with naringin
significantly induced Notch protein expression in the SANFH rabbit,
compared with the SANFH model group (Fig. 9).
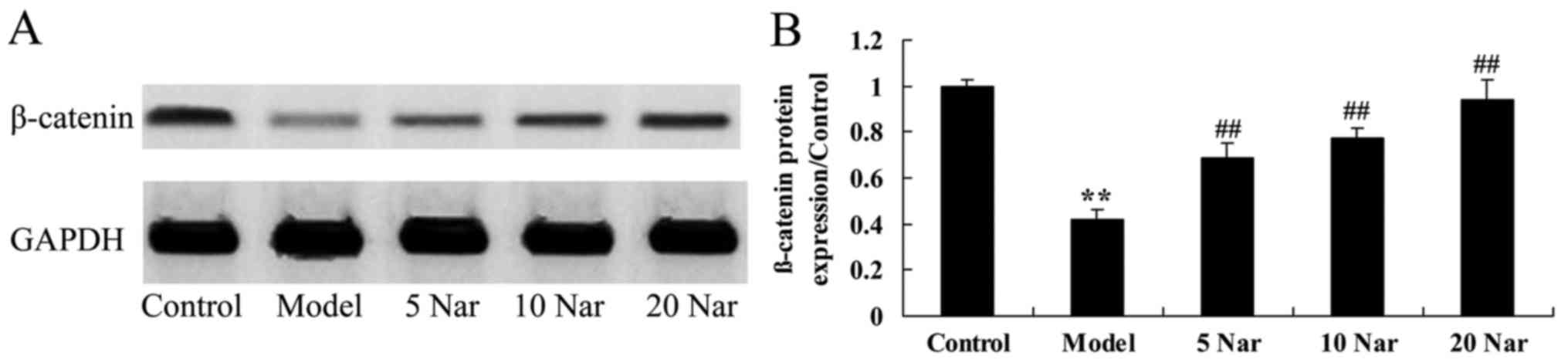
Protective effects of naringin on
β-catenin protein expression
In the SANFH rabbit model group, β-catenin protein
expression was significantly suppressed compared with the control
group (Fig. 10). The inhibition
of β-catenin protein expression in SANFH rabbit was significantly
reversed by naringin, compared with the SANFH model group (Fig. 10).
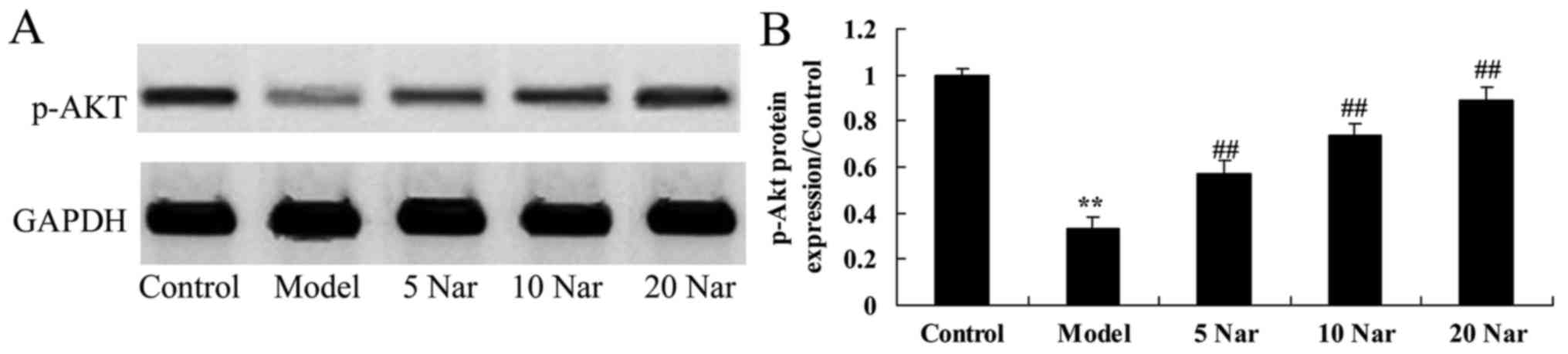
Protective effects of naringin on
p-AKT protein expression
Compared with the control group, p-Akt protein
expression in the SANFH rabbit model group was decreased (Fig. 11). Naringin significantly enhanced
the p-Akt protein expression in the SANFH rabbit, compared with the
SANFH model group (Fig. 11).
Discussion
Clinically, treatment with large doses of hormones
in the long-term is frequently associated with underlying diseases,
including systemic lupus erythematosus, anti-phospholipid antibody
syndrome, rheumatoid arthritis, leukemia and other diseases, all of
which share a common pathological process; namely, that they may
result in vascular endothelial injury and blood alterations,
leading to a hypercoagulable state (17). Therefore, the pathogenesis of SANFH
may be associated with large doses of hormones used to treat the
original vascular disease, which eventually leads to bone necrosis
(18). A previous study
demonstrated that the use of hormones alone is insufficient to
induce the animal model of FH (19). A previous study regarding animal
model establishment demonstrated that a variety of drugs in
combination with hormones results in the ideal animal model of
SANFH (20). The results of the
present study indicated that naringin significantly protected
against steroid-induced increases in serum osteocalcin levels,
osteonecrosis incidence, total cholesterol and LDL/HDL ratio in
SANFH rabbits.
Glucocorticoids are frequently used to treat serious
infectious diseases clinically, including rheumatoid arthritis and
systemic lupus erythematosus (21). However, for patients with long-term
use of corticosteroids, severe osteoporosis and FH may occur
(22). During the in vitro
culture of osteoblasts, dexamethasone exhibits two-way regulation
of osteoblast differentiation and mineralization, which depends on
the maturation stage, the number of cells, and the drug
concentration and time of treatment (23). Therefore, glucocorticoids are an
inhibitor of osteoblast differentiation and maturation in
vitro (24). An in vivo
study into necrosis of the femoral head in steroid-induced FH rats
demonstrated that gene and protein expression of RUNX and OSX is
altered and its association with bone synthesis metabolism
subsequently investigated (24).
The FH rat model was established via gluteal injection of
corticosteroids and forced load exercises, and the results
demonstrated that the gene expression and protein synthesis of RUNX
and OSX decrease over time, indicating that, in the early stages of
SANFH, the gene and protein expression of RUNX and OSX in the rat
femoral head decrease, inhibiting bone formation (25). Additionally, the results of the
present study suggested that naringin significantly increased RUNX2
and OSX mRNA expression and ALP activity in the SANFH rabbit.
Studies have demonstrated that the adipogenesis of
bone marrow stromal cells is additionally characterized by a marked
increase in the expression of PPARγ, consistent with other lipocyte
differentiation models (5,8). The increased expression of PPARγ
directly activates or induces the gene expression profile of the
majority of adipocyte phenotypes, including fatty acid synthase,
glutamic acid 4, acetyl coenzyme A and shuttle enzymes (26). However, the overexpression of PPARγ
in vitro inhibits the formation of fat cells and promotes
the formation of osteoblasts, leading to an increase in trabecular
bone (27). Studies have
demonstrated that when PPARγ genes are selectively knocked out of
fat cells, the function of fat cells is severely degraded, leading
to progressive lipodystrophy, insulin resistance and other
metabolic diseases (27,28). Fat formation is impaired in
PPARγ-knockout mice. In the process of fat formation, PPARγ
requires the involvement of transcription factor ligands in order
to increase in expression, and these ligands include natural
ligands and synthetic ligands (29). ARY is the essential transcription
factor for the differentiation of bone marrow stromal cells into
adipocytes, and serves an important role (5). PPARγ is regulated by a variety of
cytokines in the regulation of adipocyte formation, and these
factors promote increased expression of PPARγ, in order to increase
the differentiation of bone marrow fat cells and result in a
decrease in the differentiation of bone marrow stromal cells to
osteoblasts which eventually results in necrosis of the femoral
head (25). In the present study,
naringin was observed to significantly promote PPARγ protein
expression in the SANFH rabbit model.
However, the mechanisms underlying the effect of
estrogen treatment on postmenopausal osteoporosis, and the
regulation of mesenchymal stem cell osteogenic differentiation,
remain unclear (30). The Notch
signaling pathway is a highly-conserved signaling pathway involved
in cell differentiation, self-renewal and apoptosis (31). At present, research into estrogen
and the Notch signaling pathway has predominantly focused on human
tumors and brain development (32). There are relatively few studies
regarding the effect of estrogen on differentiation and
proliferation via its impact on the Notch signaling pathway, in the
differentiation of human bone marrow mesenchymal stem cells
(33). The results of the present
study demonstrated that naringin significantly suppressed Notch
protein expression and promoted the β-catenin and p-Akt protein
expression in the SANFH rabbit.
In the present study, it was demonstrated that
naringin notably protected against steroid-induced decreased serum
osteocalcin level, and increased osteonecrosis incidence, total
cholesterol and LDL/HDL in the SANFH rabbit. It was additionally
demonstrated that naringin significantly inhibited caspase-3
activity, increased RUNX2 and OSX mRNA expression, promoted ALP
activity and upregulated collagen I protein expression in the SANFH
rabbit, through regulation of PPARγ, Notch, β-catenin and p-AKT
protein expression. The results of the present study suggested that
naringin may be potentially applied in the clinic to achieve
effective treatment of FH.
Acknowledgements
This study was supported by Zhejiang Provincial
Natural Science Foundation of China (LY17H270006).
References
|
1
|
Aoyama T, Goto K, Kakinoki R, Ikeguchi R,
Ueda M, Kasai Y, Maekawa T, Tada H, Teramukai S, Nakamura T and
Toguchida J: An exploratory clinical trial for idiopathic
osteonecrosis of femoral head by cultured autologous multipotent
mesenchymal stromal cells augmented with vascularized bone grafts.
Tissue Eng Part B Rev. 20:233–242. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma Y, Wang T, Liao J, Gu H, Lin X, Jiang
Q, Bulsara MK, Zheng M and Zheng Q: Efficacy of autologous bone
marrow buffy coat grafting combined with core decompression in
patients with avascular necrosis of femoral head: A prospective,
double-blinded, randomized, controlled study. Stem Cell Res Ther.
5:1152014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lubbers M, Coenen A, Bruning T, Galema T,
Akkerhuis J, Krenning B, Musters P, Ouhlous M, Liem A, Niezen A, et
al: Sex differences in the performance of cardiac computed
tomography compared with functional testing in evaluating stable
chest pain: Subanalysis of the multicenter, randomized CRESCENT
trial (calcium imaging and selective CT angiography in comparison
to functional testing for suspected coronary artery disease). Circ
Cardiovasc Imaging. 10:pii: e0052952017. View Article : Google Scholar
|
|
4
|
Pintér Ö, Hardi P, Nagy T, Gasz B, Kovács
V, Arató E, Sínay L, Lénárd L and Jancsó G: The role of GST
polymorphism in reperfusion induced oxidative stress, inflammatory
responses and clinical complications after surgical and
percutaneous coronary intervention. Clin Hemorheol Microcirc.
66:261–272. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang J, Wang M, Li Z, Bi X, Song J, Weng
S and Fu G: NADPH oxidase activation played a critical role in the
oxidative stress process in stable coronary artery disease. Am J
Transl Res. 8:5199–5210. 2016.PubMed/NCBI
|
|
6
|
Prasad K and Dhar I: Oxidative stress as a
mechanism of added sugar-induced cardiovascular disease. Int J
Angiol. 23:217–226. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Riegersperger M, Covic A and Goldsmith D:
Allopurinol, uric acid, and oxidative stress in cardiorenal
disease. Int Urol Nephrol. 43:441–449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turan T, Menteşe Ü, Ağaç MT, Akyüz AR, Kul
S, Aykan AÇ, Bektaş H, Korkmaz L, Öztaş Menteşe S, Dursun İ and
Çelik Ş: The relation between intensity and complexity of coronary
artery lesion and oxidative stress in patients with acute coronary
syndrome. Anatol J Cardiol. 15:795–800. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao C, Hanlon A, Zhang Q, Movsas B, Ang
K, Rosenthal DI, Nguyen-Tan PF, Kim H, Le Q and Bruner DW: Risk
factors for clinician-reported symptom clusters in patients with
advanced head and neck cancer in a phase 3 randomized clinical
trial: RTOG 0129. Cancer. 120:848–854. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Y, Shi LY, Lei YM, Bao YH, Li ZY, Ding
F, Zhu GT, Wang QQ and Huang CX: Radiosensitization effect of
hsa-miR-138-2-3p on human laryngeal cancer stem cells. PeerJ.
5:e32332017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen S, Sun YY, Zhang ZX, Li YH, Xu ZM and
Fu WN: Transcriptional suppression of microRNA-27a contributes to
laryngeal cancer differentiation via GSK-3β-involved Wnt/β-catenin
pathway. Oncotarget. 8:14708–14718. 2017.PubMed/NCBI
|
|
12
|
He Q, Tian L, Jiang H, Zhang J, Li Q, Sun
Y, Zhao J, Li H and Liu M: Identification of laryngeal cancer
prognostic biomarkers using an inflammatory gene-related,
competitive endogenous RNA network. Oncotarget. 8:9525–9534.
2017.PubMed/NCBI
|
|
13
|
Li P, Liu H, Wang Z, He F, Wang H, Shi Z,
Yang A and Ye J: MicroRNAs in laryngeal cancer: Implications for
diagnosis, prognosis and therapy. Am J Transl Res. 8:1935–1944.
2016.PubMed/NCBI
|
|
14
|
Janssens GO, Rademakers SE, Terhaard CH,
Doornaert PA, Bijl HP, van den Ende P, Chin A, Marres HA, de Bree
R, van der Kogel AJ, et al: Accelerated radiotherapy with carbogen
and nicotinamide for laryngeal cancer: Results of a phase III
randomized trial. J Clin Oncol. 30:1777–1783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geng J, Liu Y, Jin Y, Tai J, Zhang J, Xiao
X, Chu P, Yu Y, Wang SC, Lu J, et al: MicroRNA-365a-3p promotes
tumor growth and metastasis in laryngeal squamous cell carcinoma.
Oncol Rep. 35:2017–2026. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu J, Wang L, Akinyi M, Li Y, Duan Z, Zhu
Y and Fan G: Danshensu protects isolated heart against ischemia
reperfusion injury through activation of Akt/ERK1/2/Nrf2 signaling.
Int J Clin Exp Med. 8:14793–14804. 2015.PubMed/NCBI
|
|
18
|
Zhong Y, Cheng CF, Luo YZ, Tian CW, Yang
H, Liu BR, Chen MS, Chen YF and Liu SM: C-reactive protein
stimulates RAGE expression in human coronary artery endothelial
cells in vitro via ROS generation and ERK/NF-κB activation. Acta
Pharmacol Sin. 36:440–447. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang J, Zeini M, Lin CY, Lin CJ, Xiong Y,
Shang C, Han P, Li W, Quertermous T, Zhou B and Chang CP:
Epicardial calcineurin-NFAT signals through Smad2 to direct
coronary smooth muscle cell and arterial wall development.
Cardiovasc Res. 101:120–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Crobu F, Palumbo L, Franco E, Bergerone S,
Carturan S, Guarrera S, Frea S, Trevi G, Piazza A and Matullo G:
Role of TGF-beta1 haplotypes in the occurrence of myocardial
infarction in young Italian patients. BMC Med Genet. 9:132008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakata Y, Ijichi K, Hanai N, Nishikawa D,
Suzuki H, Hirakawa H, Kodaira T, Fujimoto Y, Fujii T, Miyazaki T,
et al: Treatment results of alternating chemoradiotherapy with
early assessment for advanced laryngeal cancer: A
multi-institutional phase II study. Auris Nasus Larynx. 44:104–110.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weng M, Ding M, Xu Y, Yang X, Li L, Zhong
J and Miao C: An evaluation of thyromental distance-based method or
weight-based method in determining the size of the laryngeal mask
airway supreme: A randomized controlled study. Medicine
(Baltimore). 95:e29022016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang J, Kuai X, Song M, Chen X, Yu Z,
Zhang H and Mao Z: microRNA-32 inhibits the proliferation and
invasion of the SGC-7901 gastric cancer cell line in vitro. Oncol
Lett. 7:270–274. 2014.PubMed/NCBI
|
|
24
|
Andreadis C, Iliopoulou C, Sidiras T,
Boutis A, Diamantopoulos N, Vahtsevanos K, Gennatas K and
Mouratidou D: Neoadjuvant chemotherapy followed by radiotherapy
versus concurrent chemoradiotherapy for larynx preservation in
patients with advanced laryngeal cancer. J BUON. 12:341–347.
2007.PubMed/NCBI
|
|
25
|
Saito I, Azuma K, Kakikawa T, Oshima N,
Hanson ME and Tershakovec AM: A randomized, double-blind,
placebo-controlled study of the effect of ezetimibe on glucose
metabolism in subjects with type 2 diabetes mellitus and
hypercholesterolemia. Lipids Health Dis. 14:402015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tuomi L, Andréll P and Finizia C: Effects
of voice rehabilitation after radiation therapy for laryngeal
cancer: A randomized controlled study. Int J Radiat Oncol Biol
Phys. 89:964–972. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang H, Jiang Y, Nguyen HD, Poo DC and
Wang W: The effect of a smartphone-based coronary heart disease
prevention (SBCHDP) programme on awareness and knowledge of CHD,
stress, and cardiac-related lifestyle behaviours among the working
population in Singapore: A pilot randomised controlled trial.
Health Qual Life Outcomes. 15:492017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salfati E, Nandkeolyar S, Fortmann SP,
Sidney S, Hlatky MA, Quertermous T, Go AS, Iribarren C, Herrington
DM, Goldstein BA and Assimes TL: Susceptibility loci for clinical
coronary artery disease and subclinical coronary atherosclerosis
throughout the life-course. Circ Cardiovasc Genet. 8:803–811. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ammenwerth E, Woess S, Baumgartner C, Fetz
B, van der Heidt A, Kastner P, Modre-Osprian R, Welte S and Poelzl
G: Evaluation of an integrated telemonitoring surveillance system
in patients with coronary heart disease. Methods Inf Med.
54:388–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kapral M, Strzalka B, Kowalczyk M, Jurzak
M, Mazurek U, Gierek T, Paluch J, Markowski J, Swiatkowska L and
Weglarz L: Transforming growth factor beta isoforms (TGF-beta1,
TGF-beta2, TGF-beta3) messenger RNA expression in laryngeal cancer.
Am J Otolaryngol. 29:233–237. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park SJ, Jin ML, An HK, Kim KS, Ko MJ, Kim
CM, Choi YW and Lee YC: Emodin induces neurite outgrowth through
PI3K/Akt/GSK-3β-mediated signaling pathways in Neuro2a cells.
Neurosci Lett. 588:101–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li H, Yang T, Zhou H, Du J, Zhu B and Sun
Z: Emodin combined with nanosilver inhibited sepsis by
anti-inflammatory protection. Front Pharmacol. 7:5362017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang YQ, Huang GR, Wu MH, Tang HY, Huang
ZS, Zhou XH, Yu WQ, Su JW, Mo XQ, Chen BP, et al: Inhibitory
effects of emodin, baicalin, schizandrin and berberine on hefA
gene: Treatment of Helicobacter pylori-induced multidrug
resistance. World J Gastroenterol. 21:4225–4231. 2015. View Article : Google Scholar : PubMed/NCBI
|