Introduction
Copper is a trace element that is important for
neuronal functions. Additionally, it is a cofactor of enzymes and
proteins that are required for a number of physiological functions,
including neural transmission, the scavenging of free radicals, the
production of energy and the mobilization of iron (1,2). As
an important cofactor of metalloproteins, Cu ions act as an active
or structural site. However, excessive exposure to Cu has been
associated with central nervous system (CNS) dysfunction, including
memory loss, multiple neuritis and neurasthenia syndrome (3,4).
Additionally, post-mortem studies have demonstrated that Cu levels
are markedly elevated in the brains of patients with Alzheimer's
disease (AD) (5). The
neurotoxicity caused by excessive Cu is thought to be an important
risk factor for cognitive impairment in the ageing population,
particularly learning and memory impairment (6).
Numerous studies have associated chronic Cu
intoxication with AD-like pathology (7). For example, treating primary
hippocampal neurons (10–14 days in vitro) with
CuCl2 (up to 10 µM) for 3 h was observed to
significantly increase the amplitude, frequency and time constants
of synaptic events (8). In
addition, Cu has been demonstrated to be involved in the synthesis
of phosphatidyl-L-serine and phosphatidyl inositide complexes
through a process that required ATP-mediated regulation (9).
Mitochondria are an energy-producing organelle and
are thus vital for cell survival. Cu is involved in a number of
signalling cascades and has been hypothesised to serve important
roles in neurodegenerative processes associated with respiratory
chain dysfunction and the generation of reactive oxygen species
(ROS). Cu is considered to be a co-factor for complex IV of the
mitochondrial electron transport chain and for cytochrome c
oxidase. Notably, Cu deficiency has been observed to impair brain
development, as it impaired mitochondrial function and led to a
disorder of brain energy metabolism (10). Conversely, previous studies have
reported Cu concentrations as high as 200 or 400 µM in
neurodegenerative diseases (11,12).
As co-factors of certain enzymes, Cu ions are involved in the
generation of ATP and the degradation of ROS in mitochondria.
γ-aminobutyric acid (GABA) is recognized as an
important inhibitory neurotransmitter in the brain, while glutamate
(Glu) functions as an excitatory neurotransmitter; in the brain,
these transmitters serve a role in cognitive functions, including
learning and memory. Cu has been observed to dampen
GABAA and Glu receptor function (13–15).
A previous study proposed a link between Cu and the activity of the
N-methyl-D-aspartic acid subtype of Glu receptors (GluNRs), with a
functional link between Cu homeostasis and GluNR activity (16). In the present study,
neurometabolites in the hippocampus of rats acutely exposed to
various doses of CuCl2 were measured via proton magnetic
resonance spectroscopy (1H-MRS) and high-performance
liquid chromatography (HPLC), and the spatial learning and memory
of the animals were evaluated. The spatial learning and memory of
the rats were affected in a dose-dependent manner and the
beneficial effect of a relatively low dose of CuCl2 was
associated with a mild increase in Glu in the hippocampus of the
rats.
Materials and methods
Animals and experimental design
A total of 60 male Sprague-Dawley (S-D) rats (age, 7
weeks; weight, 200±34 g) were used in the present study. The
animals were purchased from the Experimental Animal Center of
Shantou University Medical College (Shantou, China) and were group
housed (7–8 rats/cage) under standard laboratory conditions (22±1°C
temperature and 5±4% humidity) on a 12-h light/dark cycle (7:00
a.m. on; 7:00 p.m. off). Standard rat diet and water were given
ad libitum. Following 7 days of acclimatization, the rats
were randomly and equally divided into six groups (10 rats/group).
Various doses (0, 0.5, 1.0, 2.0, 4.0 and 6.0 mg/kg) of
CuCl2 (Yuanye Biotechnology, Co., Ltd., Shanghai, China)
in sterilized saline were given to the rats by intraperitoneal
injection three times every other day for a 6-day period. The
injection volume was 1.0 ml/kg. A total of 2 days subsequent to the
last injection, all rats were subjected to the Morris water maze
(MWM) test to evaluate hippocampus-dependent spatial learning and
memory abilities. One training test was performed each day for 4
consecutive days, followed by one probe test on the 5th day. A
total of 24 h subsequently, the rats were subjected to magnetic
resonance imaging (MRI) and the 1H-MRS procedure.
Subsequently, the rats were sacrificed and their brains were
removed. The right hippocampus was dissected out on ice and stored
at −80°C for subsequent HPLC analysis. All animal procedures were
performed in accordance with the guidelines set up by the Animal
Care and Use Committee of Shantou University Medical College and
were approved by the committee.
MWM test
The conventional MWM test was performed to evaluate
the spatial learning and reference memory abilities of the rats
(17). As previously described,
the rats were placed in a circular white tank with a diameter of
120 cm and a depth of 50 cm. The tank was filled with opacified
water (25±1°C) to a height of 38 cm and was surrounded by dark
geometric cues affixed to white curtains (18,19).
The tank was divided into four imaginary quadrants (quadrants I,
II, III and IV), and an escape platform was positioned 2 cm under
the horizontal plane in the middle of quadrant II. The MWM test
consisted of two phases: The place navigation test and the spatial
probe test.
All rats were habituated to the maze 1 day prior to
training. During the place navigation training session, each rat
was placed into the tank at a randomly selected position and
allowed to explore the pool using a systematic or random search
strategy. The behaviour of the rats in the water maze was
videotaped using a video camera suspended above the maze that
interfaced with a computer-based video tracking system. One
navigation test was completed each day for 4 consecutive days,
followed by one spatial probe test on the 5th day.
The platform was submerged during the training.
During the place navigation trial, the rats were placed in the
water and were allowed to search for the submerged platform for 60
sec. If the rat failed to find the platform, the operator moved it
to the platform, where it remained for 20 sec. The escape latency
was measured as described previously (18).
On the first day following 4 days of training
sessions, a probe trial was completed. The platform was removed,
and the rat was placed into the water in the quadrant opposite the
target quadrant (quadrant II) and allowed to swim for 120 sec. Rats
that failed to locate the platform within 120 sec were manually
guided to the platform and kept there for 20 sec. As the rats swam
around the pool, various parameters, including the number of
platform crossings, the time required to reach the platform, the
ratio of distance travelled in the target quadrant, the percentage
time spent in the target platform quadrant, the total distance and
the average travel speed were recorded using the DigBehav-Morris
Water Maze Video Analysis System (Jiliang Software Technology Co.,
Ltd., Shanghai, China). When all tasks had been completed, the rats
were dried and placed back into the housing facility once their
body temperature had returned to a normal level (36–37°C).
MRI/MRS acquisition
The in vivo MRI/MRS experiments were
performed using a horizontal bore (bore size, 160 mm) Agilent 7.0
Tesla animal MRI scanner (Agilent Technologies, Inc., Santa Clara,
CA, USA), equipped with a 20-mm standard one-channel 1H
volume coil for radio frequency transmission and reception. The
rats were initially anaesthetised with 5% isoflurane (Abbott
Pharmaceutical Co., Ltd., Lake Bluff, IL, USA) in oxygen and
continuously anaesthetised with the 1–2% isoflurane (for
maintenance), which was delivered through a nasal mask for
spontaneous respiration. Following anaesthetisation, each rat was
placed in a prone position in the centre region of the horizontal
bore with its head fixed on a palate holder equipped with an
adjustable nose cone for MRI/MRS acquisition. Body temperature was
recorded through the respiration system and was maintained at
36–37°C using a plastic membrane.
In order to ensure high image quality, the target
must be placed in the centre of the magnet. In order to achieve
correct positioning, images of the head in three planes were
acquired with a gradient echo sequence. Subsequently, T2-weighted
images in coronal, sagittal and axial positions were obtained using
a fast spin echo multi-slice pulse sequence with the following
parameters: Field of view=23×19 mm2; matrix
size=256×256; repetition time (TR)=2,000 msec; effective echo time
(TE)=31.58 msec; slice thickness=2 mm; slice gap=0.5 mm; and
acquisition time=4 min 20 sec. The high-resolution T2-weighted
images were used to position the 1H-MRS voxels in the
hippocampus.
Following morphological imaging, the volume of
interest (VOI) was located by referring to a digital rat brain
atlas (20). All MR spectra were
acquired using a single-voxel, ultra-short echo-time stimulated
echo acquisition mode pulse sequence, with TR=3,000 msec and TE
=2.35 msec. The total number of acquisitions was 256. The 3×3×3
mm3 VOI primarily contained the right dorsal hippocampus
in addition to some adjacent tissue. Outer volume suppression was
performed around the VOI to inhibit any non-hippocampal contaminant
signals. Automated shimming was performed for the VOI to yield a
water spectrum width of 15–20 Hz. The B0 field was shimmed using a
3D gradient shimming method. Shortly afterwards, a manual shim
based on second-order 3D gradient shimming was adopted to reduce
any signal in homogeneities in the B0 field. Localized voxel
shimming was performed using the FASTMAP technique prior to
spectral acquisition (21). Outer
volume suppression and water suppression with variable pulse power
and optimized relaxation delays were used to acquire proton
spectra. In order to correct for small variations in coil
sensitivity, the unsuppressed water signal from the prescribed
voxel was used as a reference (22). The total scan duration of one MRS
measurement was 13 min and 54 sec. The raw spectral data were
exported to an external VnmrJ v4.0 workstation (Agilent
Technologies, Inc.) for post-processing.
MRS data processing and analysis
The MRS-based quantification of metabolite
concentrations was performed using LCModel software (version
6.2–4E; LCModel Inc., Oakville, ON, Canada) (23). The raw data were processed using
LCModel. Quantitative metabolite concentrations were obtained from
the raw data following processing using LCModel and scaled to the
water signal. The quantitative analysis algorithm of LCModel, which
is based on linear combinations, was used to calculate the optimal
fitting of the objective spectra to the model spectra. The tissue
water concentration was used as the internal standard. Only spectra
with a full width at half maximum <20, metabolite concentration
fitting results with Cramer-Rao lower bound <20% (24) and a signal-to-noise ratio ≥10 were
used for data analysis.
The base set included the following 17 metabolites:
Alanine, aspartate, Cr, GABA, glucose, Glu, glutamine (Gln),
glutathione, glycerophosphorylcholine (GPC), lactate, myo-inositol
(mI), N-acetyl-L-aspartate (NAA), N-acetylaspartylglutamate (NAAG),
phosphorylcholine (PCh), phosphocreatine (PCr), scyllo-inositol and
taurine (Tau). The signal intensities were processed with water
scaling for absolute quantification of the metabolic
concentrations. Additionally, the sums of certain metabolites,
including NAA+NAAG, Cr+PCr, Glu+Gln and GPC+PCh, were measured.
Preparation of tissue samples for HPLC
analysis
The right hippocampus was homogenized (10% w/v) in
ice-cold phosphate buffer solution (0.1 M; pH 7.2; Yuanye
Biotechnology, Co., Ltd.). The homogenate was centrifuged at 12,000
× g for 20 min at 4°C. The supernatant was mixed with an equivalent
volume of methanol for overnight protein precipitation at 4°C, and
the resulting samples were frozen at −80°C following membrane
filtration.
Quantification of GABA and Glu in
vitro
The HPLC procedure was performed according to a
previously described method (25).
Liquid chromatography was performed using an Agilent 1100 HPLC
system (Agilent Technologies, Inc.). In order to detect the
concentrations of GABA and Glu, a standard curve was obtained for
each under standard chromatographic conditions, with concentration
as the horizontal axis and the product peak area as the vertical
axis. Chromatographic separation was obtained on a phase column
with an Agilent C18 guard column (250×4.6 mm2; 5 µm;
Agilent Technologies, Inc.). The temperature of the column was
maintained at 25°C. The mobile phase consisted of a mixture of 0.1
mol/l sodium acetate (pH 6.8 with 2% tetrahydrofuran), methanol and
water at a flow rate of 0.75 ml/min. The GABA and Glu levels were
determined using a scanning fluorescence detector with the
excitation and emission wavelengths set at 338 nm and 425 nm,
respectively. The standard lines of GABA and Glu were
yGABA=9.94×+54.24 (R2=0.99) and
yGlu=12.06×+122.2 (R2=0.99) The HPLC system
was connected to a computer to quantify the mixture of GABA and Glu
by comparing the area under each peak with the corresponding
measure of each reference standard, using the Agilent chemical
workstation software (ChemStation for LC 3D, Rev. A.10.01; Agilent
Technologies, Inc.). The Cu concentration in the brain tissue of
the rats was not measured in the present study since Zhang et
al (26) demonstrated that
intranasal delivery of Cu nanoparticles at 1 and 10 mg/kg for 15
days did not change the Cu concentration in different tissues,
including liver, lung, spleen, kidney and brain, in mice.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS software
(version 16.0; SPSS, Inc., Chicago, IL, USA), and P<0.05 was
considered to indicate a statistically significant difference. For
the behavioural tests, the escape latency in the MWM test was
analysed by repeated measures generalised linear model and
multivariate analysis of variance (ANOVA) procedures, and one-way
ANOVA with a Fisher's least significant difference post hoc test
was performed for the other data. For the 1H-MRS and
HPLC brain metabolite data, the normality and homogeneity of the
data were verified. The data were statistically analysed by one-way
ANOVA as long as they satisfied the normality and homogeneity
assumptions; otherwise, Mann-Whitney and Kruskal-Wallis
non-parametric tests were performed. Correlations between the brain
metabolite concentrations detected through 1H-MRS and
HPLC, and the behavioural test results, were investigated using
Pearson and Spearman tests.
Results
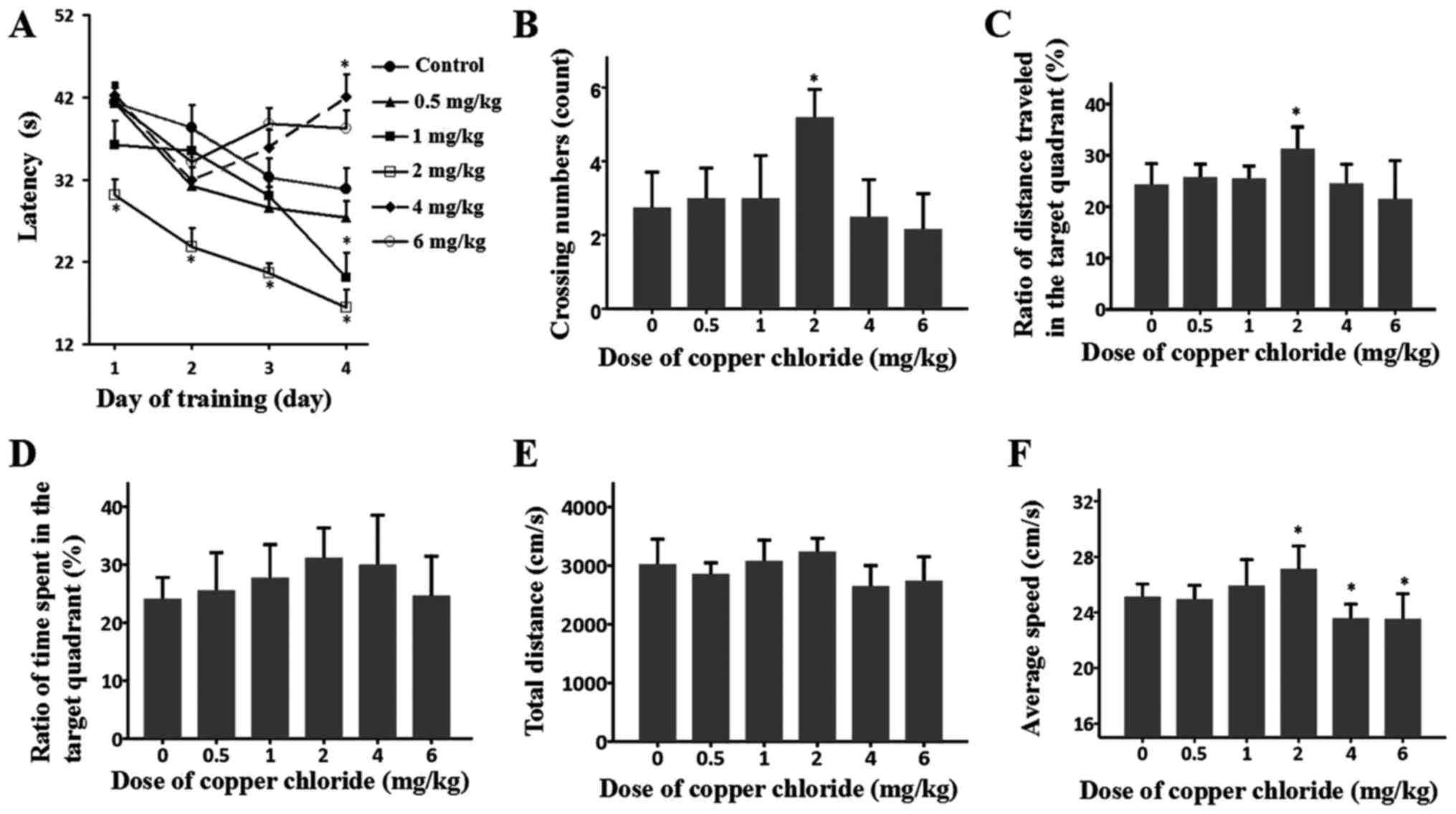
Behavioural test
In order to examine the effects of exogenous Cu
exposure on the spatial learning and memory of S-D rats, the MWM
test was performed with subjects that were intraperitoneally
injected with various doses of CuCl2 (0, 0.5, 1.0, 2.0,
4.0 and 6.0 mg/kg). Overall, there was a significant effect of day
and latency (F(3, 236)=8.28; P<0.01), and an
interaction between day and group was observed (F(5,
234)=14.49; P<0.01). During the training phase, on the
first day, all groups spent a comparable amount of time finding the
submerged platform, except for the group treated with 2.0 mg/kg
CuCl2; this group spent less time finding the platform
(P=0.002). On the following 3 days, the rats treated with 4.0 and
6.0 mg/kg CuCl2 exhibited no progress in the learning
phase in terms of the time spent finding the platform; by contrast,
all of the other groups exhibited typical progress in the water
maze test, as they spent less and less time finding the platform
(Fig. 1A). During the probe test,
the performance of the 0.5 and 1.0 mg/kg groups was comparable with
that of the control group, although the 2.0 mg/kg group crossed the
target quadrant more frequently than the control group (P<0.05).
By contrast, the 4.0 and 6.0 mg/kg groups visited the target
quadrant markedly fewer times (Fig.
1B). These results indicated deficient memory retrieval in the
groups that received higher doses and a potential beneficial effect
on memory retrieval in the 2.0 mg/kg CuCl2 group. The
ratios of distance travelled in the target quadrant (Fig. 1C), the ratios of time spent in the
target quadrant (Fig. 1D), the
total distance (Fig. 1E) and the
average speed data (Fig. 1F)
additionally suggested an adverse or beneficial effect of the
different doses of CuCl2 on the MWM performance of the
various groups.
1H-MRS analysis
The 1H-MRS analysis was performed
subsequent to the MWM test. A 3×3×3 mm3 VOI was
positioned in the right side of the dorsal hippocampus of the rat,
as presented in the localization images (Fig. 2A). Representative spectra acquired
from this VOI in each group are presented in Fig. 2B. The absolute concentrations of
brain metabolites, including Cr, PCr, Cr+PCr, GABA, Glu, Gln,
Glu+Gln, NAA, NAAG, GPC+PCh, mI and Tau, were calculated using
LCModel. For Cr, all rats exposed to CuCl2 were observed
to have levels comparable to those of the control group. In terms
of PCr, no difference was noted between the control group and any
other group, except for the 2.0 mg/kg group, which exhibited a
significantly increased level (P<0.01). Therefore, Cr + PCr
levels exhibited the same pattern as PCr levels. The effects of
CuCl2 on the levels of NAA and NAA+NAAG exhibited
typical bell-shaped curves; significantly increased levels of NAA
were observed only in the 2.0 mg/kg group (P=0.018), while NAA+NAAG
levels were increased in the 1.0 and 2.0 mg/kg groups (P=0.011 and
P=0.01, respectively). The CuCl2-induced alterations in
GABA, Glu, Gln, and Glu+Gln produced similar bell curves, although
the alterations were not significant. No differences in mI and
GPC+PCh levels were observed between the control group and any
other group. The only significant difference in Tau levels was
observed between the 2.0 mg/kg and control groups (P=0.021;
Table I).
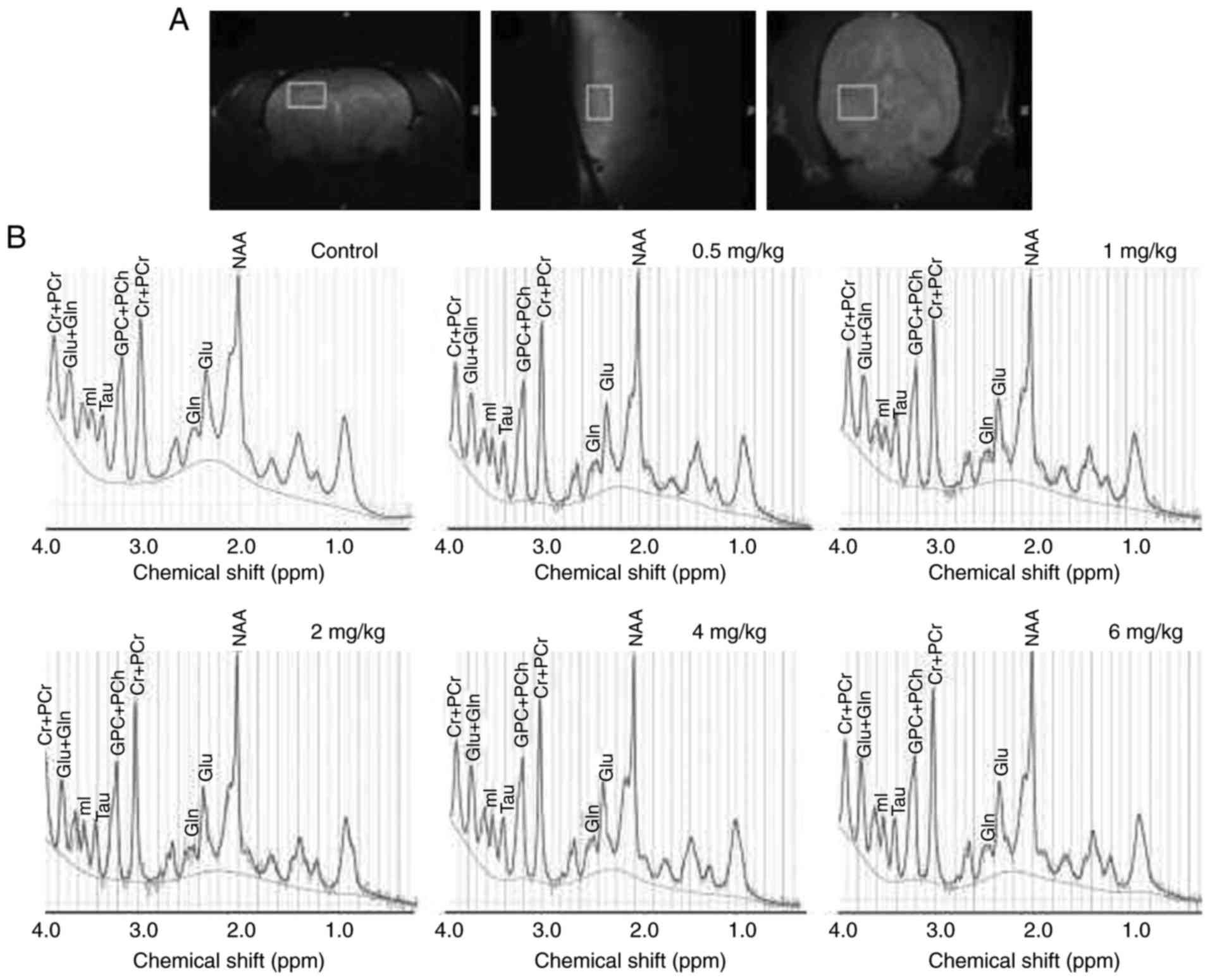 | Figure 2.Location of the VOI and
representative 1H-MRS spectra for all groups. (A) The
VOI was located in the right dorsal hippocampus of the rats, in the
coronal, saggital and axial directions. (B) Representative
1H-MRS spectra obtained from the control and 0.5, 1.0,
2.0, 4.0 and 6.0 mg/kg CuCl2 exposure groups. Cr,
creatine; PCr, phosphocreatine; Glu, glutamate; Gln, glutamine; mI,
myo-inositol; Tau, taurine; PCh, phosphorylcholine; NAA,
N-acetylaspartate; VOI, volume of interest; 1H-MRS,
proton magnetic resonance spectroscopy. |
 | Table I.Brain metabolite concentrations
determined by in vivo proton magnetic resonance spectroscopy
in hippocampal tissue (mmol/l). |
Table I.
Brain metabolite concentrations
determined by in vivo proton magnetic resonance spectroscopy
in hippocampal tissue (mmol/l).
| Metabolite | Control | CuCl2
(0.5 mg/kg) | CuCl2
(1.0 mg/kg) | CuCl2
(2.0 mg/kg) | CuCl2
(4.0 mg/kg) | CuCl2
(6.0 mg/kg) |
|---|
| Cr | 2.91±0.15 | 2.80±0.24 | 2.84±0.15 | 2.61±0.06 | 2.67±0.49 | 2.56±0.12 |
| PCr | 3.30±0.28 | 3.35±0.76 | 3.44±0.22 |
4.37±0.57b | 3.28±0.20 | 3.23±0.35 |
| Cr+PCr | 5.65±0.50 | 5.87±0.76 | 6.12±0.24 |
6.73±0.27b | 5.54±0.17 | 5.51±0.47 |
| NAA | 5.70±0.61 | 5.85±0.96 | 6.27±0.43 |
6.63±0.20a | 6.18±0.44 | 5.80±0.64 |
| NAA+NAAG | 6.45±0.62 | 6.57±0.99 |
7.13±0.44a |
7.49±0.51b | 6.71±0.28 | 6.29±0.33 |
| GABA | 1.63±0.39 | 1.65±0.23 | 1.64±0.19 | 1.66±0.27 | 1.60±0.20 | 1.54±0.22 |
| Glu | 7.22±0.40 | 7.41±0.31 | 7.46±0.55 | 7.57±0.76 | 7.20±0.49 | 7.15±0.05 |
| Gln | 1.74±0.34 | 1.82±0.28 | 1.95±0.35 | 1.93±0.62 | 1.71±0.22 | 1.74±0.36 |
| Glu+Gln | 8.90±0.61 | 9.12±0.15 | 9.49±0.87 | 9.63±1.19 | 8.81±0.40 | 8.79±0.25 |
| GPC+PCh | 1.31±0.09 | 1.21±0.20 | 1.35±0.11 | 1.27±0.17 | 1.27±0.20 | 1.43±0.10 |
| mI | 4.22±0.68 | 4.33±0.87 | 4.51±0.28 | 4.65±0.28 | 4.34±0.46 | 3.99±0.38 |
| Tau | 3.97±0.43 | 4.09±0.65 | 4.41±0.45 |
4.65±0.32a | 4.23±0.43 | 4.35±0.62 |
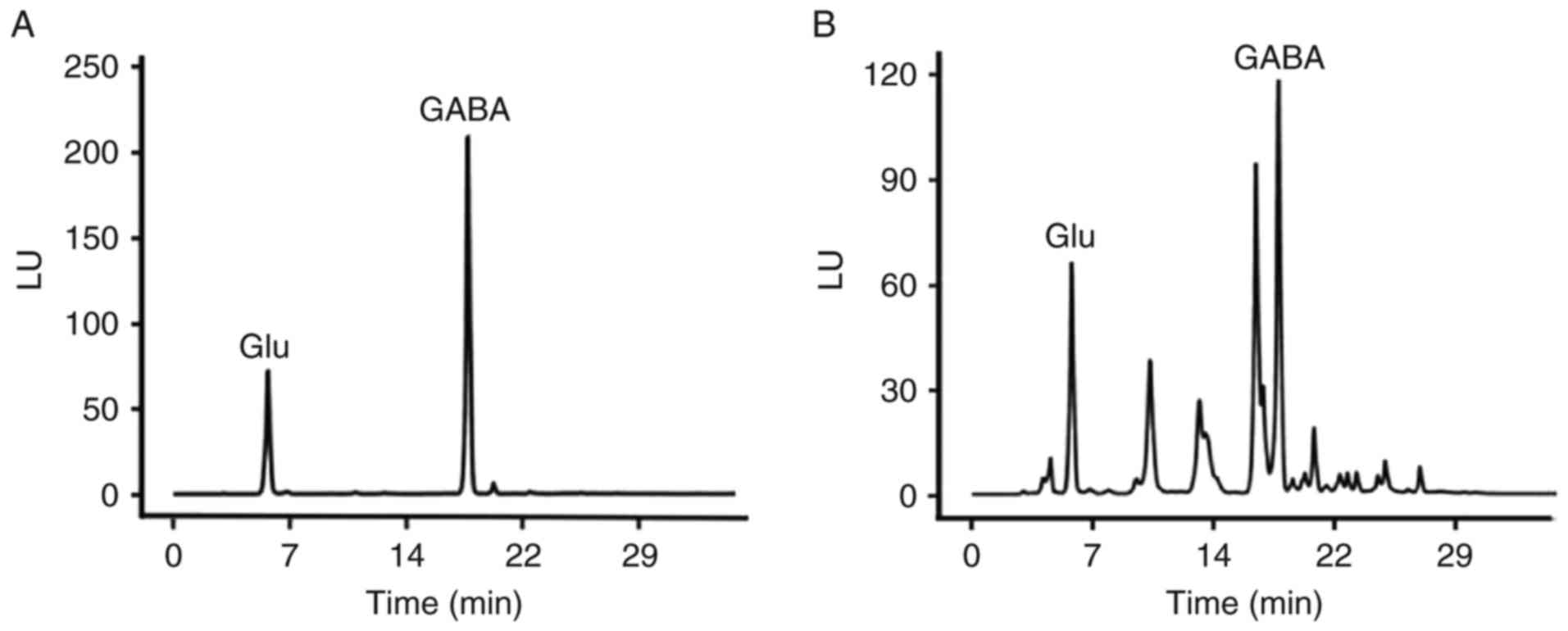
HPLC analysis
HPLC analysis, which may avoid certain confounding
factors that may affect 1H-MRS, including breathing,
inhomogeneity of the B0 field and volume contamination, was
performed to further evaluate the levels of two neurotransmitters,
GABA and Glu, which were of interest. The absolute concentrations
of these two neurotransmitters were determined subsequent to a
standard curve being obtained for each. GABA and Glu peaks for a
standard sample and a typical base peak chromatogram obtained for a
hippocampus sample from a control rat are presented in Fig. 3. Similar to the 1H-MRS
results, the effects of CuCl2 on GABA and Glu levels
were shaped like bell curves. The highest GABA level was observed
in the 2.0 mg/kg group, while comparable, although decreased,
levels were observed in the other groups (P=0.028). However, the
bell curve of the effect on Glu levels was unique. The curve
increased until it reached the highest level for the 2.0 mg/kg
group and subsequently declined (P=0.039). A remarkable decrease
was observed for the 4.0 and 6.0 mg/kg groups (P=0.042 and
P<0.01) compared with the control group (Table II).
 | Table II.Concentrations of GABA and Glu
detected by high-performance liquid chromatography (mmol/g). |
Table II.
Concentrations of GABA and Glu
detected by high-performance liquid chromatography (mmol/g).
| Metabolite | Control | CuCl2
(0.5 mg/kg) | CuCl2
(1.0 mg/kg) | CuCl2
(2.0 mg/kg) | CuCl2
(4.0 mg/kg) | CuCl2
(6.0 mg/kg) |
|---|
| GABA | 2.83±0.17 | 3.01±0.34 | 3.35±0.39 |
3.53±0.83b | 2.97±0.34 | 2.75±0.57 |
| Glu | 6.00±0.48 | 6.14±0.56 | 6.31±0.75 |
6.63±0.74a |
5.42±0.72a |
4.08±0.53b |
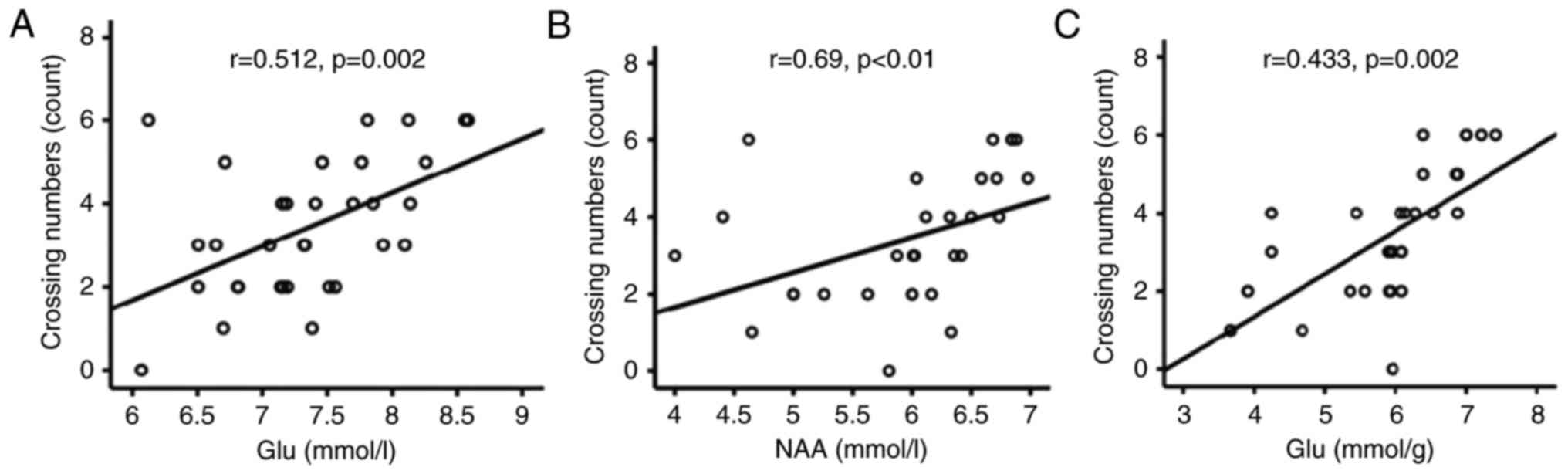
Positive correlations between spatial
memory and levels of Glu and NAA
In order to examine the association between the
CuCl2-induced effects on spatial memory and metabolite
concentrations detected by 1H-MRS and HPLC, the
correlation between metabolite concentrations and crossing numbers
was analysed using Pearson and Spearman correlation tests. This
analysis revealed that the number of platform crossings on probe
trials was positively correlated with the hippocampal levels of Glu
(r=0.512; P=0.002; Fig. 4A) and
NAA (r=0.69; P<0.001; Fig. 4B)
detected by 1H-MRS. In accordance with the
1H-MRS results, a similar positive correlation was
identified between the number of crossings and the Glu levels
revealed by HPLC analysis (r=0.433; P=0.002; Fig. 4C).
Discussion
An increasing number of studies have reported on the
effects of chronic Cu exposure on brain functions. However, the
reported results have been inconsistent; Cu excess and deficiency
have been demonstrated to impair the spatial memory capacity of the
brain. For example, chronic exposure to Cu in drinking water has
been demonstrated to impair spatial memory in mice (27), and chronic Cu exposure has been
additionally observed to accelerate memory impairment in 3×Tg-AD
mice (28). In another study,
compared with control rats, Cu-administered animals exhibited
impaired spatial memory in addition to significantly decreased
serum acetylcholinesterase activity (29). However, Cu deficiency is
detrimental to the development of brain function. In support of
this hypothesis, following water-mediated Zn supplementation for 4
months, S-D rats exhibited significantly increased levels of
anxiety compared with controls raised on lab water. The Zn-treated
rats took a markedly longer time to reach the platform in the MWM,
suggesting a spatial memory deficiency. These behavioural changes
are thought to be relevant to Cu deficiency as the addition of Cu
to the Zn-supplemented water returned freezing and latency values
closer to those of controls (30).
The authors additionally examined the effect of increasing Zn
levels in the drinking water in Tg2576 mice. Compared with mice
raised on lab water, the Zn-supplemented mice exhibited a
significant increase in latency and fewer platform crossings on
probe trials in the MWM test. However, no significant differences
were observed between the Zn+Cu group and the group raised on lab
water. The authors suggested that the negative consequences of Zn
may result in reduced Cu levels and that the effects may be due to
an imbalance of these metal ions, rather than a direct effect of
increased Zn (31). Similarly, a
recent in vitro study observed biphasic effects of Cu on rat
learning and memory in the MWM test. Free hippocampal Cu was
demonstrated to increase at a lower concentration of Cu (II)
acetate [Cu(OAc)2] (0.2 mg/kg), which improved learning
and memory. However, increased doses of Cu decreased superoxide
dismutase-1 (SOD1) activity, increased malondialdehyde (MDA) levels
and impaired spatial cognition (32).
Dysregulation of Cu metabolism contributes to an
interruption of neuronal function, and further leads to
neurodegeneration, necrosis and gliocyte hyperplasia (32). The effects of acute Cu exposure on
the spatial learning and memory of rats in vivo were
examined for the first time, to the best of our knowledge, in the
present study. The following results were observed in the present
study: i) 0.5 and 1.0 mg/kg CuCl2 exerted no effects;
ii) 2.0 mg/kg CuCl2 promoted the learning process in the
training phase of the MWM test and benefited spatial learning and
memory in the probe test; and iii) 4.0 and 6.0 mg/kg
CuCl2 impaired the learning process and the spatial
memory of the subjects. These findings suggested that the spatial
learning and memory functions of the brain may tolerate Cu
exposure. In support of this hypothesis, a previous animal study
demonstrated that Cu suppressed hippocampal long-term potentiation
in rats, although it did not alter learning or memory in the MWM
test (33). In a mouse study,
intranasal administration of Cu nanoparticles at 1 and 10 mg/kg for
15 days did not alter the Cu concentrations in various tissues,
including the liver, lung, spleen, kidney and brain, indicating
that the animals were capable of removing or metabolizing the
inhaled Cu nanoparticles (26).
Although the CuCl2 was intraperitoneally injected in the
present study, the three lower doses (0.5, 1.0 and 2.0 mg/kg) were
unlikely to be toxic to brain cells, based on the results of the
studies referred to above. Additionally, 2.0 mg/kg CuCl2
exerted a beneficial effect on the learning and spatial memory of
the rats. The beneficial effect that was observed in the 2.0 mg/kg
group is unlikely to be an artificial phenomenon, considering that
the rats which received this dose of CuCl2 performed
better than all of the other groups in the training phase and probe
test. The increased travel speed of the 2.0 mg/kg group during the
test period of the water maze additionally indicates that the rats
in this group were more energetic than the rats in the other
groups. This energy may have contributed to the improved
performance of these rats on the other indexes. The spatial memory
impairments of the rats treated with 6.0 mg/kg CuCl2 are
consistent with the excess Cu-mediated cognitive function
deficiency reported in previous animal studies. The result was
correspondent with a previous in vitro study, which
demonstrated that low concentrations of Cu (II) (1–100 nM) improved
neuronal firing rate, although higher concentrations (1–5 µM)
reduced the firing rate (32). It
may be hypothesised that increased doses of CuCl2 may
decrease the activity of SOD1 and the levels of MDA, and impair
spatial cognition. However, lower doses of CuCl2 may
have the opposite effect.
The second significant finding of the present study
is that the effect of CuCl2 on PCr, Cr+PCr, NAA and
NAA+NAAG levels exhibited a typical bell curve, with a
significantly increased level in the 2.0 mg/kg group. All of these
indexes are associated with mitochondrial function. The increased
levels in the 2.0 mg/kg CuCl2 group suggested that this
treatment had a beneficial effect on mitochondrial function; it may
be suggested that this dose of CuCl2 enhanced
mitochondrial function in neurons. This hypothesis may account for
the increased energetic status of the 2.0 mg/kg group, as
illustrated in the MWM test. Notably, NAA levels in the brain
impact the viability of neurons and are additionally associated
with neuronal-oligodendroglial integrity, which is necessary for
the normal function of neuronal circuits in the brain. In this
context, the increased NAA level in the 2.0 mg/kg CuCl2
group may explain the improved performance of this group of rats in
the MWM test. In support of this explanation, 8 weeks of social
isolation decreased the NAA and PCr levels in the dorsal
hippocampus of rats and impaired spatial working memory (34).
Glu, the main excitatory neurotransmitter in the
CNS, is not only associated with cognitive and emotional
activities; it is also an excitotoxin. An overdose of extracellular
Glu may cause the excessive accumulation of neurons, leading to
cell death, and ultimately to learning and memory impairment.
Dysfunction of glutamatergic transmission is considered to be a
predominant feature and fundamental pathological mechanism of
neurodegenerative disorders that involve impaired spatial memory.
Previous in vivo human 1H-MRS studies reported
decreased Glu levels in the hippocampus of patients with
neurodegenerative diseases (35).
In the present study, the 1H-MRS results indicated a
mild, although not statistically significant, increase in
hippocampal Glu levels in rats that were exposed to 2.0 mg/kg
CuCl2 compared with those in the control group. This
increase was confirmed via HPLC analysis; in this analysis, the
difference reached significance. By contrast, the two higher doses
(4.0 and 6.0 mg/kg) of CuCl2 significantly decreased the
levels of Glu. In the present study, the potential mechanisms of
the Cu-induced changes in Glu levels were not studied. However, it
may be hypothesised that the increase in Glu levels induced by
treatment with 2.0 mg/kg CuCl2 may reflect a
compensatory effect to counteract the blockade of certain receptors
of this neurotransmitter. In line with this speculation, CNS
neurons possess the machinery to take up Cu and to subsequently
release it at the synaptic cleft (36), where it may modulate excitatory and
inhibitory neurotransmission. Indeed, Cu blocks GABAergic and
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) ergic
neurotransmission when it is acutely applied to cultured rat
olfactory bulb neurons (13). Cu
was demonstrated to inhibit AMPAergic neurotransmission in rat
cortical neurons (15) and
GABAergic neurotransmission in acutely isolated rat cerebellar
Purkinje cells (37). However, the
compensatory response may reach its upper limit and even break down
when the dose of CuCl2 is increased, as observed in the
rats that received 4.0 and 6.0 mg/kg CuCl2 in the
present study. In agreement with this interpretation, glutamic acid
concentrations were decreased in certain brain regions, including
the hippocampus, in mice that inhaled a high dose of Cu
nanoparticles (38).
GABA is a principal inhibitory neurotransmitter in
the CNS and is known to be associated with cognitive function
(39). In a recent study, Cu was
demonstrated to affect the holding current of neurons by affecting
GABA-mediated signalling (40). In
another study, extra-synaptic GABA receptors were observed to be
susceptible to Cu modulation (41). In agreement with these previous
studies, the results of the present study demonstrated that
hippocampal GABA levels were increased in the group of rats treated
with 2.0 mg/kg CuCl2, while this treatment had a
beneficial effect on the learning and spatial memory capacity of
the subjects.
Notably, hippocampal Glu levels in the rats that
received the various doses of CuCl2 were positively
correlated with the frequency of platform crossings in the target
quadrant, suggesting a beneficial effect of higher Glu levels on
the retention of spatial reference memory. This suggestion appears
to argue against the commonly accepted notion that increased Glu
levels in the hippocampus impair spatial memory. In order to
interpret this apparent conflict, the following points are of
importance: i) The increase in Glu observed in the rats that were
given 2.0 mg/kg CuCl2 was mild (approximately 10% higher
compared with the level in the normal controls); ii) this mild
increase reflected a compensatory response of hippocampal neurons
to the blockade of AMPAergic neurotransmission, suggesting that the
increase was unlikely to be toxic to the neurons, as mentioned
above; and iii) the 2.0 mg/kg dose of CuCl2 is
relatively low and within the safe range for rats, as discussed
above. In line with this claim, the WHO Provisional Maximum
Tolerable Daily Intake upper limit for Cu was set to 0.5 mg/kg per
day (42), based on the fact that
Cu does not appear to be a cumulative toxic hazard for humans
(International Programme on Chemical Safety, 1982) (43,44).
In addition, in previous in vivo studies, a suspension of Cu
nanoparticles was administered to animals at a dose of 30 mg/kg
body weight, or at an even higher dose of 200 mg/kg to study the
adverse effect of Cu nanoparticles (45,46).
In a previous study, S-D rats were given once-daily intraperitoneal
injections of Cu(OAc)2 at doses of 0.2, 2, or 20 mg/kg
for 5 days to examine the effects of hippocampal Cu concentration
on learning and memory and observed biphasic dose-dependent effects
of Cu on rat learning and memory (32). Considering all these factors, it is
plausible to conclude that the 2.0 mg/kg dose of CuCl2
that was administered to rats according to the procedure of the
present study induced a mild increase in hippocampal Glu levels,
and thus facilitated spatial learning and memory. In support of the
beneficial effect of this dose of CuCl2, hippocampal NAA
levels were positively correlated with the number of times the rats
crossed the target quadrant in the water maze.
Notably, the region of interest in the
1H-MRS analysis was located in the right dorsal
hippocampus of the rats, whereas the HPLC analysis measured
metabolite levels in homogenates of the whole right hippocampus. In
addition, 1H-MRS results may be affected by a number of
confounding factors, including breathing, inhomogeneity of the B0
field and volume contamination, which may decrease the sensitivity
of the analysis and increase the variance of measurements. Indeed,
HPLC, although not 1H-MRS, revealed significant
alterations in GABA and Glu levels in the hippocampal tissue of the
rats that were treated with 2.0 and 6.0 mg/kg CuCl2.
In conclusion, the present study provided the first
report, to the best of our knowledge, of a beneficial effect of 2.0
mg/kg CuCl2 on the spatial learning and memory of rats.
The same treatment mildly increased Glu, GABA, NAA, PCr, and Cr+PCr
levels in the hippocampus. The concurrent mild increases in these
brain metabolites in the hippocampus suggested that the
administration of CuCl2 in accordance with the regimen
used in the present study may cause a neurotrophin-like alteration.
These results provided further evidence of the essential role of Cu
in brain function.
Acknowledgements
The present study was funded in part by the Natural
Science Foundation of Guangdong Province (grant no. 2016KZDXM013),
National Science Foundation of China (grant no. 60971075) and the
Scientific Research Foundation of Shantou University (grant no.
NTF10010).
Glossary
Abbreviations
Abbreviations:
|
CNS
|
central nervous system
|
|
GABA
|
γ-aminobutyric acid
|
|
Glu
|
glutamate
|
|
HPLC
|
high performance liquid
chromatography
|
|
VOI
|
volume of interest
|
|
MWM
|
Morris water maze
|
|
TR
|
repetition time
|
|
TE
|
echo time
|
|
Cr
|
creatine
|
|
Gln
|
glutamine
|
|
GPC
|
glycerophosphorylcholine
|
|
mI
|
myo-inositol
|
|
NAA
|
N-acetyl-L-aspartate
|
|
NAAG
|
N-acetylaspartylglutamate
|
|
PCh
|
phosphorylcholine
|
|
PCr
|
phosphocreatine
|
|
Tau
|
taurine
|
|
MRS
|
magnetic resonance spectroscopy
|
|
AD
|
Alzheimer's disease
|
|
ROS
|
reactive oxygen species
|
|
GluNR
|
N-methyl-D-aspartic acid glutamate
receptor
|
|
S-D
|
Sprague-Dawley
|
|
Tau
|
taurine
|
|
SOD1
|
superoxide dismutase
|
|
MDA
|
malondialdehyde
|
|
AMPA
|
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
|
References
|
1
|
Cortese BM, Mitchell TR, Galloway MP,
Prevost KE, Fang J, Moore GJ and Uhde TW: Region-specific
alteration in brain glutamate: Possible relationship to risk-taking
behavior. Physiol Behav. 99:445–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prohaska JR: Role of copper transporters
in copper homeostasis. Am J Clin Nutr. 88 Suppl:826S–829S.
2008.PubMed/NCBI
|
|
3
|
Squitti R, Lupoi D, Pasqualetti P, Dal
Forno G, Vernieri F, Chiovenda P, Rossi L, Cortesi M, Cassetta E
and Rossini PM: Elevation of serum copper levels in Alzheimer's
disease. Neurology. 59:1153–1161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Squitti R, Pasqualetti P, Cassetta E, Dal
Forno G, Cesaretti S, Pedace F, Finazzi-Agrό A and Rossini PM:
Elevation of serum copper levels discriminates Alzheimer's disease
from vascular dementia. Neurology. 60:2013–2014. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bush AI: Copper, zinc and the
metallobiology of Alzheimer disease. Alzheimer Dis Assoc Disord.
17:147–150. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brewer GJ: The risks of copper toxicity
contributing to cognitive decline in the aging population and to
Alzheimer's disease. J Am Coll Nutr. 28:238–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pal A and Prasad R: Regional distribution
of copper, zinc and iron in brain of Wistar rat model for
non-Wilsonian brain copper toxicosis. Indian J Clin Biochem.
31:93–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peters C, Muñoz B, Sepúlveda FJ, Urrutia
J, Quiroz M, Luza S, De Ferrari GV, Aguayo LG and Opazo C: Biphasic
effects of copper on neurotransmission in rat hippocampal neurons.
J Neurochem. 119:78–88. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maas JW and Colburn RW: Co-ordination
chemistry and membrane function with particular reference to the
synapse and catecholamine transport. Nature. 208:41–46. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mercer JF: Menkes syndrome and animal
models. Am J Clin Nutr. 67 Suppl:1022S–1028S. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mathie A, Sutton GL, Clarke CE and Veale
EL: Zinc and copper: Pharmacological probes and endogenous
modulators of neuronal excitability. Pharmacol Ther. 111:567–583.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salazar-Weber NL and Smith JP: Copper
inhibits NMDA receptor-independent LTP and modulates the
paired-pulse ratio after LTP in mouse Hippocampal slices. Int J
Alzheimers Dis. 2011:8647532011.PubMed/NCBI
|
|
13
|
Trombley PQ and Shepherd GM: Differential
modulation by zinc and copper of amino acid receptors from rat
olfactory bulb neurons. J Neurophysiol. 76:2536–2546. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vlachová V, Zemková H and Vyklický L Jr:
Copper modulation of NMDA responses in mouse and rat cultured
hippocampal neurons. Eur J Neurosci. 8:2257–2264. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Weiser T and Wienrich M: The effects of
copper ions on glutamate receptors in cultured rat cortical
neurons. Brain Res. 742:211–218. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schlief ML and Gitlin JD: Copper
homeostasis in the CNS: A novel link between the NMDA receptor and
copper homeostasis in the hippocampus. Mol Neurobiol. 33:81–90.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morris R: Developments of a water-maze
procedure for studying spatial learning in the rat. J Neurosci
Methods. 11:47–60. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi L, Adams MM, Long A, Carter CC,
Bennett C, Sonntag WE, Nicolle MM, Robbins M, D'Agostino R and
Brunso-Bechtold JK: Spatial learning and memory deficits after
whole-brain irradiation are associated with changes in NMDA
receptor subunits in the hippocampus. Radiat Res. 166:892–899.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shi L, Olson J, D'Agostino R Jr, Linville
C, Nicolle MM, Robbins ME, Wheeler KT and Brunso-Bechtold JK: Aging
masks detection of radiation-induced brain injury. Brain Res.
1385:307–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Witter MP and Amaral DG: Hippocampal
formationPaxinos G: The rat nervous system. 3rd. Academic Press;
San Diego: pp. 637–703. 2004
|
|
21
|
Gruetter R: Automatic, localized in vivo
adjustment of all first- and second-order shim coils. Magn Reson
Med. 29:804–811. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harris JL, Yeh HW, Choi IY, Lee P, Berman
NE, Swerdlow RH, Craciunas SC and Brooks WM: Altered neurochemical
profile after traumatic brain injury: (1)H-MRS biomarkers of
pathological mechanisms. J Cereb Blood Flow Metab. 32:2122–2134.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Provencher SW: Estimation of metabolite
concentrations from localized in vivo proton NMR spectra. Magn
Reson Med. 30:672–679. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cavassila S, Deval S, Huegen C, van
Ormondt D and Graveron-Demilly D: Cramer-Rao bounds: An evaluation
tool for quantitation. NMR Biomed. 14:278–283. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu W, Masaki T, Cheung AK and Kern SE:
In-vitro release of rapamycin from a thermosensitive polymer for
the inhibition of vascular smooth muscle cell proliferation. J
Bioequiv Availab. 1:3–12. 2009.PubMed/NCBI
|
|
26
|
Zhang L, Bai R, Liu Y, Meng L, Li B, Wang
L, Xu L, Le Guyader L and Chen C: The dose-dependent toxicological
effects and potential perturbation on the neurotransmitter
secretion in brain following intranasal instillation of copper
nanoparticles. Nanotoxicology. 6:562–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma Q, Ying M, Sui X, Zhang H, Huang H,
Yang L, Huang X, Zhuang Z, Liu J and Yang X: Chronic copper
exposure causes spatial memory impairment, selective loss of
hippocampal synaptic proteins, and activation of PKR/eIF2α pathway
in mice. J Alzheimers Dis. 43:1413–1427. 2015.PubMed/NCBI
|
|
28
|
Yu J, Luo X, Xu H, Ma Q, Yuan J, Li X,
Chang RC, Qu Z, Huang X, Zhuang Z, et al: Identification of the key
molecules involved in chronic copper exposure-aggravated memory
impairment in transgenic mice of Alzheimer's disease using
proteomic analysis. J Alzheimers Dis. 44:455–469. 2015.PubMed/NCBI
|
|
29
|
Pal A, Badyal RK, Vasishta RK, Attri SV,
Thapa BR and Prasad R: Biochemical, histological, and memory
impairment effects of chronic copper toxicity: A model for
non-Wilsonian brain copper toxicosis in Wistar rat. Biol Trace Elem
Res. 153:257–268. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Railey AM, Micheli TL, Wanschura PB and
Flinn JM: Alterations in fear response and spatial memory in pre-
and post-natal zinc supplemented rats: Remediation by copper.
Physiol Behav. 100:95–100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Railey AM, Groeber CM and Flinn JM: The
effect of metals on spatial memory in a transgenic mouse model of
Alzheimer's disease. J Alzheimers Dis. 24:375–381. 2011.PubMed/NCBI
|
|
32
|
Zhang Y, Lu W, Han M, Li H, Luo H, Li W
and Lin Z: Biphasic effects of copper on rat learning and memory in
the morris water maze. Ann Clin Lab Sci. 46:346–352.
2016.PubMed/NCBI
|
|
33
|
Leiva J, Palestini M, Infante C,
Goldschmidt A and Motles E: Copper suppresses hippocampus LTP in
the rat, but does not alter learning or memory in the morris water
maze. Brain Res. 1256:69–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shao Y, Yan G, Xuan Y, Peng H, Huang QJ,
Wu R and Xu H: Chronic social isolation decreases glutamate and
glutamine levels and induces oxidative stress in the rat
hippocampus. Behav Brain Res. 282:201–208. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rupsingh R, Borrie M, Smith M, Wells JL
and Bartha R: Reduced hippocampal glutamate in Alzheimer disease.
Neurobiol Aging. 32:802–810. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barnea A, Cho G and Hartter DE: A
correlation between the ligand specificity for 67copper uptake and
for copper-prostaglandin E2 stimulation of the release of
gonadotropin-releasing hormone from median eminence explants.
Endocrinology. 122:1505–1510. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sharonova IN, Vorobjev VS and Haas HL:
High-affinity copper block of GABA(A) receptor-mediated currents in
acutely isolated cerebellar Purkinje cells of the rat. Eur J
Neurosci. 10:522–528. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang L, Bai X, Tian H, Zhong L, Ma C,
Zhou Y, Chen S and Li D: Synthesis of antibacterial film
CTS/PVP/TiO2/Ag for drinking water system. Carbohydr Polym.
89:1060–1066. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lanctôt KL, Herrmann N, Mazzotta P, Khan
LR and Ingber N: GABAergic function in Alzheimer's disease:
Evidence for dysfunction and potential as a therapeutic target for
the treatment of behavioural and psychological symptoms of
dementia. Can J Psychiatry. 49:439–453. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gaier ED, Rodriguiz RM, Zhou J, Ralle M,
Wetsel WC, Eipper BA and Mains RE: In vivo and in vitro analyses of
amygdalar function reveal a role for copper. J Neurophysiol.
111:1927–1939. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
McGee TP, Houston CM and Brickley SG:
Copper block of extrasynaptic GABAA receptors in the mature
cerebellum and striatum. J Neurosci. 33:13431–13435. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Goldhaber SB: Trace element risk
assessment: Essentiality vs. toxicity. Regul Toxicol Pharmacol.
38:232–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Montesano R, Rajewsky MF, Pegg AE and
Miller E: Development and possible use of immunological techniques
to detect individual exposure to carcinogens: International Agency
for Research on Cancer/International Programme on Chemical Safety
Working Group Report. Cancer Res. 42:5236–5239. 1982.PubMed/NCBI
|
|
44
|
Rosival L and Vargová M: Chemization and
human health. Czech Med. 5:131–136. 1982.PubMed/NCBI
|
|
45
|
Lei R, Wu C, Yang B, Ma H, Shi C, Wang Q,
Wang Q, Yuan Y and Liao M: Integrated metabolomic analysis of the
nano-sized copper particle-induced hepatotoxicity and
nephrotoxicity in rats: A rapid in vivo screening method for
nanotoxicity. Toxicol Appl Pharmacol. 232:292–301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sharma M: Understanding the mechanism of
toxicity of carbon nanoparticles in humans in the new millennium: A
systemic review. Indian J Occup Environ Med. 14:3–5. 2010.
View Article : Google Scholar : PubMed/NCBI
|