Introduction
The dysregulation of glucose homeostasis is a
critical cause for type 2 diabetes mellitus (1). Decreased insulin secretion and
insulin sensitivity is hall marks of type 2 diabetes mellitus
(2). Insulin secreted by pancreas
can decrease glucose production and increase uptake of blood
glucose into glycogen in liver. Therefore, hepatic insulin
resistance leads to increased glucose production and decreased
glycogenesis, resulting in elevated blood glucose levels (3). MicroRNAs are a cluster of endogenous
small non-coding RNAs, which can negatively regulate genes
expression at the post-transcriptional level, either by inhibiting
translation or by degrading the target mRNAs (4). In our previous study, we indicated
many miRNAs involved in regulation of glycogenesis in hepatocytes,
such as miR-200s (5), miR-301a
(6), miR-152-3p (7) and miR-20a-5p (8). Moreover, we found an important role
of miR-19a in regulating of glycogenesis in hepatocytes (9). It was reported that miR-19a is a
member of miR-17-92 family, which is located on chromosome 13q31.3
and is related with the pathogenesis of cancer (10). MiR-19a could affect the activation
of PI3K/AKT pathway by targeting phosphatase and tensin homolog
(PTEN) in mouse liver cells. However, whether miR-19a serves an
important role in glucose production in hepatocytes remains
unknown.
Here, we define the impact of miR-19a on
gluconeogenesis and its underlying mechanisms. Our findings suggest
that miR-19a plays an important role in gluconeogenesis via
targeting PTEN to regulate the activation of AKT/FOXO1 pathway.
Methods and materials
Cell culture
The HEP1-6 murine liver cell line (American Type
Culture Collection) was cultured in low-glucose Dulbecco's modified
Eagle's medium (L-DMEM; Invitrogen Life Technologies, Carlsbad, CA,
USA) supplemented with 10% fetal serum (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA), 100 units/ml penicillin (Invitrogen Life
Technologies), and 0.1 mg/ml streptomycin (Hyclone) at 37°C with
humidified air and 5% CO2.
Transfection of miR-19a mimic and
inhibitor
The sequences of miR-19a mimic and inhibitor were as
follows (5′-3′): miR-19a mimic sense, UGUGCAAAUCUAUGCAAAACUGA and
antisense, AGUUUUGCAUAGAUUUGCACAUU; miR-19a inhibitor,
UCAGUUUUGCAUAGAUUUGCACA. MicroRNA oligos were purchased from
Genepharma (Shanghai, China). According to the manufacturer's
instruction, negative miRNA mimic control (NC), miR-19a mimic
(19am), negative miRNA inhibitor control (NCi) and miRNA-19a
inhibitor (19ai) were transfected into HEP1-6 cells by using
Hiperfect transfection reagent (Qiagen, Hilden, Germany). Before
transfection, seeded HEP1-6 cells in 6-well plate at
1.0×105 cell per well. Diluted 37.5 ng miRNA and 3 µl
Hiperfect transfection reagent in 100 µl L-DMEM and mixed by
vortexing. The mixture was added into the cell culture medium and
incubated for 48 h.
Quantification of miR-19a expression
by reverse transcription-quantified polymerase chain reaction
Total RNA was harvested using TRIzol (Invitrogen
Life Technologies) after transfection for 48 h. Quantification of
miR-19a levels was using by real-time PCR according the protocol of
SYBR-Green II kit (Takara Bio, Inc., Otsu, Japan). The sequences of
reverse transcription primers were as follows: (5′-3′) miR-19a,
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACTCAGTT; U6,
GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAAAAATATG. The
nucleotide primers used for real-time PCR were as follows (5′-3′):
miR-19a forward, GCGTGTGCAAATCTATGCAA; U6 forward,
GCGCGTCGTGAAGCGTTC; universal reverse primer, GTGCAGGGTCCGAGGT.
Western blot
After transfection for 48 h, cell lysates (15 µg of
protein) were harvest and separated by 10% SDS-PAGE, transferred to
PVDF membranes (EMD Millipore, Billerica, MA, USA), blocked with 8%
nonfat dry milk, and probed with 1:1,000 primary antibodies at 4°C
overnight. The blots were incubated with 1:5,000 HRP-conjugated
anti-IgG for 1 h at room temperature, followed by detection with
ECL (EMD Millipore). The antibodies against AKT (9272S),
phosphorylated AKT (ser473) (4060S), FOXO1 (2880), phosphorylated
FOXO1 (ser256) (9461), PEPCK (6924) and GAPDH (5174) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). The
antibodies against PGC1α (ab54481) and G6Pase (ab83690) were
purchased from Abcam (Cambridge, UK).
Immunofluorescence
HEP1-6 cells were seed on coverslips and fixed with
4% paraformaldehyde for 20 min at room temperature. Washed the
slides with PBS for three times, and permeabilized with 0.2% Triton
X-100/PBS for 10 min at room temperature. Then blocked with 3%
BSA/PBS for 20 min at room temperature and incubated with 1:300
primary antibody against FOXO1 over night at 4°C. The slides were
washed three times with PBS and incubated with 1:100 secondary
antibody for 1 h at 37°C. To stain cellular nuclear, the cells were
incubated with 10 mM Hoechest 333442 to stain DNA. Then the
coverslips were mounted in glycerol. The cells were analyzed by
using a ZEISS LSM700 immunofluorescence microscope. The images were
captured with CCD and Axiovision image software.
Glucose production assay
The cells were washed five times with PBS and the
stimulated with 2 mmol/l sodium pyruvate and 20 mmol/l sodium
lactate in glucose- and serum-free DMEM medium for 18 h. The
glucose concentration in the medium was analyzed by using a glucose
assay kit (Sigma) and normalized to the total protein content
determined from the whole cell extracts.
Statistical analysis
All data were presented as mean ± SEM. The
two-tailed unpaired student's t-test was used for comparisons of
two groups. The ANOVA multiple comparison test (SPSS 3.0; SPSS,
Inc., Chicago, IL, USA) followed by Turkey post hoc test were used
for comparisons of two more groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
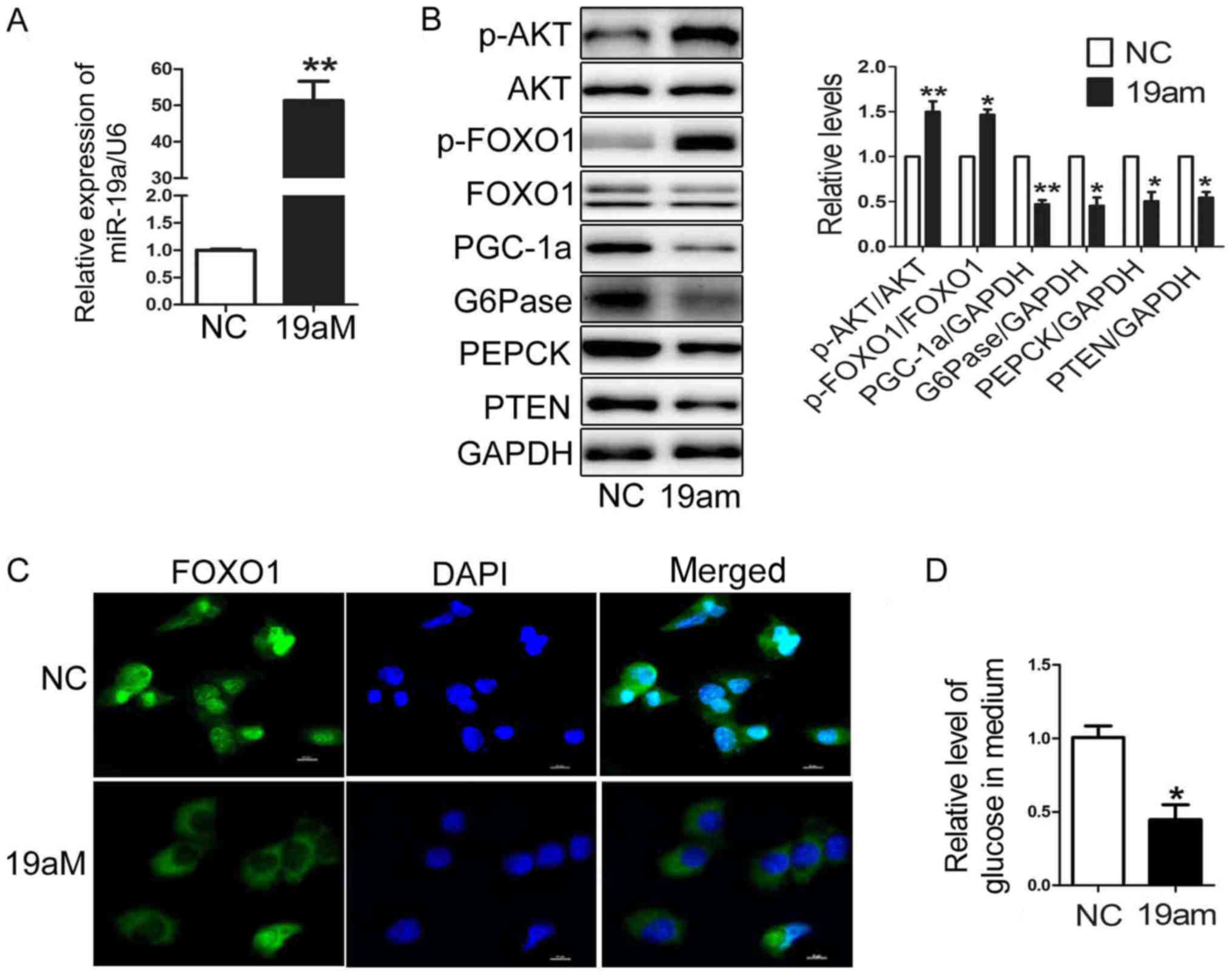
Upregulation of miR-19a leads to
decreased glucose production and expression of gluconeogenetic
genes, increased level of p-AKT and p-FOXO1 in HEP1-6 cells
In our previous study, we found that miR-19a
elevated the activation of AKT/GSK pathway and the glycogenesis in
mouse hepatic cells. However, the effects of miR-19a on
gluconeogenesis in hepatic cells still unknown. To determine the
effects of miR-19a on the glucose production and the expression of
gluconeogenetic genes including PGC-1α, G6Pase and PEPCK, miR-19a
mimic was transfected into the HEP1-6 cells. As shown in Fig. 1A, the level of miR-19a was
increased to almost 50-fold in HEP1-6 cells transfected with
miR-19a mimic. Moreover, over-expression of miR-19a suppressed the
expression of PGC-1α, G6Pase and PEPCK, accompanied by elevated
levels of p-AKT and p-FOXO1 in the HEP1-6 cells transfected with
miR-19a mimic (Fig. 1B). The
confocal analysis showed that FOXO1 located in cytoplasm in HEP1-6
cells transfected with miR-19a mimic (Fig. 1C). The gluconeogenesis level was
decreased in HEP1-6 cells transfected with miR-19a mimic (Fig. 1D). Taken together, Upregulation of
miR-19a impaired glucose production by downregulating expression of
gluconeogenetic genes and stimulating activation AKT/FOXO1 pathway
in HEP1-6 cells.
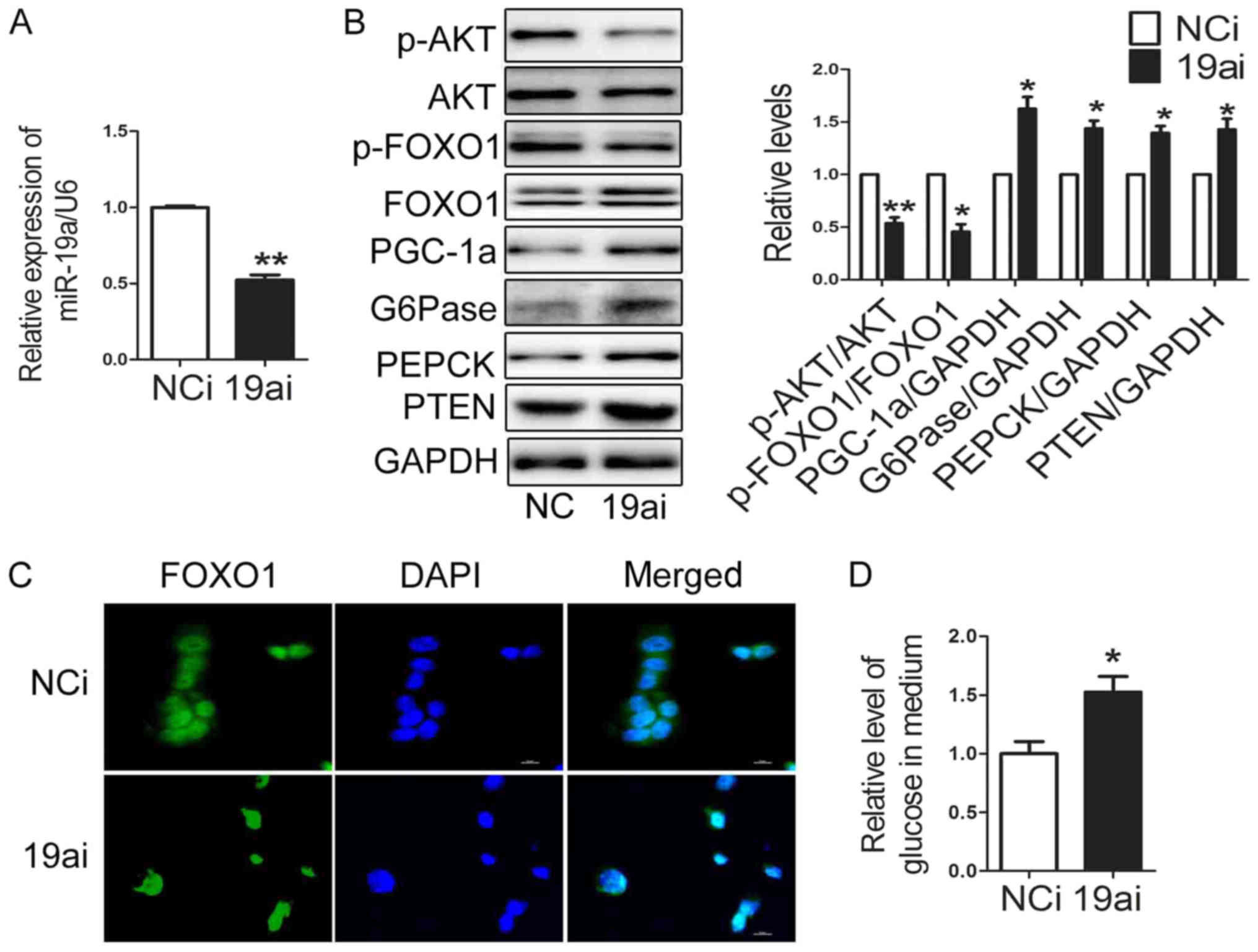
Downregulation of miR-19a promotes
glucose production and expression of gluconeogenetic genes and
suppresses activation of AKT/FOXO1 pathway in HEP1-6 cells
To gain further insight into the significance of
miR-19a in regulating gluconeogenesis, miR-19a inhibitor was
transfected into HEP1-6 cells. The level of miR-19a was decreased
to 50% in HEP1-6 cells transfected with miR-19a inhibitor (Fig. 2A). Importantly, the expression of
PGC-1α, G6Pase and PEPCK was enhanced significantly, while
activation of AKT/FOXO1 pathway was suppressed in HEP1-6 cells
transfected with miR-19a inhibitor (Fig. 2B). FOXO1 located in nuclear in
HEP1-6 cells transfected with miR-19a inhibitor (Fig. 2C). The glucose production was
increased in HEP1-6 cells transfected with miR-19a inhibitor
(Fig. 2D). These results suggest
that downregulation of miR-19a promoted glucose production through
blocking activation of AKT/FOXO1 signaling and increasing
expression of gluconeogenetic genes in HEP1-6 cells.
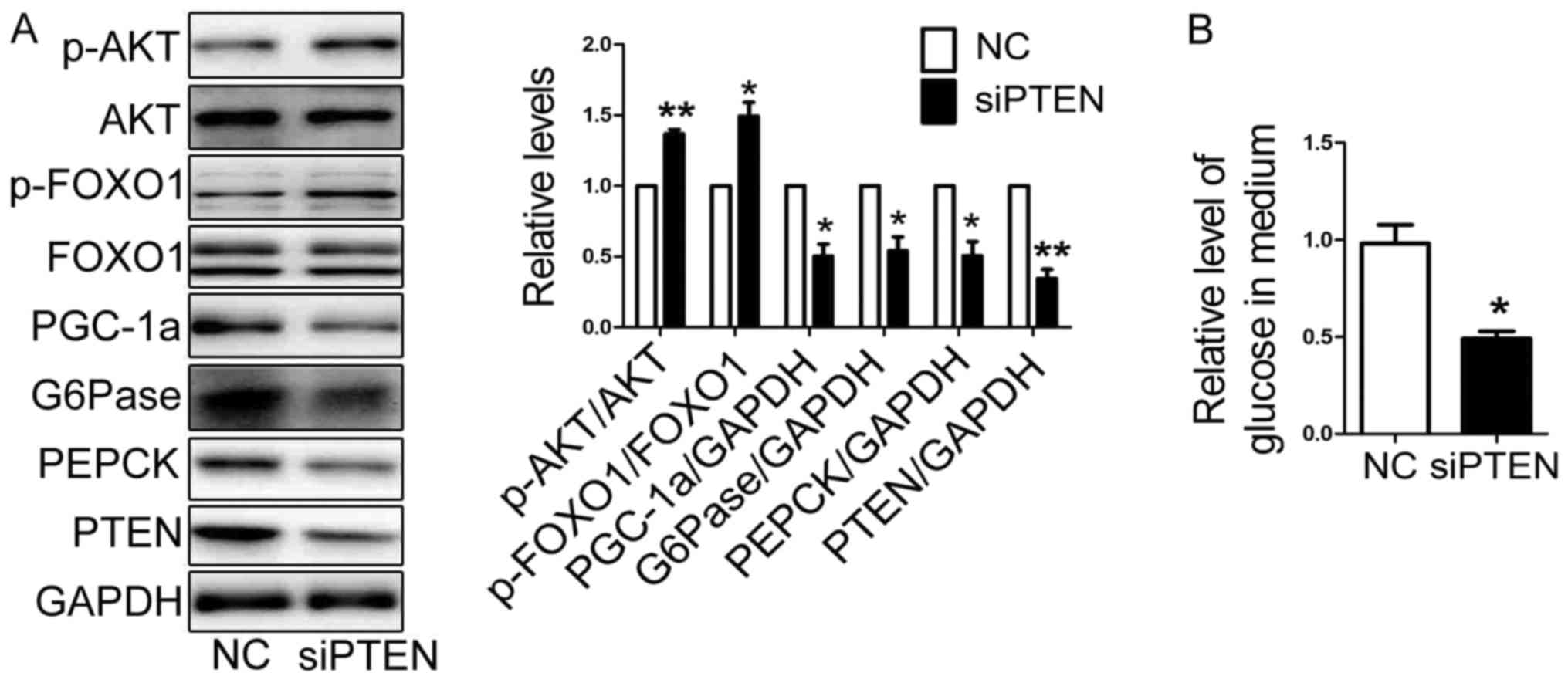
Silence of PTEN impairs glucose
production and expression of gluconeogenetic genes
In our previous study, we found that PTEN is a
target gene of miR-19a. And PTEN could regulate activation of
PI3K/AKT pathway. To explore the effect of PTEN on glucose
production and expression of gluconeogenetic genes, a siRNA
specifically targeted PTEN (si-1519) was transfected into HEP1-6
cells for 48 h (9). The protein
level of PTEN was decreased significantly (Fig. 3A). And the expression of PGC-1α,
G6Pase and PEPCK were reduced, while the activation of AKT/FOXO1
pathway were elevated in HEP1-6 cells transfected with siPTEN
(Fig. 3A). The glucose production
was decreased in HEP1-6 cells transfected with siPTEN (Fig. 3B).
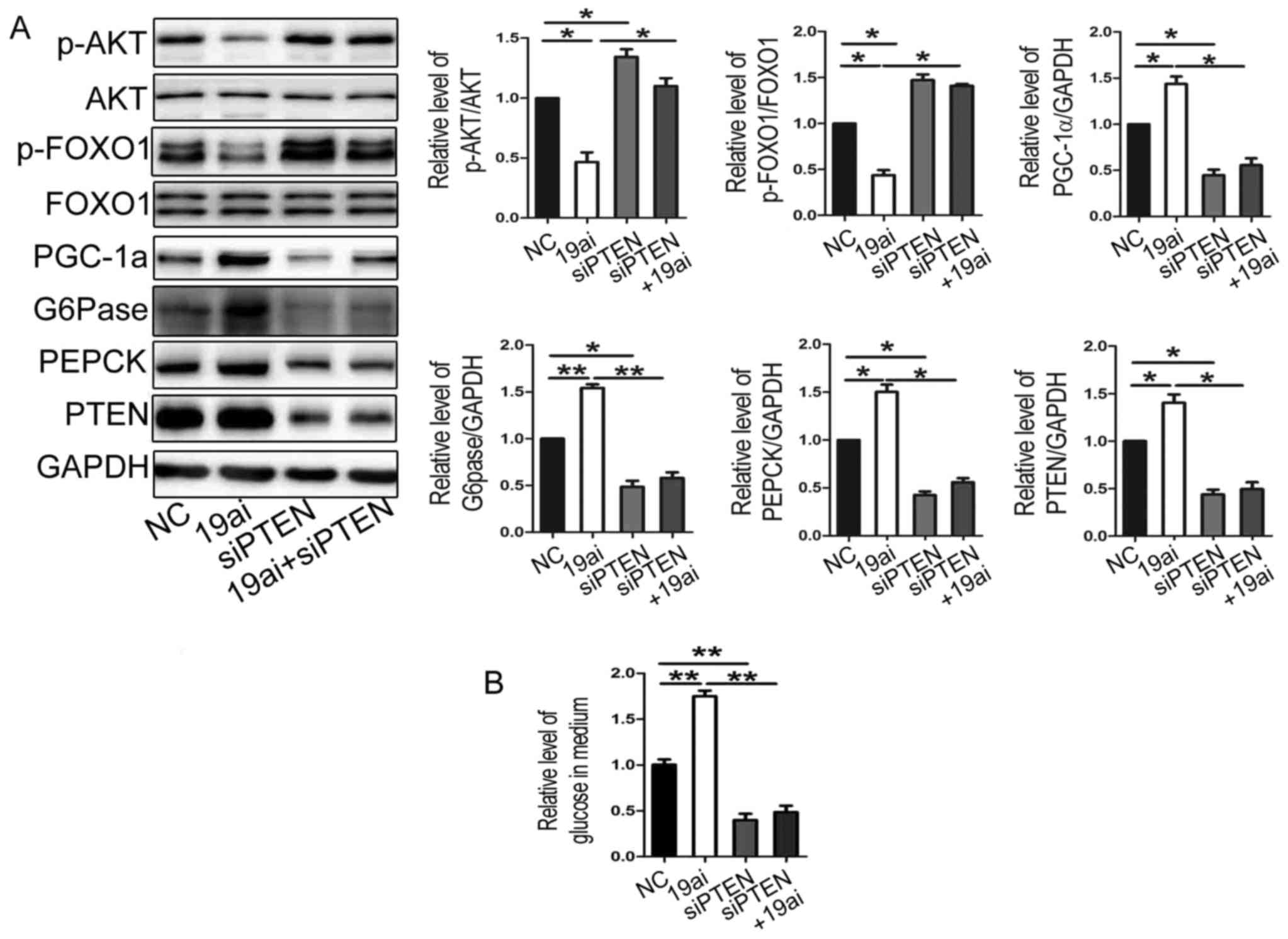
MiR-19a regulates glucose production
and expression of gluconeogenetic genes via targeting PTEN
Next, to verify that miR-19 regulates glucose
production and expression of gluconeogenetic genes via targeting
PTEN, the miR-19a inhibitor and siPTEN were co-transfected into
HEP1-6 cells. As shown in Fig. 4
that transfection of siPTEN reversed the effect of miR-19a
inhibitor on expression of gluconeogenetic genes (Fig. 4A) and glucose production (Fig. 4B). Taken together, miR-19a might
regulate glucose production and expression of gluconeogenetic genes
and via targeting PTEN.
Discussion
The impaired hepatic insulin sensitivity contributes
to increased hepatic glucose production, which may lead to
dysregulation of glucose metabolism (11,12).
There increasing evidence that miRNAs are involved in the
regulation of glucose metabolism (4). MiR-19a, a member of mir-17-92 miRNA
family, is a oncogenic miRNA, which could promote proliferation and
angiogenesis of cancer cell (10).
In our previous study, we verified that over-expression of miR-19a
increased the activation of PI3K/AKT/GSK pathway and glycogenesis
via targeting PTEN. In the present study, we focused on the
critical role of miR-19a in regulating hepatic glucose production.
Our results suggested that over-expression of miR-19a elevated the
phosphorylation of FOXO1 and suppressed expression of
gluconeogenesis-related genes, such as PGC1α, PEPCK and G6Pase.
Most importantly, glucose production was impaired in HEP1-6 cells
transfected with miR-19a mimic. It was reported that PI3K/AKT
pathway is the main insulin signal pathway in hepatocytes, which
can regulate hepatic glucose metabolism including gluconeogenesis
and glycogenesis (1). Activated
AKT can inactivate glycogen synthase kinase 3β (GSK3β), which
permits the activation of glycogen synthase and leads to
glycogenesis (13,14). Moreover, activated AKT also can
promote the phosphorylation and nuclear exclusion of FOXO1 to lower
expression of gluconeogenetic enzymes and gluconeogenesis (15). In hepatic insulin resistance,
suppressed activation of PI3K/AKT led to increased hepatic
gluconeogenesis through reduced inactivation of FOXO1. Furthermore,
PGC1α is another transcriptional co-activator which is activated
under fasting conditions (16).
PGC1α co-activates FOXO1, leading to PEPCK and G6Pase
transcription. Insulin impaired PGC1α by increasing its
phosphorylation at ser570 by activated AKT (17).
In previous study, PTEN was verified as a direct
target of miR-19a to mediate the activation of PI3K/AKT/GSK
pathway. There are several binding sites for miR-19a at the PTEN
3′-UTR. PTEN is expressed in all tissues and contains a tensin-like
domain and a phosphatase catalytic domain (18,19).
Moreover, PTEN is a negative regulator of PI3K/AKT pathway by
catalyzing PIP3 dephosphorylation and converting it into PIP2
(19–21). In the present study, we found that
PTEN could regulate phosphorylation of FOXO1. Silencing PTEN could
reverse the effects of miR-19a inhibition on phosphorylation of
FOXO1 and expression of gluconeogenesis-related genes. Therefore,
PTEN participated in miR-19a-mediated gluconeogenesis in
hepatocytes via regulating AKT/FOXO1 pathway.
In conclusion, these findings provide mechanistic
insight into the effects of miR-19a on gluconeogenesis and
regulation of AKT/FOXO1 pathway in hepatocytes. MiR-19a might
mediate gluconeogenesis via downregulating PTEN expression.
Acknowledgements
The present study was supported by grants (81570789
and 81600618) from National Natural Science Foundation of
China.
References
|
1
|
Rines AK, Sharabi K, Tavares CD and
Puigserver P: Targeting hepatic glucose metabolism in the treatment
of type 2 diabetes. Nat Rev Drug Discov. 15:786–804. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bugianesi E, McCullough AJ and Marchesini
G: Insulin resistance: A metabolic pathway to chronic liver
disease. Hepatology. 42:987–1000. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leclercq IA, Da Silva Morais A, Schroyen
B, Van Hul N and Geerts A: Insulin resistance in hepatocytes and
sinusoidal liver cells: Mechanisms and consequences. J Hepatol.
47:142–156. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fernandez-Valverde SL, Taft RJ and Mattick
JS: MicroRNAs in beta-cell biology, insulin resistance, diabetes
and its complications. Diabetes. 60:1825–1831. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dou L, Zhao T, Wang L, Huang X, Jiao J,
Gao D, Zhang H, Shen T, Man Y, Wang S and Li J: miR-200s contribute
to interleukin-6 (IL-6)-induced insulin resistance in hepatocytes.
J Biol Chem. 288:22596–22606. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dou L, Wang S, Sui X, Meng X, Shen T,
Huang X, Guo J, Fang W, Man Y, Xi J and Li J: MiR-301a mediates the
effect of IL-6 on the AKT/GSK pathway and hepatic glycogenesis by
regulating PTEN expression. Cell Physiol Biochem. 35:1413–1424.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang S, Wang L, Dou L, Guo J, Fang W, Li
M, Meng X, Man Y, Shen T, Huang X and Li J: MicroRNA 152 regulates
hepatic glycogenesis by targeting PTEN. FEBS J. 283:1935–1946.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang W, Guo J, Cao Y, Wang S, Pang C, Li
M, Dou L, Man Y, Huang X, Shen T and Li J: MicroRNA-20a-5p
contributes to hepatic glycogen synthesis through targeting p63 to
regulate p53 and PTEN expression. J Cell Mol Med. 20:1467–1480.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dou L, Meng X, Sui X, Wang S, Shen T,
Huang X, Guo J, Fang W, Man Y, Xi J and Li J: MiR-19a regulates
PTEN expression to mediate glycogen synthesis in hepatocytes. Sci
Rep. 5:116022015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen L, Li C, Zhang R, Gao X, Qu X, Zhao
M, Qiao C, Xu J and Li J: miR-17-92 cluster microRNAs confers
tumorigenicity in multiple myeloma. Cancer Lett. 309:62–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lanthier N and Leclercq IA: Liver and
systemic insulin resistance. Hepatology. 60:1113–1114. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Meshkani R and Adeli K: Hepatic insulin
resistance, metabolic syndrome and cardiovascular disease. Clin
Biochem. 42:1331–1346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Samue VT and Shulman GI: The pathogenesis
of insulin resistance: Integrating signaling pathways and substrate
flux. J Clin Invest. 126:12–22. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wan M, Leavens KF, Hunter RW, Koren S, von
Wilamowitz-Moellendorff A, Lu M, Satapati S, Chu Q, Sakamoto K,
Burgess SC and Birnbaum MJ: A noncanonical, GSK3-independent
pathway controls postprandial hepatic glycogen deposition. Cell
Metab. 18:99–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu M, Wan M, Leavens KF, Chu Q, Monks BR,
Fernandez S, Ahima RS, Ueki K, Kahn CR and Birnbaum MJ: Insulin
regulates liver metabolism in vivo in the absence of hepatic Akt
and Foxo1. Nat Med. 18:388–395. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoon JC, Puigserver P, Chen G, Donovan J,
Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, et al:
Control of hepatic gluconeogenesis through the transcriptional
coactivator PGC-1. Nature. 413:131–138. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Puigserver P, Rhee J, Donovan J, Walkey
CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D
and Spiegelman BM: Insulin-regulated hepatic gluconeogenesis
through FOXO1-PGC-1alpha interaction. Nature. 423:550–555. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gupta A and Dey CS: PTEN, a widely known
negative regulator of insulin/PI3K signaling, positively regulates
neuronal insulin resistance. Mol Biol Cell. 23:3882–3898. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Horie Y, Suzuki A, Kataoka E, Sasaki T,
Hamada K, Sasaki J, Mizuno K, Hasegawa G, Kishimoto H, Iizuka M, et
al: Hepatocyte-specific Pten deficiency results in steatohepatitis
and hepatocellular carcinomas. J Clin Invest. 113:1774–1783. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guenzl PM, Raim R, Kral J, Brunner J,
Sahin E and Schabbauer G: Insulin hypersensitivity induced by
hepatic PTEN gene ablation protects from murine endotoxemia. PLoS
One. 8:e670132013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsuda S, Kobayashi M and Kitagishi Y:
Roles for PI3K/AKT/PTEN pathway in cell signaling of nonalcoholic
fatty liver disease. ISRN endocrinol. 2013:4724322013. View Article : Google Scholar : PubMed/NCBI
|