Introduction
Cancer is one of the leading causes of mortality
worldwide. Among the various types of cancer, osteosarcoma is one
of the most commonly diagnosed primary bone tumors, often occurring
in children and adults and arising in the metaphysis (1,2).
Since the systemic spread of osteosarcoma is common, the prognosis
for affected patients is poor (3).
Previously, combination chemotherapy was demonstrated to
substantially increase the survival rates of osteosarcoma patients
by 60–70% (4); however, 40% of
osteosarcoma patients still exhibit a poor response. A high risk of
recurrence and metastasis exists despite thorough chemotherapy
treatment and curative resection of the tumor (5). Failure of chemotherapy is primarily
due to drug resistance. Therefore, an improved understanding of the
molecular mechanisms underlying osteosarcoma invasion, as well as
the development of novel potential treatments, are necessary for
osteosarcoma therapy.
Chemoprevention is the pharmacological use of
synthetic compounds or phytochemicals that prevent or reverse
carcinogenesis or tumor development. Flavonoids are a class of
phytochemical agents that are rich in naturally occurring
polyphenolic compounds. They are abundant in medicinal herbs,
vegetables, fruits and nuts. Flavonoids comprise of flavones,
isoflavones, flavanones, flavonols, chalcones and anthocyanins
(6,7). The functions of dietary flavonoids in
the prevention and treatment of cancer have been studied
extensively in clinical trials and laboratory studies (7,8). The
chemical structure of flavonoids is similar to that of quercetin,
and flavonoids have been reported to exhibit multiple
pharmacological activities (6,8). The
biochemical function of flavonoids and their corresponding
metabolites depend on the precise chemical structure of the
compound and the relative positioning of different functional
groups in the molecule.
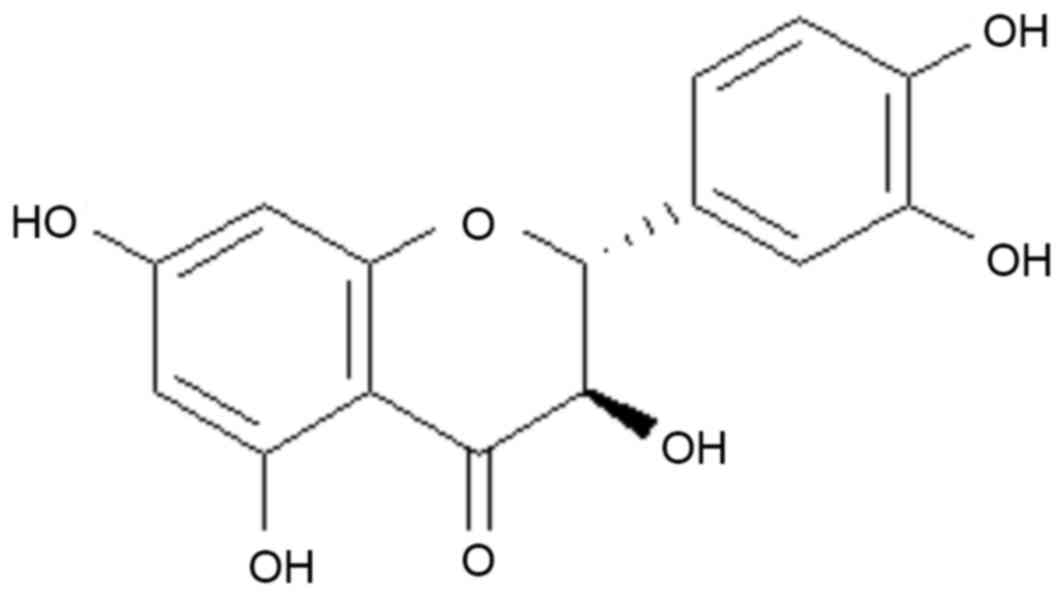
Taxifolin (Fig. 1)
is a characteristic plant flavonoid, which naturally occurs in
onion (8), citrus fruits, and milk
thistle. Taxifolin, isolated from the barks of Cedrus
brevifolia, Cedrus brevifolia (Hooker fil.), Laric
siberica (ledeb.) and Texus chinensis, has long been
used in the treatment of cerebrovascular and cardiovascular
disorders (9). Taxifolin, also
known as dihydroquercetin, exhibits strong antioxidant abilities
Taxifolin, also known as dihydroquercetin, exhibits strong
antioxidant ability (10). The
properties of taxifolin are similar to quercetin, which has been
demonstrated to induce apoptosis in cell and animal models
(10,11).
Previous studies have demonstrated that taxifolin
exerts multiple biological effects, including anti-oxidative,
anti-inflammatory, anti-proliferative and anti-coagulative effects
(12,13). Rogovskiĭ et al (14) demonstrated that taxifolin exerts
anti-proliferative properties in human breast cancer cells and in
murine fibroblasts. In addition, Vrba et al (15) demonstrated that mitogen activated
protein kinase and protein kinase C-dependent signal transduction
is regulated and maintained by taxifolin. However, to the best of
the author's knowledge, no studies performed to date have assessed
the in vitro and in vivo anti-cancer effects of
taxifolin in osteosarcoma. Therefore, the aim of the current study
was to investigate the anti-cancer properties of taxifolin in
osteosarcoma. To achieve this, the effect of taxifolin on the
viability and proliferation of U2OS and Saos-2 osteosarcoma cells
was evaluated, and the signaling factors involved in mediating
these effects were investigated.
Materials and methods
Reagents
Taxifolin was purchased from Shanghai Huicheng
Technology Ltd. (Shanghai, China). MTT, cell culture dishes and
Hoechst 33258 were purchased from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). Matrigel was purchased from BD Biosciences
(Franklin Lakes, NJ, USA). The primary antibodies, mouse monoclonal
anti-Akt serine/threonine kinase 1 (Akt1; cat. no. sc-135829;
1:1,200), rabbit polyclonal anti-phosphorylated (p-Ser473) Akt
(cat. no. sc-7985-R, 1:1,000), mouse monoclonal anti-v-myc avian
myelocytomatosis viral oncogene homolog (c-myc; cat. no. sc-70469,
1:800), mouse monoclonal anti-S-phase kinase associated protein 2
[SKP-2; cat. no. sc-74477 (p45), 1:1,000] and mouse and rabbit
monoclonal anti-β-actin (cat. no. sc-8432/sc-130656, 1:1,000) as
well as secondary antibody, rabbit anti-mouse IgG HRP (cat. no.
sc-358914, 1:10,000) were obtained from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Cell culture
The human osteosarcoma cell lines, U2OS and Saos-2,
were obtained from the American Type Cell Culture Collection
(Manassas, VA, USA). These cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum, 1% penicillin-streptomycin and 1 mM L-glutamine
(Invitrogen; Thermo Fisher Scientific, Inc.) in a humidified
incubator with 5% CO2 and 95% air at 37°C.
Cell proliferation assay
Cells (U20S/Saos-2) were cultured at a density of
4×103 cells/well in 96-well plates for 24 h. Increasing
concentrations of taxifolin (0, 5, 10, 25 and 50 µM) were then
added to the cells in 100 µl additional medium, and cells were
cultured for a further 96 h. The medium was removed by aspiration
and 100 µl water was added into the wells and stored overnight at
−80°C. Plates were then thawed at 37°C, and cells were stained by
the addition of buffer containing 10 mM Tris (pH 7.4) and 1 mM EDTA
with Hoechst 33258 (0.2%) dye at 37°C for 1 h. The relative DNA
content was assayed by measuring Hoechst 33258 fluorescence using a
Wallac 1420 Victor2 Multilabel microplate reader (Perkin Elmer
Inc., Waltham, MA, USA) at excitation and emission wavelengths of
355 and 460 nm, respectively (16).
MTT assay
Cell viability was measured using the MTT assay.
U2OS and Saos-2 cells were seeded at a density of 4×103
cells/well in 96-well plates for 24 h, and then treated with
increasing concentrations of taxifolin (0–50 µM) for a further 48
h. A total of 50 µl MTT solution (2 mg/ml) was added to each well,
and plates were incubated for 4 h. The plates were then centrifuged
at 1,000 × g for 5 min at 37°C, to remove unconverted MTT and to
leave the resulting formazan crystals at the bottom of the plates.
The crystals were then dissolved in 100 µl dimethyl sulfoxide
(DMSO), and the absorbance was read at 570 nm using a Titertek
Multiskan MCC/340 plate reader (Titertek-Berthold, Berthold
Detection Systems GmbH, Pforzheim, Germany). The results were
presented as a relative percentage of the untreated control cells
(17).
Flow cytometry
U2OS and Saos-2 cells were seeded at
6×106 cells/dish in 100 mm dishes and cultured for 24 h
prior to taxifolin treatment. Cells were treated with taxifolin (50
µM) and incubated for 48 h. Cells were then fixed using 70%
ice-cold ethanol diluted in phosphate-buffered saline (PBS) for ~12
h at 37°C. Following fixation, cells were washed with PBS and
incubated with PBS containing RNase A (0.1 mg/ml; Sigma-Aldrich;
Merck KGaA) for 30 min at 4°C, before they were resuspended in 50
µg/ml propidium iodide (Sigma-Aldrich; Merck KGaA). The cell cycle
distribution was determined using a BD FACScan flow cytometer (BD
Biosciences) and the data were analyzed using the BD CellQuest Pro
software program (version 5.1; BD Biosciences).
Soft agar assay
In order to determine soft agar colony formation,
6×103 U2OS and Saos-2 cells were diluted in complete
DMEM medium containing agar (0.3%) in 35-mm cell culture plates.
The cells were cultured for 3 weeks in a 5% CO2
incubator at 37°C and treated with different concentration of
taxifolin (0, 25 and 50 µM). The colonies were stained with crystal
violet (0.5 mg/ml) for 10 h at 37°C.
Tumor xenografts
A total of 16 male BALB/c nude mice (age, 6–8 weeks;
weight, 15–18 g) were procured from the National Cancer Institute
at Frederick, Laboratory Animal Sciences Program (Frederick, MD,
USA). BALB/c nude mice were maintained in a steel cage, under a
12-h dark/light cycle with free access to food and water. All the
mice were subcutaneously injected with 5×103 U2OS cells
suspended in Matrigel (0.5 ml), and tumor growth was monitored
weekly from 1 week post-injection. Mice were divided into control
(8 mice with 14 tumors) and drug-treated (8 mice with 13 tumors)
groups. At 21 days post-inoculation, mice in the drug-treated group
were administered with 25 mg/kg taxifolin intraperitoneally (i.p)
once every 2 days for remaining 24 days. The control mouse group
was administered with an equal volume of saline (vehicle). The
treatment was terminated at 45 days post-inoculation. Tumor size
was measured using calipers and tumor volume was calculated using
the following formula: Volume = length × width × height × 0.52
(18). Mouse body weight and tumor
volumes were expressed as the mean ± standard deviation (SD). The
present study was approved by the Ethics Committee of Hanzhong
Centre Hospital (HCH-4665; Hanzhong, China).
Western blot analysis
U2OS and Saos-2 cells (1×104) were
treated with different concentrations of taxifolin (0, 25 and 50
µM) for 72 h at 37°C and lysed using lysis buffer containing 0.5%
sodium deoxycholate, 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1%
nonidet P-40, 0.1% SDS, 1 mM dithiothreitol, 5 mM EDTA, 50 mM
sodium fluoride, and 10 µg/ml aprotinin by incubating for 1 h at
37°C. The resultant cell lysates were centrifuged at 3,000 × g for
10 min at 37°C and total cellular protein concentration was
determined using a BCA protein assay kit (BioVision, Inc.,
Milpitas, CA, USA). Protein extracts (~60 µg) were separated by 10%
SDS-PAGE and electrotransferred onto polyvinylidene difluoride
membranes. The membrane was blocked with TBS containing Tween-20
and 5% skimmed milk for 2 h at 37°C and incubated with primary
antibodies against Akt, p-S473-Akt, c-myc, SKP-2 and β-actin at 4°C
overnight, followed by probing with secondary antibody for 2 h at
37°C. Detection of bands was performed using an enhanced
chemiluminescence detection kit (GE Healthcare Life Sciences,
Chalfont, UK), and band densities were measured using Scion Image
software (version 4.0) from Scion Co. (Frederick, MD, USA).
Overexpression of AKT and SKP-2
U2OS cells (5×105) were plated in 6-well
plates for 24 h prior transfection and then U2OS cells were
transfected with SKP-2 and AKT overexpression plasmids (1 µM) like
pcDNA3.1-SKP2, pcDNA3.1-AKT (designed by Nanjing Keygen Biotech
Co., Ltd., Nanjing, China) and an empty vector (pcDNA3.1) using
Lipofectamine 2000 (Thermo Fisher Scientific, Inc.), for 24 h.
Finally the transfected cells were treated with 0 or 25 µM of
taxifolin for 48 h. Transfection of U2OS cells with the empty
pcDNA3.1 vector served as a negative control (19).
Transwell migration assay
Briefly, U2OS cells (1×104) were plated
in 6-well plates for 24 h prior to transfection. Cells were treated
with 0, 25 and 50 µM taxifolin for 48 h, then trypsinized
(centrifuged at 600 × g for 10 min at 37°C) and washed with PBS to
remove adherent cells. A total of 1×103 treated cells
diluted in 200 µl serum-free DMEM were added to the upper
compartment of transwell migration chambers (BD Biosciences), and
DMEM containing 10% FBS was added to the lower compartment. The
chambers were then incubated at 37°C overnight. Cells that had
migrated to the underside of the transwell filters were fixed with
70% methanol and stained with 10% Giemsa solution at room
temperature for 1 h. Filters were washed with water and
quantification was performed by analyzing 6 random fields and
counting the number of cells on the filter (lower side) under phase
contrast microscopy at a magnification of ×200.
Transwell invasion assay
For the invasion assays, Matrigel-coated transwell
chambers were used (BD Biosciences). A total of U2OS cells
(1×105) were plated in 6-well plates for 24 h prior to
transfection. Cells were then treated with 0, 25, and 50 µM
taxifolin for 48 h, and trypsinized (by centrifuging at 600 × g for
10 min at 37°C to remove adherent cells) and washed with PBS. Then
the 1×104 taxifolin-treated U2OS cells were diluted in
200 µl serum-free DMEM and added to the upper compartment of
Matrigel-coated transwell chambers (BD Biosciences). DMEM
containing 10% FBS was added to the lower chamber before the plates
were incubated overnight. The non-invaded cells were removed and
the invaded cells on the underside of the filters were fixed with
70% methanol at 37°C for 1 h. Filters were washed with water and
stained with 10% Giemsa solution for 1 h at 37°C. The number of
invaded cells was quantified by counting the number of cells on the
filter (6 random fields) under phase contrast microscopy at a
magnification of ×200.
Statistical analysis
Experimental results are expressed as the mean ± SD.
Differences among groups were examined for statistical significance
using one-way analysis of variance followed by Dunnett's multiple
comparison post hoc test on SPSS software (version 21.0, IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of taxifolin on the viability
and proliferation of osteosarcoma cells
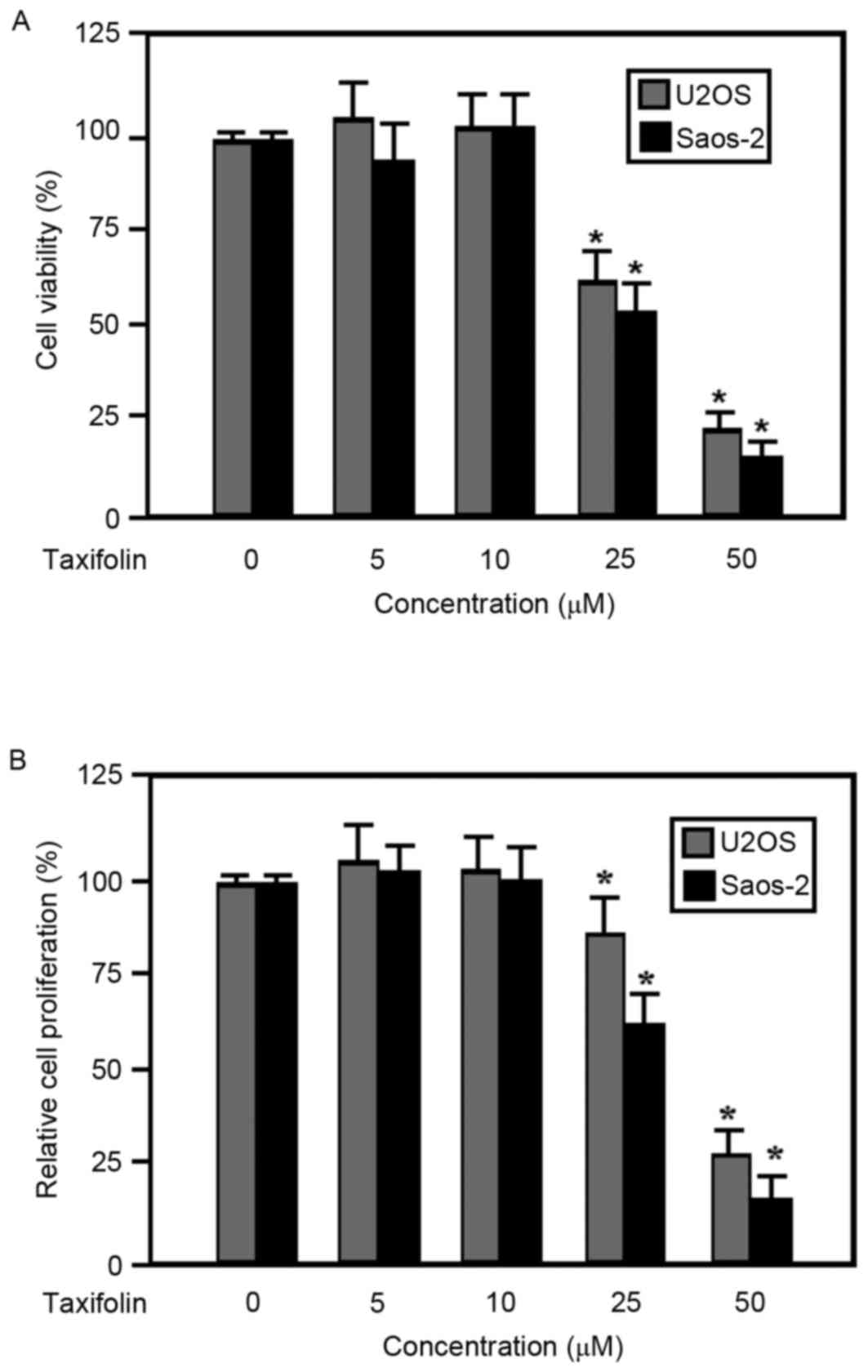
The viability and proliferation of taxifolin-treated
human U2OS and Saos-2 osteosarcoma cells were examined using MTT
and Hoechst 33258 assays, respectively. Results from the MTT assay
demonstrated that the viability of U2OS and Saos-2 cells was
inhibited by taxifolin treatment in a dose-dependent manner at
concentrations ≥25 µM compared with the untreated cells (Fig. 2A). In addition, taxifolin treatment
suppressed the proliferation of U2OS and Saos-2 cells in a
dose-dependent manner at concentrations ≥25 µM when compared with
the untreated cells (Fig. 2B). The
half maximal effective concentration of taxifolin was similar for
cell viability and proliferation (data not shown), suggesting that
the observed decrease in cell viability may have been due to the
suppression of cell proliferation.
Effect of taxifolin on soft agar
colony formation
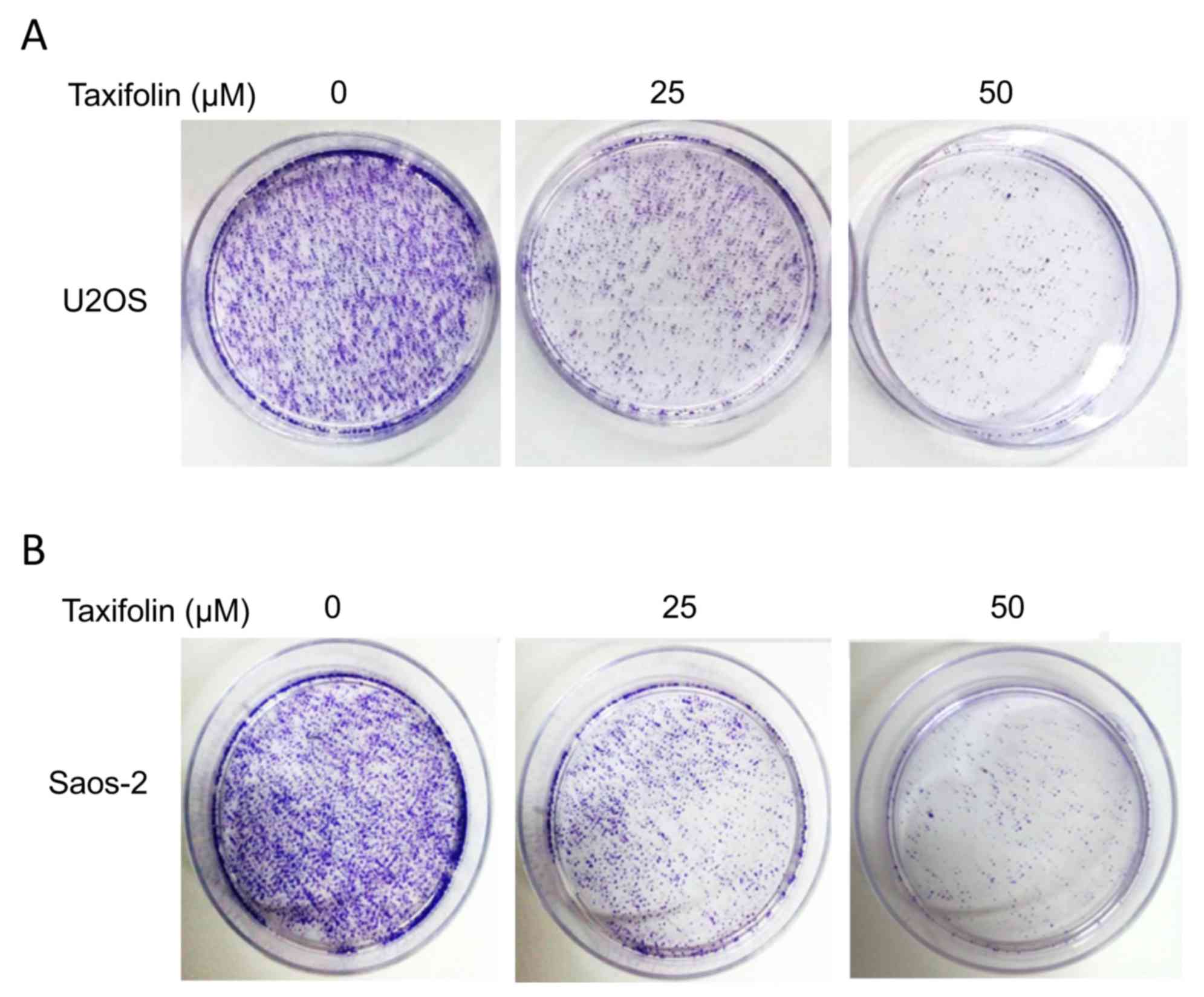
The anchorage-independent growth of U2OS and Saos-2
cells was examined using a soft agar colony formation assay. The
colony formation results demonstrated that treatment with 25 and 50
µM taxifolin markedly suppressed the ability of U2OS and Saos-2
cells to form colonies when compared with the untreated cells
(Fig. 3). These results suggest a
potential anti-tumor effect of taxifolin.
Effect of taxifolin treatment on
osteosarcoma tumor growth in vivo
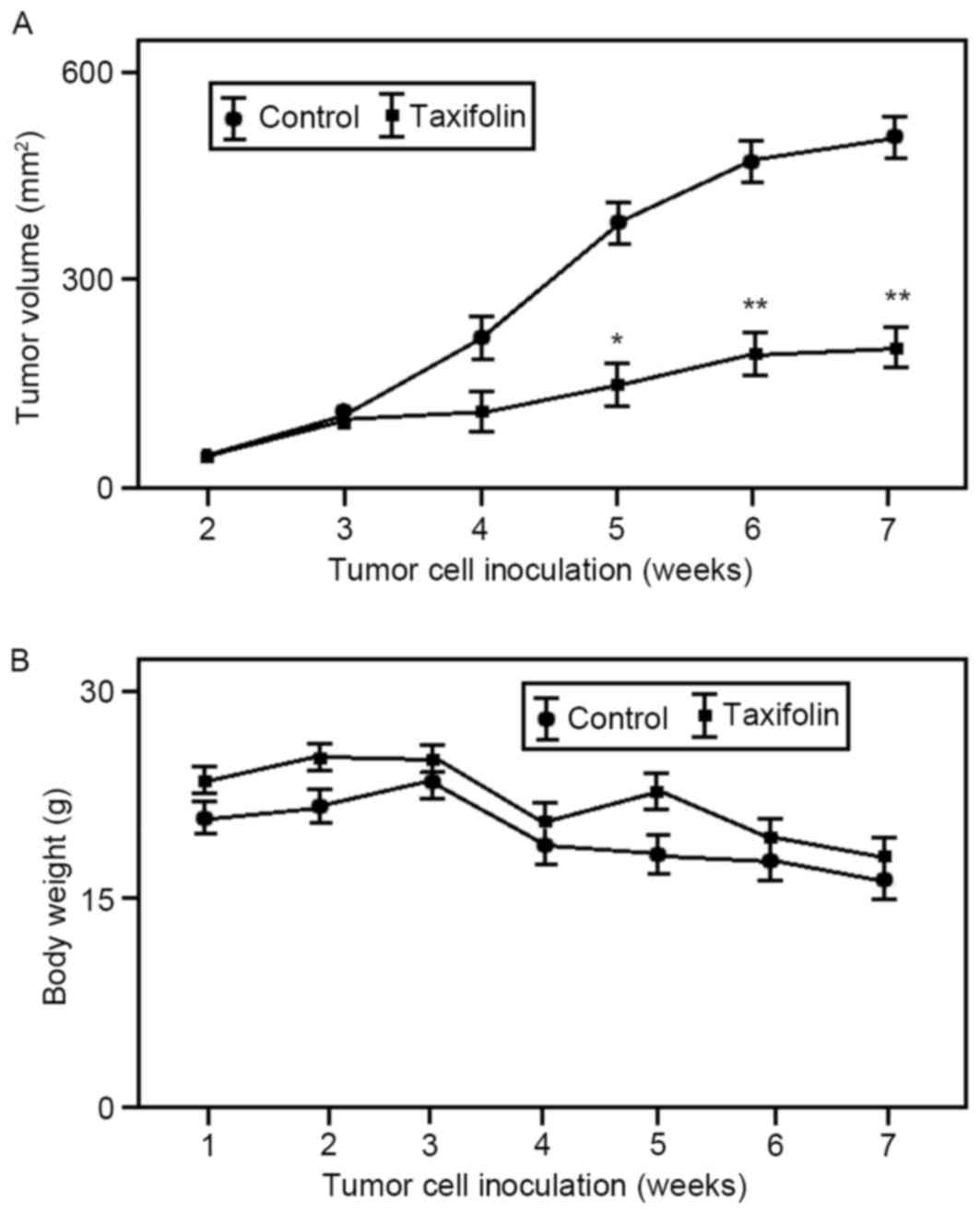
In order to determine whether taxifolin treatment
inhibits tumor growth in vivo, U2OS cells were used to
generate tumor xenograft models in nude mice. As demonstrated in
Fig. 4A, intraperitoneal
administration of taxifolin (25 mg/kg once every 2 for 24 days) was
associated with a 55% reduction (P<0.01) in the mean volume of
U2OS xenograft tumors at the end of the experiment (45 days). The
body weight of untreated and taxifolin-treated mouse groups was
gradually reduced but not significantly altered (Fig. 4B). These results suggest that
taxifolin treatment inhibited osteosarcoma tumor growth in
vivo.
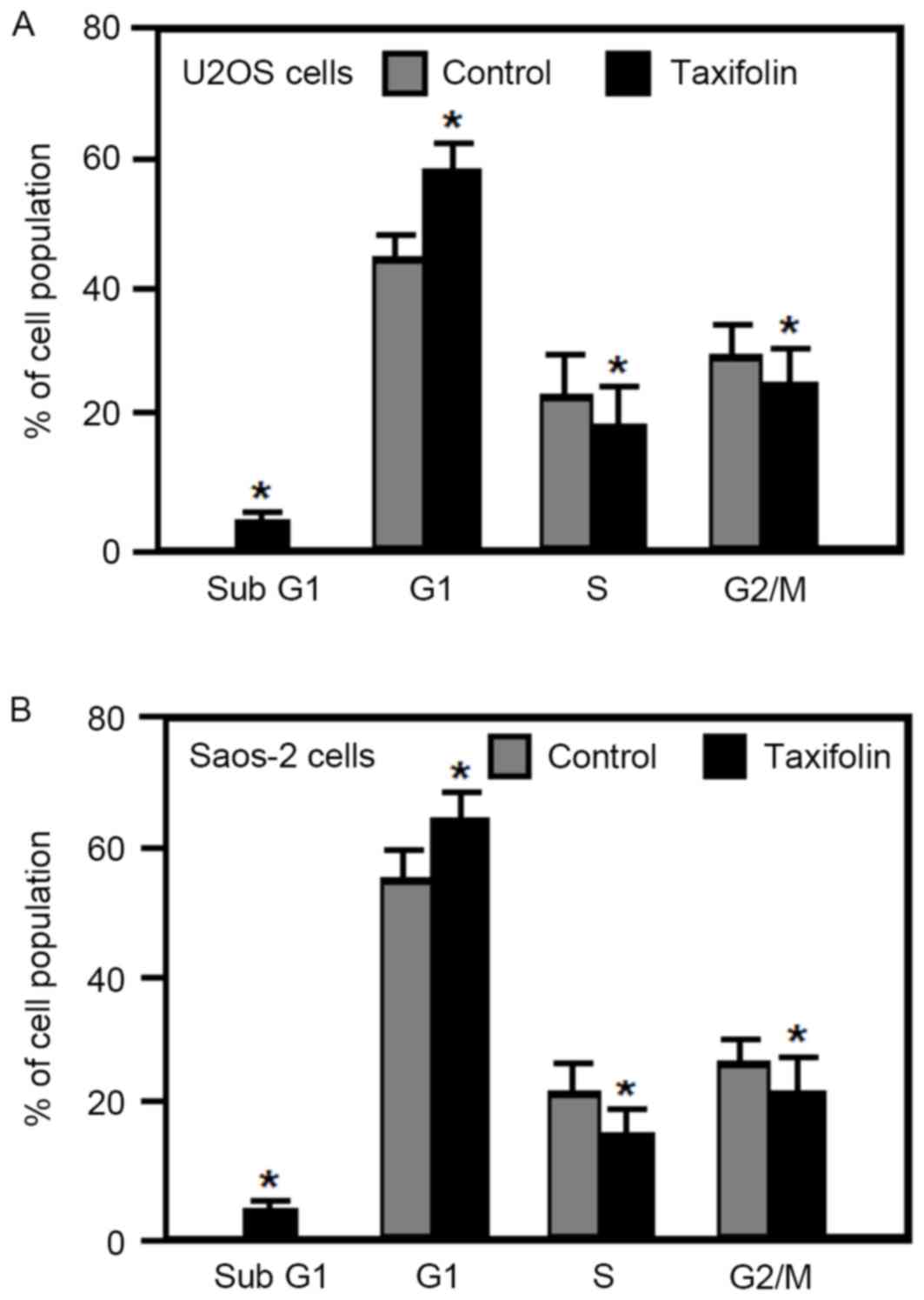
Effect of taxifolin on cell cycle
distribution and apoptosis
The effect of taxifolin on the cell cycle was
examined in human osteosarcoma U2OS and Saos-2 cells by flow
cytometry analysis. Treatment of U2OS and Saos-2 cells with 25 µM
taxifolin for 48 h significantly increased the percentage of cells
in the G1 phase, and significantly reduced the
percentage of cells in the S and G2/M phases when
compared with untreated cells (Fig.
5). In addition, taxifolin treatment increased the percentage
of U2OS and Saos-2 cells in the sub-G1 fraction (cells
undergoing apoptosis) when compared with untreated cells (Fig. 5). These results demonstrated that
taxifolin treatment induced G1 cell cycle arrest and
increased apoptosis in osteosarcoma U2OS and Saos-2 cells.
Effect of taxifolin on the expression
of cell cycle-associated proteins
Western blot analysis was performed to determine
whether taxifolin treatment altered the expression of proteins
involved in cell cycle regulation. Taxifolin treatment markedly
reduced the protein expression levels of AKT, p-Ser473-AKT, c-myc
and SKP-2 in U2OS and Saos-2 cells when compared with untreated
cells (Fig. 6A). Transfection of
U2OS cells with empty pcDNA3.1 plasmids or the pcDNA3.1-AKT vector
was performed in order to overexpress AKT and investigate its
association with c-myc and SKP-2. As demonstrated in Fig. 6B, overexpression of AKT markedly
reversed the taxifolin-induced repression of p-AKT, c-myc and SKP-2
protein expression levels, indicating that decreased AKT expression
may mediate taxifolin-induced c-myc and SKP-2 inhibition. These
results revealed that taxifolin reduced the expression levels of
signaling factors implicated in cell cycle regulation, potentially
via the AKT signaling pathway.
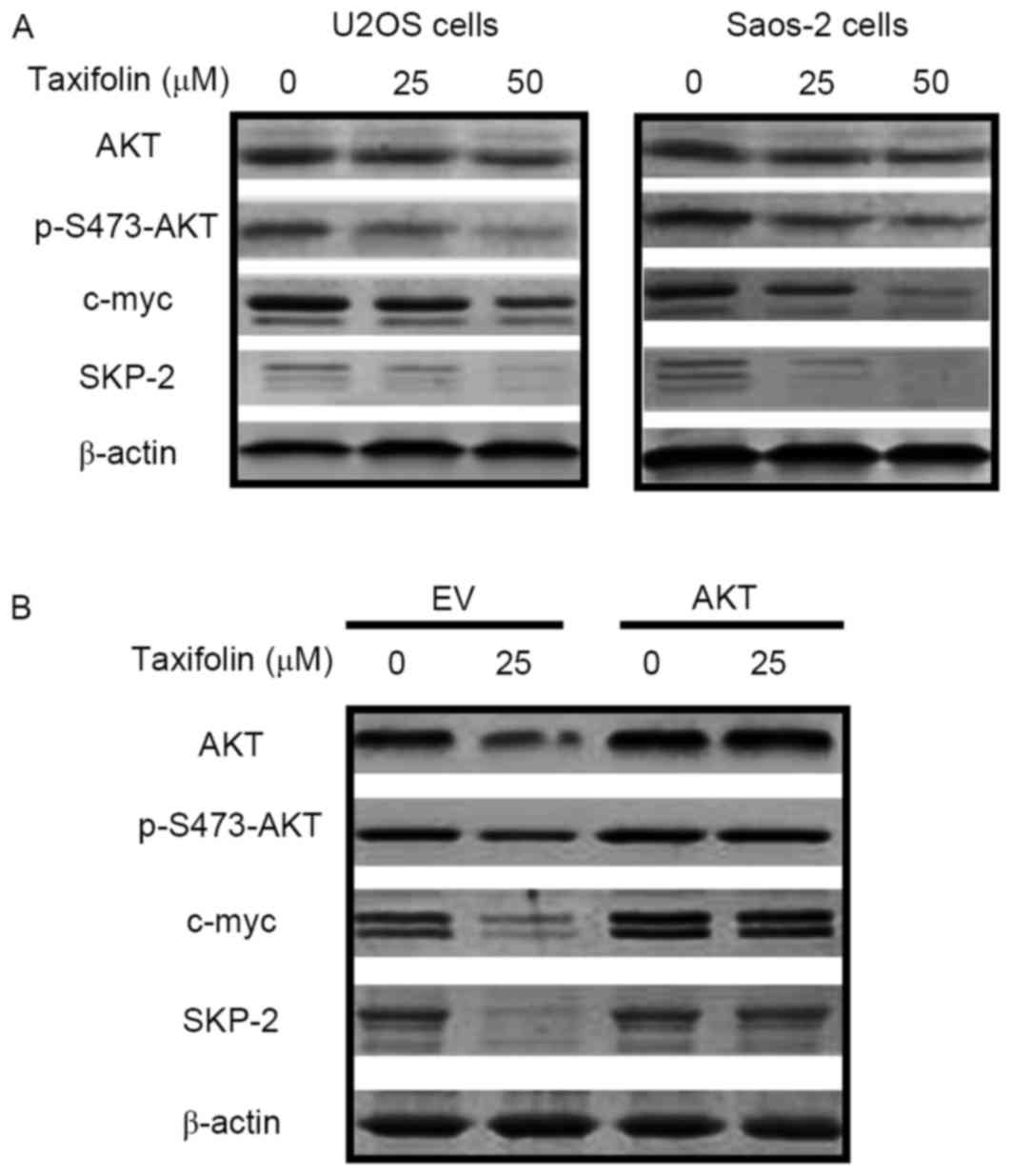 | Figure 6.Effect of taxifolin on the expression
of cell cycle proteins. The protein expression levels of AKT,
p-Ser473-AKT, c-myc, SKP-2 and β-actin were determined by western
blotting in (A) U2OS and Saos-2 cells treated with 0, 25 or 50 µM
taxifolin for 48 h, and (B) in U2OS cells transfected with an EV or
AKT-overexpressing vectors for 24 h, followed by treatment with
with 0 or 25 µM taxifolin for 48 h. AKT, AKT serine/threonine
kinase 1; p-S473-AKT, phosphorylated AKT at Ser 473; c-myc, v-myc
avian myelocytomatosis viral oncogene homolog; SKP-2, S-phase
kinase associated protein 2; EV, empty vector. |
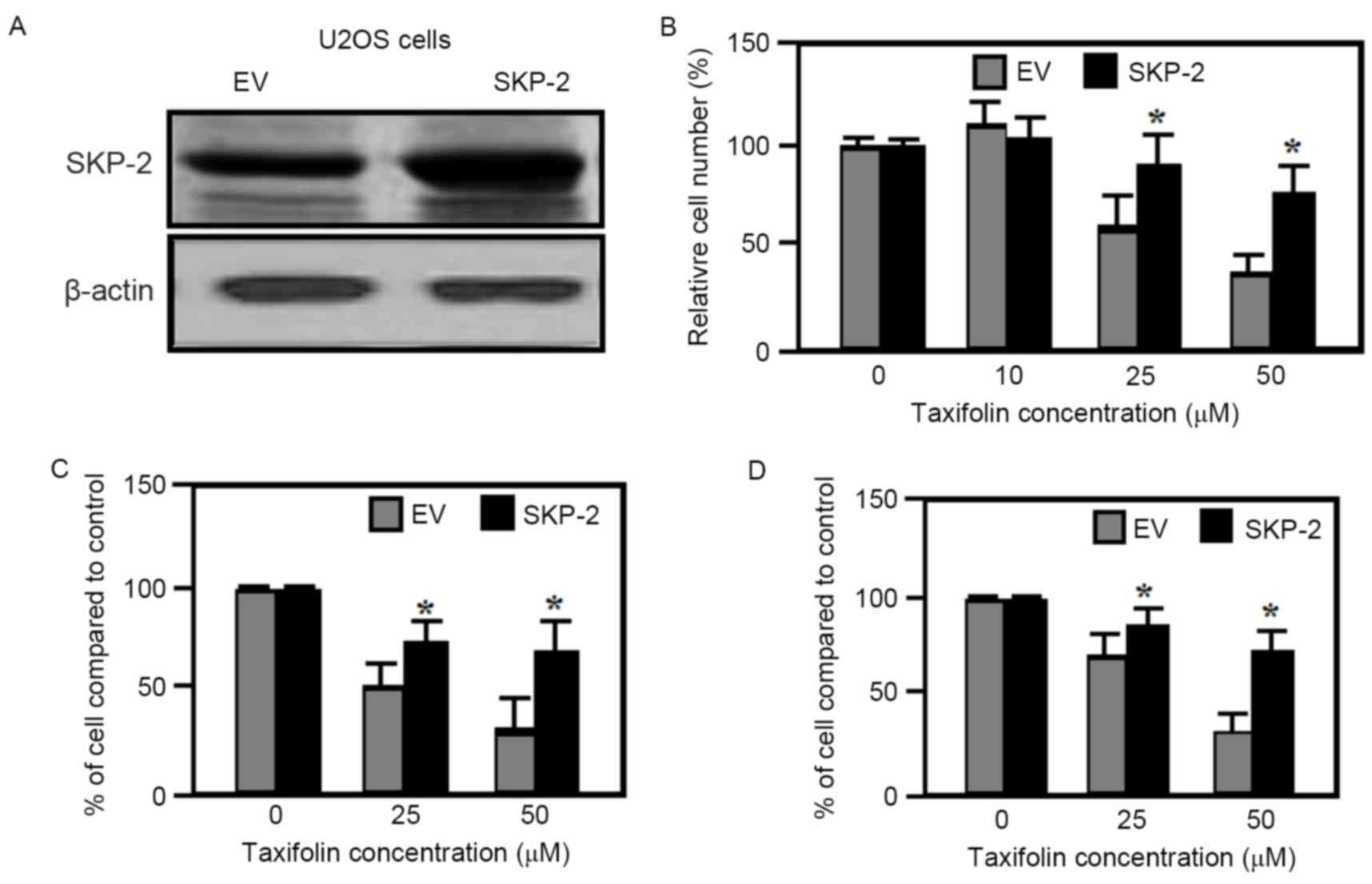
Effect of SKP-2 overexpression
As demonstrated in Fig.
6A, taxifolin treatment resulted in a gradual reduction in
SKP-2 expression levels in U2OS and Saos-2 cells. Therefore the
effect of SKP-2 overexpression on taxifolin-induced anti-cancer
properties was examined further. SKP-2 overexpression in U2OS cells
(Fig. 7A) partially inhibited the
taxifolin-induced decrease in cell viability when compared with
cells transfected with empty vectors (Fig. 7B). The migration and invasion of
U2OS cells transfected with empty vector plasmids were markedly
reduced by taxifolin treatment when compared with untreated cells
(Fig. 7C and D). By contrast,
overexpression of SKP-2 significantly attenuated the
taxifolin-mediated reduction in U2OS cell migration and invasion
when compared with empty vector-transfected cells (Fig. 7C and D). As overexpression of SKP-2
was not observed to eliminate the inhibitory effects taxifolin
completely, it is possible that additional mechanisms or signaling
pathways, other than AKT/SKP-2, may be triggered by taxifolin in
osteosarcoma cell lines.
Discussion
Flavonoids exhibit a broad range of pharmacological
and biological effects, with chemoprevention as one of their most
characterized effects (6,8). In the present study, the anti-cancer
properties of taxifolin were investigated in human U2OS and Saos-2
osteosarcoma cell lines. Treatment of osteosarcoma cells with
taxifolin resulted in G1 cell cycle arrest and increased
apoptosis. Results from the MTT assay, and transwell migration and
invasion assays revealed that taxifolin treatment inhibited cell
viability, as well as the migration and invasion abilities of human
osteosarcoma cells. In addition, the soft agar colony formation
assay revealed that taxifolin decreased the anchorage-independent
growth of osteosarcoma cells. Furthermore, taxifolin treatment
suppressed the expression of c-myc and SKP-2, and the inactivation
of AKT may have been responsible for these taxifolin-mediated
alterations in c-myc and SKP-2 expression. In vivo studies
demonstrated that taxifolin administration significantly inhibited
tumor growth in mice bearing U2OS cell-derived xenograft tumors.
These results indicate that taxifolin may present an effective
agent for the treatment of osteosarcoma.
In order to examine the mechanisms underlying the
anti-tumor effects of taxifolin further, western blotting analysis
was performed. The results indicated that the protein expression
levels of AKT, p-AKT (Ser473), c-myc and SKP-2 were markedly
reduced in U2OS and Saos-2 osteosarcoma cells treated with
taxifolin when compared to untreated cells. Phosphatidylinositol
3-kinase (PI3K)/AKT signaling activation is important during cell
proliferation and the progression of osteosarcoma (20). In addition, AKT is an important
regulator of cell survival pathways by inhibiting apoptosis, and
its kinase activation is mediated by Ser473 phosphorylation.
Overexpression of proteins in the PI3K/AKT signaling pathway is
associated with a poor clinical prognosis (21–23).
C-myc is a major transcriptional regulator. It is a
multi-functional proto-oncogene and a nuclear phosphoprotein that
mediates cell proliferation, cell cycle arrest and apoptosis
(24). Abnormal c-myc mRNA
expression promotes the invasion of osteosarcoma cells (25) and leads to cancer development
(26,27), whereas c-myc protein attenuation
leads to diminished cell proliferation and tumor growth in
osteosarcoma (28). Abnormal c-myc
function has been identified in stomach, breast, colon and lung
cancers (29). Therefore, c-myc is
considered to be a promising anti-cancer drug target (30). In the present study, taxifolin
treatment suppressed AKT, p-AKT (Ser473) and c-myc protein
expression, suggesting that c-myc repression may mediate the
inhibitory effects of taxifolin on cell growth.
SKP-2 serves an important role in regulating the
activation of cyclin-dependent kinase inhibitor 1B (CDKN1B; also
known as p27Kip1), as well as in regulating
ubiquitin-dependent processes (31,32).
CDKN1B inhibits cell proliferation by inducing cell cycle arrest at
the G1 phase (33). In
the present study, taxifolin treatment of U2OS and Saos-2 cells
reduced SKP-2 expression, promoted G1 cell cycle arrest
and induced apoptosis. SKP-2 overexpression partially inhibited the
taxifolin-induced reduction in U2OS cell viability, migration and
invasion, suggesting that SKP-2 suppression may be a major factor
in taxifolin-mediated growth inhibition of osteosarcoma cell lines.
As overexpression of SKP-2 did not completely reverse
taxifolin-mediated cell growth inhibition, it is possible that
additional signaling pathways may be induced by taxifolin to
inhibit osteosarcoma cell growth.
In conclusion, the results of the present study
revealed that taxifolin treatment inhibited the proliferation,
migration and invasion of U2OS and Saos-2 human osteosarcoma cell
lines, potentially via AKT and SKP-2 signaling pathways. The
results suggest that taxifolin may present an effective therapeutic
agent for the treatment of osteosarcoma.
Acknowledgements
This study was financially aided by Hanzhong Centre
Hospital, Shaanxi, China (grant no. HCH-17392/16).
References
|
1
|
Duong LM and Richardson LC: Descriptive
epidemiology of malignant primary osteosarcoma using
population-based registries, United States, 1999–2008. J Registry
Manag. 40:59–64. 2013.PubMed/NCBI
|
|
2
|
Savage SA, Woodson K, Walk E, Modi W, Liao
J, Douglass C, Hoover RN and Chanock SJ; National Osteosarcoma
Etiology Study Group, : Analysis of genes critical for growth
regulation identifies insulin-like growth factor 2 receptor
variations with possible functional significance as risk factors
for osteosarcoma. Cancer Epidemiol Biomarkers Prev. 16:1667–1674.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: State
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Longhi A, Fabbri N, Donati D, Capanna R,
Briccoli A, Biagini R, Bernini G, Ferrari S, Versari M and Bacci G:
Neoadjuvant chemotherapy for patients with synchronous multifocal
osteosarcoma: Results in eleven cases. J Chemother. 13:324–330.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Collins M, Wilhelm M, Conyers R, Herschtal
A, Whelan J, Bielack S, Kager L, Kühne T, Sydes M, Gelderblom H, et
al: Benefits and adverse events in younger versus older patients
receiving neoadjuvant chemotherapy for osteosarcoma: Findings from
a meta-analysis. J Clin Oncol. 31:2303–2312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Middleton E Jr, Kandaswami C and
Theoharides TC: The effects of plant flavonoids on mammalian cells:
Implications for inflammation, heart disease, and cancer. Pharmacol
Rev. 52:673–751. 2000.PubMed/NCBI
|
|
7
|
Dajas F, Rivera F, Blasina F, Arredondo F,
Echeverry C, Lafon L, Morquio A and Heizen H: Cell culture
protection and in vivo neuroprotective capacity of flavonoids.
Neurotox Res. 5:425–432. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slimestad R, Fossen T and Vågen IM:
Onions: A source of unique dietary flavonoids. J Agric Food Chem.
55:10067–10080. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo H, Zhang X, Cui Y, Zhou H, Xu D, Shan
T, Zhang F, Guo Y, Chen Y and Wu D: Taxifolin protects against
cardiac hypertrophy and fibrosis during biomechanical stress of
pressure overload. Toxicol Appl Pharmacol. 287:168–177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liang L, Gao C, Luo M, Wang W, Zhao C, Zu
Y, Efferth T and Fu Y: Dihydroquercetin (DHQ) induced HO-1 and NQO1
expression against oxidative stress through the Nrf2-dependent
antioxidant pathway. J Agric Food Chem. 61:2755–2761. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang ZR, Al Zaharna M, Wong MM, Chiu SK
and Cheung HY: Taxifolin enhances andrographolide-induced mitotic
arrest and apoptosis in human prostate cancer cells via spindle
assembly checkpoint activation. PLoS One. 8:e545772013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim YJ, Choi SE, Lee MW and Lee CS:
Taxifolin glycoside inhibits dendritic cell responses stimulated by
lipopolysaccharide and lipoteichoic acid. J Pharm Pharmacol.
60:1465–1472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahn JY, Choi SE, Jeong MS, Park KH, Moon
NJ, Joo SS, Lee CS, Choi YW, Li K, Lee MK, et al: Effect of
taxifolin glycoside on atopic dermatitis-like skin lesions in
NC/Nga mice. Phytother Res. 24:1071–1077. 2010.PubMed/NCBI
|
|
14
|
Rogovskiĭ VS, Matiushin AI, Shimanovskiĭ
NL, Semeĭkin AV, Kukhareva TS, Koroteev AM, Koroteev MP and
Nifant'ev EE: Antiproliferative and antioxidant activity of new
dihydroquercetin derivatives. Eksp Klin Farmakol. 73:39–42.
2010.(In Russian).
|
|
15
|
Vrba J, Gažák R, Kuzma M, Papoušková B,
Vacek J, Weiszenstein M, Křen V and Ulrichová J: A novel
semisynthetic flavonoid 7-O-galloyltaxifolin upregulates heme
oxygenase-1 in RAW264.7 cells via MAPK/Nrf2 pathway. J Med Chem.
56:856–866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chuu CP and Lin HP: Antiproliferative
effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on
multiple human cancer cell lines. Anticancer Res. 30:3643–3648.
2010.PubMed/NCBI
|
|
17
|
Wilson JK, Sargent JM, Elgie AW, Hill JG
and Taylor CG: Feasibility study of the MTT assay for
chemosensitivity testing in ovarian malignancy. Br J Cancer.
62:189–194. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chuu CP, Kokontis JM, Hiipakka RA, Fukuchi
J, Lin HP, Lin CY, Huo C, Su LC and Liao S: Androgen suppresses
proliferation of castration-resistant LNCaP 104-R2 prostate cancer
cells through androgen receptor, Skp2, and c-Myc. Cancer Sci.
102:2022–2028. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye J, Yin L, Xie P, Wu J, Huang J, Zhou G,
Xu H, Lu E and He X: Antiproliferative effects and molecular
mechanisms of troglitazone in human cervical cancer in vitro. Onco
Targets Ther. 8:1211–1218. 2015.PubMed/NCBI
|
|
20
|
Zhang A, He S, Sun X, Ding L, Bao X and
Wang N: Wnt5a promotes migration of human osteosarcoma cells by
triggering a phosphatidylinositol-3 kinase/Akt signals. Cancer Cell
Int. 14:152014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin HP, Lin CY, Liu CC, Su LC, Huo C, Kuo
YY, Tseng JC, Hsu JM, Chen CK and Chuu CP: Caffeic acid phenethyl
ester as a potential treatment for advanced prostate cancer
targeting akt signaling. Int J Mol Sci. 14:5264–5283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kreisberg JI, Malik SN, Prihoda TJ,
Bedolla RG, Troyer DA, Kreisberg S and Ghosh PM: Phosphorylation of
Akt (Ser473) is an excellent predictor of poor clinical outcome in
prostate cancer. Cancer Res. 64:5232–5236. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu CC, Hsu JM, Kuo LK and Chuu CP:
Caffeic acid phenethyl ester as an adjuvant therapy for advanced
prostate cancer. Med Hypotheses. 80:617–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kokontis J, Takakura K, Hay N and Liao S:
Increased androgen receptor activity and altered c-myc expression
in prostate cancer cells after long-term androgen deprivation.
Cancer Res. 54:1566–1573. 1994.PubMed/NCBI
|
|
25
|
Han G, Wang Y and Bi W: C-Myc
overexpression promotes osteosarcoma cell invasion via activation
of MEK-ERK pathway. Oncol Res. 20:149–156. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Edwards J, Krishna NS, Witton CJ and
Bartlett JM: Gene amplifications associated with the development of
hormone-resistant prostate cancer. Clin Cancer Res. 9:5271–5281.
2003.PubMed/NCBI
|
|
27
|
Grad JM, Dai JL, Wu S and Burnstein KL:
Multiple androgen response elements and a Myc consensus site in the
androgen receptor (AR) coding region are involved in
androgen-mediated up-regulation of AR messenger RNA. Mol
Endocrinol. 13:1896–1911. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Z, Zhang G, Li J, Liu J and Lv P: The
tumor-suppressive microRNA-135b targets c-myc in osteoscarcoma.
PLoS One. 9:e1026212014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Field JK and Spandidos DA: The role of ras
and myc oncogenes in human solid tumours and their relevance in
diagnosis and prognosis (review). Anticancer Res. 10:1–22.
1990.PubMed/NCBI
|
|
30
|
Matthews GM, Lefebure M, Doyle MA, Shortt
J, Ellul J, Chesi M, Banks KM, Vidacs E, Faulkner D, Atadja P, et
al: Preclinical screening of histone deacetylase inhibitors
combined with ABT-737, rhTRAIL/MD5-1 or 5-azacytidine using
syngeneic Vk*MYC multiple myeloma. Cell Death Dis. 4:e7982013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carrano AC, Eytan E, Hershko A and Pagano
M: SKP2 is required for ubiquitin-mediated degradation of the CDK
inhibitor p27. Nat Cell Biol. 1:193–199. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsvetkov LM, Yeh KH, Lee SJ, Sun H and
Zhang H: p27(Kip1) ubiquitination and degradation is regulated by
the SCF (Skp2) complex through phosphorylated Thr187 in p27. Curr
Biol. 9:661–664. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elledge SJ and Harper JW: Cdk inhibitors:
On the threshold of checkpoints and development. Curr Opin Cell
Biol. 6:847–852. 1994. View Article : Google Scholar : PubMed/NCBI
|