Introduction
Myocardial ischemia refers to the heart with blood
perfusion reduction, which occurs when the balance of myocardial
blood supply and demand is disturbed (1), resulting in abnormal metabolism of
oxygen and energy, and the abnormal pathological state of the heart
(2). The definition of myocardial
ischemia/reperfusion (I/R) injury is that the ischemic myocardium
restores blood flow following reperfusion, which affects the
prognosis of patients with myocardial infarction (3). This is followed by the disordered
synthesis of mitochondrial energy and Ca2+ homeostasis,
release of free radicals and inflammatory cytokines, and eventually
leads to myocardial cell apoptosis and organ damage (4,5).
Myocardial I/R injury, which represents a major cause of morbidity
and mortality in humans with coronary heart disease, has complex
molecular mechanisms (6,7). Furthermore, the molecular mechanisms
that regulates gene expression during myocardial I/R are not
completely understood.
Icariin (ICA) is a flavonoid extracted from
Epimedium brevicornum, a genus of flowering plants in the
family, Berberidaceae (8),
which is used in Traditional Chinese Herbal Medicine, and possesses
multiple pharmaceutical and biological activities, such as
immunoregulation (9),
antioxidation (10), anti-tumor
activity (11), neuroprotection
(12) and improves sexual function
(8). Icariin attenuates cerebral
I/R injury via inhibition of inflammatory responses mediated by
nuclear factor (NF)-κB, peroxisome proliferator-activated receptor
(PPAR)α and PPARγ in rats (13).
Furthermore, post-conditioning with icariin exerts cardioprotective
effects against myocardial I/R injury by activating the
phosphoinositide 3-kinase (PI3K)/Akt signaling pathway (14,15).
Given the cardioprotective role and anti-I/R effect of ICA, the
current study hypothesized that ICA may present as a novel
therapeutic agent for the treatment of myocardial I/R.
Increasing evidences supports a pivotal role for the
small heat shock protein (HSP) family in multiple processes
(16), including tumorigenesis
(17), cardioprotection (18), resistant to oxidative stress
(19) and apoptosis (20). HSP20 is the best characterized
small HSP compared with other small HSPs and is predominantly
upregulated in animal hearts with ischemic conditions (21). Previous studies demonstrated that
HSP20 protected hearts against cardiac myocyte apoptosis, induced
by I/R injury in vivo and in vivo (22,23).
Knockdown of endogenous miR-320 provided protection against
I/R-induced cardiomyocyte apoptosis by targeting HSP20 (24). However, the potential benefits of
HSP20 action on ICA-induced cardiac protection and its underlying
mechanism(s) remain largely unknown.
The present study was designed to further determine
the cardioprotective effect of ICA on myocardial I/R injury and the
molecular mechanism underlying HSP20.
Materials and methods
Cell culture
H9C2 cells (Institute of Biochemistry and Cell
Biology, Shanghai, China) were cultured in Dulbecco's modified
Eagle's medium (DMEM) containing 10% fetal calf serum (Invitrogen;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1% 100X
mycillin (Invitrogen; Thermo Fisher Scientific, Inc.), and
incubated in 5% CO2 at 37°C overnight. Cells were
digested and seeded into 96-well plates (3×103
cells/well). The I/R group was transferred into sugar and
serum-free DMEM and incubated in 5% CO2 and 95%
N2 at 37°C for 2 h, then transferred into normal DMEM
and incubated in 5% CO2 at 37°C for 6 h. The control
group was incubated in normal DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.) for 8 h at 37°C. In the drug-treated groups, ICA
(Xian Xiao Cao Botanical Development Co., Ltd., Xian, China) at
various concentrations (5, 10 and 15 µmol/l) was administered as a
component of the perfusion medium 10 min before ischemia and every
8 h throughout reperfusion.
Construction of recombinant
adenoviruses
The HSP20 coding sequence (commercially unavailable;
Sangon Biotech Co., Ltd., Shanghai, China) was cloned into the
pAVsi 1.1 adenovirus vector and black pAVsi 1.1 adenovirus vector
(both Sangon Biotech Co., Ltd.) served as a negative control. To
generate a high-titer adenovirus, vectors encoding the HSP20 and
the packaging plasmids were cotransfected into 293T cells
(Institute of Biochemistry and Cell Biology) using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. The packaging, purification and
titration of adenovirus were performed as previously described
(25). Following transfection (at
48 h), the cell culture medium containing viral particles was
collected via centrifugation at 1,000 × g for 5 min at 4°C.
Cell proliferation assay
H9C2 cells (1×103 cells/well) were plated
in 96-well plates. Following ICA treatment for 48 h, 10% Cell
Counting-kit 8 (CCK-8; CK04; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan) diluted in DMEM was mixed in each well for another
1 h. The absorption of each sample was measured at a wavelength of
450 nm using a Labsystems MK3 microplate reader (Thermo Fisher
Scientific, Inc.) to detect cell viability according to the
manufacturer's instruction.
Cell apoptosis assay
Following transfection, H9C2 cells were detached
using 0.25% trypsin and washed with 10% phosphate-buffered saline
(PBS), followed by the centrifugation at 1,000 × g for 5 min at
37°C. Then, the cells were incubated with 10 µl Annexin
V-fluorescein isothiocyanate (FITC) and 5 µl propidium iodide (PI)
in the dark for 15 min at 4°C. Cell apoptotic rate was measured by
Annexin V-FITC Apoptosis Detection kit (Beyotime Biotechnology,
Shanghai, China) and the data was obtained using flow cytometer (BD
Accuri C6 software version 1.0.264.21; BD Biosciences, Franklin
Lakes, NJ, USA).
Intracellular reactive oxygen species
(ROS) assay
The intracellular ROS content was determined using a
fluorescent probe, 2′,7′dichlorodihydrofluorescein-diacetate
(DCFH-DA) followed by flow cytometry. After transfection, H9C2
cells were incubated with 10 µM DCFH-DA at 37°C for 20 min in the
dark. Then, the plates were washed three times with PBS. The
fluorescent probe DCFH-DA (Thermo Fisher Scientific, Inc.), which
detected ROS production, was observed using a flow cytometer (BD
Accuri C6 software version 1.0.264.21; BD Biosciences).
mRNA quantification by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from H9C2 cells using the
TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) method,
depurated with an RNAeasy kit (Invitrogen; Thermo Fisher
Scientific, Inc.) and reversed to cDNA using the Prime-Script RT
reagent kit (Takara Bio, Inc., Otsu, Japan). qPCR was performed
using an ABI 7500 (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using SYBR Premix Ex Taq (Takara Bio, Inc.). The following
primers were used: Sense, 5′-CTGTTTTGGTGAGGGGAAGG-3′ and antisense,
5′-CTGGGGAGAAATGGGATACG-3′ for HSP20; sense,
5′-GTCGGTGTGAACGGATTTG-3′ and antisense, 5′-TCCCATTCTCAGCCTTGAC-3′
for GAPDH. The HSP20 mRNA level was normalized against internal
GAPDH mRNA. The relative quantification values for gene expression
levels were calculated using 2−ΔΔCq method (26).
Western blot analysis
Upon termination of treatment, H9C2 cells were
harvested and resuspended in ice-cold cell lysis solution and the
homogenate was centrifuged at 400 × g for 15 min at 4°C. A
bicinchoninic acid protein quantification kit (cat. no. 23225;
Thermo Fisher Scientific, Inc.) was used to quantify the protein
contents. Then, 30 µg protein was run on 12% SDS-PAGE gel and
transferred to a nitrocellulose filter membrane (Merck KGaA,
Darmstadt, Germany) electrophoretically. Blots were blocked with 5%
skimmed milk at room temperature for 1 h, followed by incubation
with anti-B-cell lymphoma 2 (Bcl-2; cat. no. sc-492; 1:150; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA), caspase-3 (cat. no.
ab2302; 1:200; Abcam, Cambridge, MA, USA), caspase-9 (cat. no.
ab2013; 1:1,000; Abcam), cytochrome complex (Cyt-c; cat. no.
ab13575; 1:1,000; Abcam), apoptotic protease activating factor 1
(APAF1; cat. no. 8969; 1:1,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA), Akt (cat. no. 2920; 1:2,000; Cell Signaling
Technology, Inc.), p-AKT (cat. no. 4060; 1:2,000; Cell Signaling
Technology, Inc.) and GAPDH (cat. no. 5174; 1:1,500; Cell Signaling
Technology, Inc.) antibodies overnight at 4°C, and incubated with
horse radish peroxidase-conjugated goat anti-mouse or anti-rabbit
secondary antibody (cat nos. A0208 and A0216, respectively;
1:1,000; Beyotime Institute of Biotechnology, Shanghai, China) for
1 h at 37°C. Enhanced chemiluminescence (Thermo Scientific,
Shanghai, China) was used to detect the blots visually and signals
were quantified by densitometry (Quantity One software version
4.62; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Experiments were performed in triplicate and data
were expressed as the mean ± standard deviation of the mean.
Statistical significance was determined by unpaired two-tailed
t-test and one-way analysis of variance followed by Tukey's post
hoc test. Statistical analyses were performed using GraphPad Prism
5 software (GraphPad Software, Inc., La Jolla, CA, USA) and
P<0.05 was considered to indicate a statistically significant
difference.
Results
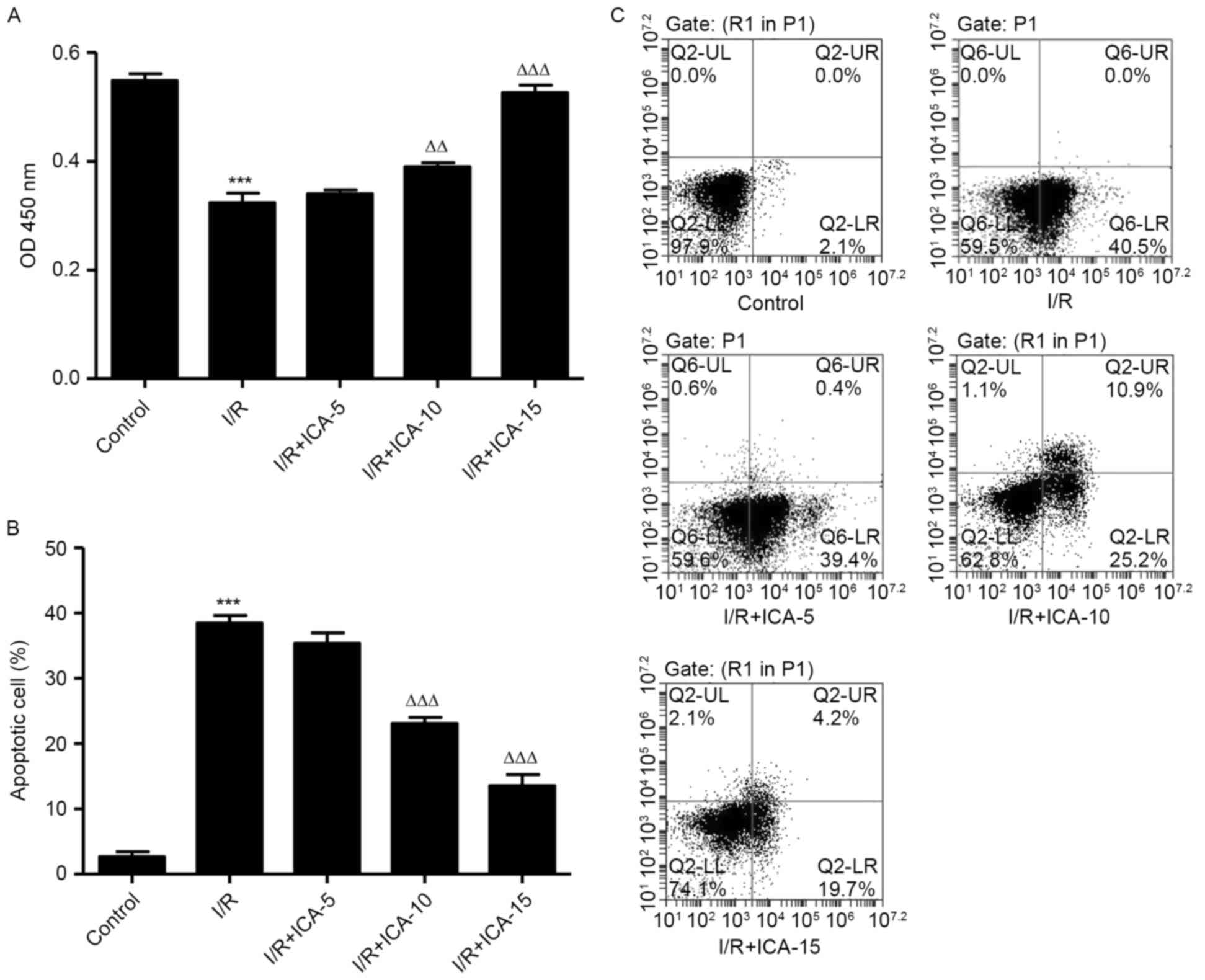
ICA improves cell viability and
inhibits cell apoptosis in H9C2 cells
In order to investigate the possible mechanisms
involved in the protective effect of ICA on cardiac cells against
I/R injury, an in vitro study was performed using H9C2
cells. CCK-8 assay was performed to evaluate the cell viability of
H9C2 cells following I/R. Compared with the I/R group, cell
proliferation in the ICA (10 and 15 µmol/l)-treated groups
increased in a dose-dependent manner (Fig. 1A), indicating the improved cell
viability of H9C2 cells following treatment with ICA. Cell
apoptosis induced by I/R was visually detected using Annexin-V
FITC/PI double staining. It was demonstrated that the apoptotic
cells of ICA (10 and 15 µmol/l)-treated H9C2 cells reduced in a
dose-dependent manner when compared with that of the I/R group
(Fig. 1B and C), revealing the
inhibitory effect of ICA against I/R-induced apoptosis.
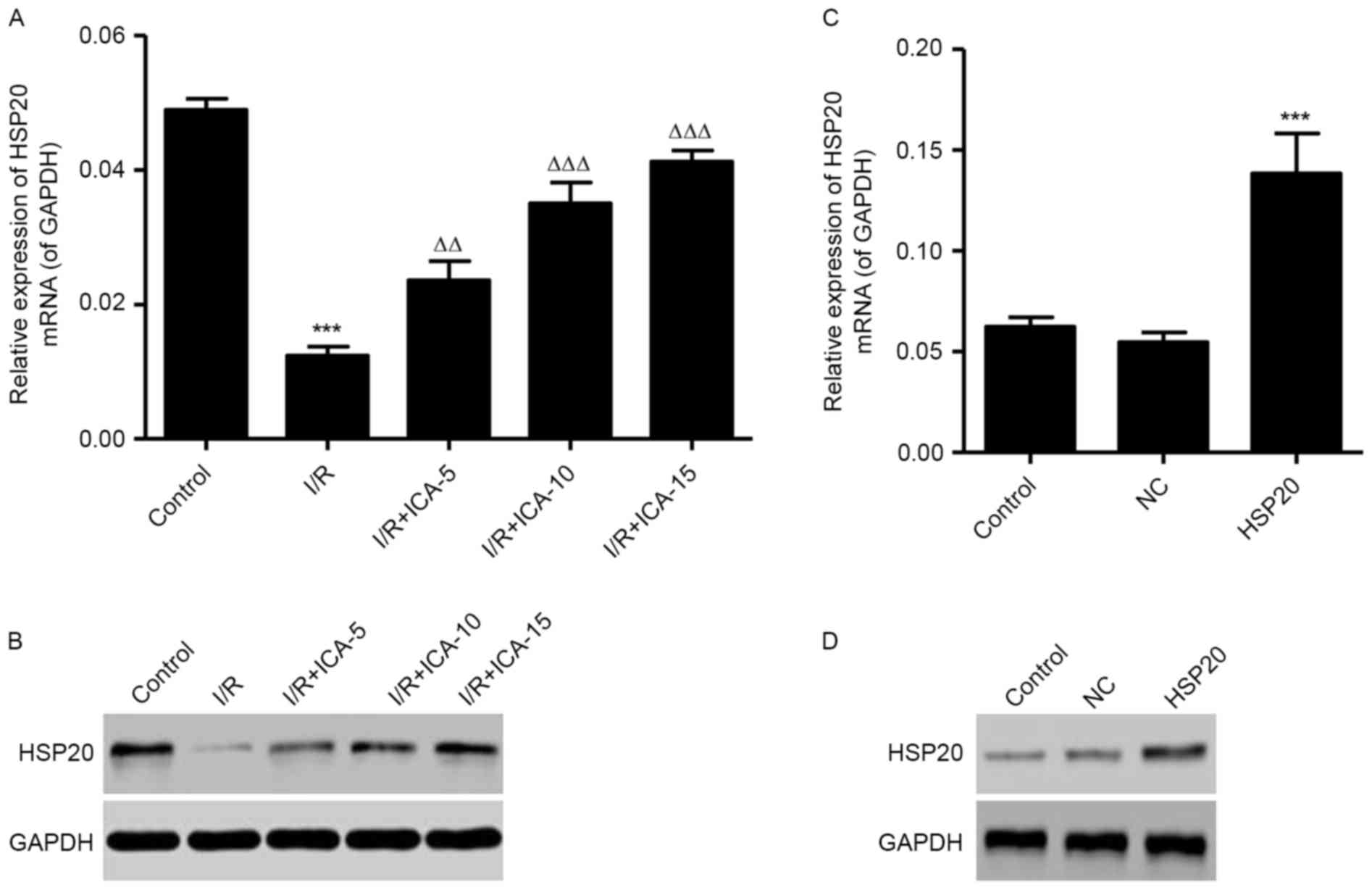
Effect of ICA on HSP20 expression
levels
To clarify the effect of I/R on HSP20 expression
levels in vitro, RT-qPCR and western blot analysis were
performed. As shown in Fig. 2A,
the mRNA expression level of HSP20 was significantly decreased in
the I/R model. Furthermore, the mRNA expression level of HSP20 was
dose-dependently increased by ICA treatment (5, 10 and 15 µmol/l).
Similarly, the protein expression levels of HSP20 were also
decreased following I/R, in which ICA dose-dependently increased
HSP20 levels were observed in the I/R induced H9C2 cells (Fig. 2B). HSP20 overexpression
significantly increased the expression level of HSP20 in
I/R-induced H9C2 cells at them RNA and protein expression levels
(Fig. 2C and D).
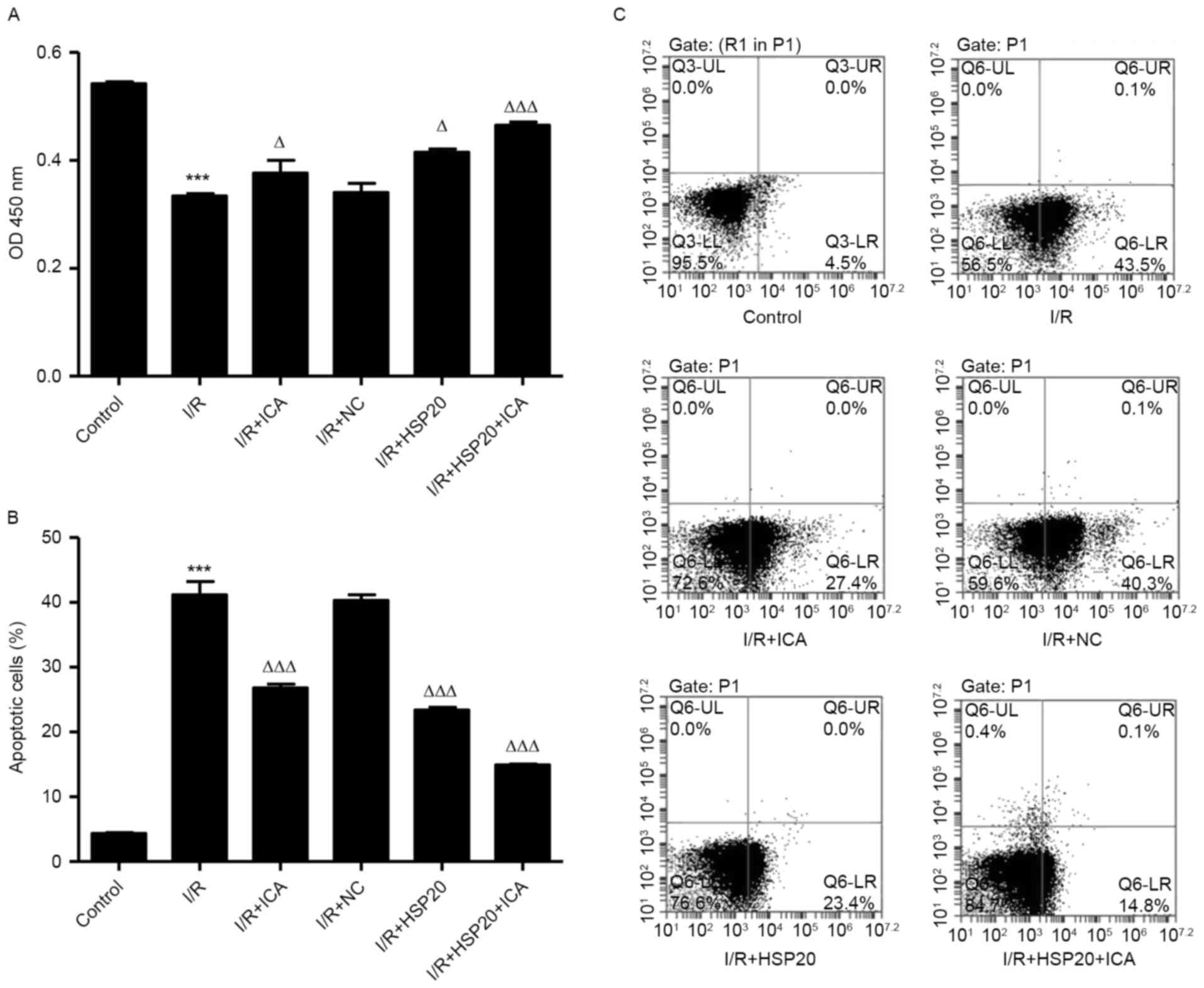
HSP20 overexpression promotes
proliferation and inhibits I/R-induced apoptosis in H9C2 cells
The effects of I/R on H9C2 cell proliferation and
apoptosis were measured by CCK-8 and flow cytometry, respectively.
As shown in Fig. 3A-C, I/R
significantly inhibited cell proliferation and increased apoptosis
in H9C2 cells compared with the control cells. Notably, HSP20
overexpression and/or ICA (10 µmol/l) treatment significantly
increased cell proliferation and decreased apoptosis in H9C2 cells
compared with the I/R group. These data indicate that ICA promotes
cell proliferation and inhibits cell apoptosis of I/R-induced H9C2
cells by upregulating HSP20.
HSP20 overexpression inhibits
I/R-induced ROS production in H9C2 cells
The effect of I/R on ROS production in H9C2 cells
was measured by flow cytometry. As exhibited in Fig. 4, I/R significantly increased ROS
production in H9C2 cells compared with the control cells. Notably,
HSP20 overexpression and/or ICA (10 µmol/l) treatment significantly
decreased ROS production in H9C2 cells compared with the I/R group.
These data demonstrate that ICA decreased ROS production in
I/R-induced H9C2 cells by upregulating HSP20.
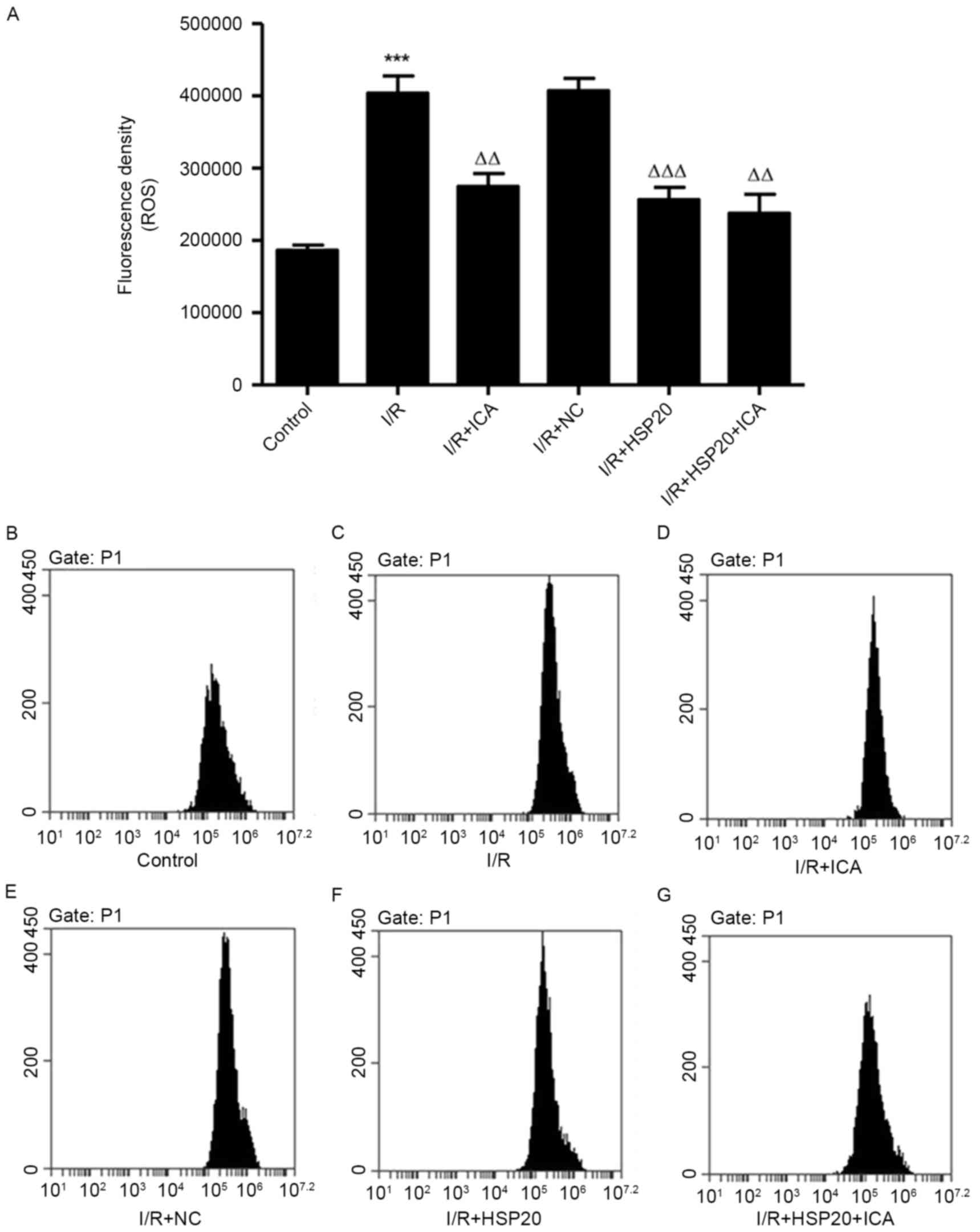 | Figure 4.Effects of HSP20 overexpression on
I/R-induced ROS production in H9C2 cells. (A) ROS production was
evaluated by flow cytometry in H9C2 cells with different
treatments, including (B) control, (C) I/R, (D) I/R+ICA, (E)
I/R+NC, (F) I/R+HSP20 and (G) I/R+HSP20+ICA. Data are presented as
the mean ± standard deviation. ***P<0.001 vs. control;
ΔΔP<0.01, ΔΔΔP<0.001 vs. I/R. HSP, heat
shock protein; I/R, ischemia/reperfusion; ROS, reactive oxygen
species; NC, negative control; ICA, icariin. |
Western blot analysis evaluated the
protective mechanisms related proteins in I/R-induced H9C2
cells
The effects of I/R on protein expression levels in
H9C2 cells were analyzed by western blotting. As presented in
Fig. 5A and B, I/R significantly
decreased the expression level of p-AKT and Bcl-2 in H9C2 cells
when compared with the control cells. HSP20 overexpression and/or
ICA (10 µmol/l) treatment significantly increased the expression
level of p-AKT and Bcl-2 in the H9C2 cells as compared with the I/R
group. In addition, I/R significantly increased the expression
levels of Cyt-c, APAF1, caspase-9 and caspase-3 in H9C2 cells
compared with the control (Fig. 5A, C
and D). Notably, HSP20 overexpression and/or ICA (10 µmol/l)
treatment significantly decreased the expression levels of Cyt-c,
APAF1, caspase-9 and caspase-3 in H9C2 cells compared with the I/R
group. These data indicate that ICA inhibits apoptosis-associated
protein expression levels in I/R-induced H9C2 cells by upregulating
HSP20.
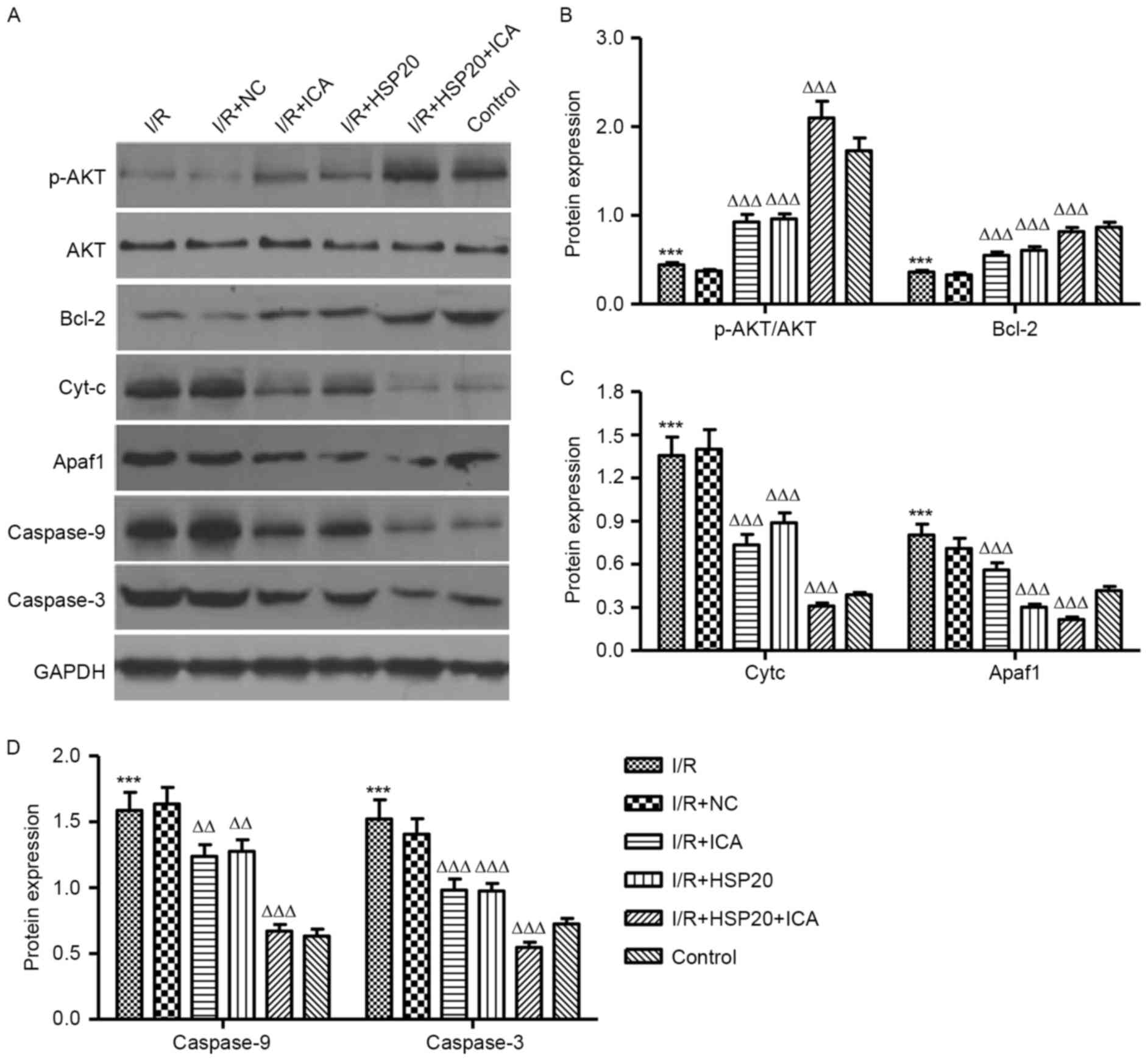 | Figure 5.Effects of HSP20 overexpression on
I/R-induced protein expression levels in H9C2 cells. Expression
levels of p-Akt, Akt, Bcl-2, Cyt-c, APAF1, caspase-9 and caspase-3
in H9C2 cells following I/R treatment were analyzed by (A) western
blotting and (B-D) quantified. Data are presented as the mean ±
standard deviation. ***P<0.001 vs. control;
ΔΔP<0.01, ΔΔΔP<0.001 vs. I/R. HSP, heat
shock protein; I/R, ischemia/reperfusion; p, phosphorylated; Bcl-2,
B-cell lymphoma 2; Cyt-c, cytochrome complex; APAF1, apoptotic
protease activating factor 1; NC, negative control; ICA,
icariin. |
Discussion
Cardiomyocyte apoptosis maybe a fundamental aspect
of the myocardial process that initiates or aggravates heart
failure. Consistent with the previously reported cardioprotective
effects of ICA (14,15), it was found that ICA pretreatment
promotes cardiomyocyte H9C2 cell proliferation, and inhibits cell
apoptosis and ROS production during the process of I/R injury.
Other studies also demonstrated that ICA significantly attenuated
cardiomyocyte apoptosis by downregulating Bcl-2/BCL2 associated X,
apoptosis regulator (Bax), matrix metallopeptidase (MMP)-2 and
MMP-9 expression levels (27). It
is well accepted that multiple genes are aberrantly expressed in
infarct hearts, which are responsible for cardiac remodeling
following I/R (21). To the best
of our knowledge, the present study is the first to demonstrate
that ICA treatment protects against I/R-induced cardiomyocyte
apoptosis and ROS production, which was associated with
overexpression of HSP20 in vitro. These data demonstrate
that HSP20 may exert a positive regulatory role in the treatment of
I/R-induced cardiomyopathy.
ICA, the major active component isolated from
Herbaepimedii, has been extensively investigated on
protection against I/R injury and other stress stimuli; however,
its possible protective effects on I/R-induced cardiotoxicity and
underlying mechanisms are less well studied. Previously, Li et
al (28) identified that
anandamide enhanced HSP72 and HSP25 expression levels in the lungs
to protect against lung inflammation, and acts as a
cardioprotective against I/R insult via its induction of HSP72. The
present study clearly demonstrates that overexpression of HSP20
significantly enhanced the protective effect of ICA on I/R-induced
apoptosis and ROS production in H9C2 cells. Furthermore, the
apoptosis-associated markers, including Bcl-2, Cyt-c, APAF1,
caspase-9 and caspase-3 were regulated by ICA treatment and HSP20
overexpression. Apoptosis is directly controlled by the Bcl-2
family, resulting in the translocation of Bax from the cytosol to
the mitochondria and the release of Cyt-c (29), following the formation of the
apoptosome together with APAF1 and caspase-9, which is followed by
the activation of caspase-3 (30).
HSP70 prevented cell apoptosis via associating with APAF1, as well
as HSP27 that binds to Cyt-c and prevents Cyt-c-mediated
interaction of APAF1 with caspase-9 (31). HSP60 and HSP10 expression in
I/R-induced cardiomyocytes decreased the apoptotic cell number,
Cyt-c release and caspase-3 activity (32). Consistent with the previous
studies, the present data indicated that the expression levels of
Cyt-c, APAF1, caspase-9 and caspase-3 were increased by I/R, but
the level of Bcl-2 expression was decreased. However, ICA treatment
and HSP20 overexpression reduced the expression levels of Cyt-c,
APAF1, caspase-9 and caspase-3 in H9C2 cells induced by I/R.
Indeed, the caspase-3 activity and percentage of myocardial
apoptosis are increased upon I/R injury, but are decreased
following ICA treatment (15). In
addition, previous data demonstrated that lactate dehydrogenase
release and caspase-3 activity in H9C2 cells infected with
recombinant adenovirus encoding wild-type HSP20 are also decreased
(31). These results indicate that
the protective effects of HSP20 are closely associated with
mitochondrial function.
PI3K/Akt is an intracellular signaling pathway,
which is particularly important following ischemic insults.
Activated Akt produces its anti-apoptotic effects via the
phosphorylation of two categories of downstream substrates: The
anti-apoptotic substrates (Bcl-2) and the pro-apoptotic substrates
(caspase-9) (7,33). Triptolide may be a potential
neuroprotective agent for cerebral I/R injury associated with the
activation of the PI3K/Akt/mechanistic target of rapamycin
signaling pathway (34). ICA
protects the heart against I/R injury and this protective effect of
ICA may be associated with its anti-oxidative and anti-apoptotic
actions involving the modulation of the PI3K-Akt signaling pathway
(15). Furthermore, overexpression
of HSP20 in the heart attenuates doxorubicin-induced cardiac
injury, which appears to be dependent on Akt activation (35). In the present study, Akt
inactivation was observed in I/R-induced H9C2 cells, which was
inhibited by ICA treatment. Notably, HSP20 overexpression enhanced
Akt activation in the H9C2 cells induced by ICA treatment.
In conclusion, the present study provides the first
evidence, to the best of our knowledge, that ICA treatment protects
the heart against I/R-induced apoptosis and ROS production, and
this protective effect of ICA may be associated with an associated
upregulation of HSP20. Further research is required to confirm the
cardioprotective effect of ICA on I/R and to clarify the molecular
mechanisms involving the Akt signaling pathway using LY294002, a
PI3K-Akt signaling pathway inhibitor. The current data indicate
that HSP20 presents as a potential therapeutic protein for ischemic
diseases and additional studies are necessary.
References
|
1
|
Donnan GA, Fisher M, Macleod M and Davis
SM: Stroke. Lancet. 371:1612–1623. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Durukan A and Tatlisumak T: Acute ischemic
stroke: Overview of major experimental rodent models,
pathophysiology, and therapy of focal cerebral ischemia. Pharmacol
Biochem Behav. 87:179–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sanz-Rosa D, Garcia-Prieto J and Ibanez B:
The future: Therapy of myocardial protection. Ann N Y Acad Sci.
1254:90–98. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eltzschig HK and Eckle T: Ischemia and
reperfusion-from mechanism to translation. Nat Med. 17:1391–1401.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang J, Upadhyay UM and Tamargo RJ:
Inflammation in stroke and focal cerebral ischemia. Surg Neurol.
66:232–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thind GS, Agrawal PR, Hirsh B, Saravolatz
L, Chen-Scarabelli C, Narula J and Scarabelli TM: Mechanisms of
myocardial ischemia-reperfusion injury and the cytoprotective role
of minocycline: Scope and limitations. Future Cardiol. 11:61–76.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ke ZP, Xu P, Shi Y and Gao AM: MicroRNA-93
inhibits ischemia-reperfusion induced cardiomyocyte apoptosis by
targeting PTEN. Oncotarget. 7:28796–28805. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang F, Li ZL, Xu XM, Hu Y, Yao JH, Xu W,
Jing HR, Wang S, Ning SL and Tian XF: Protective effects of
icariin-mediated SIRT1/FOXO3 signaling pathway on intestinal
ischemia/reperfusion-induced acute lung injury. Mol Med Rep.
11:269–276. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu J, Du J, Xu C, Le J, Liu B, Xu Y and
Dong J: In vivo and in vitro anti-inflammatory effects of a novel
derivative of icariin. Immunopharmacol Immunotoxicol. 33:49–54.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li F, Gong QH, Wu Q, Lu YF and Shi JS:
Icariin isolated from Epimedium brevicornum Maxim attenuates
learning and memory deficits induced by d-galactose in rats.
Pharmacol Biochem Behav. 96:301–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tan HL, Chan KG, Pusparajah P, Saokaew S,
Duangjai A, Lee LH and Goh BH: Anti-cancer properties of the
naturally occurring aphrodisiacs: Icariin and its derivatives.
Front Pharmacol. 7:1912016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen YJ, Zheng HY, Huang XX, Han SX, Zhang
DS, Ni JZ and He XY: Neuroprotective effects of icariin on brain
metabolism, mitochondrial functions, and cognition in
triple-transgenic Alzheimer's disease mice. CNS Neurosci Ther.
22:63–73. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiong D, Deng Y, Huang B, Yin C, Liu B,
Shi J and Gong Q: Icariin attenuates cerebral ischemia-reperfusion
injury through inhibition of inflammatory response mediated by
NF-κB, PPARα and PPARγ in rats. Int Immunopharmacol. 30:157–162.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng X, Pei H and Lan C: Icariin exerts
protective effect against myocardial Ischemia/Reperfusion injury in
rats. Cell Biochem Biophys. 73:229–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ke Z, Liu J, Xu P, Gao A, Wang L and Ji L:
The cardioprotective effect of icariin on Ischemia-reperfusion
injury in isolated rat heart: Potential involvement of the PI3K-Akt
signaling pathway. Cardiovasc Ther. 33:134–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strauch A and Haslbeck M: The function of
small heat-shock proteins and their implication in proteostasis.
Essays Biochem. 60:163–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zoubeidi A and Gleave M: Small heat shock
proteins in cancer therapy and prognosis. Int J Biochem Cell Biol.
44:1646–1656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muller P, Ruckova E, Halada P, Coates PJ,
Hrstka R, Lane DP and Vojtesek B: C-terminal phosphorylation of
Hsp70 and Hsp90 regulates alternate binding to co-chaperones CHIP
and HOP to determine cellular protein folding/degradation balances.
Oncogene. 32:3101–3110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sahebkar A, Mohammadi A, Atabati A,
Rahiman S, Tavallaie S, Iranshahi M, Akhlaghi S, Ferns GA and
Ghayour-Mobarhan M: Curcuminoids modulate pro-oxidant-antioxidant
balance but not the immune response to heat shock protein 27 and
oxidized LDL in obese individuals. Phytother Res. 27:1883–1888.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kennedy D, Jäger R, Mosser DD and Samali
A: Regulation of apoptosis by heat shock proteins. IUBMB Life.
66:327–338. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
De Celle T, Vanrobaeys F, Lijnen P,
Blankesteijn WM, Heeneman S, Van Beeumen J, Devreese B, Smits JF
and Janssen BJ: Alterations in mouse cardiac proteome after in vivo
myocardial infarction: Permanent ischaemia versus
ischaemia-reperfusion. Exp Physiol. 90:593–606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fan GC, Ren X, Qian J, Yuan Q, Nicolaou P,
Wang Y, Jones WK, Chu G and Kranias EG: Novel cardioprotective role
of a small heat-shock protein, Hsp20, against ischemia/reperfusion
injury. Circulation. 111:1792–1799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fan GC, Yuan Q, Song G, Wang Y, Chen G,
Qian J, Zhou X, Lee YJ, Ashraf M and Kranias EG: Small heat-shock
protein Hsp20 attenuates beta-agonist-mediated cardiac remodeling
through apoptosis signal-regulating kinase 1. Circ Res.
99:1233–1242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren XP, Wu J, Wang X, Sartor MA, Qian J,
Jones K, Nicolaou P, Pritchard TJ and Fan GC: MicroRNA-320 is
involved in the regulation of cardiac ischemia/reperfusion injury
by targeting heat-shock protein 20. Circulation. 119:2357–2366.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xiong S, Zheng Y, Jiang P, Liu R, Liu X
and Chu Y: MicroRNA-7 inhibits the growth of human non-small cell
lung cancer A549 cells through targeting BCL-2. Int J Biol Sci.
7:805–814. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Song YH, Li BS, Chen XM and Cai H: Ethanol
extract from Epimedium brevicornum attenuates left ventricular
dysfunction and cardiac remodeling through down-regulating matrix
metalloproteinase-2 and −9 activity and myocardial apoptosis in
rats with congestive heart failure. Int J Mol Med. 21:117–124.
2008.PubMed/NCBI
|
|
28
|
Li Q, Shi M and Li B: Anandamide enhances
expression of heat shock protein 72 to protect against
ischemia-reperfusion injury in rat heart. J Physiol Sci. 63:47–53.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuwana T, Mackey MR, Perkins G, Ellisman
MH, Latterich M, Schneiter R, Green DR and Newmeyer DD: Bid, Bax,
and lipids cooperate to form supramolecular openings in the outer
mitochondrial membrane. Cell. 111:331–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao RQ, Qi DS, Yu HL, Liu J, Yang LH and
Wu XX: Quercetin attenuates cell apoptosis in focal cerebral
ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling
pathway. Neurocheml Res. 37:2777–2786. 2012. View Article : Google Scholar
|
|
31
|
Zhu YH, Ma TM and Wang X: Gene transfer of
heat-shock protein 20 protects against ischemia/reperfusion injury
in rat hearts. Acta Pharmacol Sin. 26:1193–1200. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin KM, Lin B, Lian IY, Mestril R,
Scheffler IE and Dillmann WH: Combined and individual mitochondrial
HSP60 and HSP10 expression in cardiac myocytes protects
mitochondrial function and prevents apoptotic cell deaths induced
by simulated ischemia-reoxygenation. Circulation. 103:1787–1792.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mocanu MM and Yellon DM: PTEN, the
Achilles' heel of myocardial ischaemia/reperfusion injury? Br J
Pharmacol. 150:833–838. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li W, Yang Y, Hu Z, Ling S and Fang M:
Neuroprotective effects of DAHP and Triptolide in focal cerebral
ischemia via apoptosis inhibition and PI3K/Akt/mTOR pathway
activation. Front Neuroanat. 9:482015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fan GC, Zhou X, Wang X, Song G, Qian J,
Nicolaou P, Chen G, Ren X and Kranias EG: Heat shock protein 20
interacting with phosphorylated Akt reduces doxorubicin-triggered
oxidative stress and cardiotoxicity. Circ Res. 103:1270–1279. 2008.
View Article : Google Scholar : PubMed/NCBI
|