Introduction
Parkinson's disease (PD) is a progressive
degenerative disease of the central nervous system characterised by
the deposition of α-synuclein in the mesencephalic substantia nigra
and the progressive loss of dopaminergic neurons (1). When the loss of dopaminergic neurons
exceeds 50%, the level of secreted dopamine becomes insufficient,
resulting in the development of movement disorders, including
resting tremor, bradykinesia, muscle rigidity and gait
abnormalities, which may severely affect patient quality of life
and lead to mortality (2).
However, conventional medications, including
levodopa, are only able to increase the dopamine content in the
brain to improve symptoms, and are unable to prevent or delay
disease development (3,4). In addition, long-term use may lead to
uncontrollable movement side-effects and adverse drug reactions.
Therefore, it is important to identify drugs that may prevent the
progressive loss of dopaminergic neurons, which may help to prevent
or delay the development of PD and improve patient survival.
Although the precise mechanisms underlying the
development of PD remain unclear, research has demonstrated that
immune-mediated inflammation is involved in PD pathogenesis
(5,6). Microglia are immunocompetent cells of
the brain, and the activation of microglia leads to an increase in
the levels of tumour necrosis factor-α (TNF-α) (7). The pro-inflammatory factor
lipopolysaccharide (LPS) is a component of the cell wall in
Gram-negative bacteria that has been associated with PD. Research
has demonstrated that a systemic or intracerebral injection of LPS
may lead to progressive loss of dopaminergic neurons in the
midbrain of normal mice, resulting in Parkinsonian-type movements
as early as 28 weeks post-treatment, with increased severity by 40
weeks post-LPS exposure (8).
Notably, LPS does not directly damage nerve cells; rather, it
exerts its effects indirectly by activating microglia. These
activated microglia, in turn, release TNF-α, leading to damage to
dopaminergic neurons (9).
DL-3-n-butylphthalide (NBP) is a monomer compound
isolated from celery seeds whose neuroprotective effects have been
well-documented in animal models of cerebral ischaemia and vascular
dementia (10). Previously, a
study observed a decrease in movement and sleep disorders among 43
patients with PD who had been treated using NBP (Chen et al,
unpublished data). Therefore, it was hypothesised that NBP may
exert neuroprotective effects against the degeneration of
substantia nigra cells via an unknown mechanism.
Therefore, the present study examined the effects of
intragastric NBP administration in an LPS-induced PD mouse model.
In order to elucidate the mechanism through which NBP may aid in
the treatment of PD, the effects of NBP on behaviour, microglial
activation, TNF-α and α-synuclein levels, and the number of
tyrosine hydroxylase (TH)-positive cells in the substantia nigra
were examined in model mice.
Materials and methods
Laboratory animals and treatment
A total of 36 male C57BL/6 mice, 8 weeks-old,
weighing ~18–20 g [Jiangsu University, Zhenjiang, China; no. SCXK
(Su) 2013–0011] were raised in a specific pathogen-free grade
experimental animal facility at Bengbu Medical College [Bengbu,
China; no. YXK (Anhui) 2012–002]. Each standard rearing box
contained five mice. The room temperature was set at 22–24°C,
60–70% relative humidity, and the circadian cycle was set for 12/12
h light/dark. Mice were provided with ad libitum access to
food and water. Experimental procedures were performed in
accordance with the European Community Council Directive of 24th
November 1986 (no. 86/609/EEC), and all efforts were made to
minimise the pain and discomfort of animals. The present study was
approved by the Ethics Committee of Bengbu Medical College.
The mice were randomly divided into three groups
(n=12 mice/group). The normal control group (C) was given a single
intraperitoneal injection of normal saline (NS). The model group
was given a single intraperitoneal injection of LPS (5 mg/kg;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The NBP treatment
group was treated in the same manner as the model group with regard
to the induction of PD; however, beginning on the day of model
induction, weight-based NBP (120 mg/kg/day; NBP Pharmaceutical Co.,
Shijiazhuang, China) was given once daily via intragastric
administration for 30 days. At weeks 4 and 28 following model
induction, 6 mice from each group were randomly selected for
behavioural testing, following which they were anaesthetised using
4% chloral hydrate. Thoracotomy was performed, and the right atrium
was punctured with an infusion needle, which was immediately
inserted into the left ventricle for rapid infusion of 0.9% NS,
until the liver had turned white. Subsequently, 4% paraformaldehyde
was infused over 20 min for fixation. The mid-brain containing the
substantia nigra was removed and paraffin-embedded in preparation
for continuous coronal sectioning.
Rotarod test
A rotarod treadmill (cat. no. ENV576; Med
Associates, Inc., St. Albans, VT, USA) was used to evaluate the
motor coordination of the mice. At weeks 4 and 28 following
treatment, mice were placed on a rotarod and pre-trained at a
constant speed over a 5-min period. During testing, the speed was
increased from 2.5 to 25 rpm, and the average time of three
repeated tests in which the mice remained on the rotating bar was
recorded. The results are expressed as dwell time (sec).
Open field test
The field was contained within a custom-made wooden
box with dimensions of 40×40×30 cm (length × width × height). The
bottom and inside surface of the box were painted white, and a
camera was placed above the centre of the box. The subject mouse
was placed in the middle of the box, and its movements were
observed and recorded for 5 min. Using a motion trajectory tracking
system (Noldus Information Technology B.V., Wageningen, The
Netherlands), the bottom of the test box was divided into 16 visual
areas, each spanning 10×10 cm. Line crossing, rearing, and grooming
activities were recorded and analysed.
Immunohistochemical staining
Immunohistochemical staining was used to determine
levels of activated ionised calcium binding adaptor molecule
(Iba)-1-positive cells, α-synuclein deposition and TH-positive
cells in the midbrain. The midbrains, followed by 4%
paraformaldehyde for 20 min at 0°C, were embedded in paraffin and
sliced into coronal sections with a thickness of 4 µm each.
Sections were transferred through three washes with xylene and
rehydrated with decreasing grades of absolute alcohol (95, 75 and
50%). Endogenous peroxidases were blocked by incubating the
sections with 3% H2O2 at room temperature for
10 min. Following antigen retrieval, the sections were incubated
with the primary monoclonal antibodies anti-TH (1:1,000, cat. no.
GB11181), rabbit anti-Iba-1 (1:1,000, cat. no. GB13105), TNF-α
(1:200, cat. no. GB13188; all from Servicebio, Shanghai, China;
www.servicebio.com) or α-synuclein (1:500, cat.
no. AB1903; Abcam, Cambridge, UK) at 37°C overnight. On the
following day, the sections were rinsed with PBS and incubated with
the appropriate biotinylated goat anti-mouse/rabbit horseradish
peroxidase-labelled antibody (1:1) (cat. no. K5007; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) at 37°C for 50 min,
washed and stained with a diaminobenzidine staining kit (Dako;
Agilent Technologies, Inc.). Following Harris haematoxylin staining
for 3 min at room temperature and controlling the dying time under
a simple microscope (magnification, ×200; Nikon Corporation, Tokyo,
Japan).
Statistical analysis
All experiments were repeated twice, and all data
were processed using SPSS software version 16.0 (SPSS, Inc.,
Chicago, IL, USA), and are expressed as the mean ± standard
deviation. Multiple comparisons were performed using one-way
analysis of variance followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
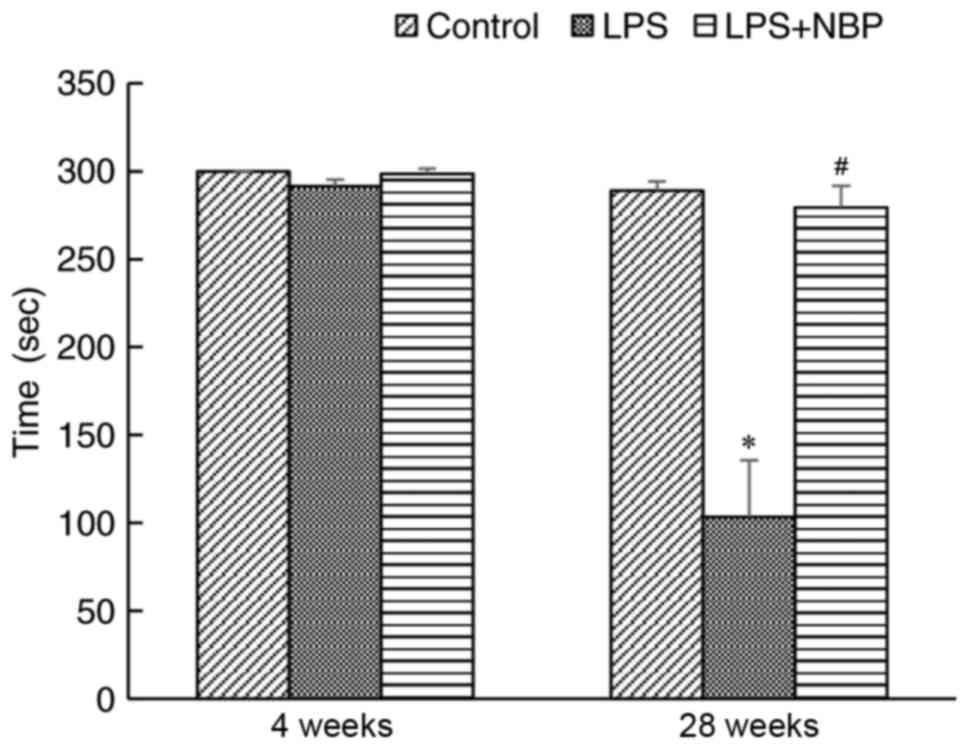
Effect of NBP on rotarod performance
in LPS-induced PD mice
In the present study, the rotarod experiment was
used to evaluate fine motor coordination and balance in PD model
mice. At 4 weeks following treatment with LPS, no significant
difference was observed in dwell time among the control, LPS, and
LPS + NBP groups. However, by 28 weeks, mice in the LPS group
exhibited significantly shorter dwell times compared with those of
the NS group (P<0.05), while NBP significantly attenuated the
effects of LPS (P<0.05) (Fig.
1).
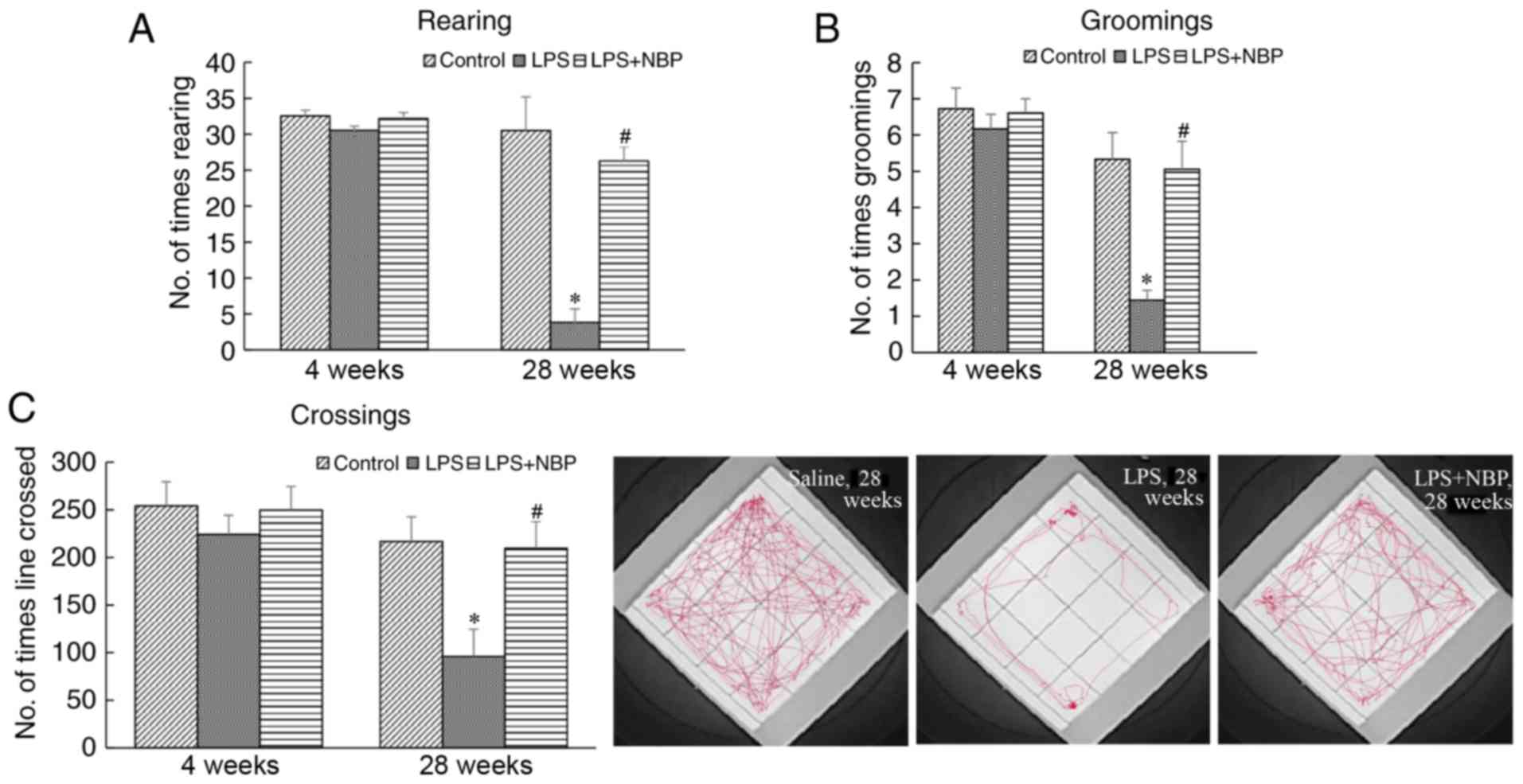
Effect of NBP on open field test
performance in LPS-induced PD mice
The open field test was used to evaluate exploratory
behaviour and spontaneous locomotor activities in PD model mice.
Line crossing, rearing and grooming activities were recorded and
analysed to determine the extent of tremor, gait instability and
bradykinesia. At 28 weeks following treatment with LPS, the
frequency of line crossing, rearing and grooming activities in the
treatment mice had decreased (P<0.05) compared with those
observed in NS mice. However, NBP significantly attenuated the
effect of LPS (P<0.05) (Fig.
2). At 4 weeks, there was no marked difference among the three
groups.
NPS prevents LPS-induced activation of
mouse microglia
Microglia serve an important role in intracerebral
inflammation. A previous study (8)
has reported that microglial activation results in the degeneration
of substantia nigra neurons in patients with PD. The results of the
present study indicated that, compared with that observed in the
control group, the number of microglia significantly increased in
mice by 4 weeks post-treatment with LPS (P<0.05). These
microglia tended to be larger, rounder, and exhibit amoeboid-like
alterations, indicative of microglial activation. These alterations
were still observed at 28 weeks following LPS treatment
(P<0.05), although NBP reduced this effect of LPS on microglia
at weeks 4 and 28 (P<0.05) (Fig.
3).
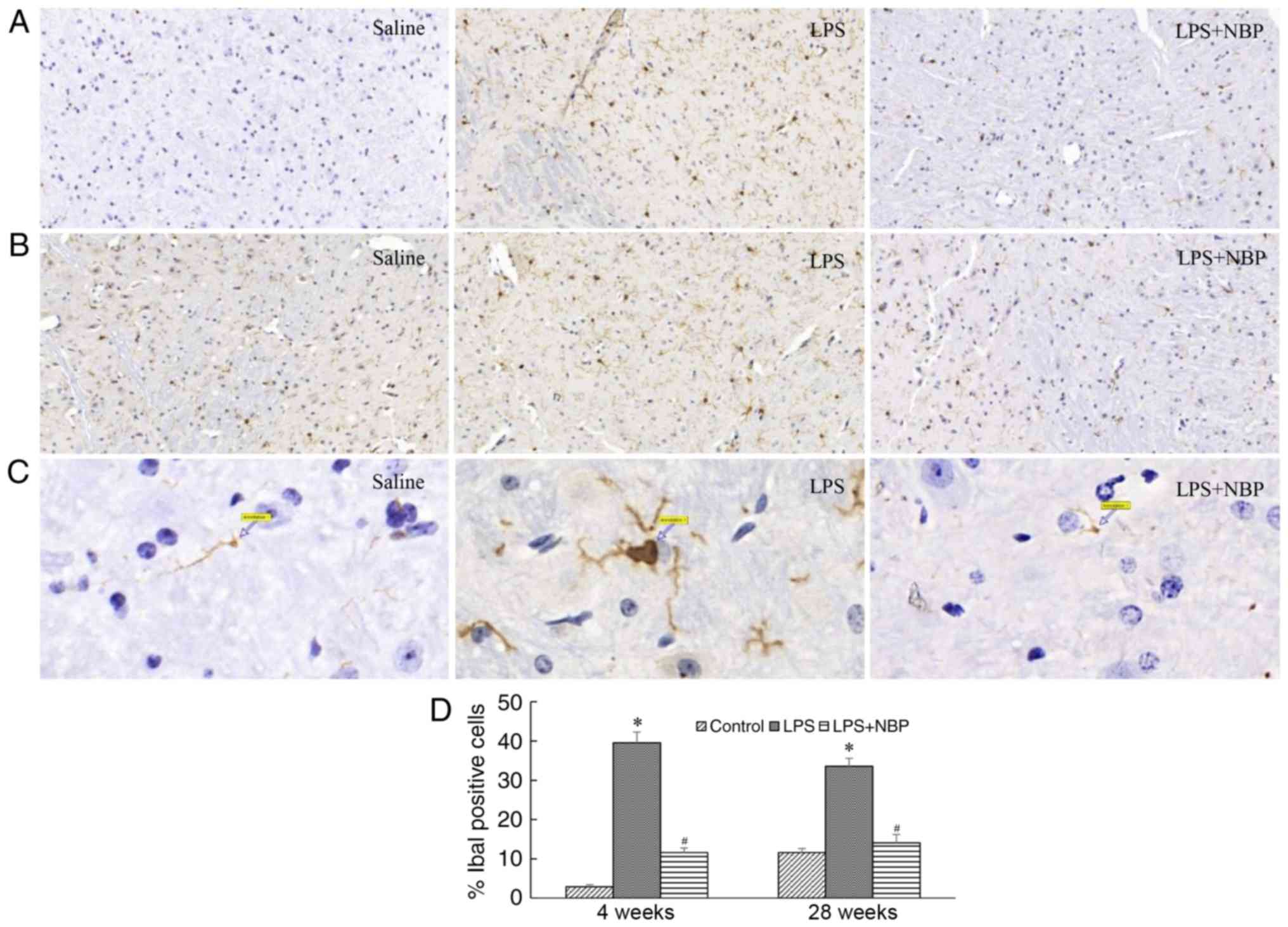 | Figure 3.NBP reduces the long-term activation
of microglia in the substantia nigra following intraperitoneal
injection of LPS. Results are expressed as a percentage of the
corresponding saline controls. (A) Visualisation of Iba-1-IR
neurons in the substantia nigra of saline-treated, LPS-treated and
LPS + NBP-treated mice at 4 weeks. (B) Visualisation of Iba-1-IR
neurons in the substantia nigra of saline-treated, LPS-treated and
LPS + NBP-treated mice at 28 weeks. (C) Arrows indicate activated
microglia in the substantia nigra of saline-treated, LPS-treated
and LPS + NBP-treated mice at 28 weeks (magnification, ×1,000). (D)
Number of Iba-1-IR neurons in the substantia nigra of
saline-treated, LPS-treated and LPS + NBP-treated mice at 4 and 28
weeks. *P<0.05 vs. control group; #P<0.05 vs. LPS
group. LPS, lipopolysaccharide; NBP, dl-3-n-butylphthalide; Iba-1,
ionised calcium binding adaptor molecule-1, IR,
immunoreactivity. |
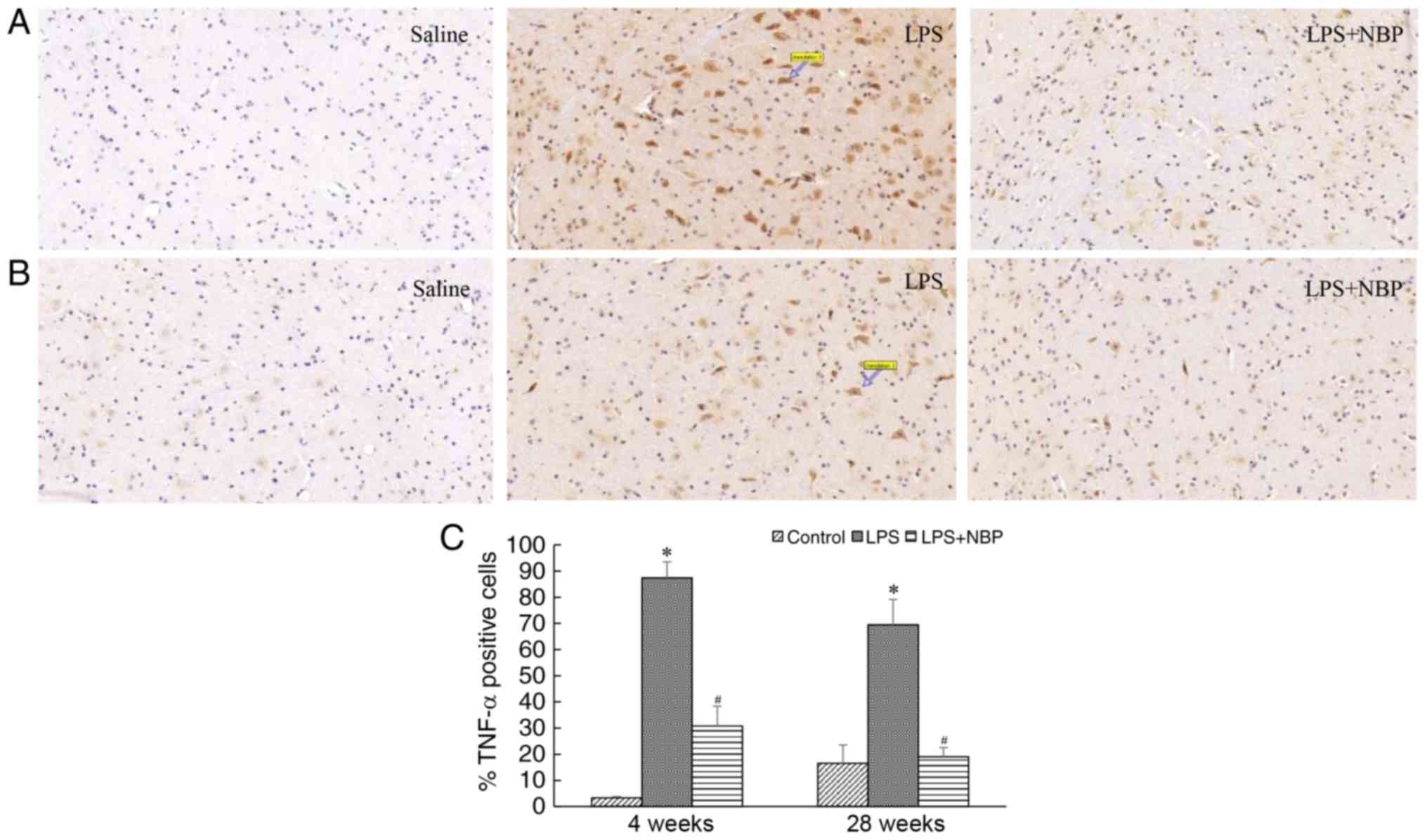
NBP prevents LPS-induced increases in
levels of TNF-α in the mouse midbrain
TNF-α released by activated microglia is among the
key inflammatory factors that lead to degeneration of substantia
nigra cells. In the present study, the expression of TNF-α was
demonstrated to be increased as the levels of activated microglia
increased. Compared with that observed in the control group, the
expression of TNF-α significantly increased in the mice of the LPS
group following 4 and 28 weeks of treatment (P<0.05). However,
NBP attenuated this effect of LPS at the two time-points
(P<0.05) (Fig. 4).
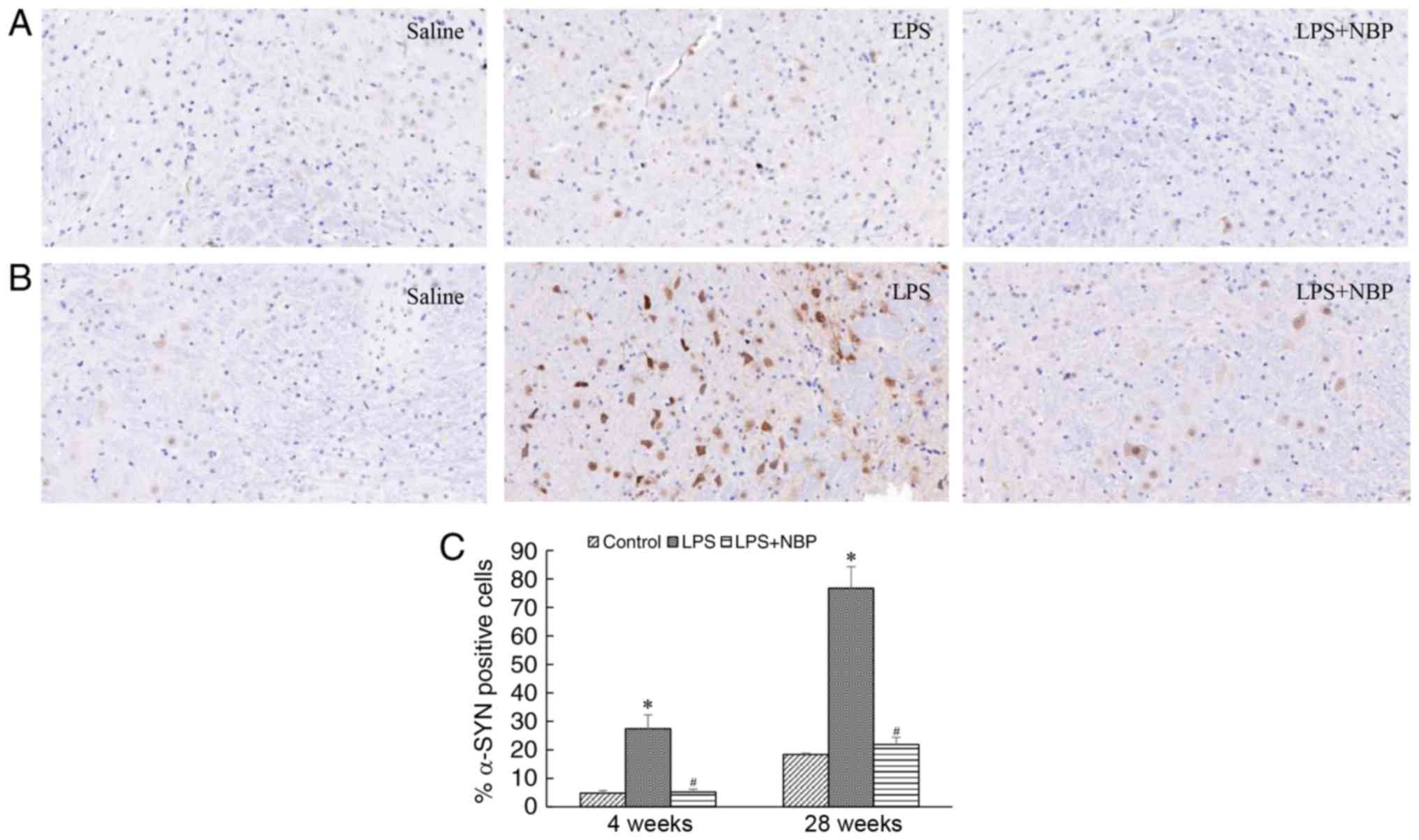
NBP reduces LPS-induced α-synuclein
deposition in the substantia nigra of the mouse midbrain
The deposition of α-synuclein is a pathognomonic
change in the midbrains of patients with PD. In the present study,
α-synuclein deposition was observed in the substantia nigra of mice
at 4 weeks following treatment with LPS and increased over time. At
week 28 post-treatment with LPS, α-synuclein deposition in the
substantia nigra had significantly increased (P<0.05), although
these effects were attenuated by NBP (P<0.05) (Fig. 5).
NBP protects against the LPS-induced
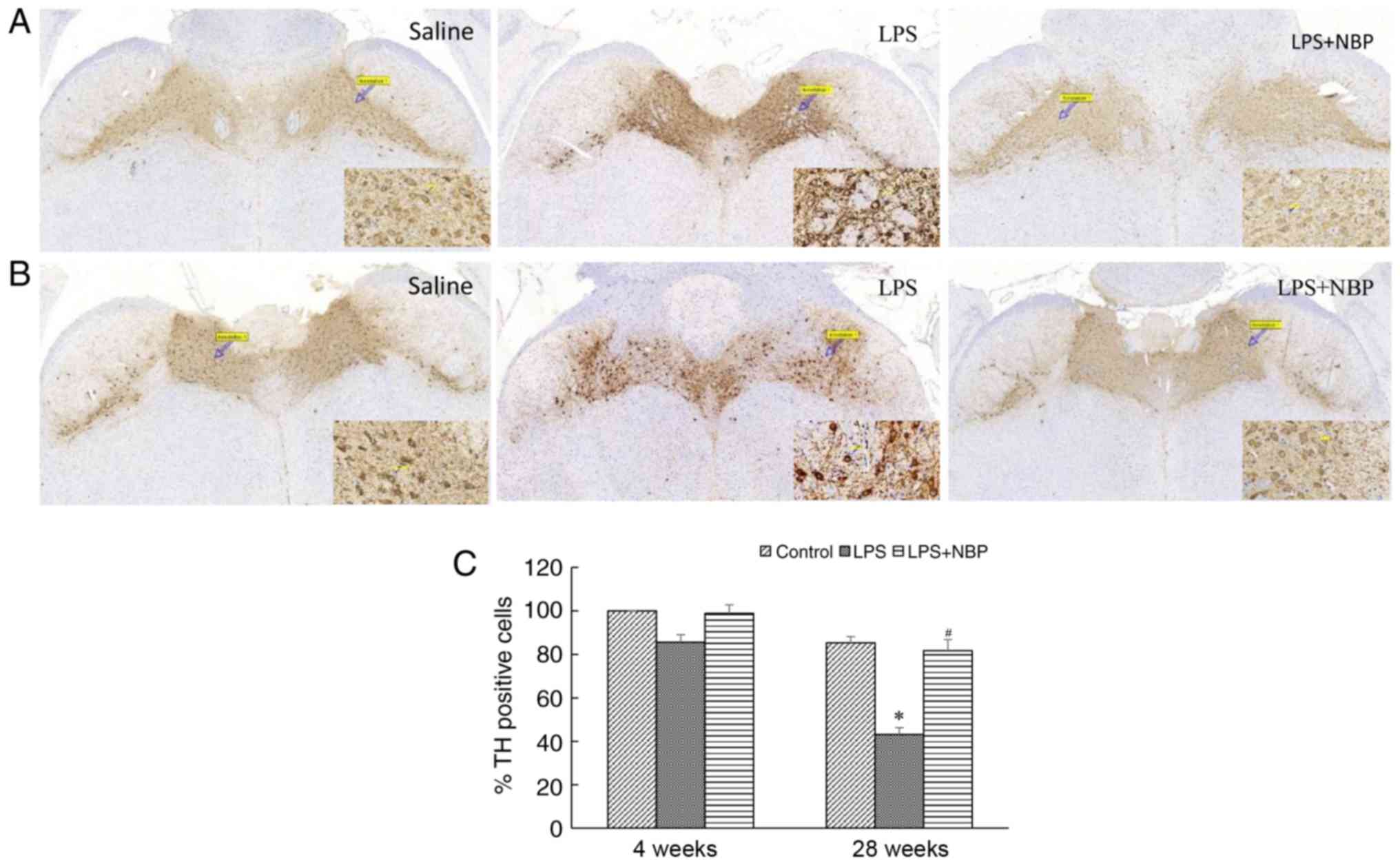
loss of dopaminergic neurons in the substantia nigra
Loss of dopaminergic neurons in the substantia nigra
represents another pathognomonic change that occurs in patients
with PD. The results of the present study indicated that the number
of dopaminergic neurons in the substantia nigra had decreased at 4
and 28 weeks following treatment with LPS, relative to that
observed in the control group (P<0.05), and that this decrease
became amplified over time. However, NBP attenuated the LPS-induced
loss of neurons (P<0.05). Notably, at 28 weeks following
treatment with LPS, the number of TH-positive cells in all three
groups of mice had decreased, although the number of TH-positive
cells in the NBP-treated group was similar to that in the control
group. The results of the present study may reflect the loss of
dopaminergic neurons in the substantia nigra during the aging
process, suggesting that NBP may decrease neuronal loss associated
with physiological aging (Fig.
6).
Discussion
In 95% of PD cases, the disease is sporadic and of
the delayed type, with an unclear aetiology and no clear pattern of
inheritance; in these cases, PD is considered to be associated with
various factors, including environmental toxins, immune-mediated
inflammation and oxidative stress (11). A previous study demonstrated that
systemic inflammation-induced cerebral microglial activation is an
important factor in the pathogenesis of PD (6). LPS, a cell wall component of
Gram-negative bacteria, is among the proinflammatory cytokines with
important roles in systemic inflammation. Previous studies have
reported that systemic and intracerebral injections of LPS may lead
to dopaminergic neuronal loss in the midbrain of normal mice
(9,12), in addition to the activation of
cerebral microglia. Such activation leads to increases in levels of
inflammatory factors, including TNF-α, interleukin (IL)-6 and IL1β,
following which Parkinsonian motor symptoms may be observed
(9,12). A previous study indicated that LPS
may cause intestinal permeability, sequential increases in
α-synuclein immunoreactivity, and pathological α-synuclein
accumulation in the colon in a manner similar to that observed in
patients with PD (13). A mouse
model of PD induced via systemic injection of LPS was selected for
the present study as this animal model presents with a progressive
loss of dopaminergic neurons. Such mice do not display classic PD
motor symptoms until 7 months following model induction, which is
more consistent with the clinical progression of PD (9). Consistent with the findings of
previous studies, no obvious motor impairment was observed in the
model mice in the early stages of the disease (4 weeks following
model induction). Apparent motor symptoms did not develop until 28
weeks following model induction. However, the corresponding
morphological alterations in the midbrain occurred 4 weeks prior to
the onset of motor symptoms in the model mice. These alterations,
which progressed over time, included α-synuclein deposition and a
decrease in the number of dopaminergic neurons in the substantia
nigra.
Notably, it was observed that microglial activation
and increased TNF-α levels occurred early in the midbrains of model
mice. Microglia are the primary intracerebral immune cells and
serve an important role in intracerebral inflammation. Recent
studies have indicated that immune-mediated inflammation is
involved in the pathogenesis of PD (12). However, LPS does not directly
damage neurons; rather, it does so by activating microglia. In a
previous study, when midbrain neurons were cultured in
vitro, oxidopamine (6-OHDA) decreased the number of TH-positive
cells by 89%, while LPS had no obvious effect on TH-positive cells.
However, when neurons and microglia were cultured together, 6-OHDA
only reduced the number of TH-positive cells by 27%, while LPS
reduced this number by 70% (14).
Subsequent studies have demonstrated that LPS may induce microglial
activation and release proinflammatory cytokines, including nitric
oxide, TNF-α, IL-6 and IL1β, which are known to cause dopaminergic
neuronal damage (15). These
previous findings suggested that LPS is among the causes of
microglial activation in the brains of patients with PD, leading to
the release of proinflammatory cytokines and cytotoxins. Such
alterations may, in turn, promote the inflammatory response of
dopaminergic neurons in the substantia nigra, resulting in
dopaminergic neuronal injury.
In the present study, it was observed that NBP
inhibited the activation of microglia and decreased α-synuclein
deposition and the loss of dopaminergic neurons in the substantia
nigra of LPS-induced PD mice. NBP, also termed butylphthalide
(C12H14O2; molecular mass, 190) is
an active ingredient isolated from the oil of celery seeds with
multiple pharmacological effects (16–18),
though the mechanisms underlying these effects remain unknown. NBP
is a national class I chemical drug with independent intellectual
property rights in China. Clinical studies have demonstrated that
NBP is a safe and effective treatment for ischaemic stroke
(10). In a mouse model of spinal
lateral sclerosis, NBP decreased the levels of glial cell
activation and the intracerebral expression of nuclear factor-κB,
transcription factor p65, and TNF-α, prolonging the survival time
of mice (16). In a murine model
of cerebral ischaemia-reperfusion, NBP decreased the intracerebral
levels of TNF-α and intracellular adhesion molecule-1, thereby
reducing damage to brain tissue (17). In a rotenone-induced PD mouse
model, NBP decreased damage to dopaminergic neurons in the midbrain
(18). In accordance with these
findings, the results indicated that 30 days of treatment with NBP
during the establishment of an LPS-induced model significantly
attenuated LPS-induced mesencephalic microglial activation in the
early stages, and decreased the loss of dopaminergic neurons in
addition to α-synuclein deposition in the substantia nigra in the
late stages. Additionally, 7 months post-treatment, the number of
dopaminergic neurons in the substantia nigra of the NBP-treated
group was significantly increased compared with the LPS-treated
group, and was similar to that of the control group, likely for two
reasons: i) NBP treatment was administered during model induction,
promptly preventing the activating effect of LPS on microglia; and
ii) the model was established when the mice were 6-weeks old, and
they had reached middle age (34-weeks old) by 7 months following
model establishment. Physiological aging is associated with a loss
of dopaminergic neurons, as aging cells cause chronic inflammatory
responses that damage nearby cells. Indeed, chronic inflammation,
aging and age-associated diseases are associated with one another.
NBP treatment was commenced when the mice were 6-weeks old and was
continued until they were 10-weeks old. Thus, the treatment may
have prevented chronic inflammation induced by the aging of
dopaminergic neurons, although the specific mechanism of action and
the targets involved require further investigation.
The present study possesses a limitation of note, as
the mechanism through which NBP blocks the LPS-induced activation
of microglia was not investigated in vivo. Future studies
are required to investigate the role of NBP in the inflammatory
pathway of microglial cells in vitro.
In conclusion, the results of the present study
demonstrated that systemic application of LPS is able to induce the
early activation of microglial cells in the mouse brain, thereby
leading to intracerebral inflammation, progressive loss of
dopaminergic neurons in the substantia nigra and α-synuclein
deposition. These alterations are associated with PD motor
symptoms. In addition, the results of the present study indicated
that NBP may prevent LPS-induced activation of microglia and
preserve dopaminergic neurons in the substantia nigra. NBP thus
exhibits potential as a treatment for neurodegenerative diseases,
including PD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81641050).
Glossary
Abbreviations
Abbreviations:
|
LPS
|
lipopolysaccharide
|
|
NBP
|
dl-3-n-butylphthalide
|
|
TH
|
tyrosine hydroxylase
|
|
PD
|
Parkinson's disease
|
|
Iba-1
|
ionised calcium binding adaptor
molecule-1
|
|
TNF-α
|
tumour necrosis factor-α
|
|
IL
|
interleukin
|
|
6-OHDA
|
oxidopamine
|
References
|
1
|
Venda LL, Cragg SJ, Buchman VL and
Wade-Martins R: α-Synuclein and dopamine at the crossroads of
Parkinson's disease. Trends Neurosci. 33:559–568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calabresi P and Di Filippo M: Multitarget
disease-modifying therapy in Parkinson's disease? Lancet Neurol.
14:975–976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peterson DS, Pickett KA and Earhart GM:
Effects of levodopa on vividness of motor imagery in parkinson
disease. J Parkinsons Dis. 2:127–133. 2012.PubMed/NCBI
|
|
4
|
Gagne JJ and Power MC: Anti-inflammatory
drugs and risk of Parkinson disease: A meta-analysis. Neurology.
74:995–1002. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lema Tomé CM, Tyson T, Rey NL, Grathwohl
S, Britschgi M and Brundin P: Inflammation and α-synuclein's
prion-like behavior in Parkinson's disease-is there a link? Mol
Neurobiol. 47:561–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grozdanov V, Bliederhaeuser C, Ruf WP,
Roth V, Fundel-Clemens K, Zondler L, Brenner D, Martin-Villalba A,
Hengerer B, Kassubek J, et al: Inflammatory dysregulation of blood
monocytes in Parkinson's disease patients. Acta Neuropathol.
128:651–663. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernández-Calle R, Vicente-Rodríguez M,
Gramage E, Pita J, Pérez-García C1, Ferrer-Alcón M, Uribarri M,
Ramos MP and Herradón G: Pleiotrophin egulates microglia-mediated
neuroinflammation. J Neuroinflammation. 14:462017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alhadidi Q and Shah ZA: Cofilin mediates
LPS-induced microglial cell activation and associated neurotoxicity
through activation of NF-κB and JAK-STAT pathway. Mol Neurobiol.
Feb 13–2017.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qin L, Wu X, Block ML, Liu Y, Breese GR,
Hong JS, Knapp DJ and Crews FT: Systemic LPS causes chronic
neuroinflammation and progressive neurodegeneration. Glia.
55:453–462. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cui LY, Zhu YC, Gao S, Wang JM, Peng B, Ni
J, Zhou LX, He J and Ma XQ: Ninety-day administration of
dl-3-n-butylphthalide for acute ischemic stroke: A randomized,
double-blind trial. Chin Med J (Engl). 126:3405–3410.
2013.PubMed/NCBI
|
|
11
|
Gan-Or Z, Dion PA and Rouleau GA: Genetic
perspective on the role of theautophagy-lysosome pathway in
Parkinson disease. Autophagy. 11:1443–1457. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoban DB, Connaughton E, Connaughton C,
Hogan G, Thornton C, Mulcahy P, Moloney TC and Dowd E: Further
characterisation of the LPS model of Parkinson's disease: A
conparison of intra-nigral and intra-striatal lipopolysaccharide
administration on motor function, microgliosis andnigrostriatal
neurodegeneration in the rat. Brain Behav Immun. 27:91–100. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kelly LP, Carvey PM, Keshavarzian A,
Shannon KM, Shaikh M, Bakay RA and Kordower JH: Progression of
intestinal permeability changes and alpha-synuclein expression in a
mouse model of Parkinson's disease. Mov Disord. 29:999–1009. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bronstein DM, Perez-Otano I, Sun V, Mullis
Sawin SB, Chan J, Wu GC, Hudson PM, Kong LY, Hong JS and McMillian
MK: Glia-dependent neurotoxicity and neuroprotection in
mesencephalic cultures. Brain Res. 704:112–116. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xing B, Xin T, Hunter RL and Bing G:
Pioglitazone inhibition of lipopolysaccharide-induced nitric oxide
synthase is associated with altered activity of p38 MAP kinase and
PI3K/Akt. J Neuroinflammation. 5:42008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Feng X, Peng Y, Liu M and Cui L:
DL-3-n-butylphthalide extends survival by attenuating glial
activation in a mouse model of amyotrophic lateral sclerosis.
Neuropharmacology. 62:1004–1010. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu HL and Feng YP: Inhibitory effects of
chiral 3-n-butylphthalide on inflammation following focal ischemic
brain injury in rats. Acta Pharmacol Sin. 21:433–438.
2000.PubMed/NCBI
|
|
18
|
Hua K, Sheng X, Li TT, Wang LN, Zhang YH,
Huang ZJ and Ji H: The edaravone and 3-n-butylphthalide
ring-opening derivative 10b effectively attenuates cerebral
ischemia injury in rats. Acta Pharmacol Sin. 36:917–927. 2015.
View Article : Google Scholar : PubMed/NCBI
|