Introduction
Osteoarthritis (OA) is a chronic disabling disease
that is induced by primary or secondary articular chondrocyte
degeneration or structural disorder. It is the most common form of
arthritis and affects millions of people worldwide, with the most
common symptoms being joint pain and stiffness (1). OA occurs when the protective
cartilage on the ends of bones wears down over time. Bone
hyperplasia is a major pathological feature of OA, which leads to
joint destruction and deformity (2). OA is most often diagnosed in
middle-aged or elderly populations. As aging populations rise, the
incidence rate of OA is now at an epidemic level worldwide,
particularly in China (3,4). Current treatments only provide
symptomatic relief and include exercise, efforts to decrease joint
stress, support groups and pain medications.
Although aging is considered to be one of the major
pathological causes for the onset of OA (5), the exact underlying mechanisms that
control OA development are yet to be elucidated. Matrix Gla protein
(MGP) is a calcification inhibitor that must be carboxylated by
vitamin K to function (6).
γ-glutamyl carboxylase (GGCX) is a key enzyme that regulates the
carboxylation of cartilage MGP, and carboxylated (c)MGP and
uncarboxylated (un)MGP are detectable in cartilage cells. It has
been suggested previously that circulating ucMGP may be elevated in
patients with OA (7), and GGCX was
apparently decreased in cartilage tissue from patients with OA
(8,9).
Inflammation has been identified as an important
process in OA. Proinflammatory cytokines, including interleukin-1β
(IL-1β) and tumor necrosis factor α (TNF-α), are critical markers
for the diagnosis and treatment of OA (10). Collagen type X expression is
typically upregulated in OA cartilage tissue, whereas collagen type
II expression has been reported to be decreased in OA, which may be
caused by the degeneration of cartilage cells (11). Matrix metalloproteinase (MMP)-13 is
expressed in the skeleton and is required to restructure the
collagen matrix for bone mineralization. In the present study, an
OA model was produced by anterior cruciate ligament transection
(ACLT) in rabbits. A GGCX-overexpression plasmid was locally
injected into the joint fluid and the protection of GGCX
overexpression against OA was investigated and inflammatory factors
and collagen levels were detected. This study may provide
experimental evidence for the clinical application of virus
injection in the treatment of OA.
Materials and methods
Animal model and treatments
A total of 48 male Japanese white rabbits (age, 3
months; weight, 3±0.5 kg) were obtained from the Animal Center of
Nanchang University (Nanchang, China) and raised in a 12 h
light/dark cycle at 22±3°C and a humidity of 40–60%, with access to
food and water ad libitum. All the experiments were approved
by Ethics Committee of Nanchang University (Nanchang, China). The
rabbits were divided into 4 groups (n=12/group): i) OA model + GGCX
overexpression plasmid (GGCX); ii) OA model + saline (Model); iii)
OA model + empty vector (Vector); and iv) Sham negative control
(Sham). The OA model was produced by ACLT surgery, as previously
described (12); briefly,
following anesthesia by 3% pentobarbital sodium (30 mg/kg) ear vein
injection, the right knee joint in each rabbit was exposed and the
anterior cruciate ligament was cut. The joint cavity was blocked by
suture and disinfected with iodophor. Sham control rabbits received
surgery, but the anterior cruciate ligament was not cut. At 8 days
post-surgery, the rabbits received lentivirus-carried GGCX
overexpression plasmid, empty vector or saline treatments, locally
injected into the joint cavity (0.2 ml). Joint fluid was obtained
using a syringe from each rabbit at weeks 2, 4, 6 and 8 and stored
at −80°C until use. At week 8 following the injections, all animals
were sacrificed by decapitation following anesthesia by 3%
pentobarbital sodium (30 mg/kg). Joint fluid and articular tissues
were collected for enzyme linked immunosorbent assay (ELISA),
histological staining and RNA/protein extraction.
ELISA
ucMGP (cat. no. JL32168, J&L Biological,
Shanghai, China), cMGP (cat. no. JL32118, J&L Biological),
MMP-13 (cat. no. SEA099Rb, Cloud-Clone Corp., Wuhan, China),
collagen type X (cat. no. MBS725868; MyBioSource, Inc., San Diego,
CA, USA), collagen type II (cat. no. SEA572Rb, Cloud-Clone Corp.),
TNF-α (cat. no. SEA133Rb, Cloud-Clone Corp.) and IL-1β (cat. no.
E-EL-RB0013c, Elabscience, Houston, TX, USA) were detected in joint
fluid (10 µl) by ELISA method, according to the manufacturer's
instructions.
Immunohistochemistry, safranin O-fast
green staining and Giemsa staining
Articular tissues were fixed in 4% paraformaldehyde
for ~1 week at 4°C, cryoprotected in 30% sucrose for 1 h at 4°C and
sectioned into 20 µm thick sections with a frozen microtome.
Immunostaining of histological sections was performed using
monoclonal antibodies against GGCX (1:100; cat. no. ab107507;
Abcam, Cambridge, UK), ucMGP (1:100; cat. no. ab108225; Abcam),
anti-cMGP (1:100; cat. no. ab12416; Abcam), MMP-13 (1:100; cat. no.
bs-10581R; BIOSS, Beijing, China), collagen type X (1:100; cat. no.
bs-0554R; BIOSS), collagen type II (1:100; cat. no. bs-8859R;
BIOSS), TNF-α (1:100; cat. no. ab179675; Abcam) and IL-1β (1:100;
cat. no. ab200478; Abcam). Endogenous peroxidase activity was
blocked with 3% (v/v) H2O2 for 5 min at room
temperature. Subsequently, tissues were incubated with primary
antibodies overnight at 4°C, followed by incubation with
horseradish peroxidase (HRP)-labeled goat anti-rabbit IgG secondary
antibody (1:10,000; cat. no. A16104SAMPLE; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) for 30 min at room temperature
and visualized with 3,3′-diaminobenzidine chromogen for 3 min at
room temperature.
Safranin O-fast green staining was carried out to
detect surface cartilage damage and Giemsa staining was performed
to detect cartilage tissue. Tissues were fixed in 4%
paraformaldehyde for ~1 week at 4°C, cryoprotected in 30% sucrose
for 1 h at 4°C and sectioned into 20 µm thick sections with a
frozen microtome. For safranin O-fast green staining, slides were
first stained with Weigert's iron hematoxylin working solution for
10 min. Slides were subsequently washed in running tap water for 10
min and stained with fast green solution for 5 min. Slides were
then rinsed quickly with 1% acetic acid solution for no more than
10–15 sec and stained with 0.1% safranin O solution for 5 min. For
Giemsa staining, fixed slides were stained with Giemsa stain for 5
min. Both stains were performed at room temperature and images were
taken under a light microscope in at least five fields of view with
magnification, ×200.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from articular tissues (10
mg) using a TRIzol kit (Thermo Fisher Scientific, Inc.). RNA
concentrations were determined spectrophotometrically, and 1 µg
total RNA was reverse transcribed into cDNA using an Avian
Myeloblastosis Virus Reverse-Transcriptase kit (Promega
Corporation, Madison, WI, USA). RT-qPCR was performed using the TB
Green™ Fast qPCR Mix (Takara Biotechnology Co., Ltd.,
Dalian, China). PCR primer sequences were as follows: GAPDH
forward, 5′-GTCTGCCACGATAACACC-3′ and reverse,
5′-CAATACAACAAGCCCACTC-3′; GGCX forward, 5′-CTTGTTGCGAAAGCTCTAT-3′
and reverse, 5′-GATTTGACTCAGGAGGATTAG-3′; MMP-13 forward,
5′-CCCCAACCCTAAACATCC-3′ and reverse, 5′-AACAGCTCCGCATCAACC-3′;
TNF-α forward, 5′-GCCGGATCGTGCAGTTCG-3′ and reverse,
5′-TCCAAGGTAGCGGTCGTG-3′; IL-1β forward,
5′-GCTGAACCTTAGTACCCTTGT-3′ and reversem, 5′-AGTTTCTGTGGCGTCTGG-3′;
collagen type X forward, 5′-TCAAAGGGCACTATCAACT-3′ and reverse,
5′-TGTTTGGTATCGCTCAGTA-5′; collagen type II forward,
5′-CAACAACCAGATCGAGAGCA-3′ and reverse 5′-CGGTCTCCATGTTGCAGAA-3′.
The amplification reactions were performed with an Applied
Biosystems 7500 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), with initial
denaturation at 95°C for 10 min, followed by 40 cycles of a
two-step PCR at 95°C for 15 sec and 60°C for 1 min. The
2−ΔΔCq method was used to determine the amount of
target, normalized to the endogenous reference, GAPDH, as
previously described (13).
Western blotting
Protein was extracted from articular tissue for
western blotting as previously described (14). Protein was isolated from 10 mg
articular tissue using a protein isolation kit (ReadyPrep; GE
Healthcare Life Sciences). Protein concentration was determined
using a bicinchoninic assay kit (Thermo Fisher Scientific, Inc.). A
total of 20 µg protein was loaded into each lane and separated via
SDS-PAGE on a 12% gel and transferred onto nitrocellulose
membranes. Subsequently, membranes were blocked in 5% skim milk for
2 h in room temperature and incubated with the following primary
antibodies overnight at 4°C: Anti-GGCX (1:200; cat. no. ab107507;
Abcam), anti-ucMGP (1:200; cat. no. ab108225; Abcam), anti-cMGP
(1:200; cat. no. ab12416, Abcam) and anti-β-actin (1:1,000; cat.
no. 4970; Cell Signaling Technology, Inc., Danvers, USA); membranes
were incubated with primary antibodies overnight at 4°C. The
nitrocellulose membranes were washed three times and incubated with
HRP-labeled goat anti-rabbit IgG secondary antibody (1:10,000, cat.
no. A16104SAMPLE; Thermo Fisher Scientific, Inc.) at 4°C for 2 h.
Protein bands were visualized using an enhanced chemiluminescence
kit (Thermo Fisher Scientific, Inc.) and the blots were scanned
using a ChemiDoc XRS (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Protein expression was normalized to β-actin and
densitometric analysis was performed by ImageJ Software version 7.0
(National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Data are presented as the mean ± standard deviation.
One-way analysis of variance with Bonferroni post-hoc test for
multiple comparisons was performed. P<0.05 was considered to
indicate a statistically significant difference.
Results
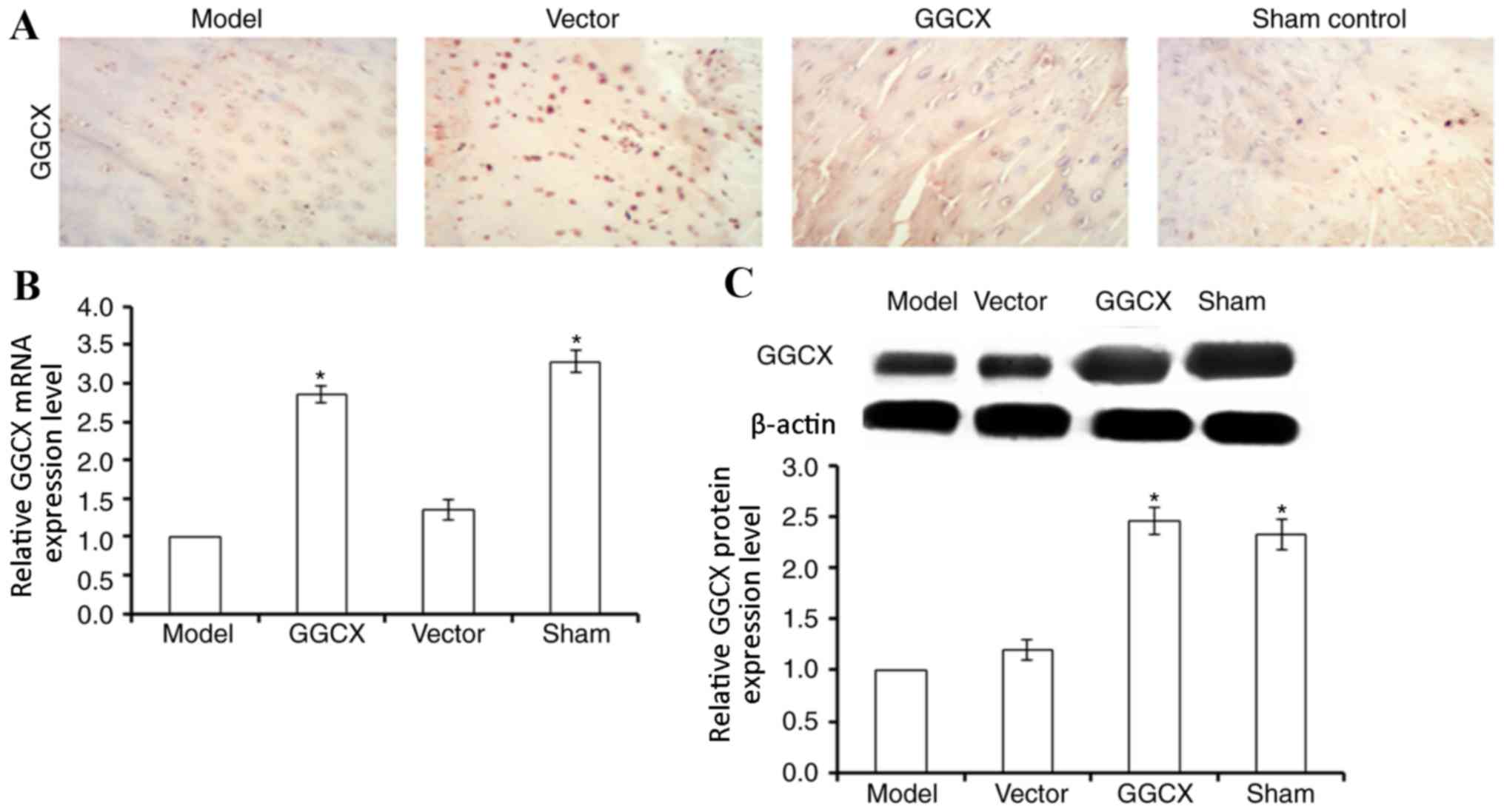
GGCX overexpression promotes GGCX
expression in OA model rabbits
The potential roles of GGCX in OA were examined in
an ACLT-induced OA rabbit model by locally injecting lentivirus
carrying GGCX overexpression vector into the joint fluid. GGCX mRNA
expression was detected by RT-qPCR, and protein expression was
detected by immunohistochemistry (Fig.
1A) and western blot analysis. GGCX protein and mRNA expression
was significantly decreased in the OA model group compared with
expression levels in Sham control rabbit articular cartilage
(Fig. 1B and C). No significant
difference was identified for GGCX mRNA or protein expression in OA
Model rabbits treated with the empty vector compared with untreated
Model rabbits, whereas those receiving injections of the
GGCX-overexpression vector exhibited significantly increased GGCX
expression at both the mRNA and protein levels in articular
cartilage compared with the Model group. These data suggested that
local injection of GGCX overexpression plasmid was a useful
approach for promoting GGCX expression.
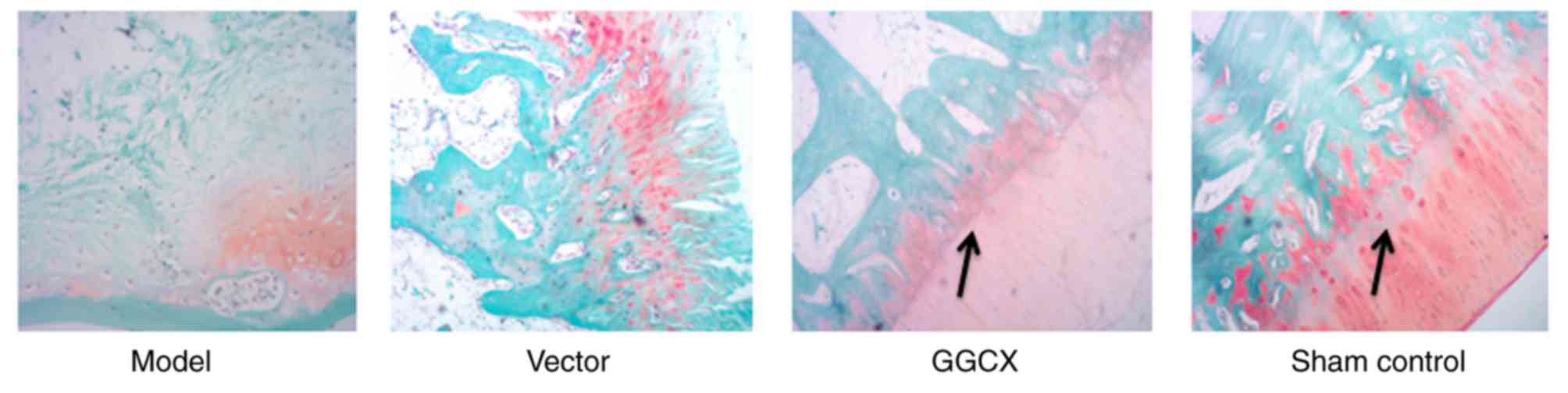
Morphological changes
Articular cartilage from rabbits in each of the four
groups was stained with safranin O-fast green. The articular
cartilage appeared normal in the Sham control group, which was
characterized by a smooth surface and with evenly organized
cartilage cells in clear layers; aggregated cells were not observed
and tide line was complete with matrix evenly stained (Fig. 2). By contrast, morphological
changes were clearly observed in the OA Model and the empty vector
groups, in which severe cartilage injury and large-scale cartilage
fibrosis was observed, and matrix was damaged in most of the
layers. GGCX overexpression appeared to reduce the morphological
changes caused by ACLT (Fig. 2).
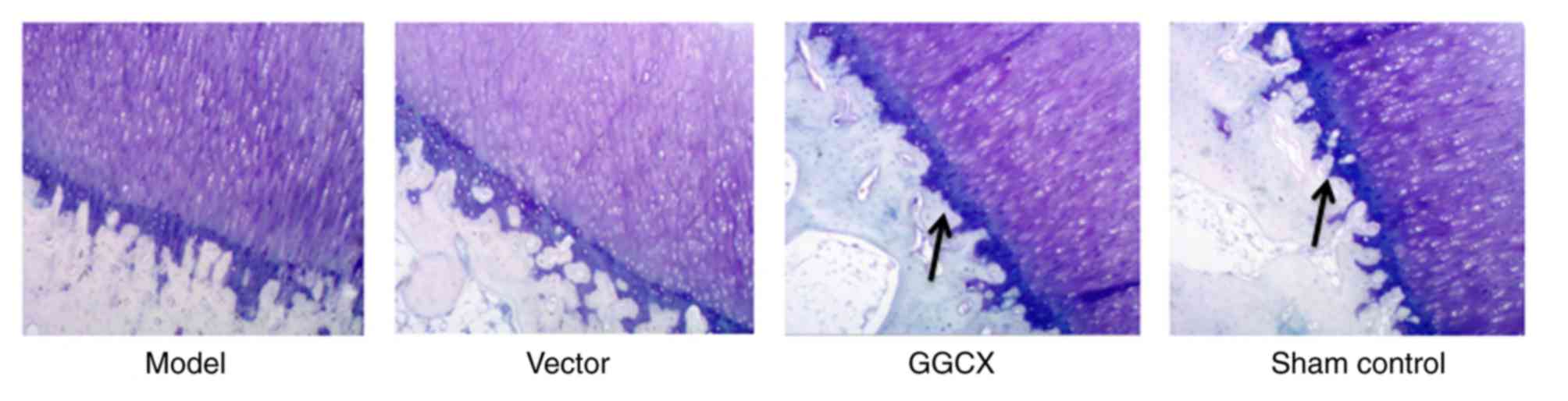
Cartilage cells in tibia were normally distributed in Sham control
group (Fig. 3); the cells were
evenly and orderly arranged with clear layers, a complete tide line
and normal staining of matrix. In the OA Model group and empty
vector group, although the tide line was complete, most layers of
the matrix exhibited mild injury. However, GGCX overexpression
appeared to attenuate the morphological changes caused by ATLC.
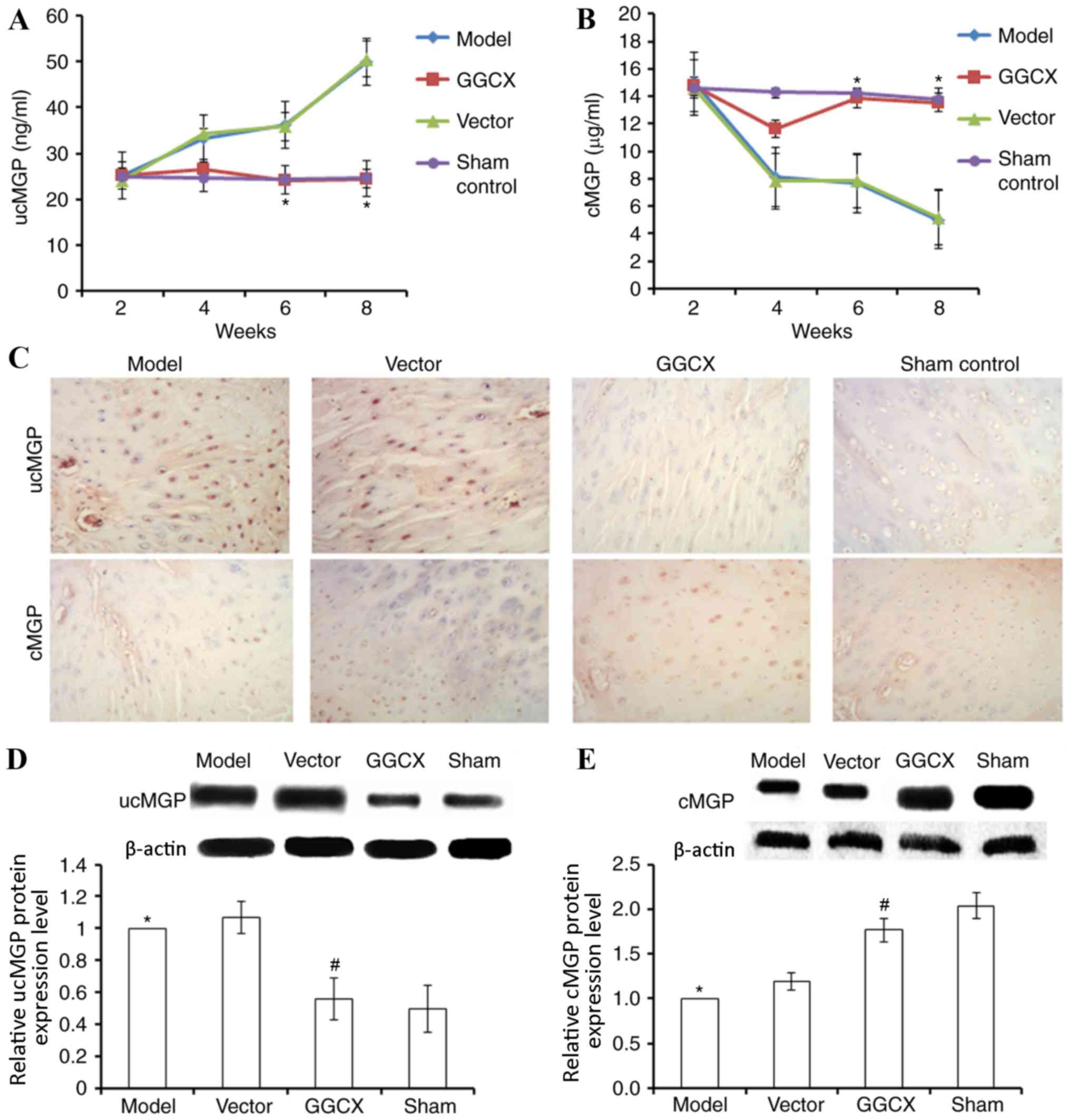
GGCX overexpression decreases ucMGP
and increases cMGP expression levels in OA model rabbits
Injection of the GGCX overexpression plasmid was
demonstrated to increase GGCX expression in articular cartilage and
joint fluid, as determined by ELISA. Thus, it was investigated
whether MGP levels were also affected by GGCX overexpression. In OA
Model rabbits and in rabbits treated with the empty vector, ucMGP
expression levels gradually increased between week 2 and week 8,
compared with the control (Fig.
4A). Rabbits overexpressing GGCX exhibited a significant
decrease in ucMGP expression level at week 8, compared with rabbits
in the Model group. By contrast, OA Model rabbits exhibited
gradually decreasing cMGP expression levels between weeks 2 and 8
(Fig. 4B). GGCX overexpression
vector injection promoted the recovery of cMGP to normal level
(week 8), whereas the empty vector did not influence cMGP, compared
with Model group.
Immunohistochemistry and western blot analysis
confirmed the results obtained from ELISA. OA model rabbits
exhibited an increase in ucMGP expression (Fig. 4C and D) and a decrease in cMGP
expression levels at week 8 post-surgery (Fig. 4C and E). By contrast, GGCX
overexpression reversed the abnormalities caused by the ATLC
model.
GGCX overexpression reduces
inflammation induced by ATLC
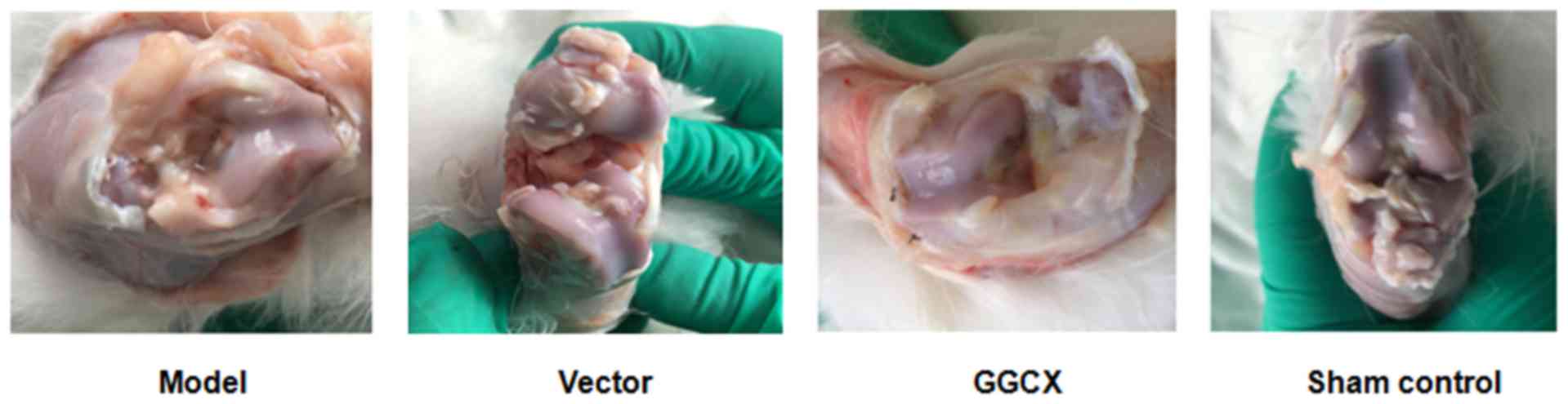
Representative images of the joints in each group
are presented in Fig. 5. The
articular cartilage of the sham group was observed to be intact
without injury. By contrast, the joint fluid in the model group was
cloudy and the articular cartilage was damaged with evidence of
inflammation. The vector and model group morphologies were
indistinguishable. In the GGCX group, the injured joints appeared
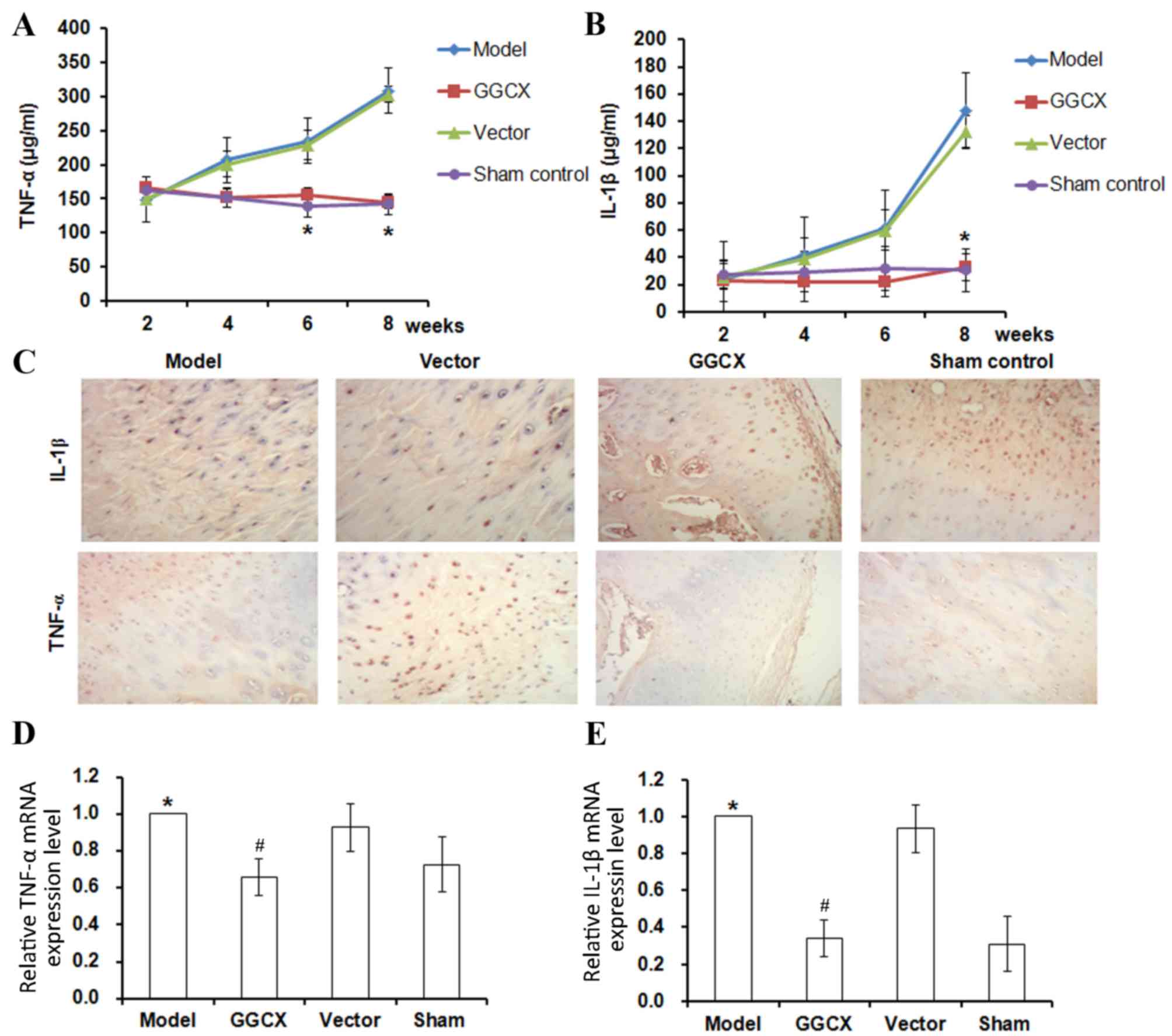
to have recovered to a normal level. TNF-α (Fig. 6A) and IL-1β (Fig. 2B) expression levels were detected
by ELISA, immunohistochemistry (Fig.
6C) and RT-qPCR (Fig. 6D).
TNF-α concentration increased between week 2 and week 8
post-surgery in the OA Model and empty vector groups (Fig. 6A). At week 8, TNF-α mRNA and
protein expression levels were increased in the OA Model group
(Fig. 6C). By contrast, GGCX
overexpression, but not empty vector, caused a decrease in
ATLC-induced TNF-α expression (Fig.
6A, C and D). A similar trend was identified for IL-1β
expression levels; OA Model rabbits exhibited increased IL-1β
synovial fluid concentration levels, protein expression and mRNA
expression levels (Fig. 6B, C and
E, respectively).
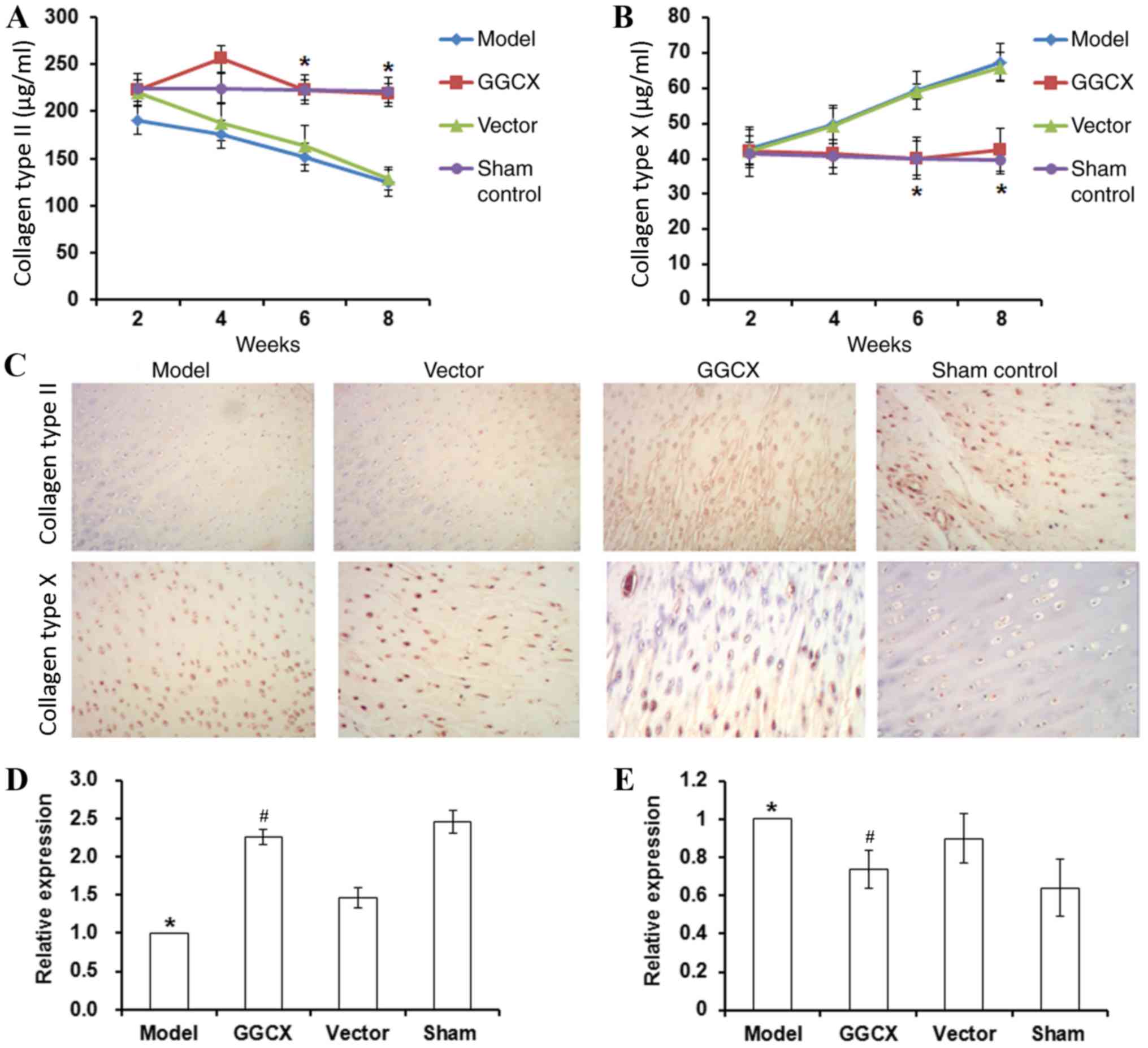
GGCX overexpression decreases collagen
type X and MMP-13 expression, but increases collagen type II
expression in ATLC model rabbits
In the present study, OA Model rabbits exhibited a
decrease in collagen type II synovial fluid concentration levels,
but an increase in collagen type X concentration levels between
week 2 and week 8 post-surgery (Fig.
7A and B, respectively). At week 8 post-surgery, OA Model
rabbits also exhibited a decrease in collagen type II and an
increase in collagen type X protein and mRNA expression levels
(Fig. 7C-E). By contrast, GGCX
overexpression reversed the abnormalities caused by ATLC-induced
OA.
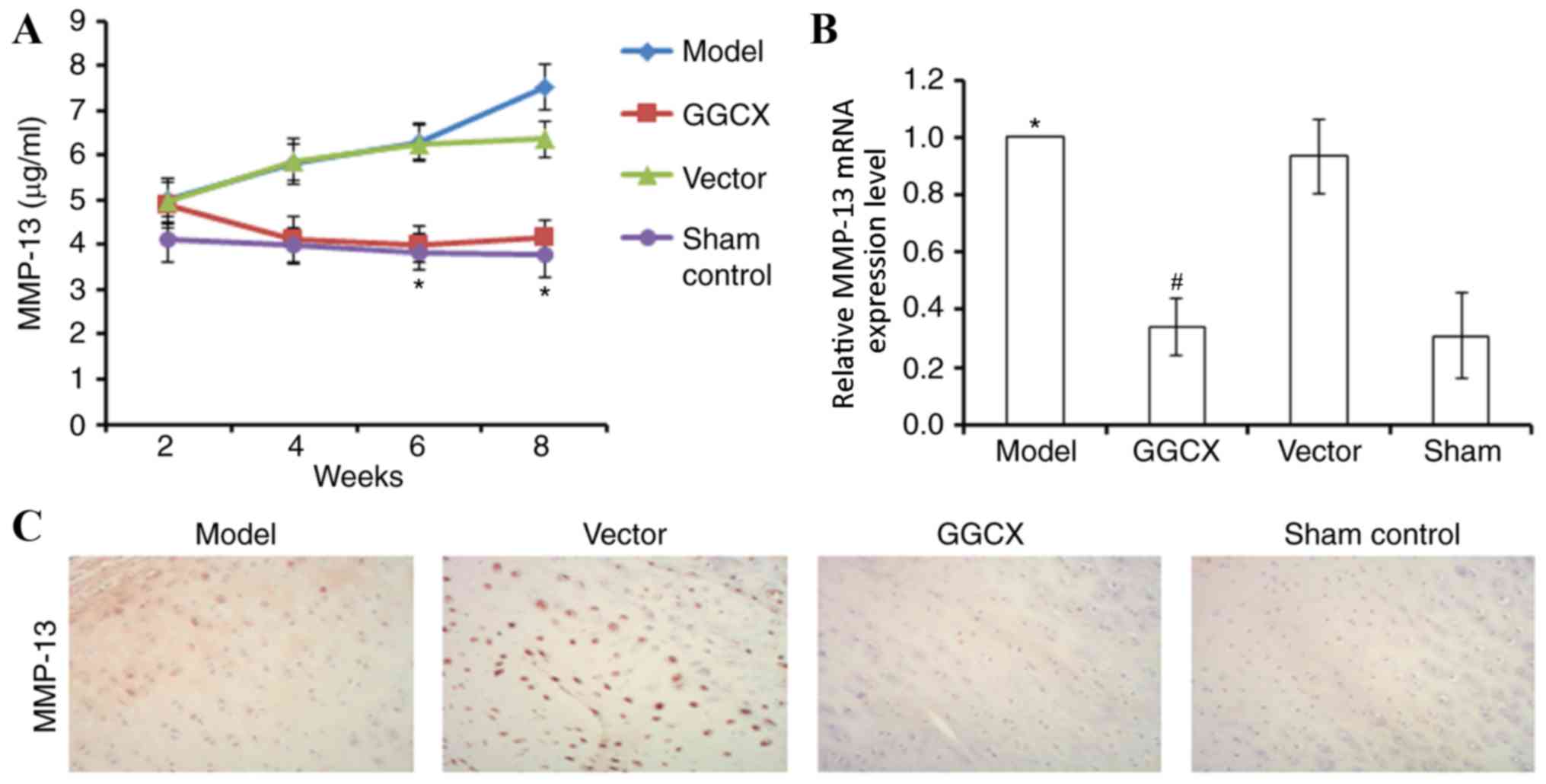
The levels of MMP-13 expression were also examined
using similar methods. OA Model rabbits exhibited gradually
increasing MMP-13 concentration levels in the synovial fluid
between weeks 2 and 8 post-surgery compared with the Sham group
(Fig. 8A). At week 8, OA Model
rabbits also exhibited increased MMP-13 mRNA and protein expression
levels compared with the Sham group (Fig. 8B and C, respectively). By contrast,
GGCX overexpression reversed the abnormalities caused by ATLC
surgery.
Discussion
Primary OA is characterized by restricted articular
activity, pain and hyperosteogeny (15). Certain treatments, including
non-steroidal analgesic and anti-inflammatory drugs, and amino
glucose capsules, were reported to alleviate the symptoms, but with
only temporal effects (16,17).
Therefore, effective treatment for OA is still urgently required.
In the present study, the overexpression of GGCX was demonstrated
to mitigate ATLC-induced damage of articular cartilage. The
potential mechanisms may be related to the reduction of
inflammation, as well as an increase in cMGP and a decrease in
ucMGP concentration in joint fluid. GGCX is a key enzyme
responsible for the carboxylation of cartilage MGP (18). In normal articular cartilage, cMGP
binds to calcium and fetuin to form an MGP-fetuin-calcium complex.
This complex inhibits cartilage calcification and the formation of
calcium crystals, which was reported to elicit the degeneration of
cartilage cells (19). The present
study demonstrated that GGCX overexpression promoted the
carboxylation of MGP in OA model rabbits.
Inflammation has been reported be an important
process in OA (20). IL-1β and
TNF-α are the major inflammatory cytokines in OA development. As
previously reported, IL-1β and TNF-α levels were apparently
elevated in OA (21,22). In the present study, mRNA and
protein levels of IL-1β and TNF-α were increased in OA Model rabbit
joint synovial fluid. GGCX overexpression was revealed to reduce
the expression of these inflammatory factors and joint inflammation
in the ATLC Model rabbits.
In a previous study, collagen type X expression was
revealed to be increased in OA cartilage tissue (23). In the present study, three
approaches consistently demonstrated that GGCX overexpression in OA
Model rabbits led to increased collagen type II and decreased
collagen type X expression levels. MMPs are capable of degrading
all kinds of extracellular matrix proteins, including collagen type
II, and increased MMP expression may have detrimental effects on
cartilage cells (24). The present
study used three methods to demonstrate that MMP-13 synthesis was
increased in OA Model rabbits, whereas it was reduced by GGCX
overexpression.
The present study also detected IL-1β, TNF-α,
Collagen type X, Collagen type II and MGPat different time points
by ELISA. The expression of these proteins was not clearly affected
in the second week post-surgery; however, the differences were
apparent at weeks 6 and 8. In addition, the protective effects of
GGCX overexpression against ATLC-induced articular injury were also
demonstrated. A number of previous studies also indicated that
growth arrest-specific 6 (Gas6) functioned in promoting the
proliferation of cartilage cells (25,26).
GGCX promotes the carboxylation of MGP, and cMGP may exert its
function through Gas6 to inhibit cartilage cell apoptosis (27). This data is consistent with the
findings of the present study and indicates that GGCX
overexpression has the potential to ameliorate OA injury.
Morphological analysis by safranin O-fast green and Giemsa staining
revealed alterations in articular cartilage and tibia. Results from
the present study indicated that GGCX overexpression may ameliorate
the morphological changes induced by ATLC.
In conclusion, the present study investigated the
effects of GGCX overexpression on ATLC-induced articular
impairments. GGCX expression was demonstrated to be decreased in
the OA Model, whereas subsequent overexpression of GGCX in OA Model
rabbits was able to reduce inflammation, reduce collagen type X and
MMP-13 expression, promote MGP carboxylation and increase collagen
type II expression. Results from the present study indicate that
GGCX may serve as an effective target for OA treatment.
Acknowledgements
This research was supported by The National Natural
Science Foundation of China (grant no. 81301561).
References
|
1
|
Loeser RF, Goldring SR, Scanzello CR and
Goldring MB: Osteoarthritis: A disease of the joint as an organ.
Arthritis Rheum. 64:1697–1707. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crema MD, Roemer FW, Felson DT, Englund M,
Wang K, Jarraya M, Nevitt MC, Marra MD, Torner JC, Lewis CE and
Guermazi A: Factors associated with meniscal extrusion in knees
with or at risk for osteoarthritis: The multicenter osteoarthritis
study. Radiology. 264:494–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Michael JW, Schlüter-Brust KU and Eysel P:
The epidemiology, etiology, diagnosis, and treatment of
osteoarthritis of the knee. Dtsch Arztebl Int. 107:152–162.
2010.PubMed/NCBI
|
|
4
|
Zhang Y and Jordan JM: Epidemiology of
osteoarthritis. Clin Geriatr Med. 26:355–369. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Geusens PP and van den Bergh JP:
Osteoporosis and osteoarthritis: Shared mechanisms and
epidemiology. Curr Opin Rheumatol. 28:97–103. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shea MK, O'Donnell CJ, Vermeer C,
Magdeleyns EJ, Crosier MD, Gundberg CM, Ordovas JM, Kritchevsky SB
and Booth SL: Circulating uncarboxylated matrix gla protein is
associated with vitamin K nutritional status, but not coronary
artery calcium, in older adults. J Nutr. 141:1529–1534. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Silaghi CN, Fodor D, Cristea V and Craciun
AM: Synovial and serum levels of uncarboxylated matrix Gla-protein
(ucMGP) in patients with arthritis. Clin Chem Lab Med. 50:125–128.
2012. View Article : Google Scholar
|
|
8
|
Xi XF, Li XZ, Liu F, Fu NN, Ren Y, Yang XG
and Zhang Y: Effects of short thrust needing plus
Electroacupuncture intervention on cartilage tissue in rabbits with
knee osteoarthritis. Zhen Ci Yan Jiu. 41:124–130. 2016.(In
Chinese). PubMed/NCBI
|
|
9
|
Shi Y, Chen W and Yan S: Study on effect
of GGCX in knee osteoarthritis pathogenesis. Int J Clin Exp Med.
9:13657–13663. 2016.
|
|
10
|
Sokolove J and Lepus CM: Role of
inflammation in the pathogenesis of osteoarthritis: Latest findings
and interpretations. Ther Adv Musculoskelet Dis. 5:77–94. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bello AE and Oesser S: Collagen
hydrolysate for the treatment of osteoarthritis and other joint
disorders: A review of the literature. Curr Med Res Opin.
22:2221–2232. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boulocher C, Duclos ME, Arnault F,
Roualdes O, Fau D, Hartmann DJ, Roger T, Vignon E and Viguier E:
Knee joint ultrasonography of the ACLT rabbit experimental model of
osteoarthritis: Relevance and effectiveness in detecting meniscal
lesions. Osteoarthritis Cartilage. 16:470–479. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kurz B, Domm C, Jin M, Sellckau R and
Schünke M: Tissue engineering of articular cartilage under the
influence of collagen I/III membranes and low oxygen tension.
Tissue Eng. 10:1277–1286. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Arden N and Nevitt MC: Osteoarthritis:
Epidemiology. Best Pract Res Clin Rheumatol. 20:3–25. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phillips S, Silvia Li C, Phillips M,
Bischoff M, Ali P, Chahal J, Snider M and Bhandari M: Treatment of
osteoarthritis of the Knee with bracing: A scoping review. Orthop
Rev (Pavia). 8:62562016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Anandacoomarasamy A and March L: Current
evidence for osteoarthritis treatments. Ther Adv Musculoskelet Dis.
2:17–28. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Viegas CS, Cavaco S, Neves PL, Ferreira A,
João A, Williamson MK, Price PA, Cancela ML and Simes DC: Gla-rich
protein is a novel vitamin K-dependent protein present in serum
that accumulates at sites of pathological calcifications. Am J
Pathol. 175:2288–2298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lorenzen JM, Martino F, Scheffner I,
Bröcker V, Leitolf H, Haller H and Gwinner W: Fetuin, matrix-Gla
protein and osteopontin in calcification of renal allografts. PLoS
One. 7:e520392012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berenbaum F, Griffin TM and Liu-Bryan R:
Metabolic regulation of inflammation in osteoarthritis. Arthritis
Rheumatol. 69:9–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martin MU and Wesche H: Summary and
comparison of the signaling mechanisms of the Toll/interleukin-1
receptor family. Biochim Biophys Acta. 1592:265–280. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chambers MG, Kuffner T, Cowan SK, Cheah KS
and Mason RM: Expression of collagen and aggrecan genes in normal
and osteoarthritic murine knee joints. Osteoarthritis Cartilage.
10:51–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Eerola I, Salminen H, Lammi P, Lammi M,
von der Mark K, Vuorio E and Säämänen AM: Type X collagen, a
natural component of mouse articular cartilage: Association with
growth, aging, and osteoarthritis. Arthritis Rheum. 41:1287–1295.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu P, Takai K, Weaver VM and Werb Z:
Extracellular matrix degradation and remodeling in development and
disease. Cold Spring Harb Perspect Biol. 3:pii: a0050582011.
View Article : Google Scholar
|
|
25
|
Loeser RF, Varnum BC, Carlson CS, Goldring
MB, Liu ET, Sadiev S, Kute TE and Wallin R: Human chondrocyte
expression of growth-arrest-specific gene 6 and the tyrosine kinase
receptor axl: Potential role in autocrine signaling in cartilage.
Arthritis Rheum. 40:1455–1465. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melaragno MG, Cavet ME, Yan C, Tai LK, Jin
ZG, Haendeler J and Berk BC: Gas6 inhibits apoptosis in vascular
smooth muscle: Role of Axl kinase and Akt. J Mol Cell Cardiol.
37:881–887. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Stenhoff J, Dahlbäck B and Hafizi S:
Vitamin K-dependent Gas6 activates ERK kinase and stimulates growth
of cardiac fibroblasts. Biochem Biophys Res Commun. 319:871–878.
2004. View Article : Google Scholar : PubMed/NCBI
|