Introduction
Osteosarcoma is one of the most common primary bone
malignancies in young adults and adolescents, with an estimated 5.6
per million children suffering from osteosarcoma yearly (1,2).
Osteosarcoma occurs mostly in long extremity bone and around
regions with active bone growth (3,4). In
spite of the recent advances in the treatment of osteosarcoma,
which were mainly based on chemotherapy, radiotherapy and surgery,
the 5-year survival rate remains still dim for patients suffering
from osteosarcoma (1,5). Thus, it is an imperative to uncover
the molecular mechanisms underlying the development and progression
of osteosarcoma and to identify novel biomarkers for the treatment,
diagnosis and prognosis of this malignancy (6).
Spen paralogue and orthologue C-terminal (SPOC)
domain containing 1 (SPOCD1) is a recently identified
novel gene that encodes a protein belonging to the transcription
factor S-II (TFIIS) family of transcription factors (7). SPOCD1 was initially found to interact
with testis protein phosphatase 1 which is a major eukaryotic
serine/threonine-specific phosphatase regulating cellular signaling
(8). It was hypothesized that
SPOCD1 might be involved in the regulation of cell developmental
since it contains the SPOC domain which is normally involved in
developmental signaling (8).
However, the exact roles of SPOCD1 in biological processes have
remained mysterious and received no experimental exploration.
Recently SPOCD1 has been recognized as a
tumor-related factor. An illumine microarray study has shown that
SPOCD1 independently discriminated progressive from non-progressive
bladder cancer patients with a sensitivity of 79% and a specificity
of 86% (AUC=0.83) (9). SPOCD1 was
overexpressed in gastric tumors and knockout of SPOCD1 reduced
gastric cancer cell proliferation, invasion and migration
activities in vitro and in vivo (10). An exome array analysis has further
identified that a low-frequency missense variant (rs1127549280) in
the SPOCD1, at 1p35.2, was reproducibly associated with
reduced risk of gastric cancer (odds ratio=0.56;
P=3.48×10−8) (10). In addition, it has been recently
found that SPOCD1 is significantly upregulated in human masticatory
mucosa during wound healing (11),
raising a hypothesis that SPOCD1 might regulate cell proliferation
and migration processes. These pioneer reports implicated the
biological significance of SPOCD1 in human tumorigenesis.
The present study aimed to evaluate the expression
profile and functional roles of SPOCD1 in osteosarcoma. Expression
of SPOCD1 in clinical osteosarcoma tissues and in a series of
osteosarcoma cell lines were initially examined. Loss-of-function
approach was utilized to assess the effects of SPOCD1 modulation on
cell aggressive activities. Molecular mechanisms of how SPOCD1
exerts its function in osteosarcoma were also explored. Our study
might pave novel insights into the treatment of osteosarcoma in
clinic.
Materials and methods
Human samples
A total of 100 osteosarcoma tissues and their
adjacent non-cancerous tissues were collected from Department of
Orthorpaedic Surgery, Peking Union Medical College Hospital during
2013–2015. All of the tissues were frozen into liquid nitrogen as
soon as dissected from human body during the surgeries. The
clinical data were also recorded for statistical analysis. Each
patient showed their full consent to participate in our study. The
present study was approved by the Ethics Committee of Peking Union
Medical College Hospital.
Cell culture and transfection
Human osteosarcoma cell line SAOS2 and MG63 were
purchased from the cell bank of Shanghai Biological Institute,
Chinese Academy of Science (Shanghai, China). Other two
osteosarcoma cell lines U2OS, as well as the normal cell line
HFOB1.19 were purchased from American Type Tissues Collection
(American Type Culture Collection, Manassas, VA, USA). All culture
media (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) were supplied with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.). Cell were maintained at 37°C in a
humidified atmosphere of 5% CO2. Cell transfection was
conducted with lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufactures' instructions. The
culture medium was replaces every two days, otherwise as
stated.
RNA isolation and RT-PCR
Total RNAs from human tissues and cultured cells
were extracted using a standard Trizol reagent (Takara
Biotechnology Co., Ltd., Dalian, China). RNAs were quantified by
Nanodrop™ 2000 by collecting the OD260 absorbance and then
immediately reversely transcribed into cDNA using Prime Script TM
Master Mix (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions. Then, qRT-PCR was performed with
SYBR® Premix EX Taq II™ (Takara Biotechnology Co., Ltd.)
according to its product manual on the real-time PCR detection
system ABI7900 (Thermo Fisher Scientific, Inc.). GAPDH was used as
the internal reference, and gene mRNA expression were calculated by
2−ΔΔCt method (12).
The thermocycling protocol was listed as below: Initial
denaturation at 95°C for 5 min, followed by 45 repeats of a
three-step cycling program consisting of 10 sec at 95°C
(denaturation), 10 s at 60°C (primer annealing) and 10 sec at 72°C
(elongation), and a final extension step for 10 min at 72°C. The
primer sequences used for qPCR are listed below: SPOCD1: Forward:
5′-CTCCCCAAGTTGCTGACCTG-3′ and Reverse:
5′-CCCCTGTAGCGGGCAAATATC-3′. VEGF-A: Forward:
5′-AGGGCAGAATCATCACGAAGT-3′ and Reverse:
5′-AGGGTCTCGATTGGATGGCA-3′. GAPDH: Forward:
5′-GTGGACATCCGCAAAGAC-3′ and Reverse:
5′-AAAGGGTGTAACGCAACTA-3′.
Western blot analysis
Cells or tissues were lysed in lysis buffer (2%
mercaptoethanol, 20% glycerol, 4% SDS in 100 mM Tris-HCl buffer, pH
6.8) with a freshly added protease inhibitor cocktail (Thermo
Fisher Scientific, Inc.). The total protein extracts were
quantified using a BCA assay kit (Thermo Fisher Scientific, Inc.).
An equal amount of 40 ug protein extracts were loaded to 12% sodium
dodecyl sulfatepolyacrylamide gel electrophoresis (SDS-PAGE)
apparatus and transferred to a PVDF membrane (EMD Millipore,
Billerica, MA, USA). Next, proteins were detected by specific
antibodies using an enhanced chemiluminescence (EDM Millipore). The
immunoreactive bands were quantified by the densitometry with
ImageJ software when necessary. The primary antibodies against
SPOCD1 (ab122188), VEGF-A (ab46154), PCNA (ab29) and Ki67 (ab16667)
were commercially purchased from Abcam (Cambridge, CA, USA). The
primary antibodies against caspase-3 (sc271759), caspase-9
(sc-7885) and secondary antibodies were purchased from Santa Cruz
Biotechnology, Inc., (Dallas, TX, USA). All of the antibodies were
diluted with a ratio of 1:1,000.
Colony formation assay
To observe the relative long-term effect of SPOCD1
on cell proliferation, colony formation assays were performed. In
brief, SAOS2 and MG63 cells were transfected with scramble shRNA
(shNC) or SPOCD1 shRNA (shSPOCD1) for 48 h and re-seeded in 6-well
plates at an initial density of 900 cells/well and allowed to grow
for 14 days to form natural colonies. At the end of monitored time,
cells were washed by PBS for three times, treated with crystal
violet for 30 min at room temperature, and washed twice by
deionized water. Then, colonies in each group of cells were
photographed using a digital camera (Nikon Corporation, Tokyo,
Japan) and the number of colonies was counted.
Cell proliferation analysis
Cell viability was determined using the
methylthiazoletetrazolium (MTT) assay. SAOS2 and MG63 cells were
transfected with shRNAs in the presence or absence of SPOCD1
knockdown for 48 h and then trypsinized and reseeded in triplicate
in 96-well plates at an initial density of 4,000 cells per well.
Cell numbers were monitored for a total of 5 consecutive days. At
each indicated time points, cells culture were added with 10 µl of
MTT solution (5 mg/ml) per well. After 2 h incubation at room
temperature, the absorbance of plate was recorded at 570 nm. Cell
viability was defined as the cell number ratio of experimental
groups to control cells.
Cell apoptosis
The annexin V/PI assay was performed according to
the manufacturer's instructions (Invitrogen; Thermo Fisher
Scientific, Inc.). Briefly, SAOS2 and MG63 cells were plated into
6-well plates and transfected with control or specific shRNA
against SPOCD1. Afterwards, cells were washed with pre-cold PBS,
trypsinized, and re-suspended in 100 µl of binding buffer with 2.5
µl FITC conjugated annexin-v and 1 µl PI (100 µg/ml). Cells were
then incubated at room temperature for 15 min in darkness. A total
of at least 10,000 events were collected and calculated by flow
cytometry (BD Biosciences, San Jose, CA, USA).
Relative caspase activities
The activities of caspase-3, caspase-8 and caspase-9
were determined by the caspase activity kits (Beyotime Institute of
Biotechnology, Nantong, China) based on the instructions. Briefly,
cells were transfected with shRNAs for the 48 h. Afterwards, cell
lysates were collected by low speed centrifuge (1,000 g, 5 min,
4°C). An equal amount of 10 µl proteins from each sample were added
into 96-well plates and mixed with an aliquot of 80 µl reaction
buffer supplied with caspase substrates (2 mM). After incubated at
37°C for 4 h, caspase activities were determined by the TECAN
reader at an absorbance of 450 nm.
Statistical analysis
GraphPad Prism v5.0 (GraphPad Software, Inc., La
Jolla, CA, USA) software was used for statistical analysis. Data
are shown as mean ± standard deviation (SD). The two-tailed
Student's t-test was used for comparisons between groups.
Chi-square test was used to compare differences among categorical
variables. P<0.05 was considered to indicate a statistically
significant difference.
Results
SPOCD1 was overexpressed in human
osteosarcoma in vivo and in vitro
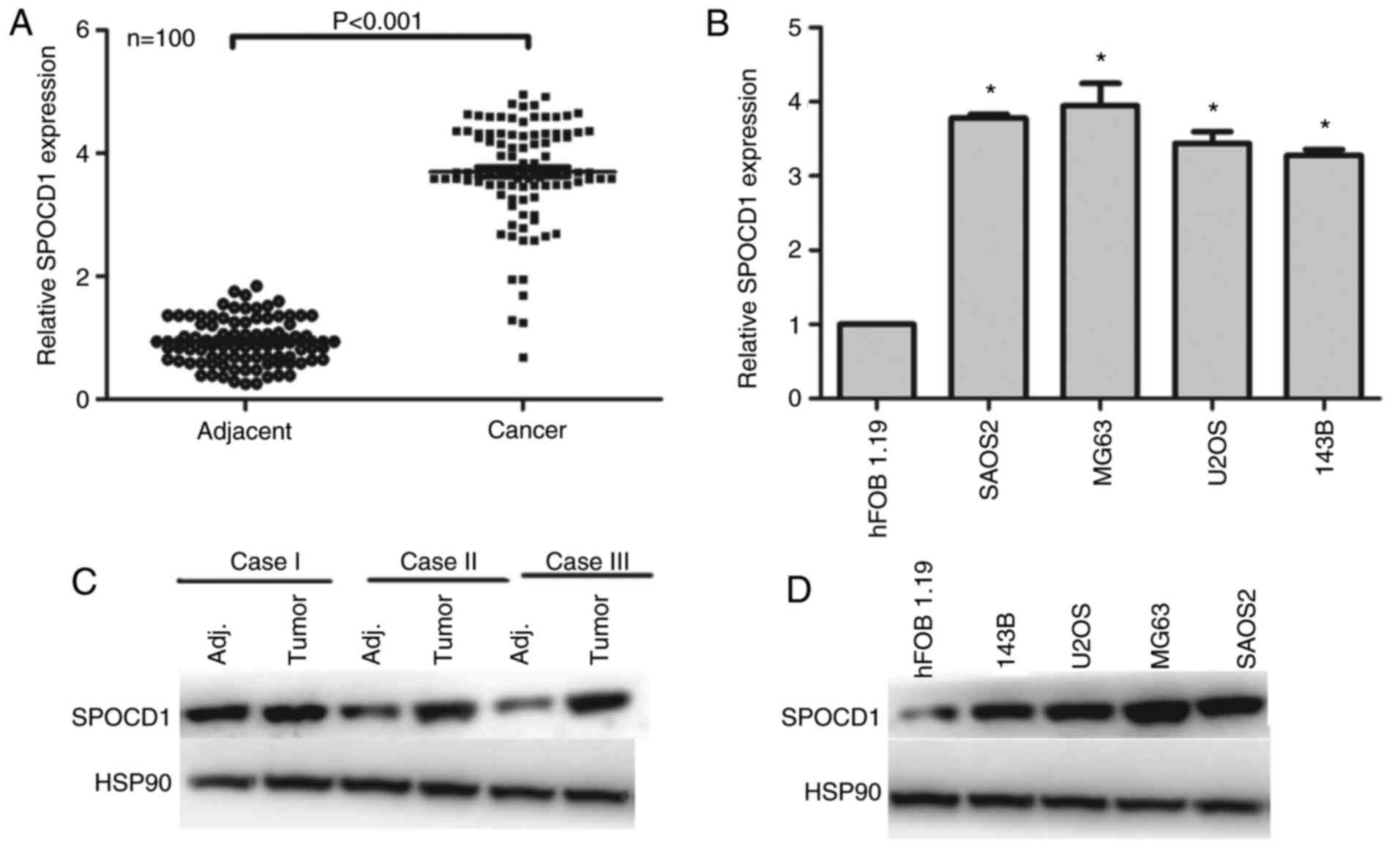
First, we examined the expression of SPOCD1 in 100
of osteosarcoma patients. As shown in Fig. 1A, the expression of SPOCD1 was
increased in 98% of cases compared with their adjacent
non-cancerous tissues (P<0.001). Of note, we identified those,
which had a higher expression of SPOCD1 than the median level as
high expression of SPOCD1 (n=56), otherwise as low expression of
SPOCD1 (n=44). The clinical variables were also analyzed and it was
shown that the expression of SPOCD1 was associated with tumor size
and TNM stages, but not age, sex and lymph node metastasis
(Table I). Afterwards, four of the
osteosarcoma cell lines were chosen for RT-PCR analysis and hFOB
1.19 was included as internal control. It was shown that all four
osteosarcoma showed higher expression of SPOCD1 than the control
cell, of which SAOS2 and MG63 showed the highest expression, thus,
were chosen for subsequent analysis (Fig. 1B). As for the protein levels, most
of the tumor tissues showed higher SPOCD1 expression than their
corresponding counterparts and three of them were listed in
Fig. 1C. Similarly, the protein
levels of SPOCD1 in U2OS, MG63 and SAOS2 cells were all
significantly increased than the control hFOB1.19 cells (Fig. 1D). These data suggested that the
expression of SPOCD1 were remarkably increased in human
osteosarcoma tissues and cells.
 | Table I.Association of SPOCD1 expression with
clinical variables among 100 osteosarcoma patients. |
Table I.
Association of SPOCD1 expression with
clinical variables among 100 osteosarcoma patients.
|
|
| Expression of
SPOCD1 |
|
|---|
|
|
|
|
|
|---|
| Variable | Numbers | Low (n=44) | High (n=56) | P-value |
|---|
| Age (y) |
|
|
| 0.308 |
|
<40 | 16 | 6 | 10 |
|
|
40–50 | 24 | 14 | 10 |
|
|
>50 | 60 | 24 | 36 |
|
| Sex |
|
|
| 1 |
| Male | 53 | 23 | 30 |
|
|
Female | 47 | 21 | 26 |
|
| Tumor size (T) |
|
|
|
<0.001a |
| T1 and
T2(≤4 cm) | 39 | 33 | 6 |
|
| T3 and T4
(>4 cm or any size with distant metastasis) | 61 | 11 | 50 |
|
| Lymph node metastasis
(N) |
|
|
| 0.151 |
| N0 | 41 | 22 | 19 |
|
| N1 or
above | 59 | 22 | 37 |
|
| TNM stage |
|
|
|
<0.001a |
| I/II | 34 | 28 | 6 |
|
|
III/IV | 66 | 16 | 50 |
|
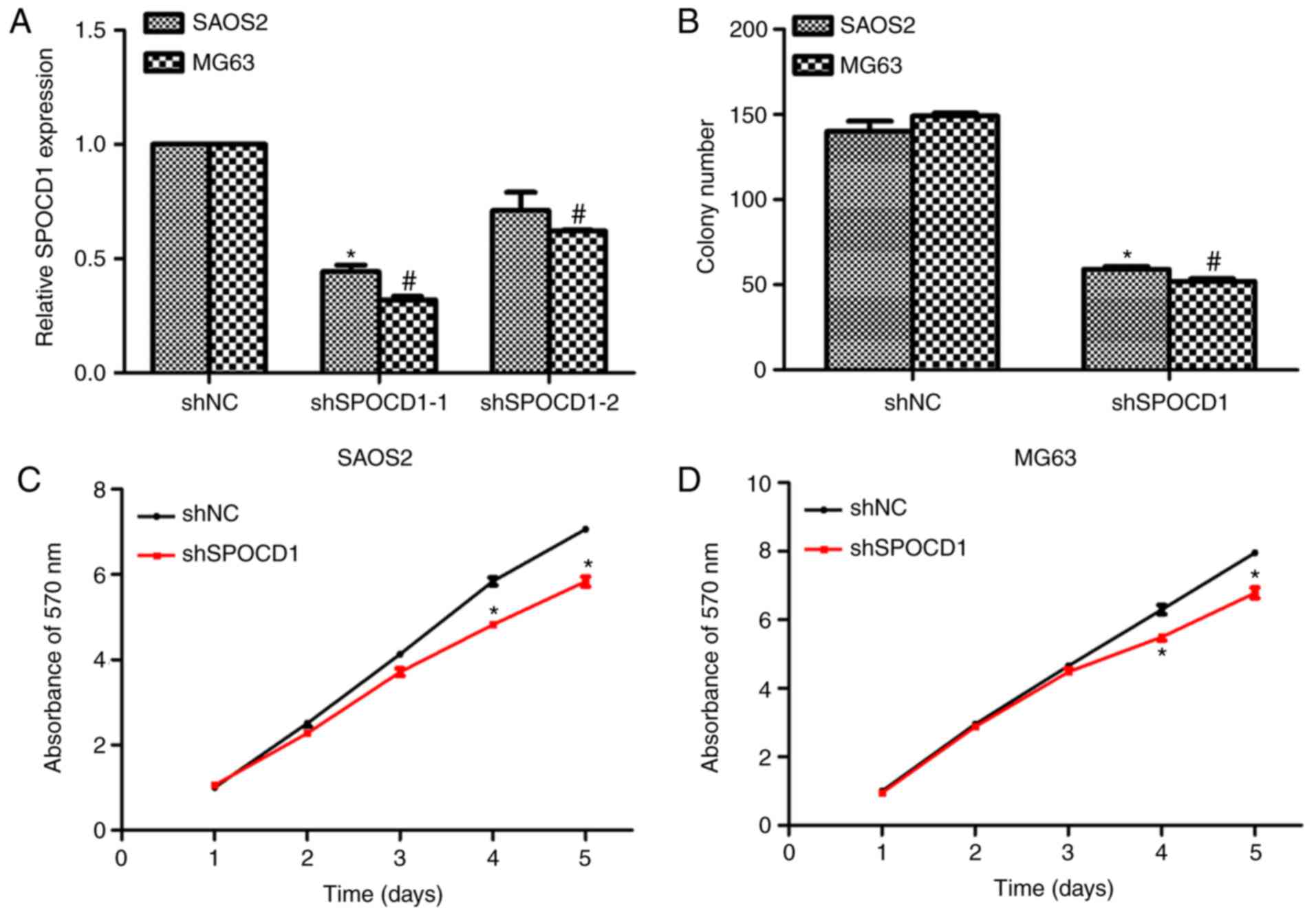
Knockdown of SPOCD1 in human
osteosarcoma cells inhibited cell proliferation
To assess the role of SPOCD1 in human osteosarcoma,
two specific shRNAs against SPOCD1 were designed, synthesized and
transfected into SAOS2 and MG63 cells. As shown in Fig. 2A, SPOCD1 expression was decreased
by more than 50% for both cell lines when shSPOCD1-1 was
transfected, however, the knockdown efficiency was not significant
when cells were treated with shSPOCD1-2. Therefore, only shSPOCD1-1
was used in later study and renamed as shSPOCD1. Colony formation
assays were performed to explore the effects of SPOCD1. Approximate
140 SAOS2 cell colonies and 150 MG63 cell colonies were observed in
control cells, while only 65 SAOS2 colonies and 55 MG63 colonies
were counted when cells were transfected with shSPOCD1 (Fig. 2B). There were no notable
differences between shNC- and shSPOCD1- treated cells in the former
three days for both SAOS2 and MG63 cells. However, the cell
proliferative rate was dramatically decreased in the fourth and
fifth day in both cell lines when SPOCD1 was knocked down (Fig. 2C and D). These results showed
knockdown of SPOCD1 in SAOS2 and MG63 cells inhibited cell
proliferation.
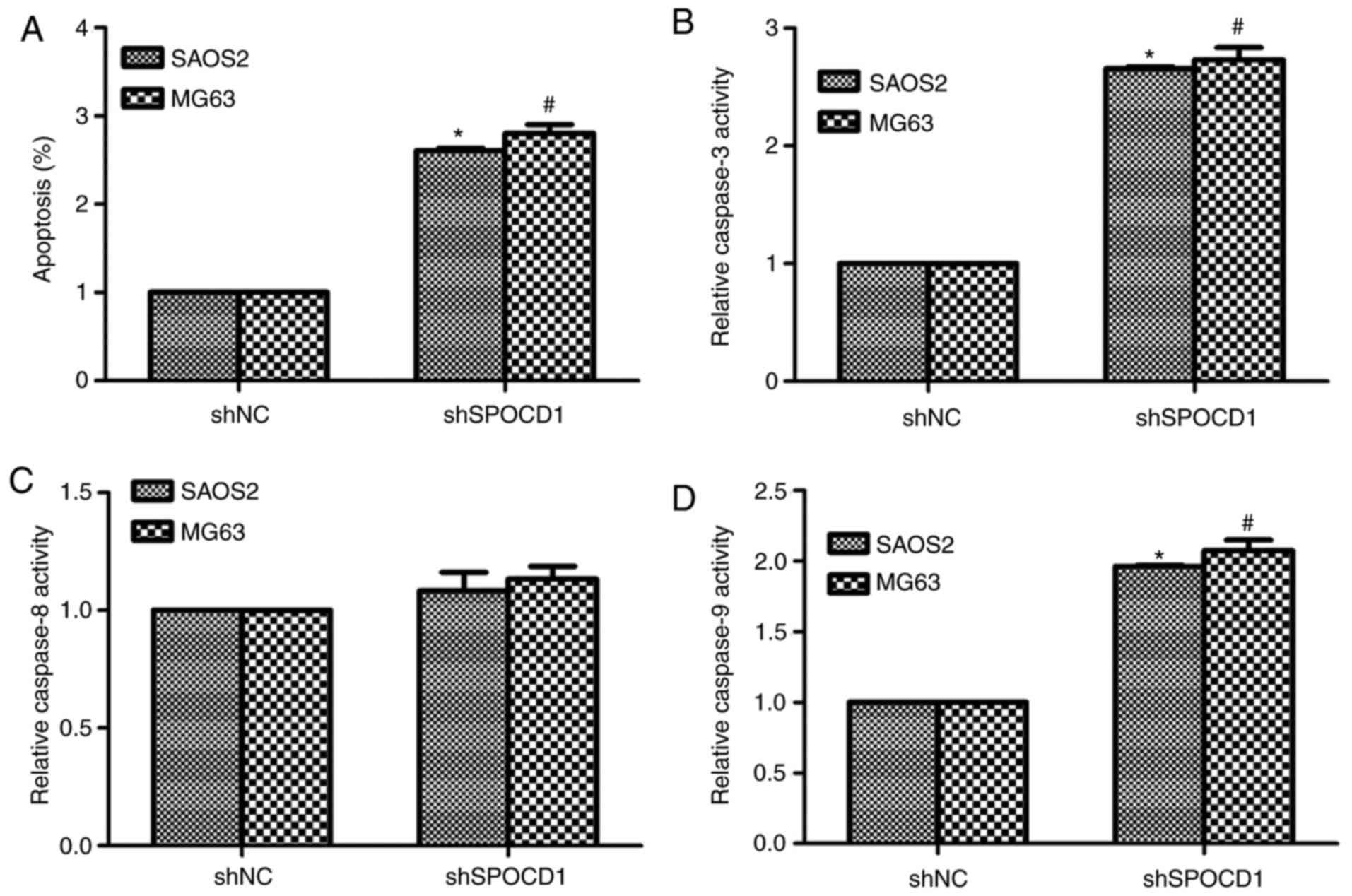
Knockdown of SPOCD1 in SAOS2 and MG63
cells increased cell apoptosis by promoting the activities of
caspase-3 and caspase-9
Based on Table I,
the expression of SPOCD1 was associated with tumor size and TNM
stages, we then examined the role of SPOCD1 in cell apoptosis.
Obviously, the cell apoptotic rate was significantly increased by
depletion of SPOCD1 in SAOS2 cells (about 2.4-fold) and MG63 cells
(2.7-fold) (Fig. 3A). Then we also
detected the relative caspase activities with a Beyotime kit. As
shown in Fig. 3B, the relative
activities of caspase-3 were increased by 2.4-fold and 2.5-fold,
respectively, in SAOS2 and MG63 cells when cells were stimulated
with shRNA against SPOCD1. However, the activity of caspase-8
remained stable with or without shSPOCD1 treatment in both cell
lines (Fig. 3C). The activities of
caspase-9 were also detected and it was shown to increase 1.75-fold
and 1.86-fold in SAOS2 and MG63 cells, separately, after
transfection of shSPOCD1 for 48 h (Fig. 3D). All of these data suggested that
depletion of SPOCD1 increased cell apoptosis by increasing the
activities of caspase-3 and caspase-9, but not caspase-8.
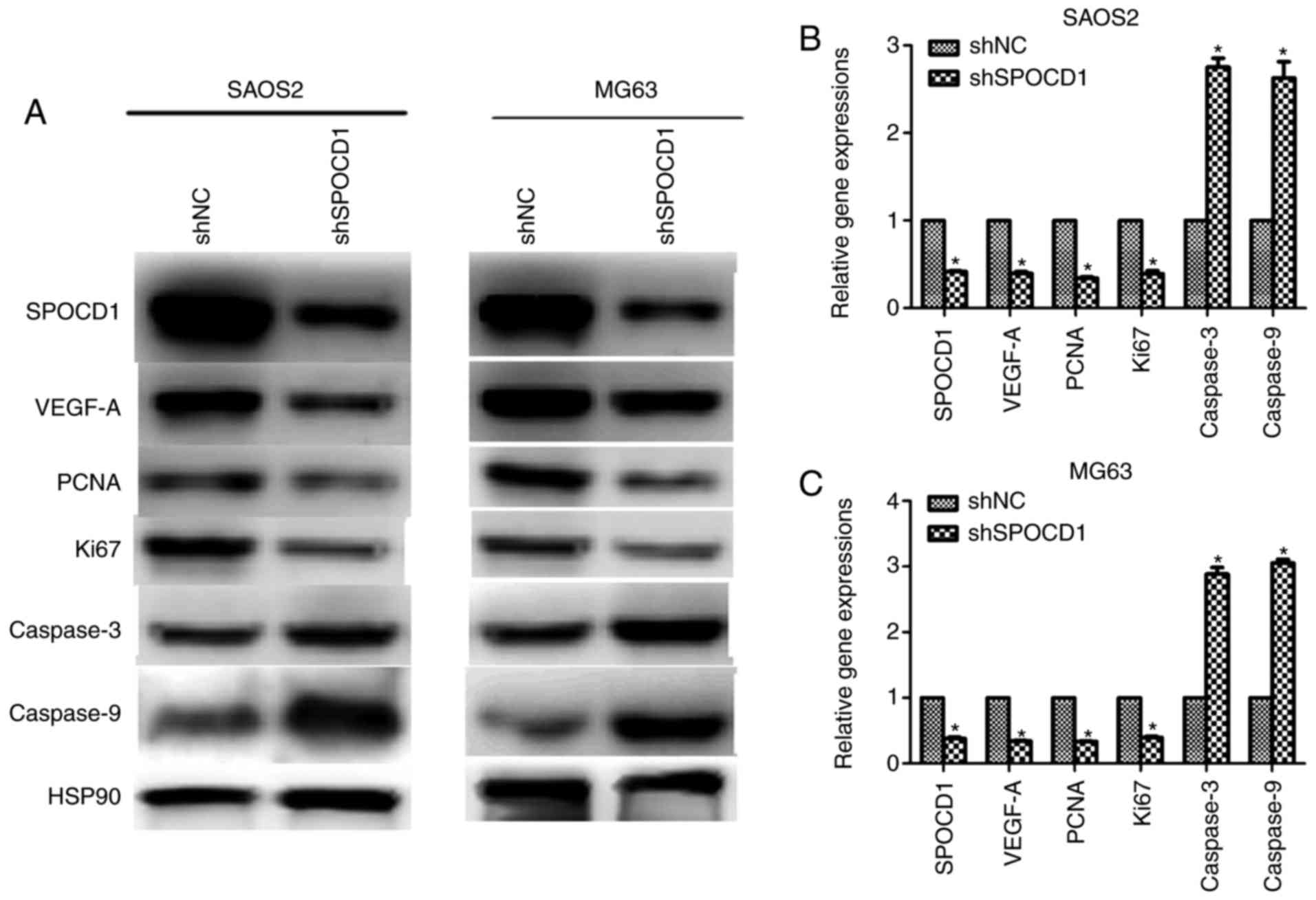
Depletion of SPOCD1 decreased the
expression of VEGF-A in mRNA and protein levels
We tried to figure out how these effects of SPOCD1
resulted from, the expression of vascular endothelial growth factor
A (VEGF-A) was examined. As shown in Fig. 4A, when SPOCD1 was knocked down in
SAOS2 and MG63, the protein levels of proliferative markers PCNA
and Ki67 were notably decreased and the expressions of caspase-3
and caspase-9 were increased, which further confirmed former
observations in Figs. 2 and
3 (Fig. 4A). Interestingly, the protein level
of VEGF-A was also decreased after depletion of SPOCD1 in both cell
lines. Meanwhile, the mRNA levels of VEGF-A, PCNA and Ki67 were
also decreased in both cells while those of caspase-3 and caspase-9
increased when cells were transfected with shSPOCD1 (Fig. 4B and C). These data altogether with
the former ones revealed that SPOCD1 regulated cell proliferation
and apoptosis by mediating the expression of VEGF-A.
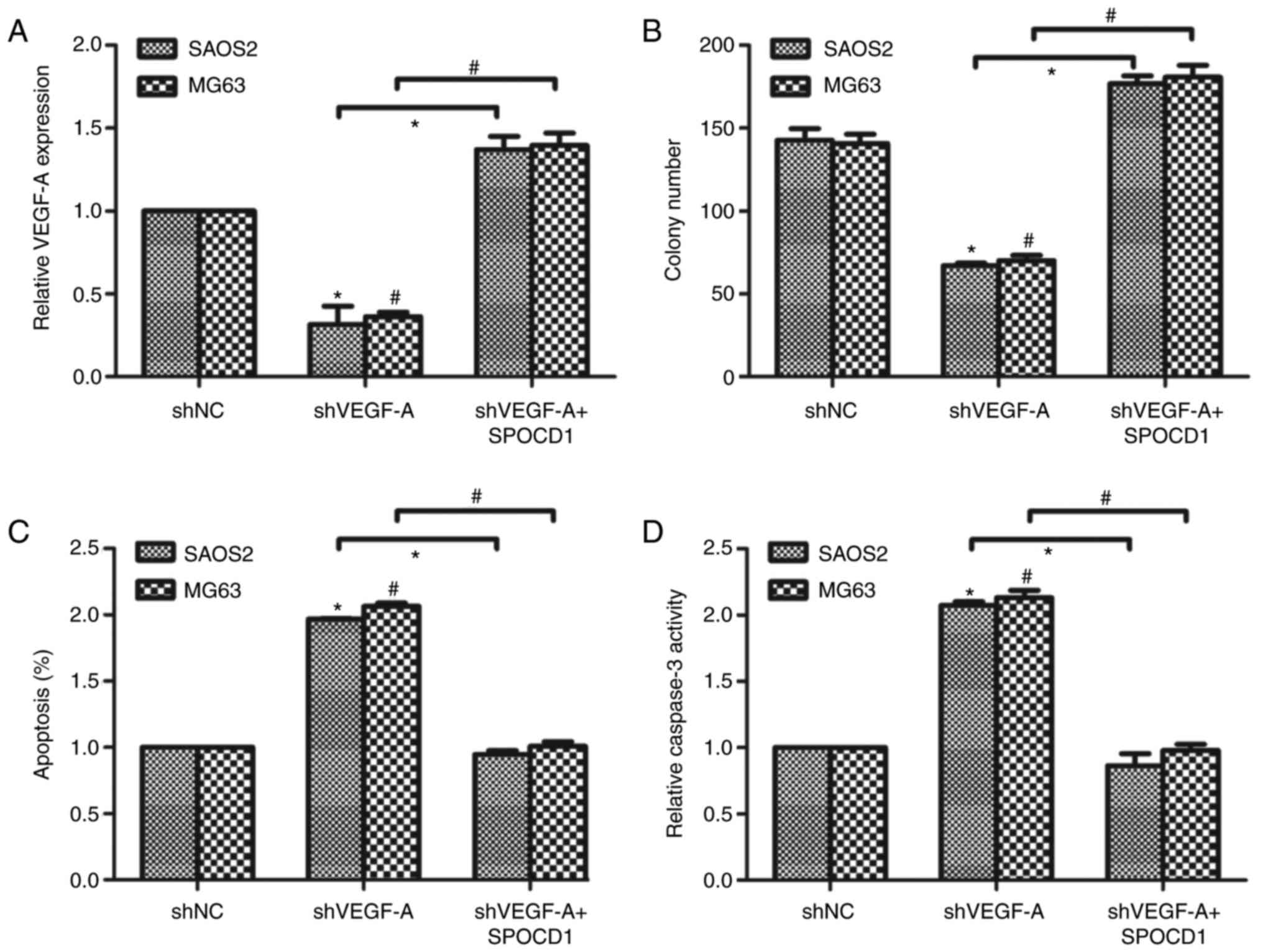
Knockdown of VEGF-A revised the
effects of SPOCD1 on cell proliferation and cell apoptosis
Next, we designed a specific shRNA against VEGF-A
and transfected it into SAOS2 and MG63 cells. It was shown that
knockdown of VEGF-A caused the mRNA level of VEGF-A to drop about
30% of the original ones. We then co-transfected the cells with
shVEGF-A and SPOCD1 plasmid and found that the mRNA level of VEGF-A
was increased to 1.4-fold compared to the control cells (Fig. 5A). Afterwards, cell proliferative
rate and cell apoptosis were again examined. As shown in Fig. 5B, knockdown of VEGF-A decreased
colony formation abilities while co-transfection of SPOCD1 plasmid
increased colony numbers. Similarly, knockdown of VEGF-A in SAOS2
and MG63 cells increased cell apoptotic rate notably, while SPOCD1
overexpression decreased cell apoptosis to basal level in both cell
lines (Fig. 5C). Then, the
relative activities of caspase-3 were detected and it was found
that depletion of VEGF-A in osteosarcoma cells increased the
caspase-3 activity and overexpression of SPOCD1 suppressed the
relative activity of caspase-3 in SAOS2 and MG63 cells (Fig. 5D). These data suggested the effects
of SPOCD1 on cell proliferation and cell apoptosis were associated
with the expression of VEGF-A.
Discussion
Osteosarcoma is responsible for approximate 20% of
all primary bone sarcomas, and associated with a relatively high
mortality rate, especially among children and adolescents (13). Osteosarcoma arises within the long
extremity bones and has high metastasis properties. Conventional
treatment strategy for osteosarcoma comprises of chemotherapy with
the assistance of surgical resections (14). However, the risk adaptation of
chemotherapy for individuals varies and the satisfaction of
postoperative clinical stratification differs due to the
differences in tumor size and sensitivity of patients to
chemotherapy (15). Therefore,
identification of novel therapeutic targets for osteosarcoma
remains an imperative in clinic.
SPOCD1 is a novel gene that belongs to the
transcription factor S-II (TFIIS) family and is implicated in
developmental regulation (7).
Emerging evidence has shown the clinical significance of SPOCD1.
The present study evaluated the functional roles of SPOCD1 in
osteosarcoma. Through examination of serial clinical osteosarcoma
tissues and osteosarcoma cell lines, it was found that SPOCD1 was
significantly overexpressed in clinical cancerous tissues as
compared with adjacent non-cancerous tissues. SPOCD1 was also
exclusively upregulated in osteosarcoma cell lines, particularity
in more invasive cell lines MG63 and SAOS2. Interestingly, clinical
statistics revealed that expression of SPOCD1 was significantly
associated with tumor size and TNM staging, but did not correlate
with patients' age or sex. Downregulation of SPOCD1 using shRNA
approach caused significant decreases in cell proliferation rate
and colony formation capacities. Considering that deregulated cell
proliferation is a hallmark of cancer (16), these data supported that SPOCD1
promoted tumor growth in osteosarcoma. Moreover, inhibition of cell
apoptosis is a good basis for tumor cell growth (17). Two major pathways are known to be
involved in the initiation of apoptosis: the mitochondria-mediated
intrinsic pathway and the death receptor-induced extrinsic pathway
(18). The mitochondria-mediated
intrinsic pathway depends on the release of cytochrome c which
leads to the caspase-9-dependent activation of caspase-3. By
contrary, the death receptor-induced extrinsic pathway signals in a
caspase-8 dependent way. Our results showed that knockdown of
SPOCD1 led to significant promotion of cell apoptosis in MG63 and
SAOS2 cells. Further evaluation of major caspase activities
revealed that caspase-3 and caspase-9, instead of caspase-8, were
modulated by SPOCD1 knockdown, indicating that SPOCD1 inhibits cell
apoptosis in an intrinsic apoptotic pathway. In all, these data
suggested that SPOCD1 promoted cell proliferation and inhibited
cell apoptosis in human osteosarcoma.
Interestingly, we identified VEGF-A as a downstream
target by which SPOCD1 exerts its cancer-promotion effects in
osteosarcoma. Expanding of tumor size relies largely on the
angiogenesis, a process termed as the formation of new blood
vessels via sprouting or splitting of the pre-existing vasculature
(19). Among the pro-angiogenic
factors, the vascular endothelial growth factor A (VEGF-A) is
especially important. VEGF-A is a potent promoter of cell survival,
growth and migration of endothelial cells and a key mediator in the
angiogenic switch from the avascular to vascular phenotype mainly
via the VEGF receptors (19).
VEGF-A also induces angiogenesis through a mechanism involving
activation of a signaling cascade in cells expressing the two major
VEGF receptors (VEGFRs), VEGFR-1 and VEGFR-2 (20). To date, VEGF and its receptors have
been considered as possible targets to inhibit tumor angiogenesis
and reduce tumor growth in several cancers. Bevacizumab, for
instance, was the first anti-angiogenic agent approved for
anti-angiogenic therapy (21), and
has shown benefit in several solid tumors (22). Especially, VEGF inhibitors were
widely studied to ameliorate the progression of osteosarcoma, such
as muramyl tripeptide as an immunotherapy drug (23), IGF-1 as targeted therapy drug
(24), Saracatinib (25) and desmopressin (26). In the present study, it was
observed that SPOCD1 positively regulated the expression of VEGF-A
at both the mRNA and protein levels. Knockdown of VEGF-A blunted
SPOCD1 overexpression-mediated cell growth and cell apoptosis
inhibition. All these data suggested that SPOCD1 promotes cell
proliferation and inhibits cell apoptosis through regulation of
VEGF in osteosarcoma.
Altogether, the present study identified SPOCD1 as
one critical mediator of cell proliferation in osteosarcoma.
Meanwhile, SPOCD1 inhibited cell apoptosis in osteosarcoma. SPOCD1
positively regulated the expression of VEGF-A. Our results
suggested that SPOCD1 promoted cell proliferation and inhibited
cell apoptosis through regulation of VEGF-A in osteosarcoma. Our
conclusions might pave novel insights into the development of novel
therapeutic strategies against osteosarcoma in clinic.
References
|
1
|
van der Deen M, Taipaleenmäki H, Zhang Y,
Teplyuk NM, Gupta A, Cinghu S, Shogren K, Maran A, Yaszemski MJ,
Ling L, et al: MicroRNA-34c inversely couples the biological
functions of the runt-related transcription factor RUNX2 and the
tumor suppressor p53 in osteosarcoma. J Biol Chem. 288:21307–21319.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang P, Wang H, Li X, Liu Y, Zhao C and
Zhu D: SRCIN1 suppressed osteosarcoma cell proliferation and
invasion. PLoS One. 11:e1555182016.
|
|
3
|
Cao Y, Wu T, Li D, Hu J and Lu H:
MicroRNA-336 directly targets Sox-2 in osteosarcoma to inhibit
tumorigenesis. Mol Med Rep. 15:4217–4224. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang C, Yao C, Li H, Wang G and He X:
Combined elevation of microRNA-196a and microRNA-196b in sera
predicts unfavorable prognosis in patients with osteosarcomas. Int
J Mol Sci. 15:6544–6555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fujiwara T, Katsuda T, Hagiwara K, Kosaka
N, Yoshioka Y, Takahashi RU, Takeshita F, Kubota D, Kondo T,
Ichikawa H, et al: Clinical relevance and therapeutic significance
of microRNA-133a expression profiles and functions in malignant
osteosarcoma-initiating cells. Stem Cells. 32:959–973. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iwasaki T, Tanaka K, Kawano M, Itonaga I
and Tsumura H: Tumor-suppressive microRNA-let-7a inhibits cell
proliferation via targeting of E2F2 in osteosarcoma cells. Int J
Oncol. 46:1543–1550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kimura K, Wakamatsu A, Suzuki Y, Ota T,
Nishikawa T, Yamashita R, Yamamoto J, Sekine M, Tsuritani K,
Wakaguri H, et al: Diversification of transcriptional modulation:
Large-scale identification and characterization of putative
alternative promoters of human genes. Genome Res. 16:55–65. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fardilha M, Esteves SL, Korrodi-Gregório
L, Vintém AP, Domingues SC, Rebelo S, Morrice N, Cohen PT, da Cruz
e Silva OA and da Cruz e Silva EF: Identification of the human
testis protein phosphatase 1 interactome. Biochem Pharmacol.
82:1403–1415. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van der Heijden AG, Mengual L, Lozano JJ,
Ingelmo-Torres M, Ribal MJ, Fernández PL, Oosterwijk E, Schalken
JA, Alcaraz A and Witjes JA: A five-gene expression signature to
predict progression in T1G3 bladder cancer. Eur J Cancer.
64:127–136. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu M, Yan C, Ren C, Huang X, Zhu X, Gu H,
Wang M, Wang S, Gao Y, Ji Y, et al: Exome array analysis identifies
variants in SPOCD1 and BTN3A2 that affect risk for gastric cancer.
Gastroenterology. 152:2011–2021. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y and Tatakis DN: Human gingiva
transcriptome during wound healing. J Clin Periodontol. 44:394–402.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Davis AM, Bell RS and Goodwin PJ:
Prognostic factors in osteosarcoma: A critical review. J Clin
Oncol. 12:423–431. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trieb K and Kotz R: Proteins expressed in
osteosarcoma and serum levels as prognostic factors. Int J Biochem
Cell Biol. 33:11–17. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luscan A, Shackleford G, Masliah-Planchon
J, Laurendeau I, Ortonne N, Varin J, Lallemand F, Leroy K, Dumaine
V, Hivelin M, et al: The activation of the WNT signaling pathway is
a Hallmark in neurofibromatosis type 1 tumorigenesis. Clin Cancer
Res. 20:358–371. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu H, Zhou Y and Tang L: Caffeine induces
sustained apoptosis of human gastric cancer cells by activating the
caspase-9/caspase-3 signalling pathway. Mol Med Rep. 16:2445–2454.
2017.PubMed/NCBI
|
|
18
|
Liu JY, Liu Z, Wang DM, Li MM, Wang SX,
Wang R, Chen JP, Wang YF and Yang DP: Induction of apoptosis in
K562 cells by dicyclohexylammonium salt of hyperforin through a
mitochondrial-related pathway. Chem Biol Interact. 190:91–101.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saharinen P, Eklund L, Pulkki K, Bono P
and Alitalo K: VEGF and angiopoietin signaling in tumor
angiogenesis and metastasis. Trends Mol Med. 17:347–362. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Germani A, Di Carlo A, Mangoni A, Straino
S, Giacinti C, Turrini P, Biglioli P and Capogrossi MC: Vascular
endothelial growth factor modulates skeletal myoblast function. Am
J Pathol. 163:1417–1428. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sullivan LA and Brekken RA: The VEGF
family in cancer and antibody-based strategies for their
inhibition. MAbs. 2:165–175. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Roberts SS, Chou AJ and Cheung NK:
Immunotherapy of childhood sarcomas. Front Oncol. 5:1812015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Armakolas N, Armakolas A, Antonopoulos A,
Dimakakos A, Stathaki M and Koutsilieris M: The role of the IGF-1
Ec in myoskeletal system and osteosarcoma pathophysiology. Crit Rev
Oncol Hematol. 108:137–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen Q, Zhou Z, Shan L, Zeng H, Hua Y and
Cai Z: The importance of Src signaling in sarcoma. Oncol Lett.
10:17–22. 2015.PubMed/NCBI
|
|
26
|
Hermo GA, Torres P, Ripoll GV, Scursoni
AM, Gomez DE, Alonso DF and Gobello C: Perioperative desmopressin
prolongs survival in surgically treated bitches with mammary gland
tumours: A pilot study. Vet J. 178:103–108. 2008. View Article : Google Scholar : PubMed/NCBI
|